Self-Assembled Monolayer of Monomercaptoundecahydro-closo-dodecaborate on a Polycrystalline Gold Surface
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Monomercaptoundecahydro-closo-dodecaborate
2.3. Gold Electrode Preparation
2.4. Electrode Modification
2.5. Electrochemical Experiments
2.6. Surface Plasmon Resonance Experiments
2.7. Theoretical Calculations
3. Results
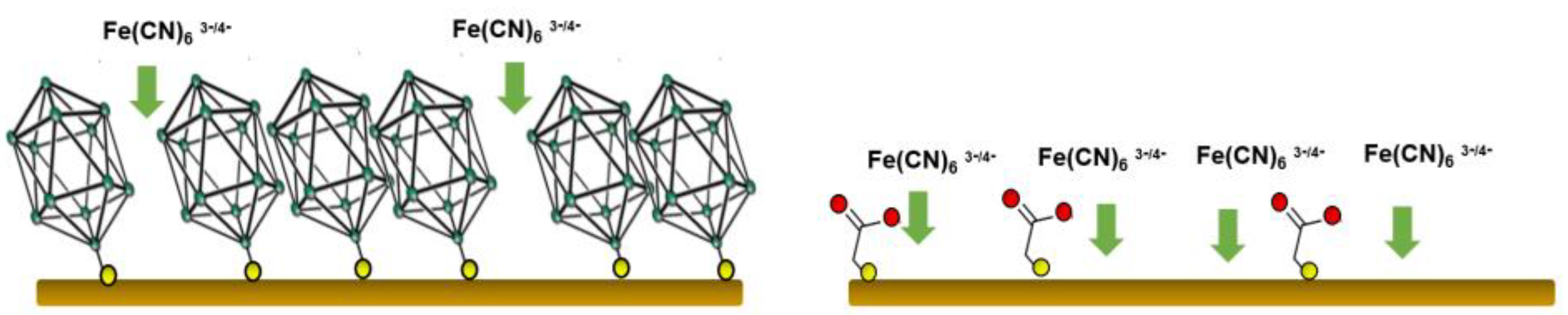
3.1. Cyclic Voltammetry Experiments
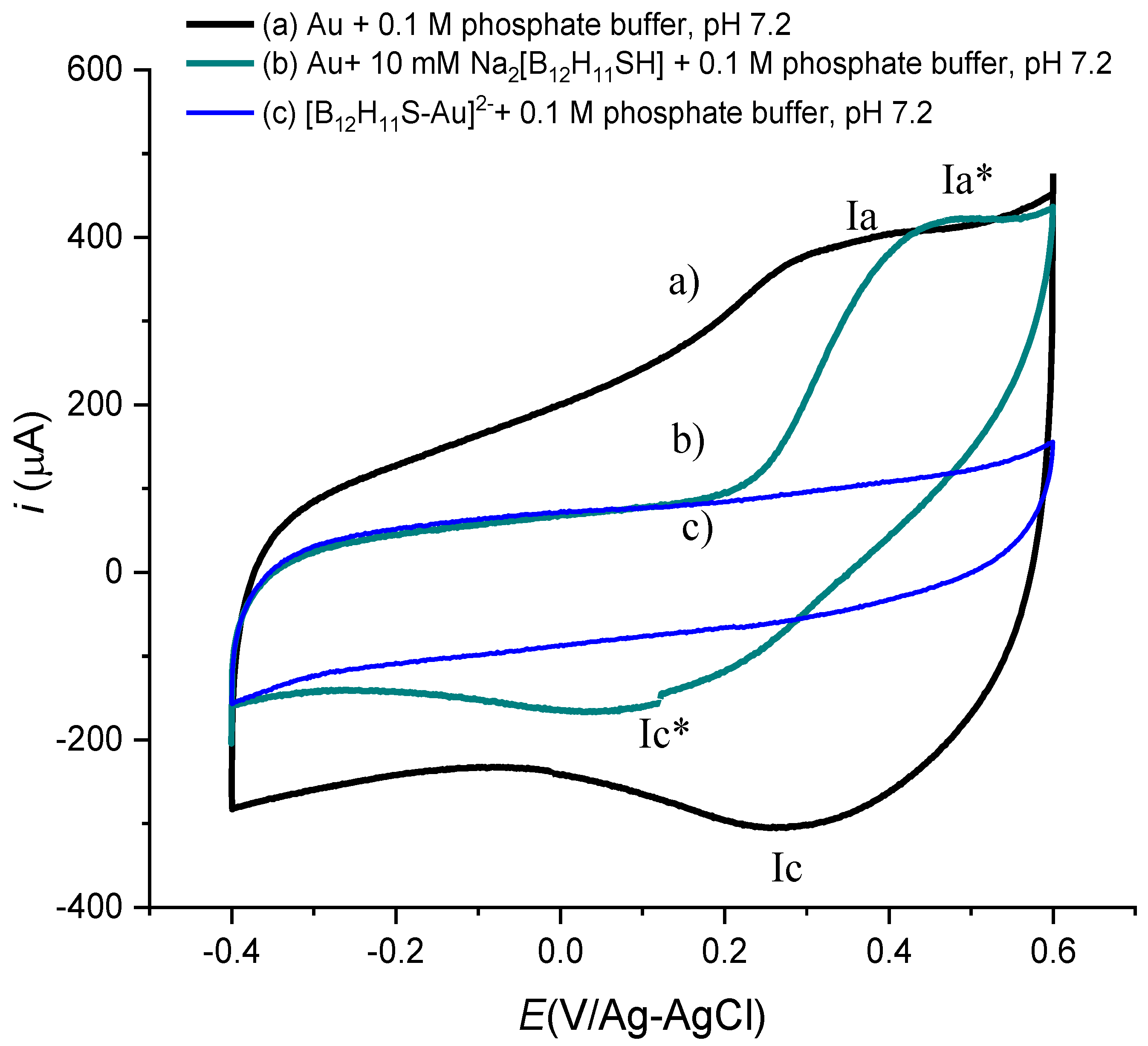
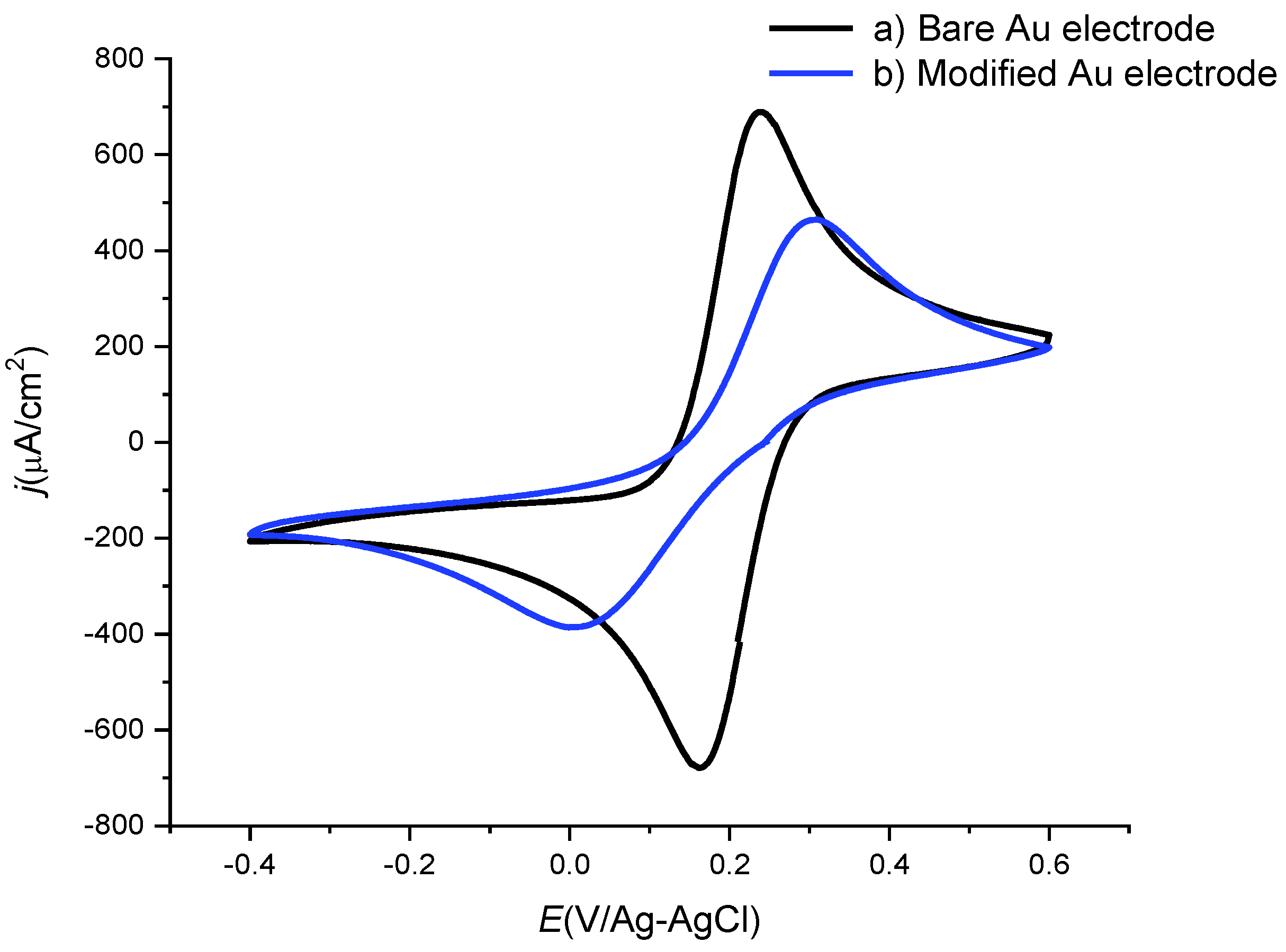
3.2. Electrochemical Impedance Spectra of [B12H11S-Au]2− Monolayer
3.3. Theoretical Calculations for [B12H11SH]2−
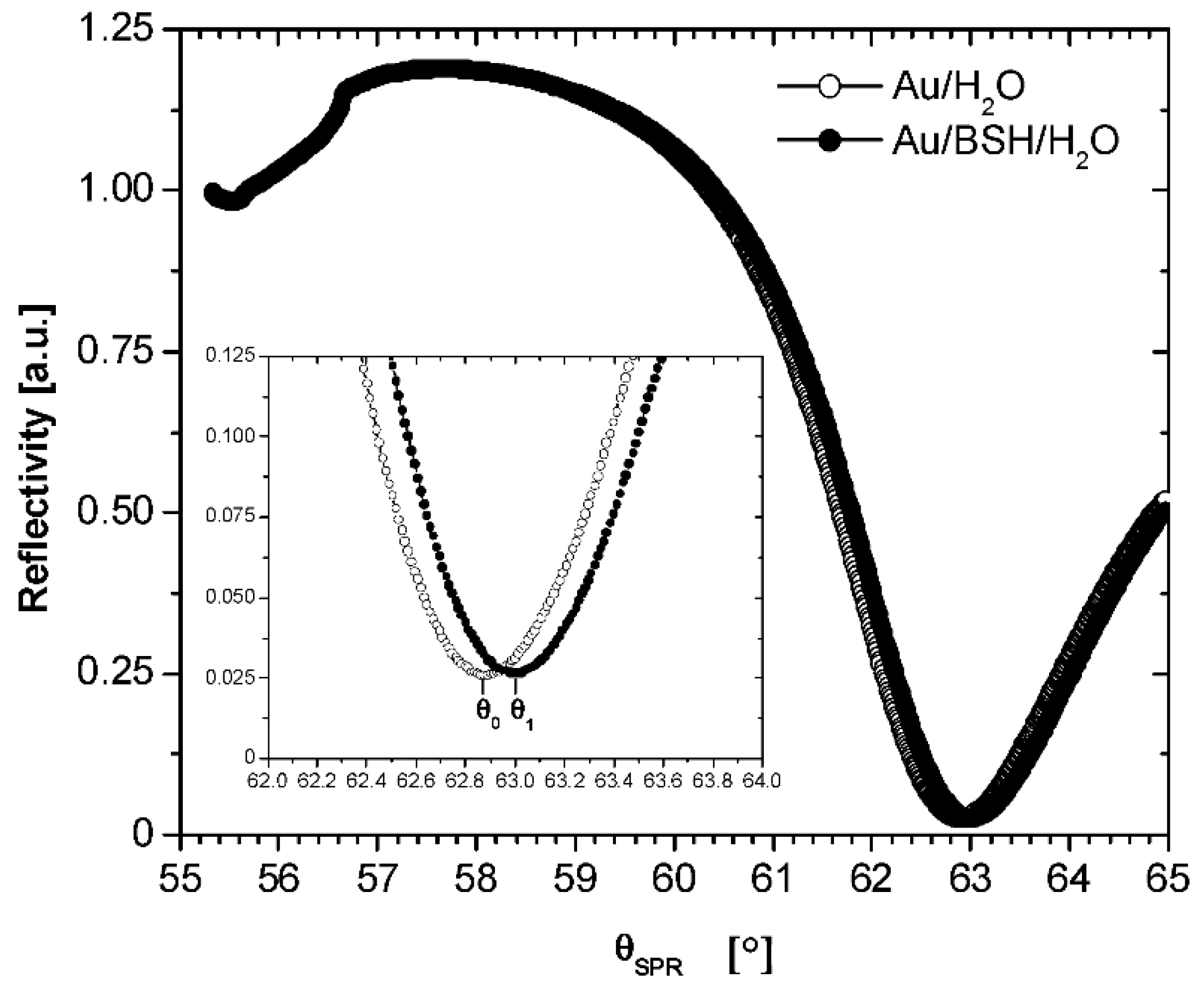
3.4. Optical Thickness by Surface Plasmon Resonance
3.5. Evaluation of the Dielectric Constant of the [B12H11S-Au]2− Monolayer
3.6. Differential Capacitance Studies of the [B12H11S-Au]2− Monolayer
3.7. Adsorption Kinetics by Surface Plasmon Resonance of the [B12H11S-Au]2− Monolayer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sweet, W.H. Early history of development of boron neutron capture therapy of tumors. J. Neuro-Oncol. 