The Complexity of Sesquiterpene Chemistry Dictates Its Pleiotropic Biologic Effects on Inflammation
Abstract
:1. Introduction
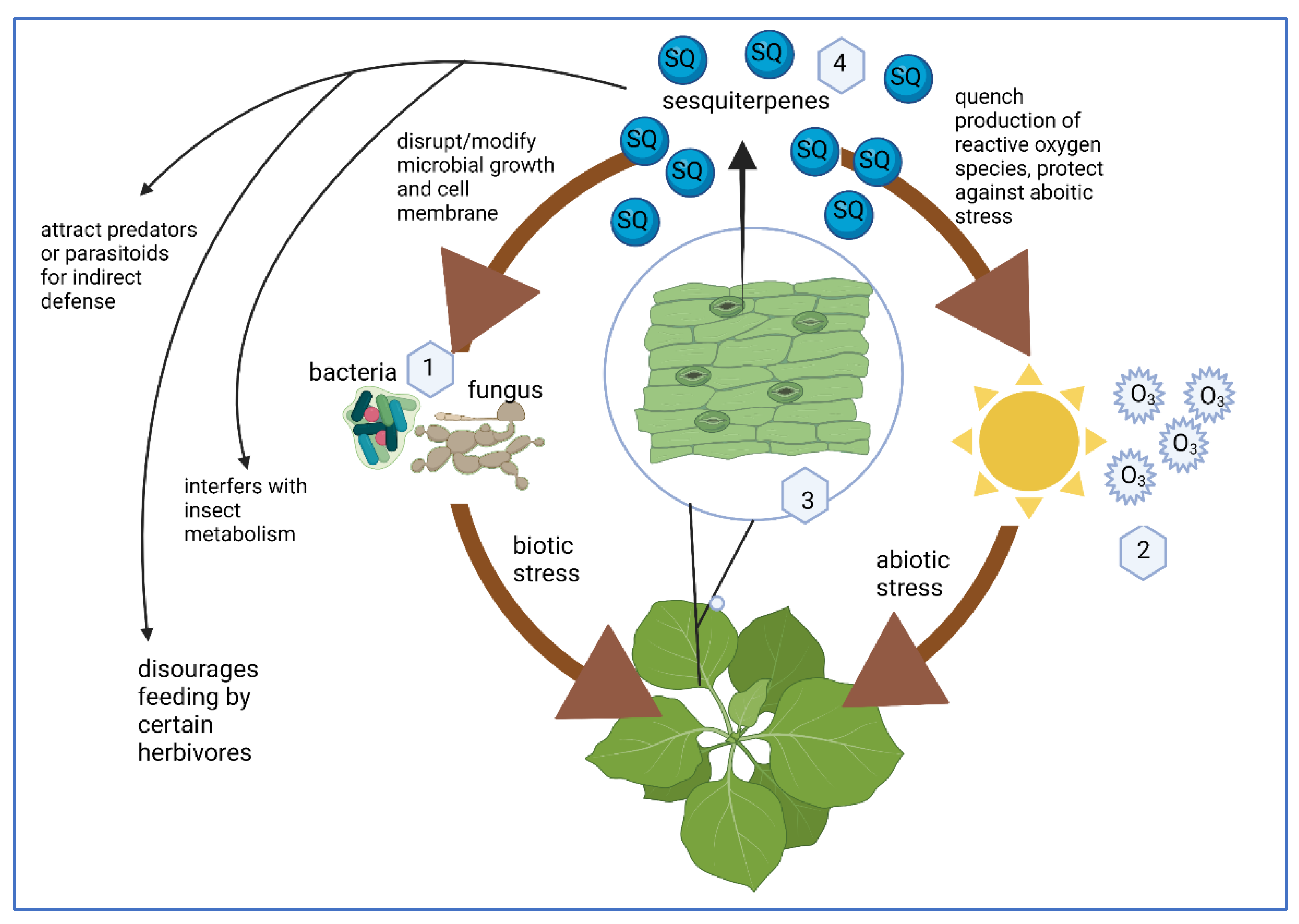
2. SQs Are Plant Metabolites That Target Several Molecular Signaling Pathways
SQs Are a Plant Defense Molecule Produced in Response to Stress
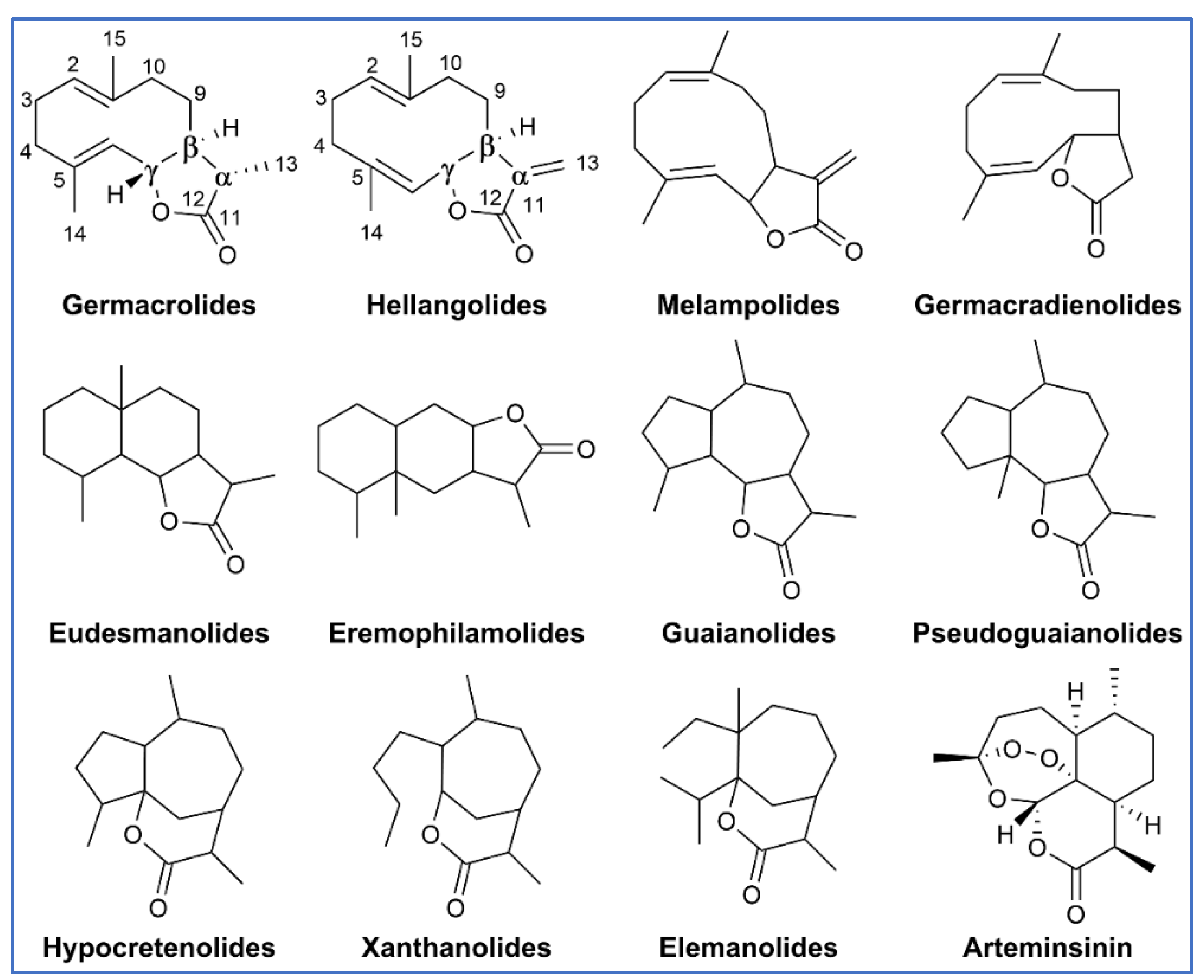
3. Chemical Structure and Biosynthesis of SQ and SQ Lactones
3.1. Structures of SQs
3.2. Structures of SQ Lactones
4. SQ as Biologically Active Molecules—Role in Inflammation
4.1. Isolation and Purification of SQ
4.2. Mechanisms of Action
4.2.1. NF-κB and NFAT Signaling in Inflammation and Its Modulation by SQs
4.2.2. Production of NO in Inflammation, Inflammatory Markers, and Its Modulation by SQ, and Subsequent Downstream Effects
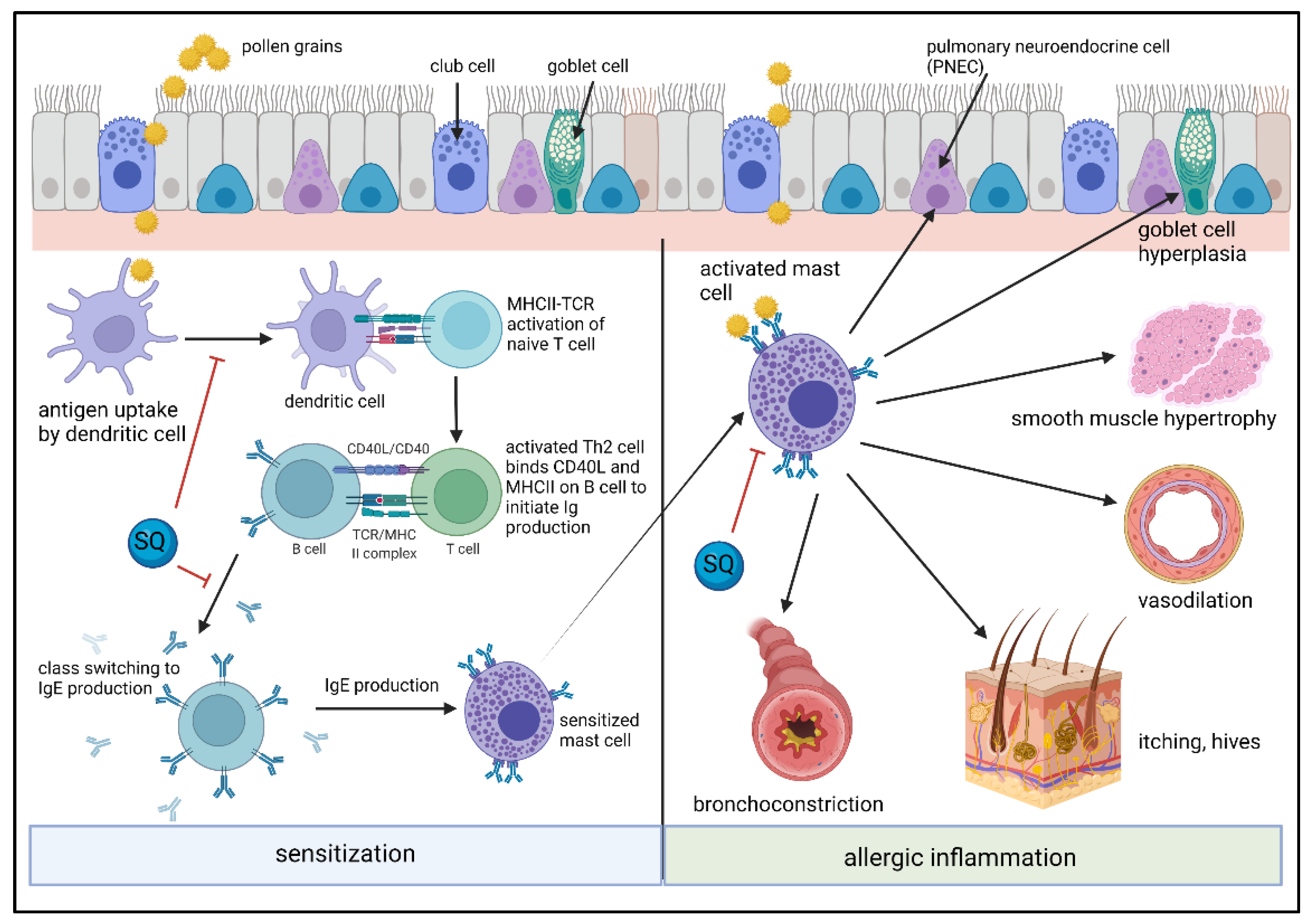
5. SQ Effects on Inflammatory Cells and Their Activation in Allergic Inflammation
5.1. SQ Effects on Dendritic Cells, Monocytes and Lymphocytes
5.2. SQ Effects on Mast Cells/Basophils and Allergic Inflammation
6. Other Important Targets of SQs That May Modulate Inflammation
6.1. Effects of SQs on Ion Channels That Regulate Inflammation
6.2. SQs as Potential Membrane Permeation Enhancers for Drug Delivery Systems
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 7HF | 7-hydroxy frullanolide |
| AKT | Protein kinase B |
| AML12 | Mouse hepatocytes |
| AP-1 | Activator protein 1 |
| BCP | β-caryophyllene |
| β-EA | β-elemonic acid |
| BMMC | Bone marrow derived mast cells |
| BV2 | Mouse microglial cells |
| C3aR, C5aR | Complement receptors |
| CCL | C-C motif ligand |
| CD | Cluster of differentiation |
| CIRI | Cerebral ischemia-reperfusion injury |
| COX-2 | Cyclooxygenase-2 |
| CXCL | C-X-C motif chemokine ligand |
| CXCR | C-X-C motif chemokine receptor |
| DEGs | Differentially expressed genes |
| DMAPP | Dimethyallyl diphosphate |
| eNOS or NOS3 | Endothelial nitric oxyde synthase |
| ERK | Extracellular signal regulated kinase |
| F-1 | 6β-angeloyloxy3β,8-dihydroxyeremophil-7(11)-en-12,8β-olide |
| Fc | Fragment crystallizable region |
| FK506 | Tacrolimus |
| FPP | Farnesyl diphosphate |
| GCSF | Granulocyte colony-stimulating factor |
| HDAC1 | Histone deacetylase 1 |
| HIF-α | Hypoxia inducible factor-1α |
| HNECs | Human nasal epithelial cells |
| HMC-1 | Human mast cell line |
| HMG-CoA | 3-hydroxy-3-methylglutaryl-CoA |
| HMGR | 3-hydroxy-3-methylglutaryl-CoA reductase |
| HMGS | 3-hydroxy-3-methylglutaryl-CoA synthase |
| HUVERCtert | Human umbilical vein endothelial cells |
| ICAM-1 | Intercellular cell adhesion molecule-1 |
| IFNγ | Interferon γ |
| IgE | Immunoglobulin E |
| IJ-5 | 1β-hydroxyalantolactone |
| IκB | Inhibitor of NF-κB |
| IKK | IκB kinase complex |
| IL | Interleukin |
| iNOS or NOS2 | Inducible nitric oxide synthase |
| IP3R | Inositol 1,4,5 thiophosphate receptor |
| IPP | Isopentyl phosphate |
| JNK | c-Jun NH2-terminal kinase |
| KEGG | Kyoto encyclopedia of genes and genomes |
| KIT | Tyrosine protein kinase |
| Kupffer | Mouse liver macrophages |
| L02 | Human hepatic cells |
| LAD2 | Laboratory of allergic diseases 2 |
| LPS | Lipopolysaccharide |
| LTC4 | Leukotriene C4 |
| LX-2 | Human hepatic stellate cells |
| Lyn | Tyrosine protein kinase |
| MAPK | Mitogen-activated protein kinase |
| MCL | Micheliolide |
| MCP-1 | Monocyte chemotactic protein 1 |
| MDD | Mevalonate diphosphate decarboxylate |
| MDM2 | Mouse double minute 2 homolog |
| MEKK | Mitogen activated protein kinase/ERK kinase kinase |
| MG6 | Mouse microglial cells |
| MIP-1α/γ | Macrophage inflammatory protein 1 α/γ |
| miRNA | Micro Ribonucleic acid |
| MMP | Matrix metalloproteinase |
| Muc-1 | Anti-adhesion mucin |
| MVA | Mevalonate |
| MVK | Mevalonate kinase |
| NFAT | Nuclear factor of activated T-cells |
| NF-κB | Nuclear factor of the κ chain in B-cells |
| NGF | Nerve growth factor |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| nNOS or NOS1 | Neuronal nitric oxide synthase |
| PAX1 | Paired box 1 |
| PCA | Pasive cutaneous anaphylaxis |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-kinase |
| PLA2 | Phospholipase A2 |
| PLC | Phospholipase C |
| PLGA | Poly lactic-co-glycolic acid |
| PMA | Phorbol myristate acetate |
| PMK | Phosphomevalonate kinase |
| PPARγ | Proliferator activator receptor γ |
| PVA | Polyvinyl alcohol |
| RAC | Ras-related C3 botulinum toxin substrate 1 |
| RAW 267.2 | Mouse monocyte/macrophage cells |
| RBL-2H3 | Rat basophilic leukemia cells |
| ROS | Reactive oxygen species |
| ROR-γt | Retineic acid receptor relatet orphan nuclear receptor γ |
| SCF | Stem cell factor |
| SERCA | Sarco/endoplasmic reticulum Ca2+-ATPase |
| SQ | Sesquiterpene |
| STAT | Signal transducer and activator of transcription |
| sTNFR1 | Soluble TNF receptor 1 |
| Syk | Spleen associated tyrosine kinase |
| TGFβ | Transforming growth factor β |
| THP-1 | Human monocyte cell line |
| TIMP | Tissue inhibitor of matrix metalloproteinases |
| TIMP1 | Tissue inhibitor of metalloprotease 1 |
| TNF | Tumor necrosis factor |
| TR | Transcription factor |
| TSLP | Thymic stromal lymphopoietin |
| TSLPR | Thymic stromal lymphopoietin receptor |
| VEGF | Vascular endothelial growth factor |
References
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.P.; Wu, G.Z.; Zhang, J.P.; Ye, J.; Liu, Q.X.; Shen, Y.H.; Li, H.L.; Zhang, W.D. Vlasouliolides A-D, four rare C17/C15 sesquiterpene lactone dimers with potential anti-inflammatory activity from Vladimiria souliei. Sci. Rep. 2017, 7, 43837. [Google Scholar] [CrossRef]
- Farmanpour-Kalalagh, K.; Beyraghdar Kashkooli, A.; Babaei, A.; Rezaei, A.; van der Krol, A.R. Artemisinins in Combating Viral Infections Like SARS-CoV-2, Inflammation and Cancers and Options to Meet Increased Global Demand. Front. Plant Sci. 2022, 13, 780257. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bian, L.; Duan, X.; Zhuang, X.; Sui, Y.; Yang, L. Alantolactone: A sesquiterpene lactone with diverse pharmacological effects. Chem. Biol. Drug Des. 2021, 98, 1131–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.F.; Li, W.; Xu, G.Y.; Wang, K.R.; Li, L.; Luo, H.; Zou, L.; Wu, J.S. Updates and advances on pharmacological properties of Taraxacum mongolicum Hand.-Mazz and its potential applications. Food Chem. 2022, 373, 131380. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.S.; Anastacio, J.D.; Nunes Dos Santos, C. Sesquiterpene Lactones: Promising Natural Compounds to Fight Inflammation. Pharmaceutics 2021, 13, 991. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.K.; Laya, M.S.; Poon, P.S. Sesquiterpenes Classified as Phytoalexins++Dedicated to Dr. Gonzalo Martin and Dr. Carlos Rivas Cols whose role as Head of the Chemistry Department (IVIC) was impressive and praiseworthy. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 33, pp. 193–237. [Google Scholar]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [Green Version]
- Jeandet, P.; Hébrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Grisebach, H.; Ebel, J. Phytoalexins, Chemical Defense Substances of Higher Plants? Angew. Chem. Int. Ed. Engl. 1978, 17, 635–647. [Google Scholar] [CrossRef]
