Psychedelics: Alternative and Potential Therapeutic Options for Treating Mood and Anxiety Disorders
Abstract
:1. Introduction
1.1. Change in Status Quo
1.2. Classification of Psychedelic Drugs
1.3. Treating Mood and Anxiety Disorders with Psychedelic Drugs
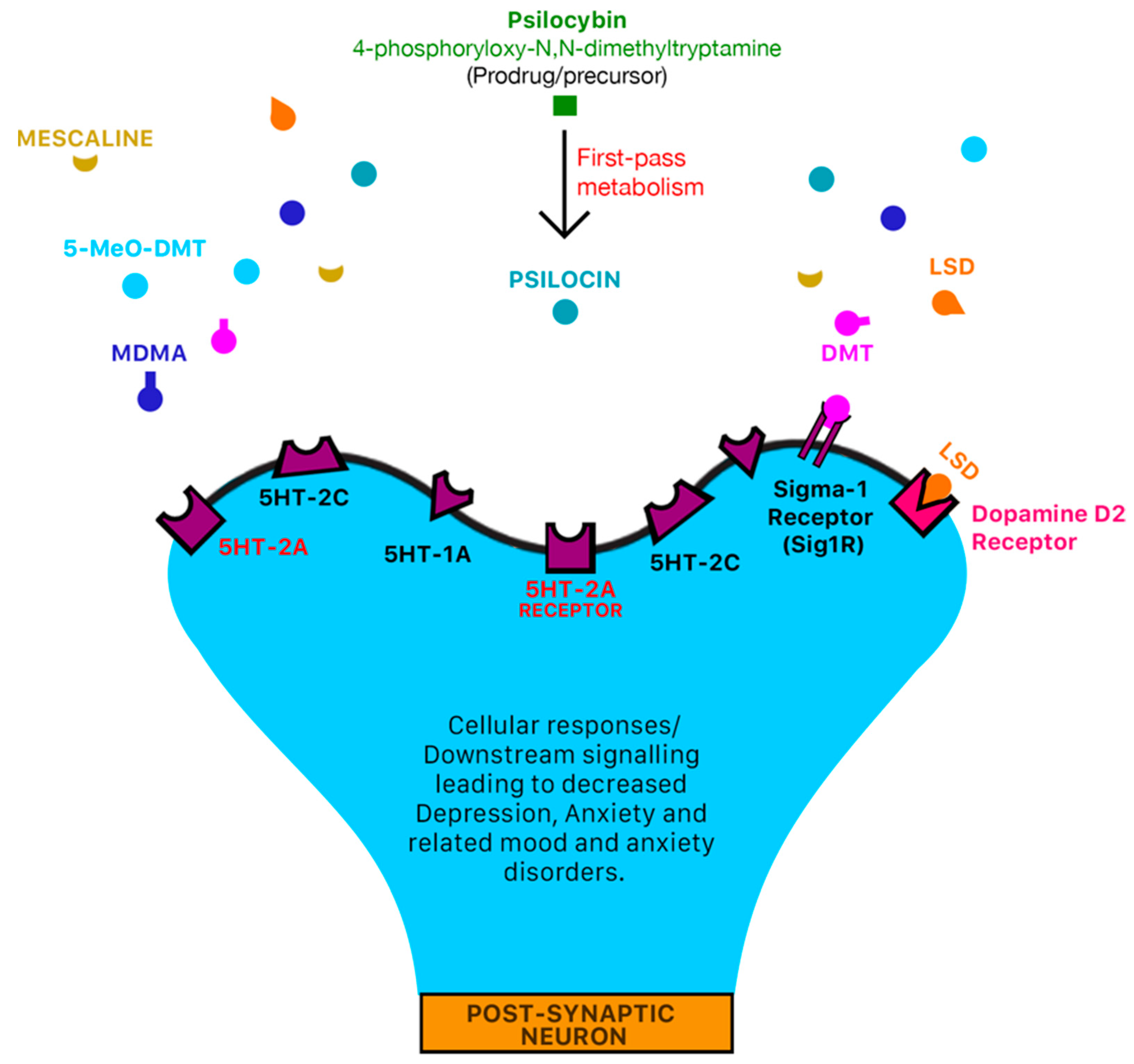
1.4. Psilocybin
1.5. N,N-DMT/DMT (The “God/Spirit Molecule”)
1.6. Ayahuasca
1.7. Ayahuasca Tourism
1.8. 5-MeO-DMT (Popularly Referred to as “Toad Venom”)
1.9. Lysergic Acid Diethylamide (LAD/LSD)
1.10. 3,4-Methylenedioxymethamphetamine (MDMA/“Ecstasy”)
2. The Economic Value of Psychedelics
3. Conclusions and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rucker, J.J.; Iliff, J.; Nutt, D.J. Psychiatry & the psychedelic drugs. past, present & future. Neuropharmacology 2018, 142, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A. Americans are Taking More Anti-Anxiety Medication and Antidepressants during CORONAVIRUS Pandemic: Report. 16 April 2020. Available online: https://thehill.com/changing-america/well-being/mental-health/493125-increase-in-anti-anxiety-medication-antidepressants (accessed on 18 February 2021).
- Harvard Health Publishing. What Are the Real Risks of Antidepressants? March 2014. Available online: https://www.health.harvard.edu/mind-and-mood/what-are-the-real-risks-of-antidepressants (accessed on 18 February 2021).
- Mayo Clinic Staff. The Most Commonly Prescribed Type of Antidepressant. 17 September 2019. Available online: https://www.mayoclinic.org/diseases-conditions/depression/in-depth/ssris/art-20044825 (accessed on 18 February 2021).
- Gable, R.S. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction 2007, 102, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Griffiths, R.R. Potential Therapeutic Effects of Psilocybin. Neurotherapeutics 2017, 14, 734–740. [Google Scholar] [CrossRef]
- Passie, T.; Seifert, J.; Schneider, U.; Emrich, H.M. The pharmacology of Psilocybin. Addict. Biol. 2002, 7, 357–364. [Google Scholar] [CrossRef]
- Oliveira e Souza, R. COVID-19 Pandemic Exacerbates Suicide Risk Factors. 10 September 2020. Available online: https://www.paho.org/en/news/10-9-2020-covid-19-pandemic-exacerbates-suicide-risk-factors (accessed on 16 January 2021).