1997, 33, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Pooh, K.; Kobayashi, T.; Kageji, T.; Uyama, S.; Matsumura, A.; Kumada, H. Clinical Review of the Japanese Experience with Boron Neutron Capture Therapy and A Proposed Strategy Using Epithermal Neutron Beams. J. Neuro-Oncol. 2003, 62, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, J.; Fukuda, H.; Kobayashi, T.; Yoshino, K.; Honda, C.; Ichihashi, M.; Mishima, Y. Long term outcome of boron neutron capture therapy for malignant melanoma 2000. J. Radiat. Res. 2020, 61, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Sivaev, I.B.; Bregadze, V.I.; Kuznetsov, N.T. Derivatives of the closo-dodecaborate anion and their application in medicine. Russ. Chem. Bull. 2002, 51, 1362–1374. [Google Scholar] [CrossRef]
- Azev, Y.; Slepukhina, I.; Gabel, D. Synthesis of boron-containing heterocyclic compounds. Appl. Radiat. Isot. 2004, 61, 1107–1110. [Google Scholar] [CrossRef]
- Lechtenberg, B.; Gabel, D. Synthesis of a (B12H11S)2− containing glucuronoside as potential prodrug for BNCT. J. Organomet. Chem. 2005, 690, 2780–2782. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.V. Polyhedral Boranes for Medical Applications: Current Status and Perspectives. Eur. J. Inorg. Chem. 2009, 2009, 1433–1450. [Google Scholar] [CrossRef]
- Schmid, G.; Pugin, R.; Meyer-Zaika, W.; Simon, U. Clusters on Clusters: Closo-Dodecaborate as a Ligand for Au55 Clusters. Eur. J. Inorg. Chem. 1999, 1999, 2051–2055. [Google Scholar] [CrossRef]
- Hohman, J.N.; Claridge, S.A.; Kim, M.; Weiss, P.S. Cage molecules for self-assembly. Mater. Sci. Eng. R Reports 2010, 70, 188–208. [Google Scholar] [CrossRef]
- Yeager, L.J.; Saeki, F.; Shelly, K.; Hawthorne, M.F.; Garrell, R.L. A new class of self-assembled monolayers: Closo-B12H11S2− on gold. J. Am. Chem. Soc. 1998, 120, 9961–9962. [Google Scholar] [CrossRef]
- Wong, E.H.J.; May, G.L.; Wilde, C.P. Oxidative desorption of thiols as a route to controlled formation of binary self assembled monolayer surfaces. Electrochim. Acta 2013, 109, 67–74. [Google Scholar] [CrossRef]
- Kolodziej, A.; Fernandez-Trillo, F.; Rodrguez, P. Determining the parameters governing the electrochemical stability of thiols and disulfides self-assembled monolayer on gold electrodes in physiological medium. J. Electroanal. Chem. 2018, 819, 51–57. [Google Scholar] [CrossRef]