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 2006, 9, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Majdi, M.; Ashengroph, M.; Abdollahi, M.R. Sesquiterpene lactone engineering in microbial and plant platforms: Parthenolide and artemisinin as case studies. Appl. Microbiol. Biotechnol. 2016, 100, 1041–1059. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Ma, L.; Wang, W.; Sun, H.; Wang, L.; Baldwin, I.T.; Wu, J. An ERF2-like transcription factor regulates production of the defense sesquiterpene capsidiol upon Alternaria alternata infection. J. Exp. Bot. 2019, 70, 5895–5908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lackus, N.D.; Morawetz, J.; Xu, H.; Gershenzon, J.; Dickschat, J.S.; Köllner, T.G. The Sesquiterpene Synthase PtTPS5 Produces (1S,5S,7R,10R)-Guaia-4(15)-en-11-ol and (1S,7R,10R)-Guaia-4-en-11-ol in Oomycete-Infected Poplar Roots. Molecules 2021, 26, 555. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, J.; Hu, Z.; Li, S.; Su, X.; Zhang, J.; Yin, Y.; Xu, T.; Zhang, Z.; Chen, H. Anti-TMV activity and functional mechanisms of two sesquiterpenoids isolated from Tithonia diversifolia. Pestic. Biochem. Physiol. 2017, 140, 24–29. [Google Scholar] [CrossRef]
- Shang, S.Z.; Zhao, W.; Tang, J.G.; Xu, X.M.; Sun, H.D.; Pu, J.X.; Liu, Z.H.; Miao, M.M.; Chen, Y.K.; Yang, G.Y. Antiviral sesquiterpenes from leaves of Nicotiana tabacum. Fitoterapia 2016, 108, 1–4. [Google Scholar] [CrossRef]
- Shen, Q.-P.; Xu, X.-M.; Li, L.; Zhao, W.; Xiang, N.-J.; Yang, G.-Y.; Chen, Y.-K.; Miao, M.-M.; Liu, C.-B.; Liu, Z.-H. Sesquiterpenes from the leaves of Nicotiana tabacum and their anti-tobacco mosaic virus activity. Chin. Chem. Lett. 2016, 27, 753–756. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Y.; Yang, L.; He, J.; Li, Y.; Xia, C. Brevilin A, a Sesquiterpene Lactone, Inhibits the Replication of Influenza A Virus In Vitro and In Vivo. Viruses 2019, 11, 835. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhou, B.; Lu, J.; Chen, Q.; Ti, H.; Huang, W.; Li, J.; Yang, Z.; Jiang, Z.; Wang, X. Inhibition of influenza virus via a sesquiterpene fraction isolated from Laggera pterodonta by targeting the NF-κB and p38 pathways. BMC Complement. Altern. Med. 2017, 17, 25. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Hayashi, T.; Ujita, K.; Takaishi, Y. Characterization of antiviral activity of a sesquiterpene, triptofordin C-2. J. Antimicrob. Chemother. 1996, 37, 759–768. [Google Scholar] [CrossRef] [Green Version]
- Kubo, I.; Ganjian, I. Insect antifeedant terpenes, hot-tasting to humans. Experientia 1981, 37, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Caputi, L.; Carlin, S.; Ghiglieno, I.; Stefanini, M.; Valenti, L.; Vrhovsek, U.; Mattivi, F. Relationship of changes in rotundone content during grape ripening and winemaking to manipulation of the ‘peppery’ character of wine. J. Agric. Food Chem. 2011, 59, 5565–5571. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, Y.; Matsuzawa, Y.; Mizutani, J. The Effect of Antifeedants against the Level of Biogenic Amines in the Central Nervous System of the Lepidopteran Insect (Spodoptera litura). Pestic. Biochem. Physiol. 1995, 52, 60–70. [Google Scholar] [CrossRef]
- Koul, O. Phytochemicals and Insect Control: An Antifeedant Approach. Crit. Rev. Plant Sci. 2008, 27, 1–24. [Google Scholar] [CrossRef]
- Holopainen, J.K. Multiple functions of inducible plant volatiles. Trends Plant Sci. 2004, 9, 529–533. [Google Scholar] [CrossRef]
- Cheng, A.X.; Xiang, C.Y.; Li, J.X.; Yang, C.Q.; Hu, W.L.; Wang, L.J.; Lou, Y.G.; Chen, X.Y. The rice (E)-beta-caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry 2007, 68, 1632–1641. [Google Scholar] [CrossRef]
- Dicke, M. Behavioural and community ecology of plants that cry for help. Plant Cell Environ. 2009, 32, 654–665. [Google Scholar] [CrossRef]
- Aljbory, Z.; Chen, M.S. Indirect plant defense against insect herbivores: A review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef]
- Ormeño, E.; Mévy, J.P.; Vila, B.; Bousquet-Mélou, A.; Greff, S.; Bonin, G.; Fernandez, C. Water deficit stress induces different monoterpene and sesquiterpene emission changes in Mediterranean species. Relationship between terpene emissions and plant water potential. Chemosphere 2007, 67, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pazouki, L.; Kanagendran, A.; Li, S.; Kännaste, A.; Memari, H.R.; Bichele, R.; Niinemets, Ü. Mono- and sesquiterpene release from tomato (Solanum lycopersicum) leaves upon mild and severe heat stress and through recovery: From gene expression to emission responses. Environ. Exp. Bot. 2016, 132, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanagendran, A.; Pazouki, L.; Li, S.; Liu, B.; Kännaste, A.; Niinemets, Ü. Ozone-triggered surface uptake and stress volatile emissions in Nicotiana tabacum ‘Wisconsin’. J. Exp. Bot. 2018, 69, 681–697. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Chan, Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [Green Version]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- Shu, Y.; Atkinson, R. Rate constants for the gas-phase reactions of O3 with a series of Terpenes and OH radical formation from the O3 reactions with Sesquiterpenes at 296 ± 2 K. Int. J. Chem. Kinet. 1994, 26, 1193–1205. [Google Scholar] [CrossRef]
- Manfredi, K.P. Terpenes. Flavors, Fragrances, Pharmaca, Pheromones By Eberhard Breitmaier (University of Bonn). Wiley-VCH, Weinheim. 2006. ix + 214 pp. 6.5 × 9.5 in. $65.00. ISBN 3-527-31786-4. J. Nat. Prod. 2007, 70, 711. [Google Scholar] [CrossRef]
- Le Bideau, F.; Kousara, M.; Chen, L.; Wei, L.; Dumas, F. Tricyclic Sesquiterpenes from Marine Origin. Chem. Rev. 2017, 117, 6110–6159. [Google Scholar] [CrossRef]
- Chappell, J.; Coates, R.M. 1.16—Sesquiterpenes. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 609–641. [Google Scholar]
- Hohmann, M.S.N.; Longhi-Balbinot, D.T.; Guazelli, C.F.S.; Navarro, S.A.; Zarpelon, A.C.; Casagrande, R.; Arakawa, N.S.; Verri, W.A. Chapter 7—Sesquiterpene Lactones: Structural Diversity and Perspectives as Anti-Inflammatory Molecules. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 243–264. [Google Scholar]
- Zwenger, S.; Basu, C. Plant terpenoids: Applications and future potentials. Biotechnol. Mol. Biol. Rev. 2008, 3, 1–7. [Google Scholar]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng./Biotechnol. 2015, 148, 63–106. [Google Scholar]
- Frey, M.; Schmauder, K.; Pateraki, I.; Spring, O. Biosynthesis of Eupatolide-A Metabolic Route for Sesquiterpene Lactone Formation Involving the P450 Enzyme CYP71DD6. ACS Chem. Biol. 2018, 13, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Vranova, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Reda, E.H.; Shakour, Z.T.A.; El-Halawany, A.M.; El-Kashoury, E.A.; Shams, K.A.; Mohamed, T.A.; Saleh, I.; Elshamy, A.I.; Atia, M.A.M.; El-Beih, A.A.; et al. Comparative Study on the Essential Oils from Five Wild Egyptian Centaurea Species: Effective Extraction Techniques, Antimicrobial Activity and In-Silico Analyses. Antibiotics 2021, 10, 252. [Google Scholar] [CrossRef]
- Blosa, M.; Uricher, J.; Nebel, S.; Zahner, C.; Butterweck, V.; Drewe, J. Treatment of Early Allergic and Late Inflammatory Symptoms of Allergic Rhinitis with Petasites hybridus Leaf Extract (Ze 339): Results of a Noninterventional Observational Study in Switzerland. Pharmaceuticals 2021, 14, 180. [Google Scholar] [CrossRef]
