Exploring Short and Efficient Synthetic Routes Using Titanocene(III)-Catalyzed Reactions: Total Synthesis of Natural Meroterpenes with Trisubstituted Unsaturations
Abstract
:1. Introduction
2. Results and Discussion
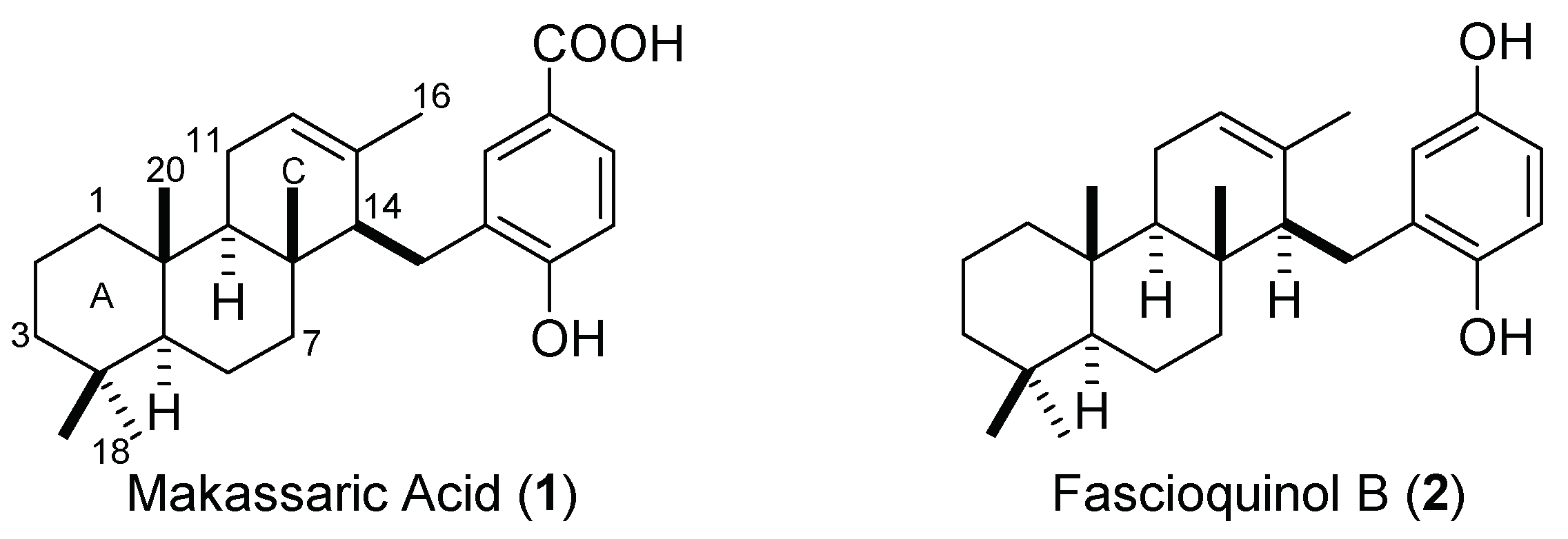
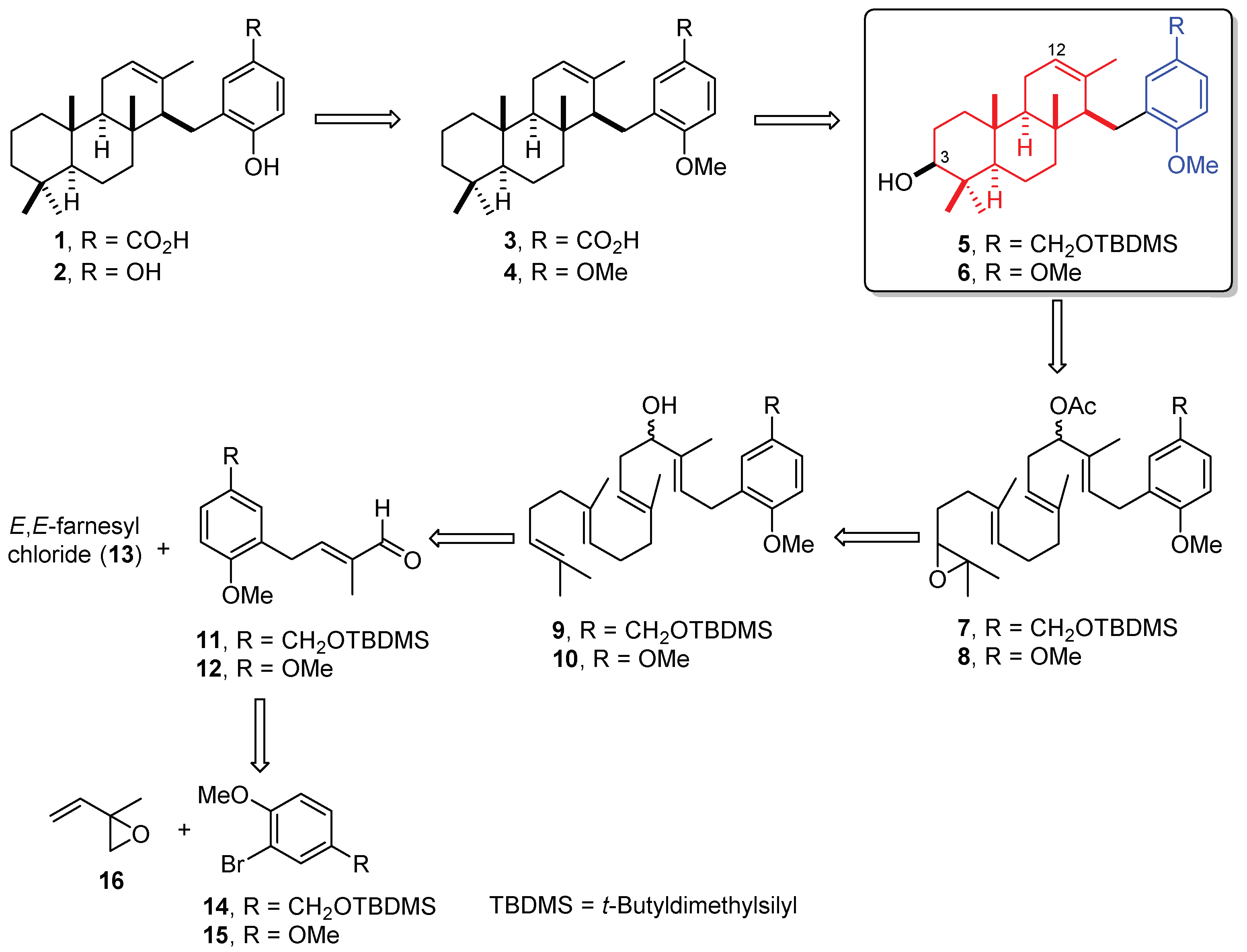
2.1. Retrosynthetic Analysis
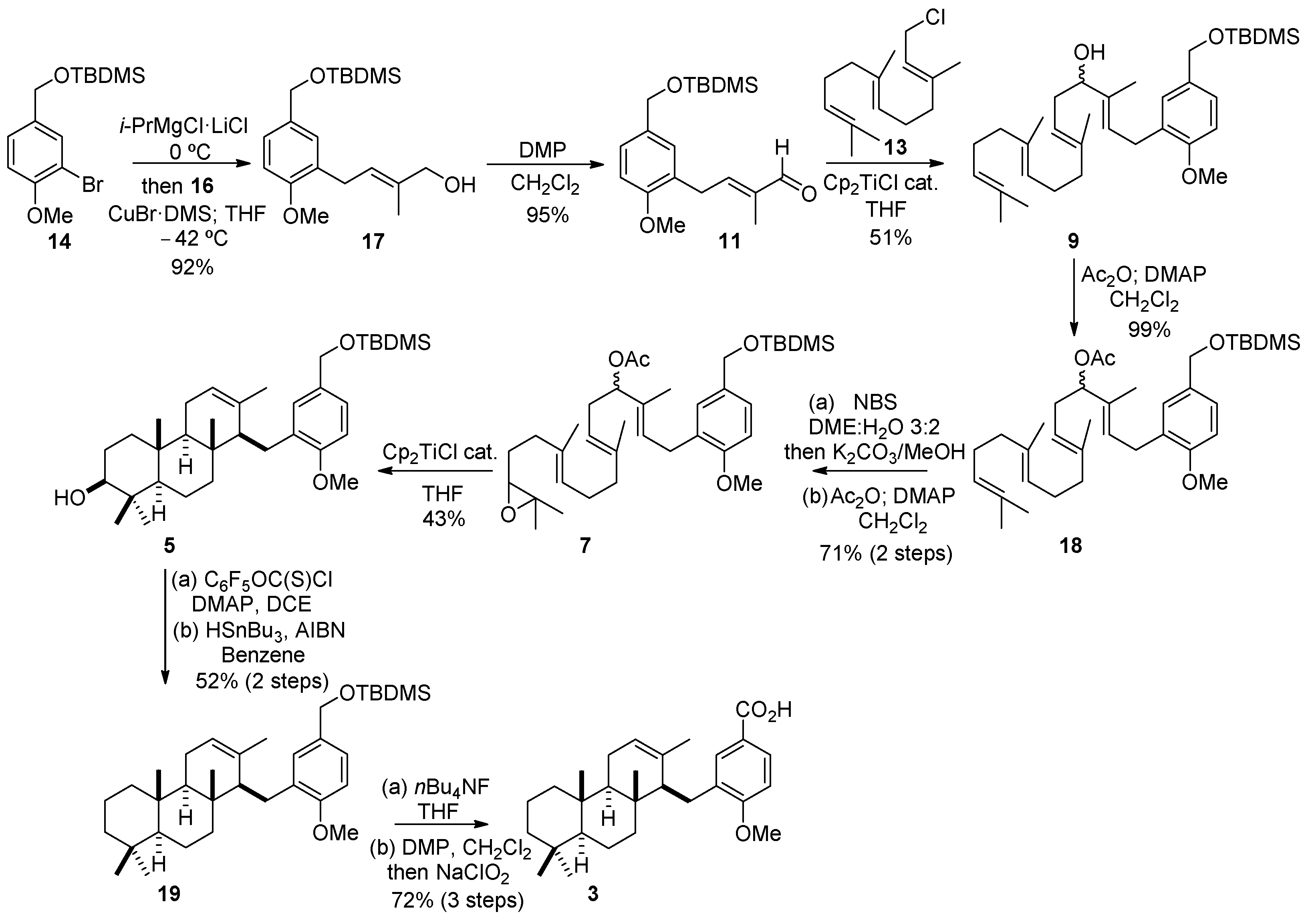
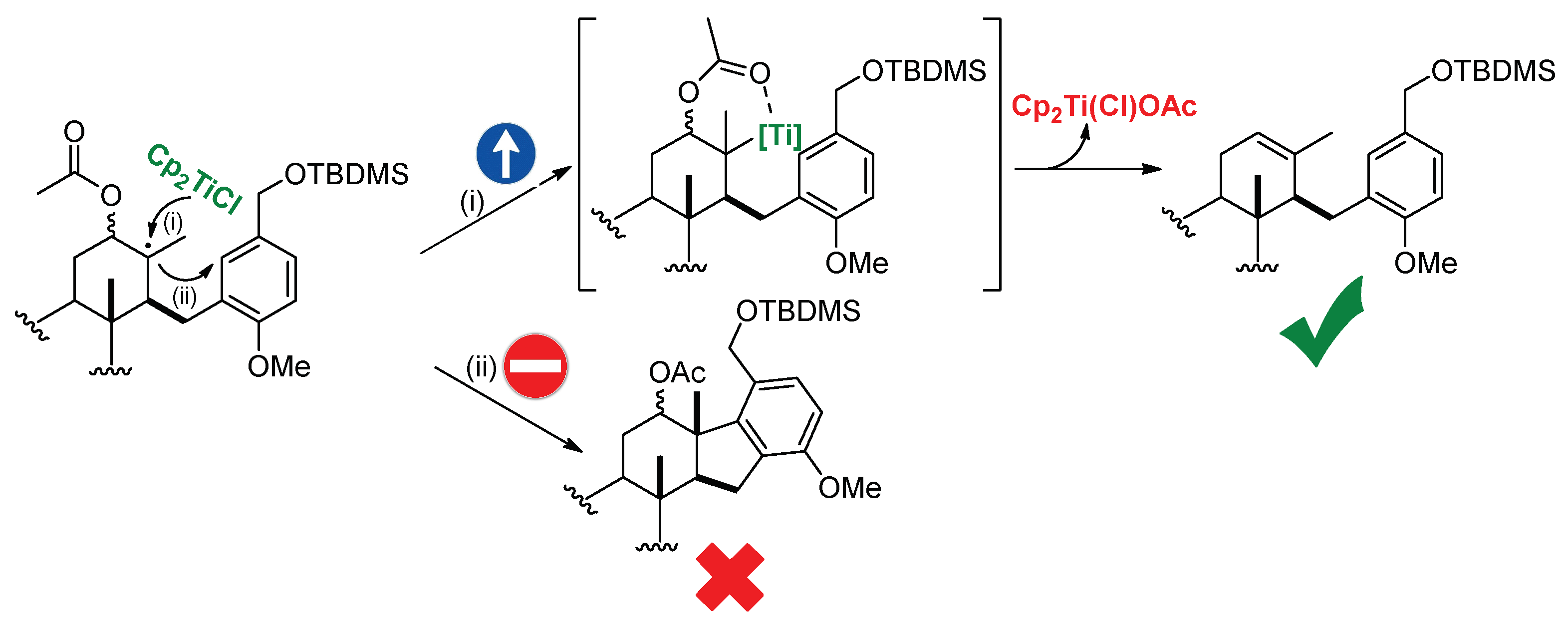
2.2. Synthesis of OMe Derivative of Makassaric Acid (3)
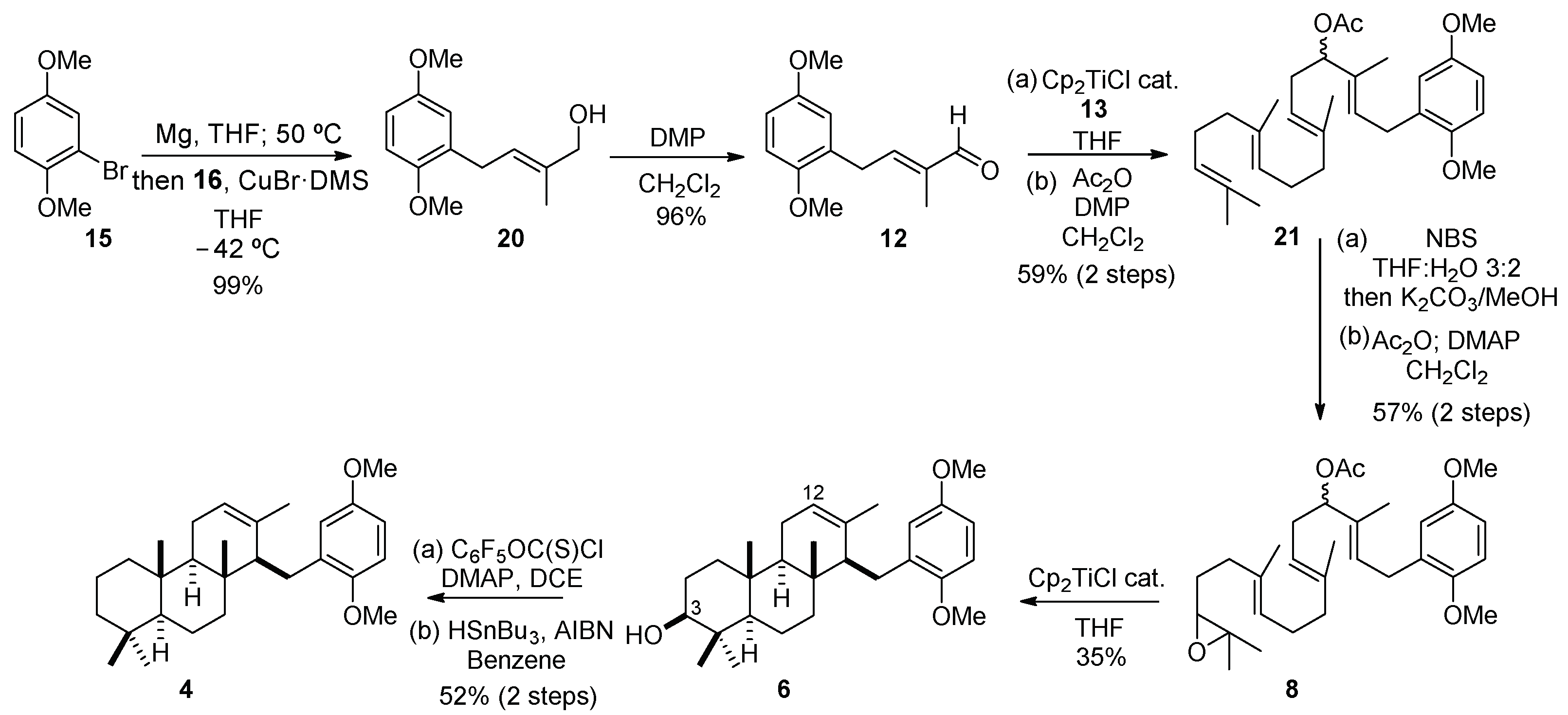
2.3. Synthesis of OMe Derivative of Fascioquinol B (4)
3. Experimental Section
3.1. General Details
3.2. Main General Procedures
3.2.1. General Procedure for Cu-Catalyzed Grignard Compound Additions (GP-1)
3.2.2. General Procedure for Barbier-Type Prenylation of Aldehydes Catalyzed by Cp2TiCl (GP-2)
3.2.3. General Procedure for Bioinspired Cp2TiCl-Catalyzed Cyclization Reactions (GP-3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Brahmachari, G. Chemistry and Pharmacology of Naturally Occurring Bioactive Compounds. In Chemistry and Pharmacology of Naturally Occurring Bioactive Compounds; Brahmachari, G., Ed.; CRC Press: Boca Ratón, FL, USA, 2013; pp. 1–8. [Google Scholar]
- Corey, E.J. The Logic of Chemical Synthesis: Multistep Synthesis of Complex Carbogenic Molecules (Nobel Lecture). Angew. Chem. Int. Ed. 1991, 30, 455–612. [Google Scholar] [CrossRef]