- Tolpin, E.I.; Wellum, G.R.; Berley, S.A. Synthesis and chemistry of mercaptoundecahydro-closo-dodecaborate (2-). Inorg. Chem. 1978, 17, 2867–2873. [Google Scholar] [CrossRef]
- Kretschmann, E.; Raether, H. Notizen: Radiative Decay of Non Radiative Surface Plasmons Excited by Light. Z. Für Naturforsch. A 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R. Development of the Colle-Salvetti correlation energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Rassolov, V.A.; Pople, J.A.; Ratner, M.A.; Windus, T.L. 6-31G* basis set for atoms K through Zn. J. Chem. Phys. 1998, 109, 1223. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, M.; Zhao, Y.; Zhang, J.; Sun, C.-C. A theoretical study on the structural and functional features of BSH Agent used in boron neutron capture therapy. J. Mol. Struct. THEOCHEM 2005, 728, 173–178. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. The Gaussian 09 Program; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Angerstein-Kozlowska, H.; Conway, B.E.; Stoicoviciu, L. Elementary steps of electrochemical oxidation of single-crystal planes of Au—I. Chemical basis of processes involving geometry of anions and the electrode surfaces. Electrochim. Acta 1986, 109, 1051–1061. [Google Scholar] [CrossRef]
- Ding, S.-J.; Chang, B.-W.; Wu, C.-C.; Lai, M.-F.; Chang, H.-C. Impedance spectral studies of self-assembly of alkanethiols with different chain lengths using different immobilization strategies on Au electrodes. Anal. Chim. Acta 2005, 554, 43–51. [Google Scholar] [CrossRef]
- Mendes, R.K.; Freire, R.S.; Fonseca, C.P.; Neves, S.; Kubota, L.T. Characterization of self-assembled thiols monolayers on gold surface by electrochemical impedance spectroscopy. J. Braz. Chem. Soc. 2004, 15, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Ramírez, J.Z.; Vargas, R.; Garza, J.; Gázquez, J.L. Simple Charge-Transfer Model for Metallic Complexes. J. Phys. Chem. A 2010, 114, 7945–7951. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Lipkowski, J. Clhoride adsorption at Au(111) electrode surface. J. Electroanal. Chem. 1996, 403, 225–239. [Google Scholar] [CrossRef]
