A Significant Fluorescence Turn-On Probe for the Recognition of Al3+ and Its Application
Abstract
:1. Introduction
2. Results and Discussion
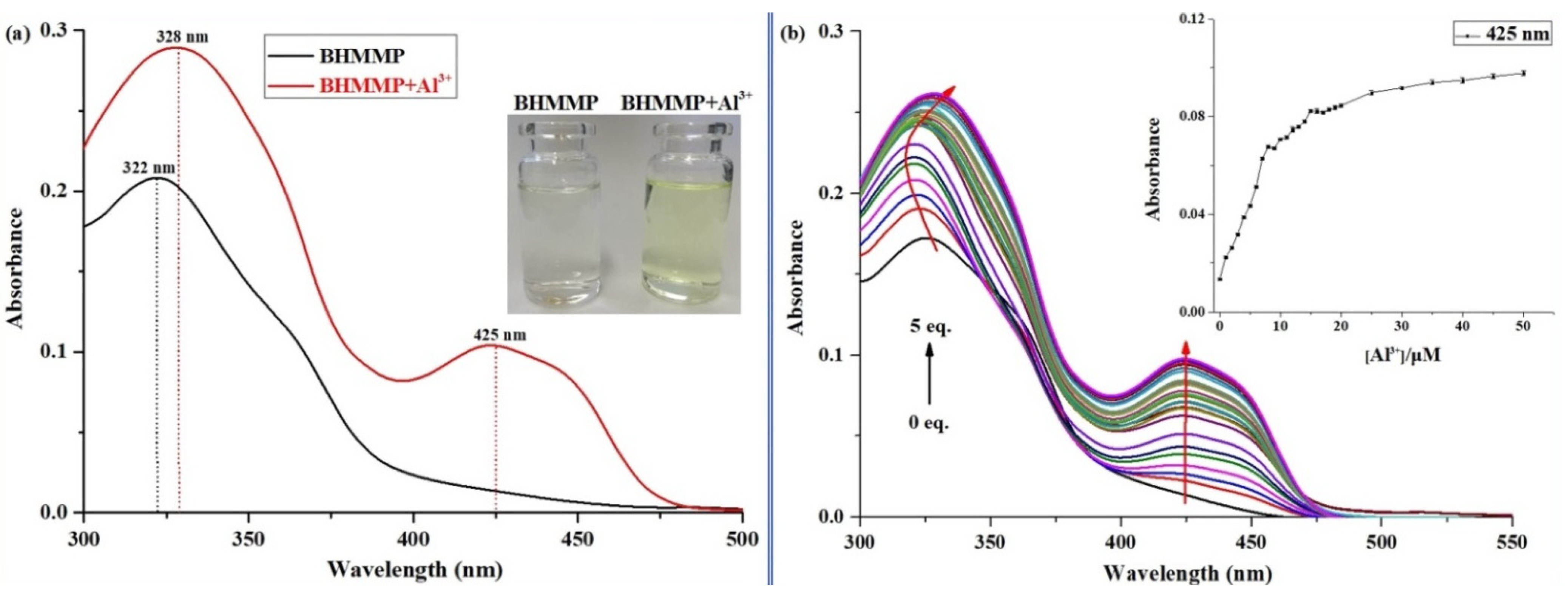