- Griffiths, R.R.; Richards, W.A.; McCann, U.; Jesse, R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 2006, 187, 268–292. [Google Scholar] [CrossRef]
- Mahapatra, A.; Gupta, R. Role of Psilocybin in the treatment of depression. Ther. Adv. Psychopharmacol. 2016, 7, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Halpern, J.H.; Pope, H.G., Jr. Do hallucinogens cause residual neuropsychological toxicity? Drug Alcohol Depend. 1999, 53, 247–256. [Google Scholar] [CrossRef]
- Lim, A. Psychedelics as Antidepressants. 30 January 2021. Available online: https://www.scientificamerican.com/article/psychedelics-as-antidepressants/ (accessed on 18 February 2021).
- Marona-Lewicka, D.; Thisted, R.A.; Nichols, D.E. Distinct temporal phases in the behavioral pharmacology of LSD: Dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology 2005, 180, 427–435. [Google Scholar] [CrossRef]
- Geyer, M.; Vollenweider, F. Serotonin research: Contributions to understanding psychoses. Trends Pharmacol. Sci. 2008, 29, 445–453. [Google Scholar] [CrossRef]
- Aghajanian, G.K.; Haigler, H.J.; Bloom, F.E. Lysergic acid diethylamide and serotonin: Direct actions on serotonin-containing neurons in rat brain. Life Sci. 1972, 11, 615–622. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 1150, Tryptamine. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tryptamine (accessed on 16 February 2021).
- Halberstadt, A.L.; Geyer, M.A. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 2011, 61, 364–381. [Google Scholar] [CrossRef] [Green Version]
- Beliveau, V.; Ganz, M.; Feng, L.; Ozenne, B.; Højgaard, L.; Fisher, P.M.; Svarer, C.; Greve, D.N.; Knudsen, G.M. A High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 120–128. [Google Scholar] [CrossRef]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar]
- Frazer, A.; Hensler, J.G. Serotonin Involvement in Physiological Function and Behavior. Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Lippincott-Raven: Philadelphia, PA, USA, 1999. Available online: https://www.ncbi.nlm.nih.gov/books/NBK27940/ (accessed on 9 March 2021).
- Su, T.P.; Hayashi, T.; Vaupel, D.B. When the endogenous hallucinogenic trace amine N,N-dimethyltryptamine meets the sigma-1 receptor. Sci. Signal. 2009, 2, pe12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontanilla, D.; Johannessen, M.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 2009, 323, 934–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canal, C.E. Serotonergic Psychedelics: Experimental Approaches for Assessing Mechanisms of Action. Handb. Exp. Pharmacol. 2018, 252, 227–260. [Google Scholar] [CrossRef]
- Berry, M.D. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev. Recent Clin. Trials 2007, 2, 3–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, M.D.; Gainetdinov, R.R.; Hoener, M.C.; Shahid, M. Pharmacology of human trace amine-associated receptors: Therapeutic opportunities and challenges. Pharmacol. Ther. 2017, 180, 161–180. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef] [Green Version]
- Simmler, L.D.; Buchy, D.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1. J. Pharmacol. Exp. Ther. 2016, 357, 134–144. [Google Scholar] [CrossRef]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, P.R.; Benkelfat, C.; Descarries, L. The neurobiology of depression--revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2012, 367, 2378–2381. [Google Scholar] [CrossRef] [Green Version]
- Lowe, H.; Toyang, N.; Steele, B.; Valentine, H.; Grant, J.; Ali, A.; Ngwa, W.; Gordon, L. The Therapeutic Potential of Psilocybin. Molecules 2021, 26, 2948. [Google Scholar] [CrossRef] [PubMed]
- Palhano-Fontes, F.; Barreto, D.; Onias, H.; Andrade, K.C.; Novaes, M.M.; Pessoa, J.A.; Mota-Rolim, S.A.; Osório, F.L.; Sanches, R.; dos Santos, R.G. Rapid antidepressant effects of the psychedelic Ayahuasca in treatment-resistant depression: A randomized placebo-controlled trial. Psychol. Med. 2019, 49, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Palhano-Fontes, F.; Alchieri, J.C.; Oliveira, J.P.M.; Soares, B.L.; Hallak, J.E.C.; Galvao-Coelho, N.; de Araujo, D.B. The Therapeutic Potentials of Ayahuasca in the Treatment of Depression. In The Therapeutic Use of Ayahuasca; Labate, B., Cavnar, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Sanches, R.F.; Osório, F.D.; Hallak, C.J.E. Long-term effects of Ayahuasca in patients with recurrent depression: A 5-year qualitative follow-up. Arch. Clin. Psychiatry (São Paulo) 2018, 45, 22–24. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, R.G.; Osório, F.L.; Crippa, J.A.; Riba, J.; Zuardi, A.W.; Hallak, J.E. Antidepressive, anxiolytic, and antiaddictive effects of Ayahuasca, Psilocybin and lysergic acid diethylamide (LSD): A systematic review of clinical trials published in the last 25 years. Ther. Adv. Psychopharmacol. 2016, 6, 193–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osório, F.; Sanches, R.F.; Macedo, L.R.; Santos, R.G.; Maia-de-Oliveira, J.P.; Wichert-Ana, L.; Araujo, D.B.; Riba, J.; Crippa, J.A.; Hallak, J.E. Antidepressant effects of a single dose of Ayahuasca in patients with recurrent depression: A preliminary report. Rev. Bras. Psiquiatr. 2015, 37, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Johnson, F.G. LSD in the treatment of alcoholism. Am. J. Psychiatry 1969, 126, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Levine, J.; Stark, L.; Lazar, R. A clinical study of LSD treatment in alcoholism. Am. J. Psychiatry 1969, 126, 59–69. [Google Scholar] [CrossRef]
- Smart, R.G.; Storm, T. The Efficacy of LSD in the Treatment of Alcoholism. Q. J. Stud. Alcohol 1964, 25, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Visotsky, H.M. LSD and alcoholism: A clinical study of treatment efficacy. J. Chronic Dis. 1971, 24, 597. [Google Scholar] [CrossRef]
- Dahlberg, C.C. Lysergic Acid Diethylamide (LSD) in the Treatment of Alcoholism. Arch. Gen. Psychiatry 1968, 19, 508. [Google Scholar] [CrossRef]
- Simmons, J.Q.; Benor, D.; Daniel, D. The variable effects of LSD-25 on the behavior of a heterogeneous group of childhood schizophrenics. Behav. Neuropsychiatry 1972, 4, 10–24. [Google Scholar] [PubMed]
- Ball, J.R.; Armstrong, J.J. The use of L.S.D. 25 (D-lysergic acid diethylamide) in the treatment of the sexual perversions. Can. Psychiatr. Assoc. J. 1961, 6, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Sigafoos, J.; Green, V.A.; Edrisinha, C.; Lancioni, G.E. Flashback to the 1960s: LSD in the treatment of autism. Dev. Neurorehabilit. 2007, 10, 75–81. [Google Scholar] [CrossRef]
- Zamaria, J.A. A Phenomenological Examination of Psilocybin and its Positive and Persisting Aftereffects. NeuroQuantology 2016, 14. [Google Scholar] [CrossRef] [Green Version]
- Mithoefer, M.C.; Grob, C.S.; Brewerton, T.D. Novel psychopharmacological therapies for psychiatric disorders: Psilocybin and MDMA. Lancet Psychiatry 2016, 3, 481–488. [Google Scholar] [CrossRef]
- Sessa, B.; Sakal, C.; O’Brien, S.; Nutt, D. First study of safety and tolerability of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy in patients with alcohol use disorder: Preliminary data on the first four participants. BMJ Case Rep. 2019, 12, e230109. [Google Scholar] [CrossRef]
- Zarley, B.D. First-of-Its-Kind Pilot Study Uses MDMA for Alcohol Addiction. 28 February 2021. Available online: https://www.freethink.com/articles/mdma-for-alcohol-addiction (accessed on 10 March 2021).