- Rosales Martínez, A.; Pozo Morales, L.; Díaz Ojeda, E.; Castro Rodríguez, M.; Rodríguez-García, I. The proven versatility of Cp2TiCl. J. Org. Chem. 2021, 86, 1311–1329. [Google Scholar] [CrossRef] [PubMed]
- Justicia, J.; Álvarez de Cienfuegos, L.; Campaña, A.G.; Miguel, D.; Jakoby, V.; Gansäuer, A.; Cuerva, J.M. Bioinspired terpene synthesis: A radical approach. Chem. Soc. Rev. 2011, 40, 3525–3537. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, S.P.; Miguel, D.; Campaña, A.G.; Álvarez de Cienfuegos, L.; Justicia, J.; Cuerva, J.M. Recent applications of Cp2TiCl in natural product synthesis. Org. Chem. Front. 2014, 1, 15–33. [Google Scholar] [CrossRef] [Green Version]
- Estévez, R.E.; Justicia, J.; Bazdi, B.; Fuentes, N.; Paradas, M.; Choquesillo-Lazarte, D.; García-Ruiz, J.M.; Robles, R.; Gansäuer, A.; Cuerva, J.M.; et al. Ti-catalyzed Barbier-type allylations and related reactions. Chem. Eur. J. 2009, 15, 2774–2791. [Google Scholar] [CrossRef]
- Paradas, M.; Campaña, A.G.; Estévez, R.E.; Álvarez de Cienfuegos, L.; Jiménez, T.; Robles, R.; Cuerva, J.M.; Oltra, J.E. Unexpected TiIII/Mn-promoted pinacol coupling of ketones. J. Org. Chem. 2009, 74, 3616–3619. [Google Scholar] [CrossRef]
- Frey, G.; Luu, H.-T.; Bichovski, P.; Feurer, M.; Streuff, J. Convenient titanium(III)-catalyzed synthesis of cyclic aminoketones and pyrrolidinones-development of a formal [4+1] cycloaddition. Angew. Chem. Int. Ed. 2013, 52, 7131–7134. [Google Scholar] [CrossRef]