- Pacheco, L.A.; Ethur, E.M.; Sheibel, T.; Buhl, B.; Weber, A.C.; Kauffmann, C.; Marchi, M.I.; Freitas, E.M.; Hoehne, L. Chemical characterization and antimicrobial activity of Campomanesia aurea against three strains of Listeria monocytogenes. Braz. J. Biol. 2021, 81, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Zeggwagh, N.A.; Ouahidi, M.L.; Lemhadri, A.; Eddouks, M. Study of hypoglycaemic and hypolipidemic effects of Inula viscosa L. aqueous extract in normal and diabetic rats. J. Ethnopharmacol. 2006, 108, 223–227. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, Y.; Deng, L.; Chen, S.; Wang, X.; Zuo, T.; Hu, Q.; Rao, J.; Wang, Q.; et al. Differentially expressed genes induced by beta-caryophyllene in a rat model of cerebral ischemia-reperfusion injury. Life Sci. 2021, 273, 119293. [Google Scholar] [CrossRef]
- Alhusayan, R.M.; Aldahmash, B.A.; El-Nagar, D.M.; Rady, A.; Ibrahim, K.E.; Alkahtani, S. Butterbur (Petasites hybridus) Extract Ameliorates Hepatic Damage Induced by Ovalbumin in Mice. Oxid. Med. Cell. Longev. 2020, 2020, 3178214. [Google Scholar] [CrossRef]
- Park, Y.J.; Jeon, M.S.; Lee, S.; Kim, J.K.; Jang, T.S.; Chung, K.H.; Kim, K.H. Anti-fibrotic effects of brevilin A in hepatic fibrosis via inhibiting the STAT3 signaling pathway. Bioorg. Med. Chem. Lett. 2021, 41, 127989. [Google Scholar] [CrossRef]
- Pathak, S.; Gokhroo, A.; Kumar Dubey, A.; Majumdar, S.; Gupta, S.; Almeida, A.; Mahajan, G.B.; Kate, A.; Mishra, P.; Sharma, R.; et al. 7-Hydroxy Frullanolide, a sesquiterpene lactone, increases intracellular calcium amounts, lowers CD4(+) T cell and macrophage responses, and ameliorates DSS-induced colitis. Int. Immunopharmacol. 2021, 97, 107655. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Xu, C.; Alejos-Gonzalez, F.; Wang, H.; Yang, J.; Judd, R.; Xie, D.Y. Overexpression of Artemisia annua Cinnamyl Alcohol Dehydrogenase Increases Lignin and Coumarin and Reduces Artemisinin and Other Sesquiterpenes. Front. Plant Sci. 2018, 9, 828. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Jeong, M.; Park, S.; Ryu, S.M.; Lee, J.; Song, Z.; Guo, Y.; Choi, J.H.; Lee, D.; Jang, D.S. Chemical Constituents of the Leaves of Butterbur (Petasites japonicus) and Their Anti-Inflammatory Effects. Biomolecules 2019, 9, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Wang, F.; Guo, J.; Xu, C.; Cao, Y.; Fang, Z.; Wang, Q. Pharmacological Mechanisms Underlying the Neuroprotective Effects of Alpinia oxyphylla Miq. on Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2071. [Google Scholar] [CrossRef] [Green Version]
- Thapa, P.; Lee, Y.J.; Nguyen, T.T.; Piao, D.; Lee, H.; Han, S.; Lee, Y.J.; Han, A.R.; Choi, H.; Jeong, J.H.; et al. Eudesmane and Eremophilane Sesquiterpenes from the Fruits of Alpinia oxyphylla with Protective Effects against Oxidative Stress in Adipose-Derived Mesenchymal Stem Cells. Molecules 2021, 26, 1762. [Google Scholar] [CrossRef]
- McKinnon, R.; Binder, M.; Zupko, I.; Afonyushkin, T.; Lajter, I.; Vasas, A.; de Martin, R.; Unger, C.; Dolznig, H.; Diaz, R.; et al. Pharmacological insight into the anti-inflammatory activity of sesquiterpene lactones from Neurolaena lobata (L.) R.Br. ex Cass. Phytomedicine 2014, 21, 1695–1701. [Google Scholar] [CrossRef]
- Chen, J.J.; Bai, W.; Gobu, F.R.; Wu, C.H.; Zeng, J.; Sun, M.; Gao, K. Sesquiterpenoids from the roots of Vladimiria muliensis. J. Asian Nat. Prod. Res. 2015, 17, 1188–1195. [Google Scholar] [CrossRef]
- Wu, Z.L.; Wang, J.X.; Chen, L.P.; Dong, H.Y.; Li, H.L.; Zhang, W.D. Five rare C32 sesquiterpene lactone dimers with anti-inflammation activity from Vladimiria souliei. Fitoterapia 2018, 125, 117–122. [Google Scholar] [CrossRef]
- Wu, Z.L.; Wang, Q.; Wang, J.X.; Dong, H.Y.; Xu, X.K.; Shen, Y.H.; Li, H.L.; Zhang, W.D. Vlasoulamine A, a Neuroprotective [3.2.2]Cyclazine Sesquiterpene Lactone Dimer from the Roots of Vladimiria souliei. Org. Lett. 2018, 20, 7567–7570. [Google Scholar] [CrossRef]
- Wu, R.F.; Zhou, B.D.; Wang, W.Q.; Chen, T.; Xu, T.T.; Zhu, S.L.; Xuan, L.J. Neolinulicin A and B from Inula japonica and their anti-inflammatory activities. Fitoterapia 2021, 152, 104905. [Google Scholar] [CrossRef]
- Abbas, M.A.; Taha, M.O.; Zihlif, M.A.; Disi, A.M. beta-Caryophyllene causes regression of endometrial implants in a rat model of endometriosis without affecting fertility. Eur. J. Pharmacol. 2013, 702, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Matsuda, H.; Sakamoto, Y.; Ueda, K.; Yoshikawa, M. New farnesane-type sesquiterpenes, hedychiols A and B 8,9-diacetate, and inhibitors of degranulation in RBL-2H3 cells from the rhizome of Hedychium coronarium. Chem. Pharm. Bull. 2002, 50, 1045–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Wang, L.; Wang, J.H.; Liu, L.L.; Zhao, T.J. Effect of Curcumol on NOD-Like Receptor Thermoprotein Domain 3 Inflammasomes in Liver Fibrosis of Mice. Chin. J. Integr. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.W. Nitric oxide: A regulator of mast cell activation and mast cell-mediated inflammation. Clin. Exp. Immunol. 2002, 129, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Gong, W.; Chen, J.; Qing, Y.; Wu, S.; Li, H.; Huang, C.; Chen, Y.; Wang, Y.; Xu, Z.; et al. Micheliolide alleviates hepatic steatosis in db/db mice by inhibiting inflammation and promoting autophagy via PPAR-gamma-mediated NF-small ka, CyrillicB and AMPK/mTOR signaling. Int. Immunopharmacol. 2018, 59, 197–208. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [Green Version]
- Li, S.Y.; Zhou, Y.L.; He, D.H.; Liu, W.; Fan, X.Z.; Wang, Q.; Pan, H.F.; Cheng, Y.X.; Liu, Y.Q. Centipeda minima extract exerts antineuroinflammatory effects via the inhibition of NF-kappaB signaling pathway. Phytomed. Int. J. Phytother. Phytopharm. 2020, 67, 153164. [Google Scholar] [CrossRef]
- Xue, P.-H.; Zhang, N.; Liu, D.; Zhang, Q.-R.; Duan, J.-S.; Yu, Y.-Q.; Li, J.-Y.; Cao, S.-J.; Zhao, F.; Kang, N.; et al. Cytotoxic and Anti-Inflammatory Sesquiterpenes from the Whole Plants of Centipeda minima. J. Nat. Prod. 2021, 84, 247–258. [Google Scholar] [CrossRef]
- Pan, L.; Hu, L.; Zhang, L.; Xu, H.; Chen, Y.; Bian, Q.; Zhu, A.; Wu, H. Deoxyelephantopin decreases the release of inflammatory cytokines in macrophage associated with attenuation of aerobic glycolysis via modulation of PKM2. Int. Immunopharmacol. 2020, 79, 106048. [Google Scholar] [CrossRef]
- Steiert, S.A.; Zissler, U.M.; Chaker, A.M.; Esser-von-Bieren, J.; Dittlein, D.; Guerth, F.; Jakwerth, C.A.; Piontek, G.; Zahner, C.; Drewe, J.; et al. Anti-inflammatory effects of the petasin phyto drug Ze339 are mediated by inhibition of the STAT pathway. BioFactors 2017, 43, 388–399. [Google Scholar] [CrossRef]
- Fonseca, L.C.; Dadarkar, S.S.; Lobo, A.S.; Mishra, P.B.; Thakkar, A.D.; Chandrababu, S.; Padigaru, M. NF-kappaB-mediated anti-inflammatory activity of the sesquiterpene lactone 7-hydroxyfrullanolide. Eur. J. Pharmacol. 2011, 657, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Butturini, E.; Cavalieri, E.; de Prati, A.C.; Darra, E.; Rigo, A.; Shoji, K.; Murayama, N.; Yamazaki, H.; Watanabe, Y.; Suzuki, H.; et al. Two naturally occurring terpenes, dehydrocostuslactone and costunolide, decrease intracellular GSH content and inhibit STAT3 activation. PLoS ONE 2011, 6, e20174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butturini, E.; Di Paola, R.; Suzuki, H.; Paterniti, I.; Ahmad, A.; Mariotto, S.; Cuzzocrea, S. Costunolide and Dehydrocostuslactone, two natural sesquiterpene lactones, ameliorate the inflammatory process associated to experimental pleurisy in mice. Eur. J. Pharmacol. 2014, 730, 107–115. [Google Scholar] [CrossRef]