2.1. Fluorescence and UV–Vis Spectral Response of BHMMP to Al3+
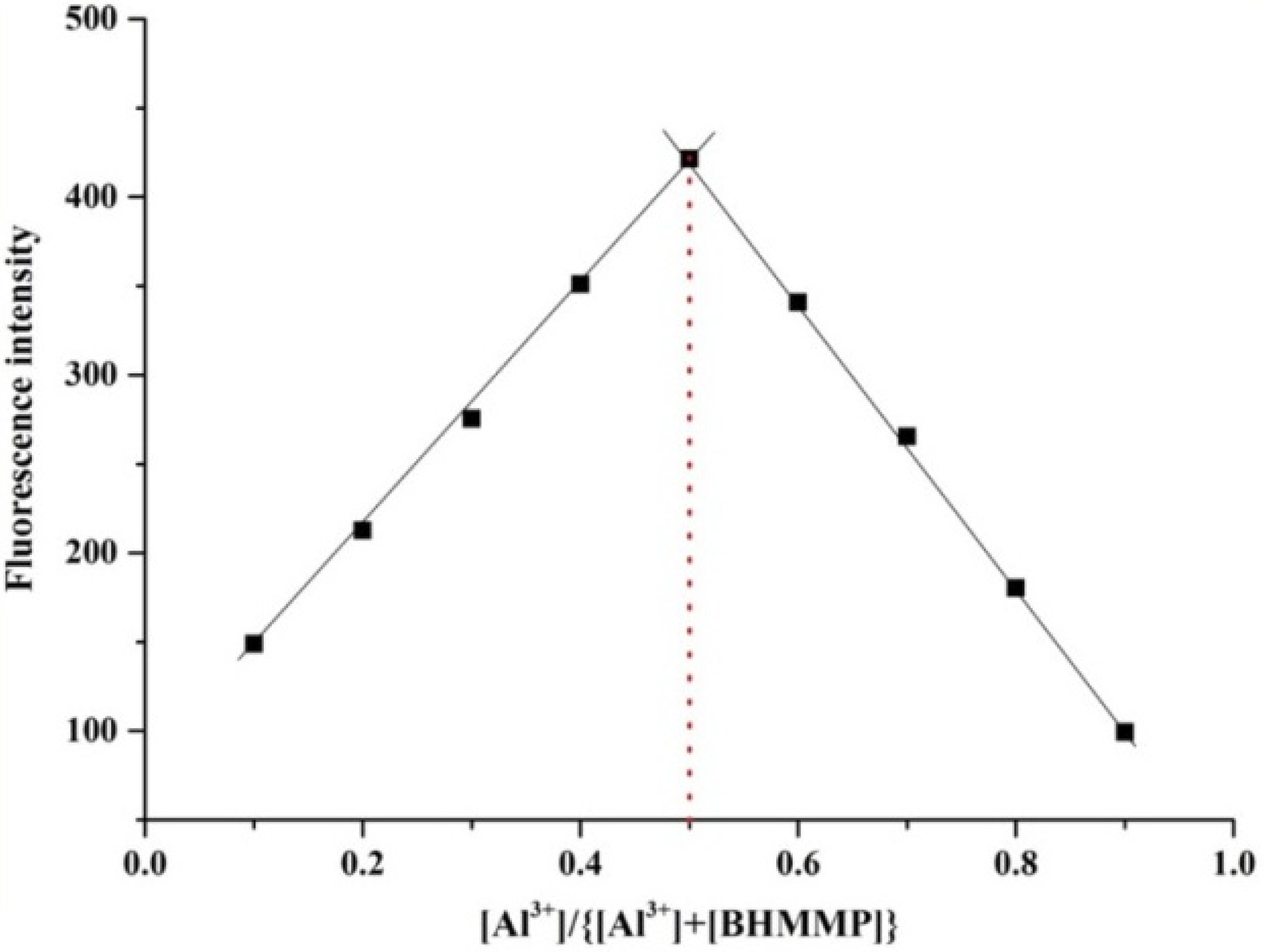