- Ot’alora, G.M.; Grigsby, J.; Poulter, B.; Van Derveer, J.W., 3rd; Giron, S.G.; Jerome, L.; Feduccia, A.A.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; et al. 3,4-Methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: A randomized phase 2 controlled trial. J. Psychopharmacol. 2018, 32, 1295–1307. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.K.; Barsuglia, J.P.; Lancelotta, R.; Grant, R.M.; Renn, E. The epidemiology of 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) use: Benefits, consequences, patterns of use, subjective effects, and reasons for consumption. J. Psychopharmacol. 2018, 32, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; So, S.; Lancelotta, R.; Barsuglia, J.P.; Griffiths, R.R. 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) used in a naturalistic group setting is associated with unintended improvements in depression and anxiety. Am. J. Drug Alcohol Abus. 2019, 45, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Lima da Cruz, R.V.; Moulin, T.C.; Petiz, L.L.; Leão, R.N. A Single Dose of 5-MeO-DMT Stimulates Cell Proliferation, Neuronal Survivability, Morphological and Functional Changes in Adult Mice Ventral Dentate Gyrus. Front. Mol. Neurosci. 2018, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Lancelotta, R.L.; Davis, A.K. Use of Benefit Enhancement Strategies among 5-Methoxy-N,N-Dimethyltryptamine (5-MeO-DMT) Users: Associations with Mystical, Challenging, and Enduring Effects. J. Psychoact. Drugs 2020, 52, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A.M.; Claveau, R.; Lancelotta, R.; Kaylo, K.W.; Lenoch, K. Synthesis and Characterization of 5-MeO-DMT Succinate for Clinical Use. ACS Omega 2020, 5, 32067–32075. [Google Scholar] [CrossRef] [PubMed]
- Bahji, A.; Forsyth, A.; Groll, D.; Hawken, E.R. Efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: A systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 96, 109735. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Osório, F.L.; Rocha, J.M.; Rossi, G.N.; Bouso, J.C.; Rodrigues, L.S.; de Oliveira Silveira, G.; Yonamine, M.; Hallak, J. Ayahuasca Improves Self-perception of Speech Performance in Subjects with Social Anxiety Disorder: A Pilot, Proof-of-Concept, Randomized, Placebo-Controlled Trial. J. Clin. Psychopharmacol. 2021. [Google Scholar] [CrossRef]
- Leonard, J. Ayahuasca: What It Is, Effects, and Usage (1182927539 885508185 Warwick, K.W., Ed.). 31 January 2021. Available online: https://www.medicalnewstoday.com/articles/Ayahuasca (accessed on 7 February 2021).
- Eisner, B. Set, setting, and matrix. J. Psychoact. Drugs 1997, 29, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Tupper, K.W. The globalization of Ayahuasca: Harm reduction or benefit maximization? Int. J. Drug Policy 2008, 19, 297–303. [Google Scholar] [CrossRef]
- Scaccia, A. What Psychedelics Really Do to Your Brain. Rolling Stone. 25 June 2018. Available online: https://www.rollingstone.com/culture/culture-news/what-psychedelics-really-do-to-your-brain-112948/ (accessed on 10 March 2021).