- Scarponi, C.; Butturini, E.; Sestito, R.; Madonna, S.; Cavani, A.; Mariotto, S.; Albanesi, C. Inhibition of inflammatory and proliferative responses of human keratinocytes exposed to the sesquiterpene lactones dehydrocostuslactone and costunolide. PLoS ONE 2014, 9, e107904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Hua, Y.; Wang, D.; Shan, L.; Zhang, Y.; Zhu, J.; Jin, H.; Li, H.; Hu, Z.; Zhang, W. A sesquiterpene lactone from a medicinal herb inhibits proinflammatory activity of TNF-alpha by inhibiting ubiquitin-conjugating enzyme UbcH5. Chem. Biol. 2014, 21, 1341–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, G.; Gao, S.; Cheng, J.; Li, Y.; Shan, L.; Hu, Z. 1beta-Hydroxyalantolactone, a sesquiterpene lactone from Inula japonica, attenuates atopic dermatitis-like skin lesions induced by 2,4-dinitrochlorobenzene in the mouse. Pharm. Biol. 2016, 54, 516–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arizmendi, N.; Hou, C.; Guo, F.; Li, Y.; Kulka, M. Bicyclic eremophilane-type petasite sesquiterpenes potentiate peroxisome proliferator-activated receptor gamma activator-mediated inhibition of dendritic cells. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418787739. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Pathare, R.S.; Gorve, D.A. Advances in Total Synthesis of Some 2,3,5-Trisubstituted Tetrahydrofuran Natural Products. Chem. Asian J. 2020, 15, 2815–2837. [Google Scholar] [CrossRef]
- Koft, E.R.; Smith, A.B. Total synthesis of hibiscone C (gmelofuran). J. Am. Chem. Soc. 1982, 104, 5568–5570. [Google Scholar] [CrossRef]
- Curran, D.P.; Chen, M.-H. Radical-initiated polyolefinic cyclizations in condensed cyclopentanoid synthesis. Total synthesis of (±)-Δ9(12)-capnellene. Tetrahedron Lett. 1985, 26, 4991–4994. [Google Scholar] [CrossRef]
- Hotta, T.; Haynes, S.E.; Blasius, T.L.; Gebbie, M.; Eberhardt, E.L.; Sept, D.; Cianfrocco, M.; Verhey, K.J.; Nesvizhskii, A.I.; Ohi, R. Parthenolide Destabilizes Microtubules by Covalently Modifying Tubulin. Curr. Biol. CB 2021, 31, 900–907.e6. [Google Scholar] [CrossRef] [PubMed]
- Ajeet, S.; Nandkishore, K. The boom in unnecessary caesarean surgeries is jeopardizing women’s health. Health Care Women Int. 2013, 34, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, S.B.; Chun, J.; Song, K.H.; Kim, Y.S.; Chung, S.J.; Cho, H.J.; Yoon, I.S.; Kim, D.D. High body clearance and low oral bioavailability of alantolactone, isolated from Inula helenium, in rats: Extensive hepatic metabolism and low stability in gastrointestinal fluids. Biopharm. Drug Dispos. 2016, 37, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.J.; Beltran, D.; Tomas-Barberan, F.A. Human Gut Microbiota Metabolism of Dietary Sesquiterpene Lactones: Untargeted Metabolomics Study of Lactucopicrin and Lactucin Conversion In Vitro and In Vivo. Mol. Nutr. Food Res. 2020, 64, e2000619. [Google Scholar] [CrossRef]
- Weng, H.; He, L.; Zheng, J.; Li, Q.; Liu, X.; Wang, D. Low Oral Bioavailability and Partial Gut Microbiotic and Phase II Metabolism of Brussels/Witloof Chicory Sesquiterpene Lactones in Healthy Humans. Nutrients 2020, 12, 3675. [Google Scholar] [CrossRef]
- Karin, M. Nuclear factor-kappaB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basilio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-kappaB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zeng, H.; Zhang, L.; Pu, Y.; Li, S.; Yuan, Y.; Zhang, T.; Wang, B. Patchouli Alcohol: A Natural Sesquiterpene Against Both Inflammation and Intestinal Barrier Damage of Ulcerative Colitis. Inflammation 2020, 43, 1423–1435. [Google Scholar] [CrossRef]
- Chen, H.D.; Yang, S.P.; Liao, S.G.; Zhang, B.; Wu, Y.; Yue, J.M. Limonoids and sesquiterpenoids from Amoora tsangii. J. Nat. Prod. 2008, 71, 93–97. [Google Scholar] [CrossRef]
- Gopal, Y.N.; Arora, T.S.; Van Dyke, M.W. Parthenolide specifically depletes histone deacetylase 1 protein and induces cell death through ataxia telangiectasia mutated. Chem. Biol. 2007, 14, 813–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopal, Y.N.; Chanchorn, E.; Van Dyke, M.W. Parthenolide promotes the ubiquitination of MDM2 and activates p53 cellular functions. Mol. Cancer Ther. 2009, 8, 552–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.Y.; Liu, P.; Chai, Y.W.; Wang, Y.; Ren, S.H.; Li, Y.Y.; Zhou, H. Artesunate attenuates 2, 4-dinitrochlorobenzene-induced atopic dermatitis by down-regulating Th17 cell responses in BALB/c mice. Eur. J. Pharmacol. 2020, 874, 173020. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Jin, S.E.; Kim, O.S.; Shin, H.K.; Jeong, S.J. Alantolactone from Saussurea lappa Exerts Antiinflammatory Effects by Inhibiting Chemokine Production and STAT1 Phosphorylation in TNF-alpha and IFN-gamma-induced in HaCaT cells. Phytother. Res. PTR 2015, 29, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Choi, R.J.; Khan, S.; Lee, D.S.; Kim, Y.C.; Nam, Y.J.; Lee, D.U.; Kim, Y.S. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-kappaB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int. Immunopharmacol. 2012, 14, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Park, S.; Zhang, H.; Park, S.; Kwon, W.; Kim, E.; Zhang, X.; Jang, S.; Yoon, D.; Choi, S.K.; et al. Targeting AKT with costunolide suppresses the growth of colorectal cancer cells and induces apoptosis in vitro and in vivo. J. Exp. Clin. Cancer Res. 2021, 40, 114. [Google Scholar] [CrossRef]
- Viennois, E.; Xiao, B.; Ayyadurai, S.; Wang, L.; Wang, P.G.; Zhang, Q.; Chen, Y.; Merlin, D. Micheliolide, a new sesquiterpene lactone that inhibits intestinal inflammation and colitis-associated cancer. Lab. Investig. 2014, 94, 950–965. [Google Scholar] [CrossRef] [Green Version]
- Matos, M.S.; Anastacio, J.D.; Allwood, J.W.; Carregosa, D.; Marques, D.; Sungurtas, J.; McDougall, G.J.; Menezes, R.; Matias, A.A.; Stewart, D.; et al. Assessing the Intestinal Permeability and Anti-Inflammatory Potential of Sesquiterpene Lactones from Chicory. Nutrients 2020, 12, 3547. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Q.; Gao, W.; Sehgal, S.A.; Wu, H. The multifaceted regulation of mitophagy by endogenous metabolites. Autophagy 2021, 1–24. [Google Scholar] [CrossRef]
- Chung, A.W.; Anand, K.; Anselme, A.C.; Chan, A.A.; Gupta, N.; Venta, L.A.; Schwartz, M.R.; Qian, W.; Xu, Y.; Zhang, L.; et al. A phase 1/2 clinical trial of the nitric oxide synthase inhibitor L-NMMA and taxane for treating chemoresistant triple-negative breast cancer. Sci. Transl. Med. 2021, 13, eabj5070. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Costa, P.P.C.; Campos, R.; Cabral, P.H.B.; Gomes, V.M.; Santos, C.F.; Waller, S.B.; de Sousa, E.H.S.; Lopes, L.G.F.; Fonteles, M.C.; do Nascimento, N.R.F. Antihypertensive potential of cis-[Ru(bpy)2(ImN)(NO)](3+), a ruthenium-based nitric oxide donor. Res. Vet. Sci. 2020, 130, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ozenver, N.; Efferth, T. Small molecule inhibitors and stimulators of inducible nitric oxide synthase in cancer cells from natural origin (phytochemicals, marine compounds, antibiotics). Biochem. Pharmacol. 2020, 176, 113792. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, Z.; Zhou, Z.; Wang, L.; Yue, F.; Wang, J.; Wang, H.; Song, L. Transcriptional activation and translocation of ancient NOS during immune response. FASEB J. 2016, 30, 3527–3540. [Google Scholar] [CrossRef] [Green Version]