- Schouten, S.; Stroeve, P.; Longo, M.L. DNA adsorption and cationic bilayer deposition on self-assembled monolayers. Langmuir 1999, 15, 8133–8139. [Google Scholar] [CrossRef]

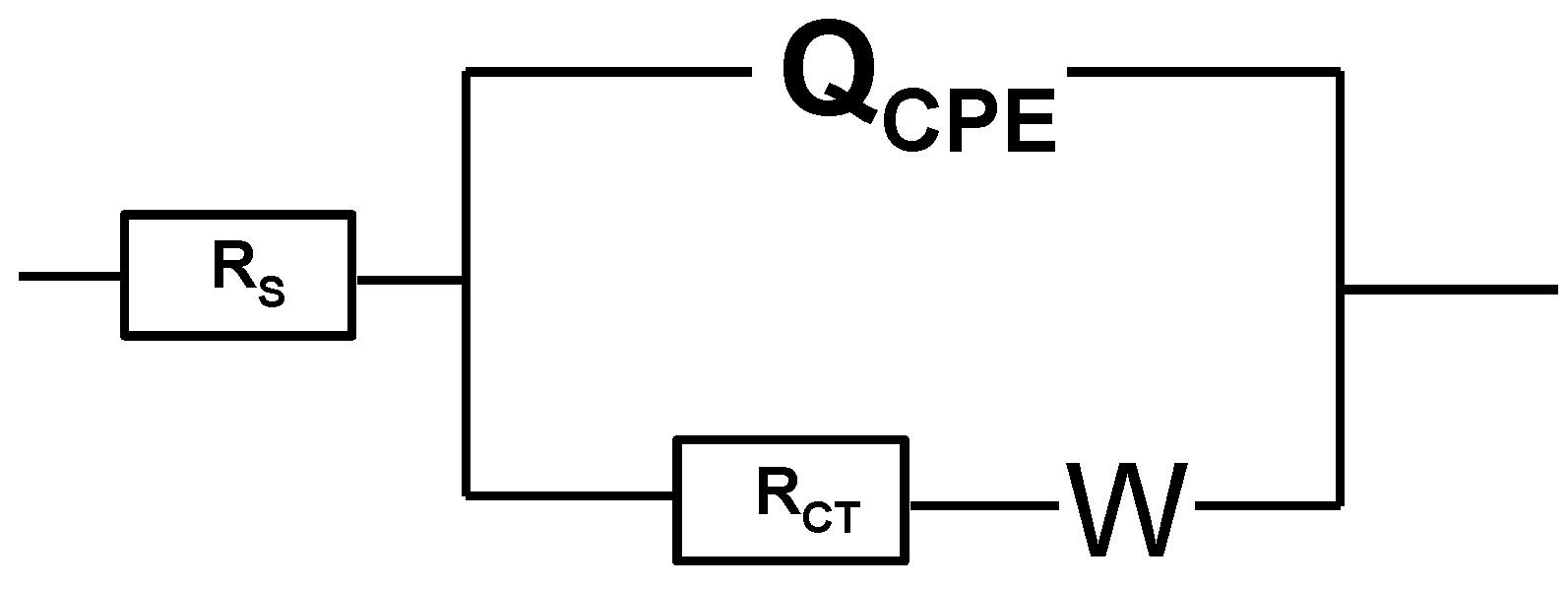
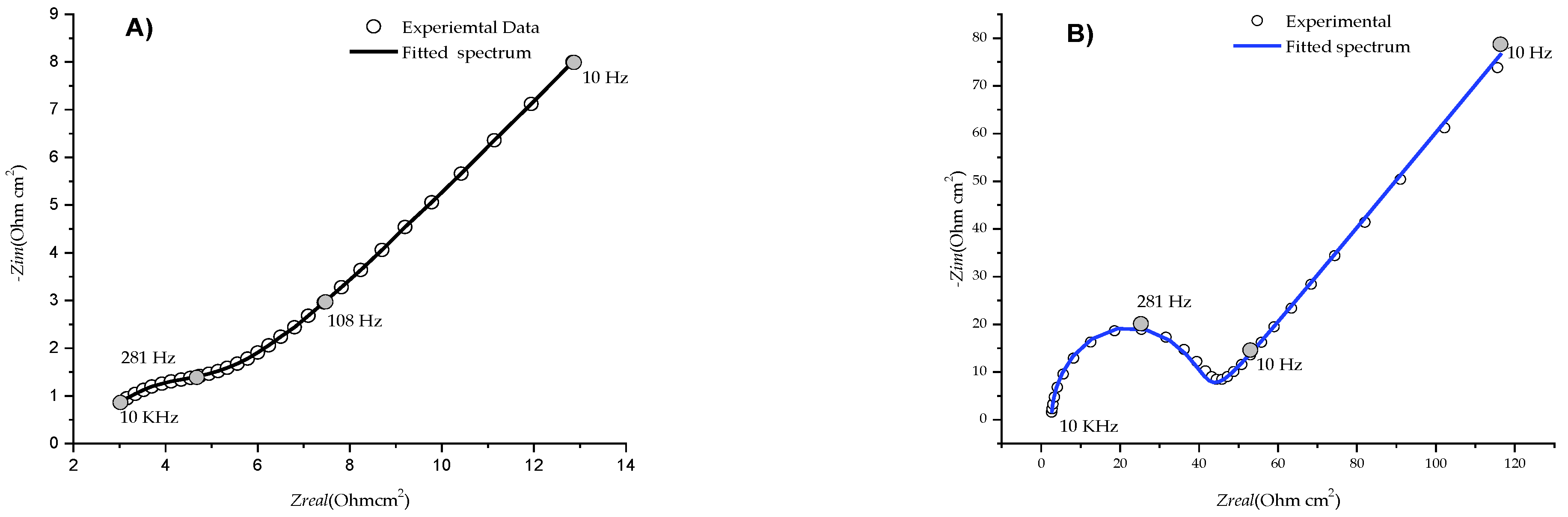



| Parameters | |
|---|---|
| Unmodified Au electrode | Rs = 117.2 Ω, Rct = 150 Ω, Y0 = 3.04 (µF)n, n = 0.7671, W= 3219 Ω/s1/2 |
| Au electrode after modification with [B12H11SH]2−. | Rs = 124.87 Ω, Rct = 1932 Ω, Y0 = 0.304 (µF)n, n =0.963, W= 2967 Ω/s1/2 |
| Chemical Descriptor | BHS | TGA |
|---|---|---|
| I | 188.83 | 153.29 |
| A | 32.34 | 1.68 |
| η | 78.24 | 75.80 |
| μ− | −149.71 | −115.39 |
| μ+ | −71.46 | −39.58 |
| ω− | 143.22 | 87.82 |
| ω+ | 32.63 | 10.33 |
| Dipole Moment (Debye) | 188.21 | 2.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-González, M.L.; Rebolledo-Chávez, J.P.F.; Cruz-Ramírez, M.; Antaño, R.; Mendoza, A.; Hosmane, N.S.; Ruiz-Azuara, L.; Hernández-López, J.L.; Ortiz-Frade, L. Self-Assembled Monolayer of Monomercaptoundecahydro-closo-dodecaborate on a Polycrystalline Gold Surface. Molecules 2022, 27, 2496. https://doi.org/10.3390/molecules27082496