- Stafford, P.G.; Bigwood, J. Psychedelics Encyclopedia; Ronin Publishing: Berkeley, CA, USA, 2013. [Google Scholar]
- Inaba, D.; Cohen, W.E. Uppers, Downers, All Arounders: Physical and Mental Effects of Psychoactive Drugs, 7th ed.; CNS Productions: Medford, OR, USA, 2014. [Google Scholar]
- Nichols, D.E. Psilocybin: From ancient magic to modern medicine. J. Antibiot. 2020, 73, 679–686. [Google Scholar] [CrossRef]
- Hollister, L.E. Some general thoughts about endogenous psychotogens. In Neuroregulators and Psychiatric Disorders; Usdin, E., Hamburg, D.A., Barchassca, J.D., Eds.; Oxford University Press: New York, NY, USA, 1977; pp. 550–556. [Google Scholar]
- Saavedra, J.M.; Axelrod, J. Psychotomimetic N-methylated tryptamines: Formation in brain in vivo and in vitro. Science 1972, 175, 1365–1366. [Google Scholar] [CrossRef]
- Checkley, S.A.; Murray, R.M.; Oon, M.C.; Rodnight, R.; Birley, J.L. A longitudinal study of urinary excretion of N,N,-dimethyltryptamine in psychotic patients. Br. J. Psychiatry J. Ment. Sci. 1980, 137, 236–239. [Google Scholar] [CrossRef]
- Dean, J.; Liu, T.; Huff, S.; Sheler, B.; Barker, S.A.; Strassman, R.J.; Wang, M.M.; Borjigin, J. Biosynthesis and Extracellular Concentrations of N,N-dimethyltryptamine (DMT) in Mammalian Brain. Sci. Rep. 2019, 9, 9333. [Google Scholar] [CrossRef] [PubMed]
- Gillin, J.C.; Kaplan, J.; Stillman, R.; Wyatt, R.J. The psychedelic model of schizophrenia: The case of N,N-dimethyltryptamine. Am. J. Psychiatry 1976, 133, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.S.; Presti, D.E. Endogenous psychoactive tryptamines reconsidered: An anxiolytic role for dimethyltryptamine. Med. Hypotheses 2005, 64, 930–937. [Google Scholar] [CrossRef]
- Murray, R.M.; Oon, M.C.; Rodnight, R.; Birley, J.L.; Smith, A. Increased excretion of dimethyltryptamine and certain features of psychosis: A possible association. Arch. Gen. Psychiatry 1979, 36, 644–649. [Google Scholar] [CrossRef]
- Pomilio, A.B.; Vitale, A.A.; Ciprian-Ollivier, J.; Cetkovich-Bakmas, M.; Gómez, R.; Vázquez, G. Ayahoasca: An experimental psychosis that mirrors the transmethylation hypothesis of schizophrenia. J. Ethnopharmacol. 1999, 65, 29–51. [Google Scholar] [CrossRef]
- Grammenos, D.; Barker, S.A. On the transmethylation hypothesis: Stress, N,N-dimethyltryptamine, and positive symptoms of psychosis. J. Neural Transm. 2015, 122, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Smythies, J.R. The transmethylation hypotheses of schizophrenia re-evaluated. Trends Neurosci. 1984, 7, 45–47. [Google Scholar] [CrossRef]
- Marazziti, D. Understanding the role of serotonin in psychiatric diseases. F1000Research 2017, 6, 180. [Google Scholar] [CrossRef] [Green Version]
- Strassman, R.J. Human psychopharmacology of N,N-dimethyltryptamine. Behav. Brain Res. 1996, 73, 121–124. [Google Scholar] [CrossRef]
- Gouzoulis-Mayfrank, E.; Heekeren, K.; Neukirch, A.; Stoll, M.; Stock, C.; Obradovic, M.; Kovar, K.A. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): A double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry 2005, 38, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Paterson, N.E.; Darby, W.C.; Sandhu, P.S. N,N-Dimethyltryptamine-Induced Psychosis. Clin. Neuropharmacol. 2015, 38, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Strassman, R.J.; Qualls, C.R. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch. Gen. Psychiatry 1994, 51, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Dmt. 26 June 2015. Available online: https://www.release.org.uk/drugs/dmt/pharmacology (accessed on 7 February 2021).
- McKenna, D.J.; Towers, G.H.; Abbott, F. Monoamine oxidase inhibitors in South American hallucinogenic plants: Tryptamine and beta-carboline constituents of Ayahuasca. J. Ethnopharmacol. 1984, 10, 195–223. [Google Scholar] [CrossRef]
- Halberstadt, A.L. Behavioral and pharmacokinetic interactions between monoamine oxidase inhibitors and the hallucinogen 5-methoxy-N,N-dimethyltryptamine. Pharmacol. Biochem. Behav. 2016, 143, 1–10. [Google Scholar] [CrossRef] [Green Version]
- An Option if Other Antidepressants Haven’t Helped. 12 September 2019. Available online: https://www.mayoclinic.org/diseases-conditions/depression/in-depth/maois/art-20043992 (accessed on 7 February 2021).
- Szabo, A.; Kovacs, A.; Riba, J.; Djurovic, S.; Rajnavolgyi, E.; Frecska, E. The Endogenous Hallucinogen and Trace Amine N,N-Dimethyltryptamine (DMT) Displays Potent Protective Effects against Hypoxia via Sigma-1 Receptor Activation in Human Primary iPSC-Derived Cortical Neurons and Microglia-Like Immune Cells. Front. Neurosci. 2016, 10, 423. [Google Scholar] [CrossRef] [Green Version]
- Frecska, E.; Bokor, P.; Winkelman, M. The Therapeutic Potentials of Ayahuasca: Possible Effects against Various Diseases of Civilization. Front. Pharmacol. 2016, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Inserra, A. Hypothesis: The Psychedelic Ayahuasca Heals Traumatic Memories via a Sigma 1 Receptor-Mediated Epigenetic-Mnemonic Process. Front. Pharmacol. 2018, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Fontanilla, D.; Gopalakrishnan, A.; Chae, Y.K.; Markley, J.L.; Ruoho, A.E. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur. J. Pharmacol. 2012, 682, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Carbonaro, T.M.; Gatch, M.B. Neuropharmacology of N,N-Dimethyltryptamine. Brain Res. Bull. 2016, 126, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Malcolm, B.J.; Lee, K.C. Ayahuasca: An ancient sacrament for treatment of contemporary psychiatric illness? Ment. Health Clin. 2018, 7, 39–45. [Google Scholar] [CrossRef]
- Ruffell, S.; Netzband, N.; Bird, C.; Young, A.H.; Juruena, M.F. The pharmacological interaction of compounds in Ayahuasca: A systematic review. Rev. Bras. Psiquiatr. 2020, 42, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Albarracin-Jordan, J.; Moore, C.; Capriles, J.M. Chemical evidence for the use of multiple psychotropic plants in a 1,000-year-old ritual bundle from South America. Proc. Natl. Acad. Sci. USA 2019, 116, 11207–11212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savoldi, R.; Polari, D.; Pinheiro-da-Silva, J.; Silva, P.F.; Lobao-Soares, B.; Yonamine, M.; Freire, F.; Luchiari, A.C. Behavioral Changes Over Time Following Ayahuasca Exposure in Zebrafish. Front. Behav. Neurosci. 2017, 11, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labate, B.C.; Macrae, E. Ayahuasca, Ritual and Religion in Brazil; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Herraiz, T.; González, D.; Ancín-Azpilicueta, C.; Arán, V.J.; Guillén, H. β-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO). Food Chem. Toxicol. 2010, 48, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Morales-García, J.A.; de la Fuente Revenga, M.; Alonso-Gil, S.; Rodríguez-Franco, M.I.; Feilding, A.; Perez-Castillo, A.; Riba, J. The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro. Sci. Rep. 2017, 7, 5309. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Garrido, D.F.; Gómez-Sousa, M.; Ona, G.; Dos Santos, R.G.; Hallak, J.; Alcázar-Córcoles, M.Á.; Bouso, J.C. Effects of Ayahuasca on mental health and quality of life in naïve users: A longitudinal and cross-sectional study combination. Sci. Rep. 2020, 10, 4075. [Google Scholar] [CrossRef] [Green Version]
- McKenna, D.J. Clinical investigations of the therapeutic potential of Ayahuasca: Rationale and regulatory challenges. Pharmacol. Ther. 2004, 102, 111–129. [Google Scholar] [CrossRef]
- Manske, R.H. A synthesis of the methyltryptamines and some derivatives. Can. J. Res. 1931, 5, 592–600. [Google Scholar] [CrossRef]
- Bigwood, J.; Ott, J. GWU Event on Colombia. for Wola if You Need Higher Resolution, Please e-mail Me, and I Will Send Them in Hi-res. November 1977. Available online: https://web.archive.org/web/20060127003553/ (accessed on 4 March 2021).