- Somasundaram, V.; Gilmore, A.C.; Basudhar, D.; Palmieri, E.M.; Scheiblin, D.A.; Heinz, W.F.; Cheng, R.Y.S.; Ridnour, L.A.; Altan-Bonnet, G.; Lockett, S.J.; et al. Inducible nitric oxide synthase-derived extracellular nitric oxide flux regulates proinflammatory responses at the single cell level. Redox Biol. 2020, 28, 101354. [Google Scholar] [CrossRef]
- Dhillon, S.S.; Mastropaolo, L.A.; Murchie, R.; Griffiths, C.; Thoni, C.; Elkadri, A.; Xu, W.; Mack, A.; Walters, T.; Guo, C.; et al. Higher activity of the inducible nitric oxide synthase contributes to very early onset inflammatory bowel disease. Clin. Transl. Gastroenterol. 2014, 5, e46. [Google Scholar] [CrossRef]
- Basudhar, D.; Bharadwaj, G.; Somasundaram, V.; Cheng, R.Y.S.; Ridnour, L.A.; Fujita, M.; Lockett, S.J.; Anderson, S.K.; McVicar, D.W.; Wink, D.A. Understanding the tumour micro-environment communication network from an NOS2/COX2 perspective. Br. J. Pharmacol. 2019, 176, 155–176. [Google Scholar] [CrossRef]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 2011, 25, 243–254. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.L.; Tian, H.; Bai, R.Y.; Bi, Y.N.; Yuan, X.M.; Sun, L.K.; Deng, Y.R.; Zhou, K. Evaluation of Anti-Inflammatory Activities of a Triterpene beta-Elemonic Acid in Frankincense In Vivo and In Vitro. Molecules 2019, 24, 1187. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ren, K.; Wu, S.; Guo, J.; Ren, S.; Pan, Y.; Wang, D.; Morikawa, T.; Hua, H.; Liu, X. Cytotoxicity evaluation and metabolism of hepatotoxicity components of Euodiae Fructus in L02 cells. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1186, 123040. [Google Scholar] [CrossRef]
- Wang, X.; Tang, S.A.; Wang, R.; Qiu, Y.; Jin, M.; Kong, D. Inhibitory Effects of JEUD-38, a New Sesquiterpene Lactone from Inula japonica Thunb, on LPS-Induced iNOS Expression in RAW264.7 Cells. Inflammation 2015, 38, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Tursunova, N.V.; Syrov, V.N.; Khushbaktova, Z.A.; Tornuev, Y.V.; Klinnikova, M.G. Monooxygenase System and NO Metabolism in Liver Microsomes of Rats with Toxic Hepatitis and the Effect of Sesquiterpene Lactones. Bull. Exp. Biol. Med. 2021, 172, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Huang, X.; Ni, B.; Zhang, R.; Niu, G.; You, H. Selective STAT3 Inhibitor Alantolactone Ameliorates Osteoarthritis via Regulating Chondrocyte Autophagy and Cartilage Homeostasis. Front. Pharmacol. 2021, 12, 730312. [Google Scholar] [CrossRef] [PubMed]
- Novianti, E.; Katsuura, G.; Kawamura, N.; Asakawa, A.; Inui, A. Atractylenolide-III suppresses lipopolysaccharide-induced inflammation via downregulation of toll-like receptor 4 in mouse microglia. Heliyon 2021, 7, e08269. [Google Scholar] [CrossRef]
- Ma, G.; Chen, J.; Wang, L.; Qian, F.; Li, G.; Wu, X.; Zhang, L.; Li, Y. Eighteen structurally diversified sesquiterpenes isolated from Pogostemon cablin and their inhibitory effects on nitric oxide production. Fitoterapia 2022, 156, 105098. [Google Scholar] [CrossRef]
- Sandhu, J.K.; Kulka, M. Decoding Mast Cell-Microglia Communication in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 1093. [Google Scholar] [CrossRef]
- Tian, X.; Liu, H.; Xiang, F.; Xu, L.; Dong, Z. beta-Caryophyllene protects against ischemic stroke by promoting polarization of microglia toward M2 phenotype via the TLR4 pathway. Life Sci. 2019, 237, 116915. [Google Scholar] [CrossRef]
- Machado, K.D.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. PTR 2018, 32, 2376–2388. [Google Scholar] [CrossRef]
- Yang, M.; Lv, Y.; Tian, X.; Lou, J.; An, R.; Zhang, Q.; Li, M.; Xu, L.; Dong, Z. Neuroprotective Effect of beta-Caryophyllene on Cerebral Ischemia-Reperfusion Injury via Regulation of Necroptotic Neuronal Death and Inflammation: In Vivo and in Vitro. Front. Neurosci. 2017, 11, 583. [Google Scholar] [CrossRef]
- Hespel, C.; Moser, M. Role of inflammatory dendritic cells in innate and adaptive immunity. Eur. J. Immunol. 2012, 42, 2535–2543. [Google Scholar] [CrossRef]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.; Kulka, M.; Zhang, J.; Li, Y.; Guo, F. Occurrence and biological activities of eremophilane-type sesquiterpenes. Mini Rev. Med. Chem. 2014, 14, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, F.; Zhang, P.; Liu, M.; Qian, L.; Lv, F.; Cheng, W.; Hou, R. Myrothecine A modulates the proliferation of HCC cells and the maturation of dendritic cells through downregulating miR-221. Int. Immunopharmacol. 2019, 75, 105783. [Google Scholar] [CrossRef] [PubMed]
- Fukuo, Y.; Yamashina, S.; Sonoue, H.; Arakawa, A.; Nakadera, E.; Aoyama, T.; Uchiyama, A.; Kon, K.; Ikejima, K.; Watanabe, S. Abnormality of autophagic function and cathepsin expression in the liver from patients with non-alcoholic fatty liver disease. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2014, 44, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lin, C.C.; Chu, Y.C.; Fu, C.W.; Sheu, J.H. Bioactive Diterpenes, Norditerpenes, and Sesquiterpenes from a Formosan Soft Coral Cespitularia sp. Pharmaceuticals 2021, 14, 1252. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Xing, Y.; Dong, D.; Hu, Y.; Chen, Q.; Zhai, L.; Hu, L.; Zhang, Y. Costunolide ameliorates colitis via specific inhibition of HIF1alpha/glycolysis-mediated Th17 differentiation. Int. Immunopharmacol. 2021, 97, 107688. [Google Scholar] [CrossRef]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef]
- Metcalfe, D.D. Mast cells and mastocytosis. Blood 2008, 112, 946–956. [Google Scholar] [CrossRef] [Green Version]
- Kubo, M. Mast cells and basophils in allergic inflammation. Curr. Opin. Immunol. 2018, 54, 74–79. [Google Scholar] [CrossRef]
- Hallgren, J.; Gurish, M.F. Mast cell progenitor trafficking and maturation. Adv. Exp. Med. Biol. 2011, 716, 14–28. [Google Scholar] [PubMed] [Green Version]
- Ohnmacht, C.; Voehringer, D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J. Immunol. 2010, 184, 344–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.P.; Kang, S.; Park, S.J.; Choi, Y.W.; Lee, Y.G.; Im, D.S. Anti-allergic and anti-inflammatory effects of bakkenolide B isolated from Petasites japonicus leaves. J. Ethnopharmacol. 2013, 148, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.W.; Lee, K.P.; Kim, J.M.; Kang, S.; Park, S.J.; Lee, J.M.; Moon, H.R.; Jung, J.H.; Lee, Y.G.; Im, D.S. Petatewalide B, a novel compound from Petasites japonicus with anti-allergic activity. J. Ethnopharmacol. 2016, 178, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pyun, H.; Kang, U.; Seo, E.K.; Lee, K. Dehydrocostus lactone, a sesquiterpene from Saussurea lappa Clarke, suppresses allergic airway inflammation by binding to dimerized translationally controlled tumor protein. Phytomedicine 2018, 43, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Park, S.J.; Nam, S.Y.; Kang, S.; Hwang, J.; Lee, S.J.; Im, D.S. Anti-allergic effects of sesquiterpene lactones from Saussurea costus (Falc.) Lipsch. determined using in vivo and in vitro experiments. J. Ethnopharmacol. 2018, 213, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Guo, G.; Li, Y.; Kulka, M. A novel eremophilane lactone inhibits FcepsilonRI-dependent release of pro-inflammatory mediators: Structure-dependent bioactivity. Inflamm. Res. 2016, 65, 303–311. [Google Scholar] [CrossRef]