- Yoder, R.A.; Johnston, J.N. A case study in biomimetic total synthesis: polyolefin carbocyclizations to terpenes and steroids. Chem. Rev. 2005, 105, 4730–4756. [Google Scholar] [CrossRef] [Green Version]
- Brunoldi, E.; Luparia, M.; Porta, A.; Zanoni, G.; Vidari, G. Biomimetic cyclizations of functionalized isoprenoid polyenes: A cornucopia of synthetic opportunities. Curr. Org. Chem. 2006, 10, 2259–2282. [Google Scholar] [CrossRef]
- Ungarean, C.N.; Southgate, E.H.; Sarlah, D. Enantioselective polyene cyclizations. Org. Biomol. Chem. 2016, 14, 5454–5467. [Google Scholar] [CrossRef]
- Jiménez, T.; Morcillo, S.P.; Martín-Lasanta, A.; Collado-Sanz, D.; Cárdenas, D.J.; Gansäuer, A.; Justicia, J.; Cuerva, J.M. Combining the power of TiIII-mediated processes for easy access to hydroxylated polycyclic terpenoids: Synthesis of sesterstatin 1 and C–D rings of aspergilloxide. Chem. Eur. J. 2012, 18, 12825–12833. [Google Scholar] [CrossRef] [PubMed]
- González-Delgado, J.A.; Arteaga, J.F.; Herrador, M.M.; Barrero, A.F. First total synthesis of (+)-apotrisporin E and (+)-apotrientriols A–B: A cyclization approach to apocarotenoids. Org. Biomol. Chem. 2013, 11, 5404–5408. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Telliez, J.