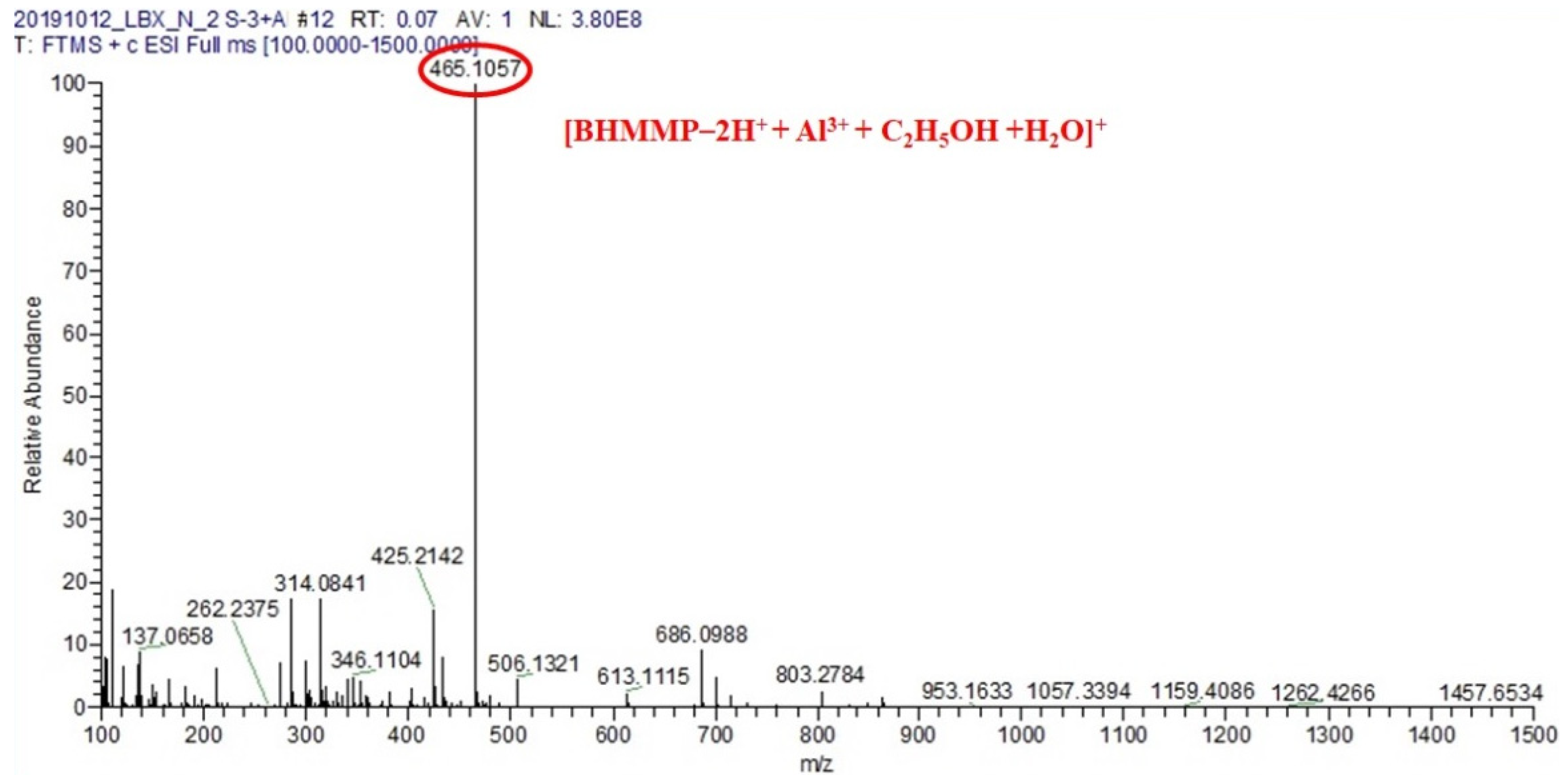
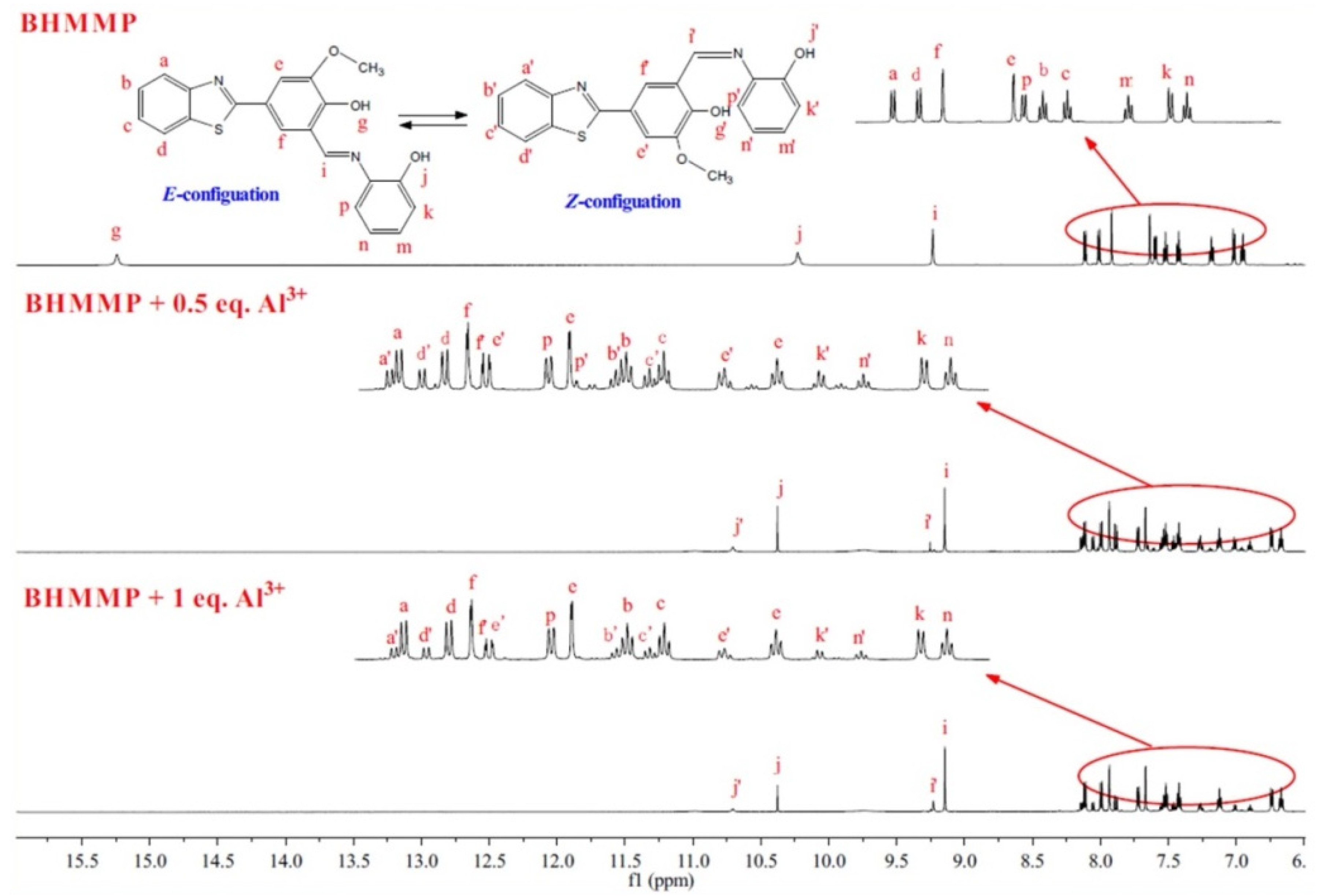