- Fujimoto, M.; Oka, T.; Murata, T.; Hori, M.; Ozaki, H. Fluvastatin inhibits mast cell degranulation without changing the cytoplasmic Ca2+ level. Eur. J. Pharmacol. 2009, 602, 432–438. [Google Scholar] [CrossRef]
- Miyata, N.; Gon, Y.; Nunomura, S.; Endo, D.; Yamashita, K.; Matsumoto, K.; Hashimoto, S.; Ra, C. Inhibitory effects of parthenolide on antigen-induced microtubule formation and degranulation in mast cells. Int. Immunopharmacol. 2008, 8, 874–880. [Google Scholar] [CrossRef]
- Lim, H.J.; Jin, H.G.; Woo, E.R.; Lee, S.K.; Kim, H.P. The root barks of Morus alba and the flavonoid constituents inhibit airway inflammation. J. Ethnopharmacol. 2013, 149, 169–175. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, T.; Oh, I.; Nam, K.W.; Kim, K.H.; Oh, K.B.; Shin, J.; Mar, W. Mast cell stabilizing effect of (-)-Elema-1,3,11(13)-trien-12-ol and thujopsene from Thujopsis dolabrata is mediated by down-regulation of interleukin-4 secretion in antigen-induced RBL-2H3 cells. Biol. Pharm. Bull. 2013, 36, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.N.; Park, D.K.; Park, H.J. The inhibitory activity of atractylenolide capital SHA, Cyrillic, a sesquiterpenoid, on IgE-mediated mast cell activation and passive cutaneous anaphylaxis (PCA). J. Ethnopharmacol. 2013, 145, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Tang, Z.; Shi, Z.; Guo, Q.; Xiong, H. New Insights into Artesunate as a Pleiotropic Regulator of Innate and Adaptive Immune Cells. J. Immunol. Res. 2022, 2022, 9591544. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Ng, D.S.; Chan, T.K.; Guan, S.P.; Ho, W.E.; Koh, A.H.; Bian, J.S.; Lau, H.Y.; Wong, W.S. Anti-allergic action of anti-malarial drug artesunate in experimental mast cell-mediated anaphylactic models. Allergy 2013, 68, 195–203. [Google Scholar] [CrossRef]
- Nugroho, A.E.; Riyanto, S.; Sukari, M.A.; Maeyama, K. Effects of aegeline, a main alkaloid of Aegle Marmelos Correa leaves, on the histamine release from mast cells. Pak. J. Pharm. Sci. 2011, 24, 359–367. [Google Scholar]
- Hong, J.; Sasaki, H.; Hirasawa, N.; Ishihara, K.; Kwak, J.H.; Zee, O.; Schmitz, F.J.; Seyama, T.; Ohuchi, K. Suppression of the antigen-stimulated RBL-2H3 mast cell activation by Artekeiskeanol A. Planta Med. 2009, 75, 1494–1498. [Google Scholar] [CrossRef]
- Jin, C.; Ye, K.; Luan, H.; Liu, L.; Zhang, R.; Yang, S.; Wang, Y. Tussilagone inhibits allergic responses in OVA-induced allergic rhinitis guinea pigs and IgE-stimulated RBL-2H3 cells. Fitoterapia 2020, 144, 104496. [Google Scholar] [CrossRef]
- Castillo-Arellano, J.I.; Gomez-Verjan, J.C.; Rojano-Vilchis, N.A.; Mendoza-Cruz, M.; Jimenez-Estrada, M.; Lopez-Valdes, H.E.; Martinez-Coria, H.; Gutierrez-Juarez, R.; Gonzalez-Espinosa, C.; Reyes-Chilpa, R.; et al. Chemoinformatic Analysis of Selected Cacalolides from Psacalium decompositum (A. Gray) H. Rob. & Brettell and Psacalium peltatum (Kunth) Cass. and Their Effects on FcepsilonRI-Dependent Degranulation in Mast Cells. Molecules 2018, 23, 3367. [Google Scholar]
- Kim, D.K.; Lee, J.H.; Kim, J.W.; Kim, H.S.; Kim, A.R.; Kim, B.K.; Yi, K.Y.; Park, H.J.; Park, D.K.; Choi, W.S. A novel imidazo[1,5-b]isoquinolinone derivative, U63A05, inhibits Syk activation in mast cells to suppress IgE-mediated anaphylaxis in mice. J. Pharmacol. Sci. 2011, 115, 500–508. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Nam, S.Y.; Hwang, S.Y.; Kim, H.M.; Jeong, H.J. Atractylone, an active constituent of KMP6, attenuates allergic inflammation on allergic rhinitis in vitro and in vivo models. Mol. Immunol. 2016, 78, 121–132. [Google Scholar] [CrossRef]
- Han, N.R.; Moon, P.D.; Nam, S.Y.; Ryu, K.J.; Yoou, M.S.; Choi, J.H.; Hwang, S.Y.; Kim, H.M.; Jeong, H.J. Inhibitory effects of atractylone on mast cell-mediated allergic reactions. Chem. -Biol. Interact. 2016, 258, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Kim, S.G.; Park, Y.N.; Lee, J.; Lee, Y.J.; Park, N.Y.; Jeong, K.T.; Lee, E. Suppressive effects of britanin, a sesquiterpene compound isolated from Inulae flos, on mast cell-mediated inflammatory responses. Am. J. Chin. Med. 2014, 42, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, X.; Park, Y.N.; Kwon, O.; Piao, D.; Chang, Y.C.; Kim, C.H.; Lee, E.; Son, J.K.; Chang, H.W. Britanin Suppresses IgE/Ag-Induced Mast Cell Activation by Inhibiting the Syk Pathway. Biomol. Ther. 2014, 22, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, M.J.; Kim, S.J.; Kang, T.H.; Rim, H.K.; Jeong, H.J.; Um, J.Y.; Hong, S.H.; Kim, H.M. The regulatory mechanism of beta-eudesmol is through the suppression of caspase-1 activation in mast cell-mediated inflammatory response. Immunopharmacol. Immunotoxicol. 2011, 33, 178–185. [Google Scholar] [CrossRef]
- Coll, R.C.; Vargas, P.M.; Mariani, M.L.; Penissi, A.B. Natural alpha, beta-unsaturated lactones inhibit neuropeptide-induced mast cell activation in an in vitro model of neurogenic inflammation. Inflamm. Res. 2020, 69, 1039–1051. [Google Scholar] [CrossRef]
- Vera, M.E.; Mariani, M.L.; Aguilera, C.; Penissi, A.B. Effect of a Cytoprotective Dose of Dehydroleucodine, Xanthatin, and 3-Benzyloxymethyl-5H-furan-2-one on Gastric Mucosal Lesions Induced by Mast Cell Activation. Int. J. Mol. Sci. 2021, 22, 5983. [Google Scholar] [CrossRef]
- Vera, M.E.; Persia, F.A.; Mariani, M.L.; Rudolph, M.I.; Fogal, T.H.; Cenal, J.P.; Favier, L.S.; Tonn, C.E.; Penissi, A.B. Activation of human leukemic mast cell line LAD2 is modulated by dehydroleucodine and xanthatin. Leuk. Lymphoma 2012, 53, 1795–1803. [Google Scholar] [CrossRef]
- Arizmendi, N.; Qian, H.; Li, Y.; Kulka, M. Sesquiterpene-Loaded Co-Polymer Hybrid Nanoparticle Effects on Human Mast Cell Surface Receptor Expression, Granule Contents, and Degranulation. Nanomaterials 2021, 11, 953. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Wang, L.; Luo, H.; Lu, Y.; Zhang, Q.; Tang, L.; Wang, Z. Farfarae Flos: A review of botany, traditional uses, phytochemistry, pharmacology, and toxicology. J. Ethnopharmacol. 2020, 260, 113038. [Google Scholar] [CrossRef]
- Song, X.Q.; Yu, J.H.; Sun, J.; Liu, K.L.; Zhang, J.S.; Zhang, H. Bioactive sesquiterpenoids from the flower buds of Tussilago farfara. Bioorganic Chem. 2021, 107, 104632. [Google Scholar] [CrossRef]