-B.; Liu, J.; Tahir, A.; van Soest, R.; Andersen, R.J. Meroterpenoid MAPKAP (MK2) inhibitors isolated from the Indonesian marine sponge Acanthodendrilla sp. J. Nat. Prod. 2004, 67, 2127–2129. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, A.; Neininger, A.; Schubert, C.; Eckert, R.; Birchmeier, C.; Volk, H.-D.; Gaestel, M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat. Cell. Biol. 1999, 1, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Khalil, Z.G.; Capon, R.J. Fascioquinols A-F: Bioactive meroterpenes from a deep-water southern Australian marine sponge, Fasciospongia sp. Tetrahedron 2011, 67, 2591–2595. [Google Scholar] [CrossRef]
- Basabe, P.; Martín, M.; Bodero, O.; Blanco, A.; Marcos, I.S.; Urones, J.G. Synthesis of (+)-makassaric acid, a protein kinase MK2 inhibitor. Tetrahedron 2010, 66, 6008–6012. [Google Scholar] [CrossRef]
- Corey, E.J.; Das, J. Total synthesis of the complement inhibitor K-76 in racemic form. Structural assignment to “K-76 monocarboxylic acid”. J. Am. Chem. Soc. 1982, 104, 5551–5553. [Google Scholar] [CrossRef]
- González, A.G.; Martín, J.D.; Rodríguez, M.L. Stereospecific total synthesis of dl-taondiol methyl ether. Tetrahedron Lett. 1973, 14, 3657–3664. [Google Scholar] [CrossRef]
- Trost, B.M. The atom economy-a search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Newhouse, T.; Baran, P.S.; Hoffmann, R.W. The economies of synthesis. Chem. Soc. Rev. 2009, 38, 3010–3021. [Google Scholar] [CrossRef]