Jiménez-González ML, Rebolledo-Chávez JPF, Cruz-Ramírez M, Antaño R, Mendoza A, Hosmane NS, Ruiz-Azuara L, Hernández-López JL, Ortiz-Frade L. Self-Assembled Monolayer of Monomercaptoundecahydro-closo-dodecaborate on a Polycrystalline Gold Surface. Molecules. 2022; 27(8):2496. https://doi.org/10.3390/molecules27082496
Chicago/Turabian StyleJiménez-González, Martha L., Juan Pablo F. Rebolledo-Chávez, Marisela Cruz-Ramírez, René Antaño, Angel Mendoza, Narayan S. Hosmane, Lena Ruiz-Azuara, José Luis Hernández-López, and Luis Ortiz-Frade. 2022. "Self-Assembled Monolayer of Monomercaptoundecahydro-closo-dodecaborate on a Polycrystalline Gold Surface" Molecules 27, no. 8: 2496. https://doi.org/10.3390/molecules27082496
APA StyleJiménez-González, M. L., Rebolledo-Chávez, J. P. F., Cruz-Ramírez, M., Antaño, R., Mendoza, A., Hosmane, N. S., Ruiz-Azuara, L., Hernández-López, J. L., & Ortiz-Frade, L. (2022). Self-Assembled Monolayer of Monomercaptoundecahydro-closo-dodecaborate on a Polycrystalline Gold Surface. Molecules, 27(8), 2496. https://doi.org/10.3390/molecules27082496