- Liu, J.; Hong, X.; Lin, D.; Luo, X.; Zhu, M.; Mo, H. Artesunate influences Th17/Treg lymphocyte balance by modulating Treg apoptosis and Th17 proliferation in a murine model of rheumatoid arthritis. Exp. Ther. Med. 2017, 13, 2267–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Li, Y.; Jin, M.; Yang, J.H.; Li, X.; Chao, G.H.; Park, H.H.; Park, Y.N.; Son, J.K.; Lee, E.; et al. Inula japonica extract inhibits mast cell-mediated allergic reaction and mast cell activation. J. Ethnopharmacol. 2012, 143, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Zamaraeva, M.V.; Hagelgans, A.I.; Azimov, R.R.; Krasilnikov, O.V. Influence of plant terpenoids on the permeability of mitochondria and lipid bilayers. Biochim. Biophys. Acta 2001, 1512, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gliozzi, M.; Scarano, F.; Musolino, V.; Carresi, C.; Scicchitano, M.; Ruga, S.; Zito, M.C.; Nucera, S.; Bosco, F.; Maiuolo, J.; et al. Role of TSPO/VDAC1 Upregulation and Matrix Metalloproteinase-2 Localization in the Dysfunctional Myocardium of Hyperglycaemic Rats. Int. J. Mol. Sci. 2020, 21, 7432. [Google Scholar] [CrossRef] [PubMed]
- Curtis, W.R.; Wang, P.; Humphrey, A. Role of calcium and differentiation in enhanced sesquiterpene elicitation from calcium alginate-immobilized plant tissue. Enzym. Microb. Technol. 1995, 17, 554–557. [Google Scholar] [CrossRef]
- Supanjani, A.R.M.; Tawaha, M.S.Y.; Lee, D.K. Calcium effects on yield, mineral uptake and terpene components of hydroponic Crysanthemum coronorium L. Int. J. Bot. 2005, 1, 196–200. [Google Scholar]
- Mendanha, S.A.; Moura, S.S.; Anjos, J.L.V.; Valadares, M.C.; Alonso, A. Toxicity of terpenes on fibroblast cells compared to their hemolytic potential and increase in erythrocyte membrane fluidity. Toxicol. Vitr. 2013, 27, 323–329. [Google Scholar]
- Mishra, S.K.; Bae, Y.S.; Lee, Y.-M.; Kim, J.-S.; Oh, S.H.; Kim, H.M. Sesquiterpene Alcohol Cedrol Chemosensitizes Human Cancer Cells and Suppresses Cell Proliferation by Destabilizing Plasma Membrane Lipid Rafts. Front. Cell Dev. Biol. 2021, 8, 1799. [Google Scholar] [CrossRef]
- Chu, X.; Yan, P.; Zhang, N.; Chen, N.; Liu, Y.; Feng, L.; Li, M.; Zhang, Z.; Wang, Q.; Wang, S.; et al. The efficacy and safety of intermittent preventive treatment with sulphadoxine-pyrimethamine vs. artemisinin-based drugs for malaria: A systematic review and meta-analysis. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 298–309. [Google Scholar] [CrossRef]

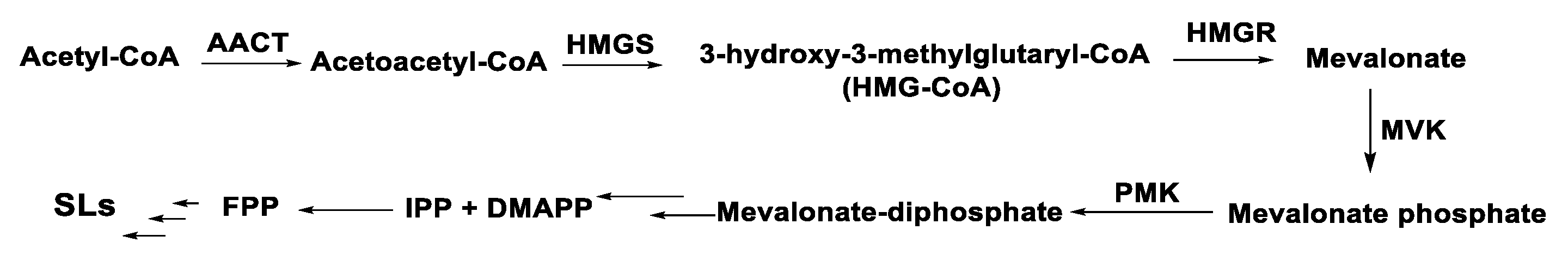

| Sesquiterpene (SQ) | Effect | Mechanism |
|---|---|---|
| Lob-1, -2, -3, -4, -5, -6, -7, -8 SQ lactones from Neurolaena lobata (74% purity) | Interfere with the induction of inflammatory cell adhesion molecules and chemokines in HUVECtert and THP-1 cells stimulated with bacterial products and cytokines. | Inhibition of LPS and TNF-induced regulation of E-selectin and IL-8 [59] |
| Vlasouliolides-A, -B, -C, -D, -E, -F, -G, -H,-I SQ lactone dimers from Vladimiria souliei (70–80% purity) | Anti-inflammatory activity against LPS-induced NO production in RAW 264.7 cells. | Potent inhibitory activity of the phosphorylation of NF-κB [59,60,61,62]. |
| Neolinulicin-A, and –B SQ dimers from Inula japonica | Anti-inflammatory activity against LPS-induced NO production in RAW 264.7 cells. | Inhibition of NO production [63]. |
| 8-0-methacryloylelephanpane, 2,4-bis-0-methyl-8-0-methacryloylelephanpane, 4-0-ethyl-8-0-methacryloylelephanpane, 8-0-methacryloylisoelephanpane, 2-0-demethyltomenphanatopin C, molephantin A, molephantin B SQ lactones from Elephantopus mollis (98% purity) | Anti-inflammatory activity against LPS-induced NO production in RAW 264.7 cells. | Inhibition of pro-inflammatory mediator production such as iNOS, IL-6, MCP-1 and IL-1β through NF-κB and AP-1 signaling pathways [64]. |
| β-elemonic acid from Boswellia carteri (94% purity) | Anti-inflammatory activity against LPS-induced NO production in RAW 264.7 cells, mice, and rats. | Inhibition of NO production [65]. Inhibit the overproduction of TNF, IL-6, MCP-1, soluble TNF receptor 1, eotaxin-2, IL-10 and GCSF [65,66]. Inhibition of the activation of NF-κB, by reduced phosphorylation of p65 and attenuates the induction of iNOS, COX-2, NADPH oxidase 2 (NOX-2), and NADPH oxidase 4 (NOX4) leading to the decrease production of NO, PGE2 and ROS. [65] |
| Dimethylaminoicheliolide (DMAMCL, 82% purity) and Micheliolide guaianolide (MCL, 90% purity)SQ lactones from Michelia compressa and Michelia champaca | Anti-inflammatory activity against LPS-induced NO production in RAW 264.7 cells. Ameliorates colitis symptoms in a mouse model of dextran sulfate sodium-induced colitis, and azoxymethane/dextran sulfate sodium model of colitis-associated cancer. Anti-inflammatory effects on diabetic kidney disease by inhibiting Mtdh mediated renal inflammation type 2 diabetic bd/db mice. | Potent inhibitory activity of the phosphorylation of NF-κB. Significant inhibition of IL-6 and IL-1β, and TNF, and significant decreased of colon tumors [67]. Attenuates inflammatory responses and lipid accumulation in lipid mixture-induced AML12 and LO2 cells by upregulating PPARγ and decreasing phosphorylation of IκBα and NF-κB/p65, inhibiting NF-κB and reducing lipotoxicity [68]. |
| 6-0-angeloylplenolin (Brevilin A) from Centipeda minima (98% purity) | Inhibition of hepatic stellate cell activation in activated LX-2 cells. Inhibit neuroinflammation in BV2 microglial cells and protects neurones from inflammatory injury. Inhibition of the activation of microglial cells in the hippocampus of mice. | Inhibition of STAT3 phosphorylation through non-SMAD JAK1/STAT3 pathway during the inflammation process following liver injury [52]. Potent inhibitory activity of the phosphorylation of NF-κB and IκB-α [69]. Decreased TNF, IL-1β, and NO, and PGE2 [69]. Inhibition of iNOS, COX-2, NADPH oxidase 2 (NOX-2), and NADPH oxidase 4 (NOX4) leading to the decrease production of NO, PGE2 and ROS [69]. |
| JEUD-38 SQ lactone from Inula japonica | Anti-inflammatory activity against LPS-induced NO production in RAW 264.7 cells. | Inhibition of the activation of NF-κB, by reduced phosphorylation of p65 and attenuates the induction of iNOS. Inhibition of the LPS-induced activation of NF-κB, by reduced translocation of p65, via abrogation of IκB-α phosphorylation and degradation. Inhibited LPS-stimulated phosphorylation of MAPKs including ERK1/2, JNK and p38 and attenuates the induction of iNOS [70,71]. |
| Deoxyelephantopin SQ lactone from Elephantopus scaber (98% purity) | Glycolysis interference, attenuates LPS-induced IL-1β and high-mobility group box 1 (HMGB1) release in RAW 264.7 cells. | Decreased expression of pyruvate dehydrogenase kinase 1 (PDK1), glucose transporter 1 (GLU1), lactate dehydrogenase A (LDHA), and reduced lactate production. Regulation of the nuclear localization of pyruvate kinase M2 (PKM2) [72]. |
| Ze339 from Petasites hybridus (99% purity) | Anti-inflammatory to acute viral infections on primary human nasal epithelial cells (HNECs). | Anti-cytokine effects by interfering with nuclear translocation of STAT-signaling pathways [73]. |
| 7-hydroxy frullanolide, SQ lactone from Sphaeranthus indicus (98% purity) | Anti-inflammatory activity upon 7HF treated followed by LPS activation of human peripheral blood mononuclear cells. | Downregulates the expression of adhesion molecules such as ICAM1, VCAM1 and E-selectin in TNF activated human endothelial cells. Inhibition of the translocation of NF-κB into the nucleus by inhibiting IKK-β phosphorylation [73,74]. |