- Barton, D.H.R.; McCombie, S.W. A new method for the deoxygenation of secondary alcohols. J. Chem. Soc. Perkin Trans. 1975, 16, 1574–1585. [Google Scholar] [CrossRef]
- van Tamelen, E.E.; Storni, A.; Hessler, E.J.; Schwartz, M. The biogenetically patterned in vitro oxidation-cyclization of farnesyl acetate. J. Am. Chem. Soc. 1963, 85, 3295–3297. [Google Scholar] [CrossRef]
- Xiao, Z.; Cai, S.; Shi, Y.; Yang, B.; Gao, S. A photo-induced C–O bond formation methodology to construct tetrahydroxanthones. Chem. Commun. 2014, 50, 5254–5257. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; D’Amico, D.C. Stereospecific rearrangement of optically active tertiary allylic epoxides to give optically active quaternary aldehydes: Synthesis of α-alkyl amino aldehydes and acids. J. Am. Chem. Soc. 1995, 117, 7379–7388. [Google Scholar] [CrossRef]
- Ziegler, D.S.; Wei, B.; Knochel, P. Improving the halogen-magnesium exchange by using new Turbo-Grignard reagents. Chem. Eur. J. 2019, 25, 2695–2703. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. A useful 12-I-5 triacetoxyperiodinane (the Dess-Martin periodinane) for the selective oxidation of primary or secondary alcohols and a variety of related 12-I-5 species. J. Am. Chem. Soc. 1991, 113, 7277–7287. [Google Scholar] [CrossRef]