- Sanches, R.F.; de Lima Osório, F.; Dos Santos, R.G.; Macedo, L.R.; Maia-de-Oliveira, J.P.; Wichert-Ana, L.; de Araujo, D.B.; Riba, J.; Crippa, J.A.; Hallak, J.E. Antidepressant Effects of a Single Dose of Ayahuasca in Patients with Recurrent Depression: A SPECT Study. J. Clin. Psychopharmacol. 2016, 36, 77–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, R.G.; Landeira-Fernandez, J.; Strassman, R.J.; Motta, V.; Cruz, A.P. Effects of Ayahuasca on psychometric measures of anxiety, panic-like and hopelessness in Santo Daime members. J. Ethnopharmacol. 2007, 112, 507–513. [Google Scholar] [CrossRef]
- Zeifman, R.J.; Palhano-Fontes, F.; Hallak, J.; Arcoverde, E.; Maia-Oliveira, J.P.; Araujo, D.B. The Impact of Ayahuasca on Suicidality: Results From a Randomized Controlled Trial. Front. Pharmacol. 2019, 10, 1325. [Google Scholar] [CrossRef]
- Uthaug, M.V.; van Oorsouw, K.; Kuypers, K.; van Boxtel, M.; Broers, N.J.; Mason, N.L.; Toennes, S.W.; Riba, J.; Ramaekers, J.G. Sub-acute and long-term effects of Ayahuasca on affect and cognitive thinking style and their association with ego dissolution. Psychopharmacology 2018, 235, 2979–2989. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.; Elices, M.; Dominguez-Clavé, E.; Pascual, J.C.; Feilding, A.; Navarro-Gil, M.; García-Campayo, J.; Riba, J. Four Weekly Ayahuasca Sessions Lead to Increases in “Acceptance” Capacities: A Comparison Study with a Standard 8-Week Mindfulness Training Program. Front. Pharmacol. 2018, 9, 224. [Google Scholar] [CrossRef] [Green Version]
- Galvão, A.; de Almeida, R.N.; Silva, E.; Freire, F.; Palhano-Fontes, F.; Onias, H.; Arcoverde, E.; Maia-de-Oliveira, J.P.; de Araújo, D.B.; Lobão-Soares, B.; et al. Cortisol Modulation by Ayahuasca in Patients with Treatment Resistant Depression and Healthy Controls. Front. Psychiatry 2018, 9, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, R.G.; Hallak, J.E. Effects of the Natural β-Carboline Alkaloid Harmine, a Main Constituent of Ayahuasca, in Memory and in the Hippocampus: A Systematic Literature Review of Preclinical Studies. J. Psychoact. Drugs 2017, 49, 1–10. [Google Scholar] [CrossRef]
- Loizaga-Velder, A.; Verres, R. Therapeutic effects of ritual Ayahuasca use in the treatment of substance dependence—qualitative results. J. Psychoact. Drugs 2014, 46, 63–72. [Google Scholar] [CrossRef]
- Thomas, G.; Lucas, P.; Capler, N.R.; Tupper, K.W.; Martin, G. Ayahuasca-assisted therapy for addiction: Results from a preliminary observational study in Canada. Curr. Drug Abus. Rev. 2013, 6, 30–42. [Google Scholar] [CrossRef]
- Palhano-Fontes, F.; Andrade, K.C.; Tofoli, L.F.; Santos, A.C.; Crippa, J.A.S.; Hallak, J.E.C.; Ribeiro, S.; de Araujo, D.B. The psychedelic state induced by Ayahuasca modulates the activity and connectivity of the default mode network. PLoS ONE 2015, 10, e0118143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, L.Q.; Kelly, A.M.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum. Brain Mapp. 2009, 30, 625–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.H.; Jung, W.H.; Kang, D.H.; Byun, M.S.; Kwon, S.J.; Choi, C.H.; Kwon, J.S. Increased default mode network connectivity associated with meditation. Neurosci. Lett. 2011, 487, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Kiviniemi, V.; Tervonen, O.; Vainionpää, V.; Alahuhta, S.; Reiss, A.L.; Menon, V. Persistent default-mode network connectivity during light sedation. Hum. Brain Mapp. 2008, 29, 839–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horovitz, S.G.; Braun, A.R.; Carr, W.S.; Picchioni, D.; Balkin, T.J.; Fukunaga, M.; Duyn, J.H. Decoupling of the brain’s default mode network during deep sleep. Proc. Natl. Acad. Sci. USA 2009, 106, 11376–11381. [Google Scholar] [CrossRef] [Green Version]
- Schrouff, J.; Perlbarg, V.; Boly, M.; Marrelec, G.; Boveroux, P.; Vanhaudenhuyse, A.; Bruno, M.A.; Laureys, S.; Phillips, C.; Pélégrini-Issac, M.; et al. Brain functional integration decreases during propofol-induced loss of consciousness. NeuroImage 2011, 57, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K.; et al. Neural correlates of the psychedelic state as determined by fMRI studies with Psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carhart-Harris, R.L.; Leech, R.; Erritzoe, D.; Williams, T.M.; Stone, J.M.; Evans, J.; Sharp, D.J.; Feilding, A.; Wise, R.G.; Nutt, D.J. Functional connectivity measures after Psilocybin inform a novel hypothesis of early psychosis. Schizophr. Bull. 2013, 39, 1343–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, M.F.; Norton, M.I.; Van Horn, J.D.; Wegner, D.M.; Grafton, S.T.; Macrae, C.N. Wandering minds: The default network and stimulus-independent thought. Science 2007, 315, 393–395. [Google Scholar] [CrossRef] [Green Version]
- Brewer, J.A.; Worhunsky, P.D.; Gray, J.R.; Tang, Y.Y.; Weber, J.; Kober, H. Meditation experience is associated with differences in default mode network activity and connectivity. Proc. Natl. Acad. Sci. USA 2011, 108, 20254–20259. [Google Scholar] [CrossRef] [Green Version]
- Sämann, P.G.; Wehrle, R.; Hoehn, D.; Spoormaker, V.I.; Peters, H.; Tully, C.; Holsboer, F.; Czisch, M. Development of the brain’s default mode network from wakefulness to slow wave sleep. Cereb. Cortex 2011, 21, 2082–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, J. Ayahuasca Tourism: The Commercialization of Culture. 2 August 2019. Available online: https://worldfootprints.com/Ayahuasca-tourism-the-commercialization-of-culture/ (accessed on 8 February 2021).