| β-caryophyllene bicyclic SQ from Asparagus falcatus (98% purity) | Anti-inflammatory effects in rat models of endometriosis. Reduction in cyst size and apoptosis in endometrial explants. Cerebral ischemia-reperfusion injury rat model. | Decreases prostaglandin E2 production, TNF release, nitric oxide synthase and COX-2 [64,69]. Potent agonists for the cannabinoid CB2 receptor [69]. Suppression of IL-1β and TNF [51]. |
| Costunolide (98% purity) and Dehydrocostuslactone (99% purity) SQ lactones from Laurus nobilis | Anti-inflammatory. | Inhibition of IL-6 induced tyrosine phosphorylation of STAT3 in human leukemic cell line THP-1. Downregulate phosphorylation of the tyrosine Janus kinases JAK1, JAK2 and Tyk2. Downregulation of NF-κB and STAT3 activation in lung injury mouse model. Counteracts the pro-inflammatory effects of IFN-γ and IL-22 on keratinocytes [75,76,77]. |
| 1β-hydroxyalantolactone (IJ-5) SQ lactone from Inula japonica (99% purity) | Suppress TNF-induced NF-κB activation and inflammatory gene transcription. Attenuate atopic dermatitis severity, IgE, IL-4, and IL-6 in serum, mRNA levels of TNF, IL-1, IL-4 and IL-6 in skin lesions in vivo. | Inhibition of the ubiquitination of receptor-interacting protein 1 and NF-κB essential modifier [78]. Inhibition of inflammatory cytokine expression through NF-κB activating pathway [79]. |
| Alantolactone (AL, 95% purity) and isoalantolactone (IAL, 95% purity) SQ lactones from Inula helenium | Inhibition of TNF-induced activation of synovial fibroblasts, and RAW 264.7 cells. | Inhibition of TNF-induced activation of NF-κB and MAPK pathways, supress the expression of MMP-3, MCP-1, and IL-1, IL-6 and iNOS [79]. |
| Fukinone and 10βH-8α,12-epidioxyeremophil-7(11)-en-8β-ol isolated from Petasites tatewakianus | Inhibit dendritic cell maturation and activation. | Dendritic cell inhibition is mediated by nuclear peroxisome-activated receptor γ (PPARγ) [80]. |
| Sesquiterpene (SQ) | Effect | Mechanism |
|---|---|---|
| Fluvastatin | Inhibited RBL-2H3 cells degranulation. | Ca2+ independent due to suppression of geranylgeranyl transferase [141]. |
| Parthenolide | Inhibited RBL-2H3, and BMMC degranulation. Inhibited passive cutaneous anaphylaxis reaction in mice. | Disrupted microtubule formation-fyn kinase dependent [142] |
| Magnolialide | Inhibited RBL-2H3 cells degranulation. | Decreased levels of IL-4 [136] |
| Bakkenolide B | Inhibited RBL-2H3 cells degranulation. | Suppressed IL-4 release [143]. Inhibited NOS2 and COX-2 [143]. Suppressed IL-4 production [136,144]. |
| (-)-Elema-1,3,11(13)-trien-12-ol | Inhibit RBL-2H3 cells degranulation. | Suppressed IL-4 production [144]. |
| Thujopsene | Inhibit RBL-2H3 cells degranulation. | Suppressed IL-4 production [144]. |
| Atractylenolide III | Inhibit RBL-2H3 cells degranulation. | Inhibit phosphorylation of Lyn, Fyn, Syk, LAT, PLCγ, Gab2, Akt, p38, and JNK kinases; Ca2+ dependent [145]. |
| Artesunate | Reduce infiltration of mast cell in mouse skin. Inhibit the release of inflammatory cytokines, downregulate Th17 cell responses in RBL-2H3 and mature human cultured mast cells. | Inhibited IgE-induced Syk and PLCγ1 phosphorylation, production of IP3, and rise in cytosolic Ca2+ level [146]. Reduce IgE and TNF concentration in serum. Suppress of IL-6, IL-17, and IL-23 expression [147]. Promote SOCS3 protein and inhibit ROR-γt protein and STAT3 phosphorylation [96]. |
| Aegeline | Inhibit degranulation and cytokine secretion of RBL-2H3 cells. | Influence the intracellular Ca2+ pool [148]. |
| Artekeiskeanol A | Inhibit degranulation and cytokine secretion of RBL-2H3 cells. | Suppress TNF, IL-13 and phosphorylation of Akt, p38 MAPK, JNK, p44/42MAPK [149]. |
| Tussilagone | Inhibit degranulation and cytokine secretion of RBL-2H3 cells. | Suppress phosphorylation of Lyn, Syk, Akt, NF-κB p65, ERK and p38 MAPK [150]. |
| SQ lactones derived from 6β-angeloyloxy3β,8-dihydroxyeremophil-7(11)-en-12,8β-olide (F-1) | Inhibit degranulation and cytokine secretion of RBL-2H3 cells. | Inhibit β-hexosaminidase release and TNF production [140]. |
| 3-butyl-1-chloro-8-(2-methoxycarbonyl)phenyl-5H-imidazo[1,5-b]isoquinolin-10-one (U63A05) | Inhibit degranulation and cytokine secretion of RBL-2H3 and BMMC. | Inhibit Syk activation; Ca2+ independent [151,152]. |
| Cacalolides | Inhibit degranulation and cytokine secretion of BMMC. | Inhibit the activity mediated by FcεRI-induced intracellular Ca2+ mobilization, ROS production, VEGFR-2, and activation of PI3K-Akt kinases, and MAPK pathway [153]. |
| Atractylone | Decrease histamine levels, IgE, IL-4, IL-5, IL-6, VEGF, and IL-13 in peritoneal mast cells of PCA-induced mice Attenuate pro-inflammatory cytokine production and mRNA expression of phorbol 12-myristate 13-acetate and calcium ionophore A23187-stimulated HMC-1, rat peritoneal mast cells, and allergic rhinitis mouse model. | Inhibit intracellular Ca2+, tryptase release, and histamine release. Decrease histidine decarboxylase activity and expression. Induce caspase-1/NF-κB/MAPKs activation. Reduce total IgE, histamine, PGD2, TSLP, IL-1β, IL-4, IL-5, IL-6, IL-13, TNF, COX-2, ICAM-1, and MIP-2 [153,154]. |
| Britanin | Inhibit pro-inflammatory cytokines and degranulation of HMC-1 and BMMC. | Suppress gene expression and secretion of pro-inflammatory cytokines [155]. Attenuate activation of NF-κB pathway. Inhibit generation of PGD2 and phosphorylation of Syk-dependent pathway [155,156]. |
| β-Eudesmol | Inhibit the production and expression of IL-6 in PMA and Ca2+ ionophore-stimulated HMC-1; suppress SCF-induced mast cell migration and morphological.alterations, reduce F-actin formation in rat peritoneal mast cells. | Suppress activation of p38 MAPKs, and NF-κB. Suppress the activation of caspase-1 and expression of receptor-interacting protein-2. Reduce activation of Fyn kinase, Rac1 GTPase, and p38 MAPKs [154,157] {Nam, 2017 #773}. |
| Dehydroleucodine xanthatin | Inhibit degranulation of LAD2, rat peritoneal mast cells and rat gastric mucosa mast cells. | Anti-inflammatory properties, with inhibition of mast cell activation [158,159,160]. |
| Fukinone | Inhibit IgE dependent degranulation. | Inhibit expression of FcεRI(α, β,γ), and Kit receptors, and tryptase expression [161]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arizmendi, N.; Alam, S.B.; Azyat, K.; Makeiff, D.; Befus, A.D.; Kulka, M. The Complexity of Sesquiterpene Chemistry Dictates Its Pleiotropic Biologic Effects on Inflammation. Molecules 2022, 27, 2450. https://doi.org/10.3390/molecules27082450
Arizmendi N, Alam SB, Azyat K, Makeiff D, Befus AD, Kulka M. The Complexity of Sesquiterpene Chemistry Dictates Its Pleiotropic Biologic Effects on Inflammation. Molecules. 2022; 27(8):2450. https://doi.org/10.3390/molecules27082450
Chicago/Turabian StyleArizmendi, Narcy, Syed Benazir Alam, Khalid Azyat, Darren Makeiff, A. Dean Befus, and Marianna Kulka. 2022. "The Complexity of Sesquiterpene Chemistry Dictates Its Pleiotropic Biologic Effects on Inflammation" Molecules 27, no. 8: 2450. https://doi.org/10.3390/molecules27082450
APA StyleArizmendi, N., Alam, S. B., Azyat, K., Makeiff, D., Befus, A. D., & Kulka, M. (2022). The Complexity of Sesquiterpene Chemistry Dictates Its Pleiotropic Biologic Effects on Inflammation. Molecules, 27(8), 2450. https://doi.org/10.3390/molecules27082450