- Justicia, J.; Oller-López, J.L.; Campaña, A.G.; Oltra, J.E.; Cuerva, J.M.; Buñuel, E.; Cárdenas, D.J. 7-endo radical cyclizations catalyzed by titanocene(III). Straightforward synthesis of terpenoids with seven-membered carbocycles. J. Am. Chem. Soc. 2005, 127, 14911–14921. [Google Scholar]
- Ishiara, K.; Ishibashi, H.; Yamamoto, H. Enantioselective biomimetic cyclization of homo(polyprenyl)arenes. A new entry to (+)-podpcarpa-8,11,13-triene diterpenoids and (−)-tetracyclic polyprenoid of sedimentary origin. J. Am. Chem. Soc. 2001, 123, 1505–1506. [Google Scholar] [CrossRef]
- Rosales, V.; Zambrano, J.L.; Demuth, M. Regioselective palladium-catalyzed alkylation of allylic halides with benzylic Grignard reagents. Two-step synthesis of abietane terpenes and tetracyclic polyprenoid compounds. J. Org. Chem. 2002, 67, 1167–1170. [Google Scholar] [CrossRef]
- Justicia, J.; Oltra, J.E.; Cuerva, J.M. General approach to polycyclic meroterpenoids based on Stille couplings and titanocene catalysis. J. Org. Chem. 2004, 69, 5803–5806. [Google Scholar] [CrossRef]
- Castillo, A.; Quílez del Moral, J.F.; Barrero, A.F. Studies in cyclization of aromatic epoxy-acyclicpolyprenes: Lewis superacids and titanocene chloride. Nat. Prod. Commun. 