- Draven, J. Hot Topic: Is Ayahuasca Tourism a Bad Trip? 8 April 2019. Available online: https://www.nationalgeographic.co.uk/travel/2017/03/hot-topic-Ayahuasca-tourism-bad-trip (accessed on 8 February 2021).
- Kavenská, V.; Simonová, H. Ayahuasca Tourism: Participants in Shamanic Rituals and their Personality Styles, Motivation, Benefits and Risks. J. Psychoact. Drugs 2015, 47, 351–359. [Google Scholar] [CrossRef]
- Hill, D. Peru’s Ayahuasca Industry Booms as Westerners Search for Alternative Healing. 7 June 2016. Available online: https://www.theguardian.com/travel/2016/jun/07/peru-Ayahuasca-drink-boom-amazon-spirituality-healing (accessed on 9 February 2021).
- Braczkowski, A.; Ruzo, A.; Sanchez, F.; Castagnino, R.; Brown, C.; Guynup, S.; Winter, S.; Gandy, D.; O’Bryan, C.J. The Ayahuasca tourism boom: An undervalued demand driver for jaguar body parts? Conserv. Sci. Pract. 2019, 1, Ee126. [Google Scholar] [CrossRef] [Green Version]
- Crisafulli, A. Ayahuasca Tourism: Shamans, Charlatans AND Thousand-Dollar Retreats. 2019. Available online: https://www.vergemagazine.com/travel-intelligence/beyond-the-guidebook/2495-is-Ayahuasca-tourism-safe-and-ethical.html (accessed on 9 February 2021).
- Babe, A. Ayahuasca Tourism is Ripping off Indigenous Amazonians. May 2016. Available online: https://www.vice.com/en/article/qbn8vq/Ayahuasca-tourism-is-ripping-off-indigenous-amazonians (accessed on 8 February 2021).
- Vaughn, C. Ayahuasca Ceremonies and Tourism Return to Costa Rica. 25 October 2020. Available online: https://news.co.cr/Ayahuasca-ceremonies-and-tourism-return-to-costa-rica/82489/ (accessed on 9 February 2021).
- Matthews, K. Visiting Brazil for an Ayahuasca Ceremony. 20 December 2019. Available online: https://thetravelmanuel.com/visiting-brazil-for-an-Ayahuasca-ceremony/ (accessed on 9 February 2021).
- Fraser, B. The Perils and Privileges of an Amazonian Hallucinogen. 8 August 2017. Available online: https://www.sapiens.org/culture/Ayahuasca-tourism-amazon/ (accessed on 9 February 2021).
- Om Spirit. Ayahuasca Retreat Sacred Valley Tribe. 29 December 2018. Available online: https://omspirit.net/retrets-centers/ayahuasca-retreat-sacred-valley-tribe/ (accessed on 9 February 2021).
- Amor, B. Ayahuasca is the Latest TRENDY Tonic for White People Problems. 21 June 2019. Available online: https://www.bitchmedia.org/article/heart-of-whiteness-spiritual-tourism-colonization-Ayahuasca (accessed on 9 February 2021).
- Hay, M. The Colonization of the Ayahuasca Experience. JSTOR Daily. 20 November 2020. Available online: https://daily.jstor.org/the-colonization-of-the-ayahuasca-experience/ (accessed on 9 February 2021).
- Krebs-Thomson, K.; Ruiz, E.M.; Masten, V.; Buell, M.; Geyer, M.A. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology 2006, 189, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.S. Psychedelics and the human receptorome. PLoS ONE 2010, 5, e9019. [Google Scholar] [CrossRef]
- Shen, H.W.; Jiang, X.L.; Winter, J.C.; Yu, A.M. Psychedelic 5-methoxy-N,N-dimethyltryptamine: Metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Curr. Drug Metab. 2010, 11, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Malaca, S.; Lo Faro, A.F.; Tamborra, A.; Pichini, S.; Busardò, F.P.; Huestis, M.A. Toxicology and Analysis of Psychoactive Tryptamines. Int. J. Mol. Sci. 2020, 21, 9279. [Google Scholar] [CrossRef]
- Hoshino, T.; Shimodaira, K. Über die synthese des bufotenin-methyl-äthers (5-methoxy-n-dimethyl-tryptamin) und bufotenins (synthesen in der indol-gruppe. xv). Bull. Chem. Soc. Jpn. 1936, 11, 221–224. [Google Scholar] [CrossRef]
- Pachter, I.J.Z.; Ribeiro, D.E.; Ribeiro, O. Indole alkaloids of acer saccharinum (the silver maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis. J. Org. Chem. 1959, 24, 1285–1287. [Google Scholar] [CrossRef]
- Weil, A.T.; Davis, W. Bufo alvarius: A potent hallucinogen of animal origin. J. Ethnopharmacol. 1994, 41, 1–8. [Google Scholar] [CrossRef]
- Guide to 5-MeO-DMT-EXPERIENCE, BENEFITS, & Side Effects. 5 January 2021. Available online: https://thethirdwave.co/psychedelics/5-meo-dmt/ (accessed on 5 February 2021).