2017, 12, 657–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mühlhaus, F.; Weissbarth, H.; Dahmen, T.; Schnakenburg, G.; Gansäuer, A. Merging regiodivergent catalysis with atom-economical radical arylation. Angew. Chem. Int. Ed. 2019, 58, 14208–14212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosales, V.; Zambrano, J.; Demuth, M. Straightforward synthesis of aromatic polycyclic terpenoids through biomimetic cascade cyclizations triggered by photochemical electron transfer. Eur. J. Org. Chem. 2004, 2004, 1798–1802. [Google Scholar] [CrossRef]
- Bal, B.S.; Childers, W.E.; Pinnick, H.W. Oxidation of α,β-unsaturated aldehydes. Tetrahedron 1981, 37, 2091–2096. [Google Scholar] [CrossRef]
- Gansäuer, A.; Justicia, J.; Rosales, A.; Worgull, D.; Rinker, B.; Cuerva, J.M.; Oltra, J.E. Transition-metal-catalyzed allylic substitution and titanocene-catalyzed epoxypolyene cyclization as a powerful tool for the preparation of terpenoids. Eur. J. Org. Chem. 2006, 2006, 4115–4127. [Google Scholar] [CrossRef]
- Manners, G.D.; Wong, R.Y. Acid-catalysed intramolecular cyclization of geranyl hydroquinone derivatives from Cordia alliodora. X-ray crystal and molecular structure of 1,2,3,3a,4,9,10,10a-octahydro-5,8-dimethoxy-3,10-dimethyl-3a,9-epoxybenz[f]azulene. J. Chem. Soc. Perkin Trans. 1981, 1, 1849–1854. [Google Scholar] [CrossRef]