- Uthaug, M.V.; Lancelotta, R.; van Oorsouw, K.; Kuypers, K.; Mason, N.; Rak, J.; Šuláková, A.; Jurok, R.; Maryška, M.; Kuchař, M.; et al. A single inhalation of vapor from dried toad secretion containing 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in a naturalistic setting is related to sustained enhancement of satisfaction with life, mindfulness-related capacities, and a decrement of psychopathological symptoms. Psychopharmacology 2019, 236, 2653–2666. [Google Scholar] [CrossRef] [Green Version]
- Araújo, A.M.; Carvalho, F.; Bastos, M.; Guedes de Pinho, P.; Carvalho, M. The hallucinogenic world of tryptamines: An updated review. Arch. Toxicol. 2015, 89, 1151–1173. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Buell, M.R.; Masten, V.L.; Risbrough, V.B.; Geyer, M.A. Modification of the effects of 5-methoxy-N,N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology 2008, 201, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.L.; Shen, H.W.; Mager, D.E.; Yu, A.M. Pharmacokinetic interactions between monoamine oxidase A inhibitor harmaline and 5-methoxy-N,N-dimethyltryptamine, and the impact of CYP2D6 status. Drug Metab. Dispos. Biol. Fate Chem. 2013, 41, 975–986. [Google Scholar] [CrossRef] [Green Version]
- Gale, E. Bufo Alvarius-Colorado River Toad Sonoran Desert Toad. 11 April 2003. Available online: https://www.erowid.org/archive/sonoran_desert_toad/deeptoad.htm (accessed on 10 March 2021).
- Pochettino, M.L.; Cortella, A.R.; Ruiz, M. Hallucinogenic snuff from Northwestern Argentina: Microscopical identification of anadenanthera colubrina var. cebil (fabaceae) in powdered archaeological material. Econ. Bot. 1999, 53, 127–132. [Google Scholar] [CrossRef]
- Carod-Artal, F.J.; Vázquez Cabrera, C.B. Usos rituales de la semilla de Anadenanthera sp entre los indígenas sudamericanos [Ritual use of Anadenanthera seeds among South America natives]. Neurologia 2007, 22, 410–415. [Google Scholar]
- Schultes, R.E. The Plant Kingdom and Hallucinogens (Part II). 1 January 1969. Available online: https://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1969-01-01_4_page004.html (accessed on 5 February 2021).
- Pipeline: GH Research. GH Research Limited (n.d.). Available online: https://www.ghres.com/pipeline (accessed on 10 March 2021).
- Lysergide (LSD) drug profile. European Monitoring Centre for Drugs and Drug Addiction. 1 January 2021. Available online: https://www.emcdda.europa.eu/publications/drug-profiles/lsd_en (accessed on 10 March 2021).
- Whelan, A.; Johnson, M.I. Lysergic acid diethylamide and Psilocybin for the management of patients with persistent pain: A potential role? Pain Manag. 2018, 8, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Brandt, S.D.; Kavanagh, P.V.; Twamley, B.; Westphal, F.; Elliott, S.P.; Wallach, J.; Stratford, A.; Klein, L.M.; McCorvy, J.D.; Nichols, D.E.; et al. Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM-775). Drug Test. Anal. 2018, 10, 310–322. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Klein, L.M.; Chatha, M.; Valenzuela, L.B.; Stratford, A.; Wallach, J.; Nichols, D.E.; Brandt, S.D. Pharmacological characterization of the LSD analog N-ethyl-N-cyclopropyl lysergamide (ECPLA). Psychopharmacology 2019, 236, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Watts, V.J.; Lawler, C.P.; Fox, D.R.; Neve, K.A.; Nichols, D.E.; Mailman, R.B. LSD and structural analogs: Pharmacological evaluation at D1 dopamine receptors. Psychopharmacology 1995, 118, 401–409. [Google Scholar] [CrossRef]
- Hoffman, A.J.; Nichols, D.E. Synthesis and LSD-like discriminative stimulus properties in a series of N(6)-alkyl norlysergic acid N,N-diethylamide derivatives. J. Med. Chem. 1985, 28, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Marona-Lewicka, D.; Pfaff, R.C.; Nichols, D.E. Drug discrimination and receptor binding studies of N-isopropyl lysergamide derivatives. Pharmacol. Biochem. Behav. 1994, 47, 667–673. [Google Scholar] [CrossRef]
- Fuentes, J.J.; Fonseca, F.; Elices, M.; Farré, M.; Torrens, M. Therapeutic Use of LSD in Psychiatry: A Systematic Review of Randomized-Controlled Clinical Trials. Front. Psychiatry 2020, 10, 943. [Google Scholar] [CrossRef] [Green Version]
- Davies, B. Lysergic acid (LSD 25) and ritalin in the treatment of neurosis. J. Psychosom. Res. 1964, 8, 164–165. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Barnwal, P.; Ramasamy, A.; Sen, S.; Mondal, S. Lysergic acid diethylamide: A drug of ‘use’? Ther. Adv. Psychopharmacol. 2016, 6, 214–228. [Google Scholar] [CrossRef] [Green Version]
- Jordy, S.S. Book Reviews-LSD and alcoholism; a clinical study of treatment efficacy. Q. J. Stud. Alcohol 1971, 32, 589–592. [Google Scholar] [CrossRef]
- McBroom, P. LSD vs. Alcoholism. Sci. News 1968, 93, 578. [Google Scholar] [CrossRef]
- Szabo, A. Psychedelics and Immunomodulation: Novel Approaches and Therapeutic Opportunities. Front. Immunol. 2015, 6, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Family, N.; Maillet, E.L.; Williams, L.T.J.; Krediet, E.; Carhart-Harris, R.L.; Williams, T.M.; Nichols, C.D.; Goble, D.J.; Raz, S. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology 2020, 237, 841–853. [Google Scholar] [CrossRef] [Green Version]
- Bender, L.; Goldschmidt, L.; Sankar, D.V.S.; Freedman, A.M. Treatment of Autistic Schizophrenic Children with LSD-25 and UML-491. In Recent Advances in Biological Psychiatry; Wortis, J., Ed.; Springer: Boston, MA, USA, 1962. [Google Scholar] [CrossRef]
- Bender, L. D-lysergic acid in the treatment of the biological features of childhood schizophrenia. Dis. Nerv. Syst. 1966, 7 (Suppl. 7), 43–46. [Google Scholar]
- Freedman, A.M.; Ebin, E.V.; Wilson, E.A. Autistic Schizophrenic Children; An Experiment in the Use of D-Lysergic Acid Diethylamide (LSD-25). Arch. Gen. Psychiatry 1962, 6, 203–213. [Google Scholar] [CrossRef]