- Masaki, Y.; Hashimoto, K.; Sakuma, K.; Kaji, K. Synthetic studies on isoprenoidquinones. I. A facile regio- and stereocontrolled synthesis of protected hydroquinones with an omega-hydroxyprenyl or hydroxygeranyl side chain. Chem. Pharm. Bull. 1984, 32, 3952–3958. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales, J.; Cabrera, G.; Justicia, J. Exploring Short and Efficient Synthetic Routes Using Titanocene(III)-Catalyzed Reactions: Total Synthesis of Natural Meroterpenes with Trisubstituted Unsaturations. Molecules 2022, 27, 2400. https://doi.org/10.3390/molecules27082400
Rosales J, Cabrera G, Justicia J. Exploring Short and Efficient Synthetic Routes Using Titanocene(III)-Catalyzed Reactions: Total Synthesis of Natural Meroterpenes with Trisubstituted Unsaturations. Molecules. 2022; 27(8):2400. https://doi.org/10.3390/molecules27082400
Chicago/Turabian StyleRosales, Jennifer, Gustavo Cabrera, and José Justicia. 2022. "Exploring Short and Efficient Synthetic Routes Using Titanocene(III)-Catalyzed Reactions: Total Synthesis of Natural Meroterpenes with Trisubstituted Unsaturations" Molecules 27, no. 8: 2400. https://doi.org/10.3390/molecules27082400
APA StyleRosales, J., Cabrera, G., & Justicia, J. (2022). Exploring Short and Efficient Synthetic Routes Using Titanocene(III)-Catalyzed Reactions: Total Synthesis of Natural Meroterpenes with Trisubstituted Unsaturations. Molecules, 27(8), 2400. https://doi.org/10.3390/molecules27082400