- Moller, H. The treatment of childhood schizophrenia in a public school system. Psychol. Sch. 1964, 1, 297–304. [Google Scholar] [CrossRef]
- Rice, M.E.; Harris, G.T.; Cormier, C.A. An evaluation of a maximum security therapeutic community for psychopaths and other mentally disordered offenders. Law Hum. Behav. 1992, 16, 399–412. [Google Scholar] [CrossRef]
- Harris, G.T.; Rice, M.E.; Cormier, C.A. Psychopaths: Is a therapeutic community therapeutic? Ther. Communities 1994, 15, 283–299. [Google Scholar]
- Reidy, D.E.; Kearns, M.C.; DeGue, S. Reducing psychopathic violence: A review of the treatment literature. Aggress. Violent Behav. 2013, 18, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Honigfeld, G. Temporal effects of LSD-25 and epinephrine on verbal behavior. PsycEXTRA Dataset. 1965, 70, 303. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.M.; Buckman, J. The Treatment of Frigidity with LSD and Ritalin. In The Psychedelic Reader: Selected from the Psychedelic Review; Weil, G.M., Metzner, R., Leary, T., Eds.; Place of publication not identified; University Books: New York, NY, USA, 1965; pp. 231–239. [Google Scholar]
- Davenport, W.J. Psychedelic and nonpsychedelic LSD and Psilocybin for cluster headache. Can. Med. Assoc. J. 2016, 188, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewell, R.A.; Halpern, J.H.; Pope, H.G. Response of cluster headache to Psilocybin and lsd. Neurology 2006, 66, 1920–1922. [Google Scholar] [CrossRef] [PubMed]
- Liechti, M.; Schmid, Y. Lysergic Acid Diethylamide (LSD) as Treatment for Cluster Headache (LCH). Identifier NCT03781128. (2 January 2019–December 2023). Available online: https://clinicaltrials.gov/ct2/show/NCT03781128 (accessed on 11 March 2021).
- Karst, M.; Halpern, J.H.; Bernateck, M.; Passie, T. The non-hallucinogen 2-bromo-lysergic acid diethylamide as preventative treatment for cluster headache: An open, non-randomized case series. Cephalalgia 2010, 30, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Schindler, E.A.; Gottschalk, C.H.; Weil, M.J.; Shapiro, R.E.; Wright, D.A.; Sewell, R.A. Indoleamine hallucinogens in cluster headache: Results of the clusterbusters medication use survey. J. Psychoact. Drugs 2015, 47, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Sicuteri, F. Prophylactic treatment of migraine by means of lysergic acid derivatives. Triangle 1963, 6, 116–125. [Google Scholar]
- Crowther, D.L. The prophylactic effect of 1-methyl-D-lysergic acid butanolamide (methysergide) in the treatment of vascular headache. A clinical study. Med. Exp. Int. J. Exp. Med. 1964, 10, 137–143. [Google Scholar]
- Fanciullacci, M.; Bene, E.D.; Franchi, G.; Sicuteri, F. Brief report: Phantom limp pain: Sub-hallucinogenic treatment with lysergic acid diethylamide (LSD-25). Headache 1977, 17, 118–119. [Google Scholar] [CrossRef]
- Liester, M.B. A review of lysergic acid diethylamide (LSD) in the treatment of addictions: Historical perspectives and future prospects. Curr. Drug Abus. Rev. 2014, 7, 146–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkelman, M. Psychedelics as medicines for substance Abuse Rehabilitation: Evaluating treatments with lsd, Peyote, ibogaine and Ayahuasca. Curr. Drug Abus. Rev. 2015, 7, 101–116. [Google Scholar] [CrossRef] [Green Version]
- Oehen, P.; Traber, R.; Widmer, V.; Schnyder, U. A randomized, controlled pilot study of MDMA (± 3,4-Methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD). J. Psychopharmacol. 2013, 27, 40–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mithoefer, M.C.; Wagner, M.T.; Mithoefer, A.T.; Jerome, L.; Doblin, R. The safety and efficacy of {+/-}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. J. Psychopharmacol. 2011, 25, 439–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoroso, T.; Workman, M. Treating posttraumatic stress disorder with MDMA-assisted psychotherapy: A preliminary meta-analysis and comparison to prolonged exposure therapy. J. Psychopharmacol. 2016, 30, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Psychedelic Drugs Market Projected to Reach $6859.95 Million by 2027. 3 June 2020. Available online: https://www.prnewswire.com/news-releases/psychedelic-drugs-market-projected-to-reach-6-859-95-million-by-2027--301069861.html (accessed on 16 February 2021).
- Europe Psychedelic Drugs Market Global Survey Report with COVID-19 Impact and Overwhelming Hike of BILLION-DOLLAR INDUSTRY: COMPASS, Johnson & JOHNSON, NeuroRX, Hikma Pharmaceuticals, Jazz Pharmaceuticals. 17 November 2020. Available online: https://apnews.com/press-release/wired-release/business-technology-lifestyle-products-and-services-mental-health-04591bf30586e804c554fe61fafddaf7 (accessed on 16 February 2021).
- Winkelman, M. Drug tourism or spiritual healing? Ayahuasca seekers in Amazonia. J. Psychoact. Drugs 2005, 37, 209–218. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lowe, H.; Toyang, N.; Steele, B.; Grant, J.; Ali, A.; Gordon, L.; Ngwa, W. Psychedelics: Alternative and Potential Therapeutic Options for Treating Mood and Anxiety Disorders. Molecules 2022, 27, 2520. https://doi.org/10.3390/molecules27082520
Lowe H, Toyang N, Steele B, Grant J, Ali A, Gordon L, Ngwa W. Psychedelics: Alternative and Potential Therapeutic Options for Treating Mood and Anxiety Disorders. Molecules. 2022; 27(8):2520. https://doi.org/10.3390/molecules27082520
Chicago/Turabian StyleLowe, Henry, Ngeh Toyang, Blair Steele, Justin Grant, Amza Ali, Lorenzo Gordon, and Wilfred Ngwa. 2022. "Psychedelics: Alternative and Potential Therapeutic Options for Treating Mood and Anxiety Disorders" Molecules 27, no. 8: 2520. https://doi.org/10.3390/molecules27082520