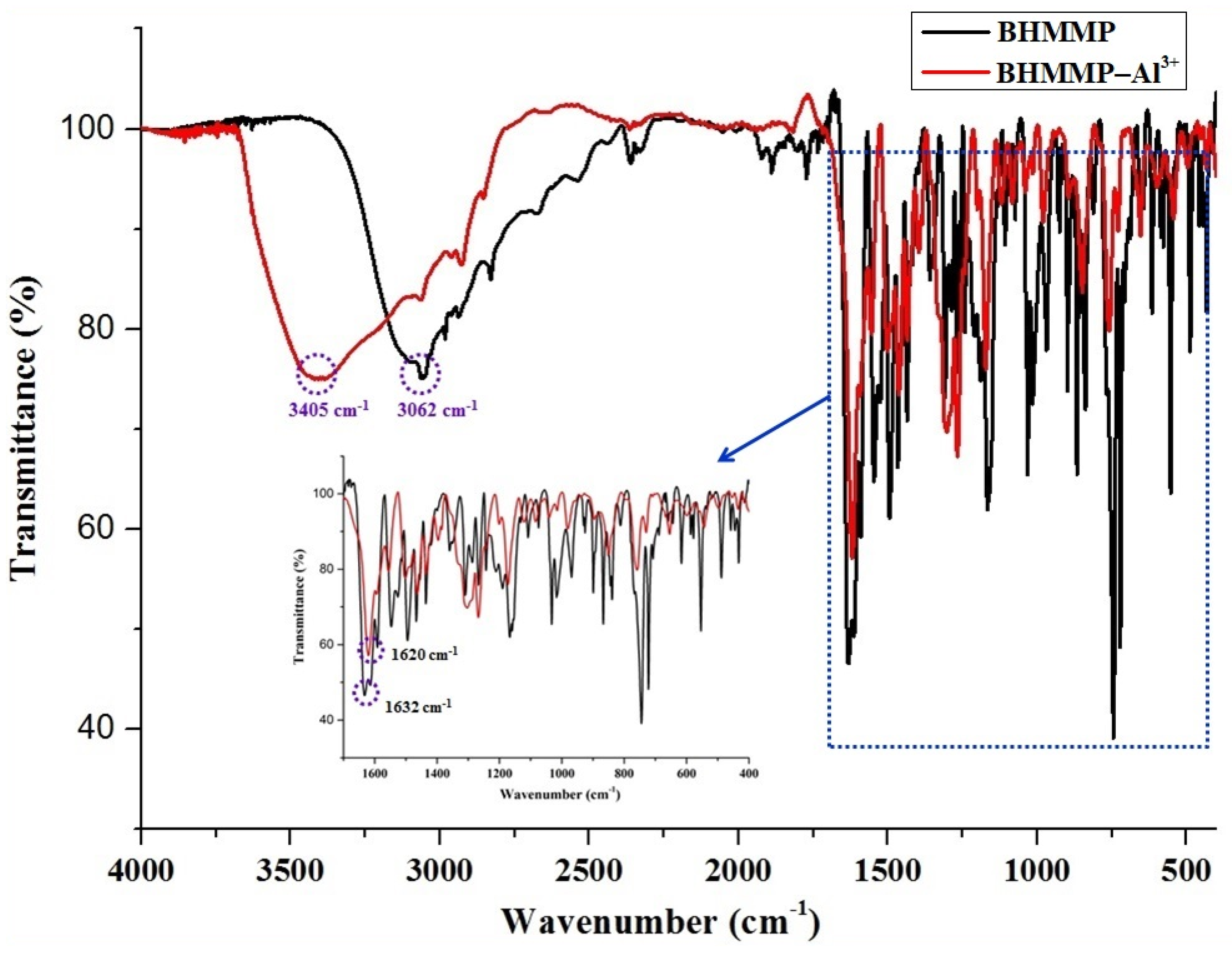
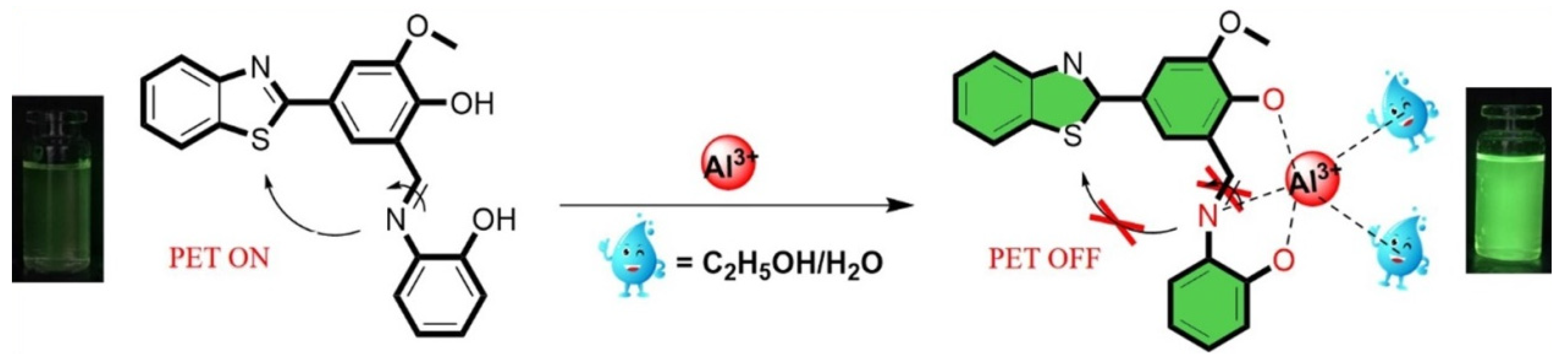
2.2. Binding Mode Studied
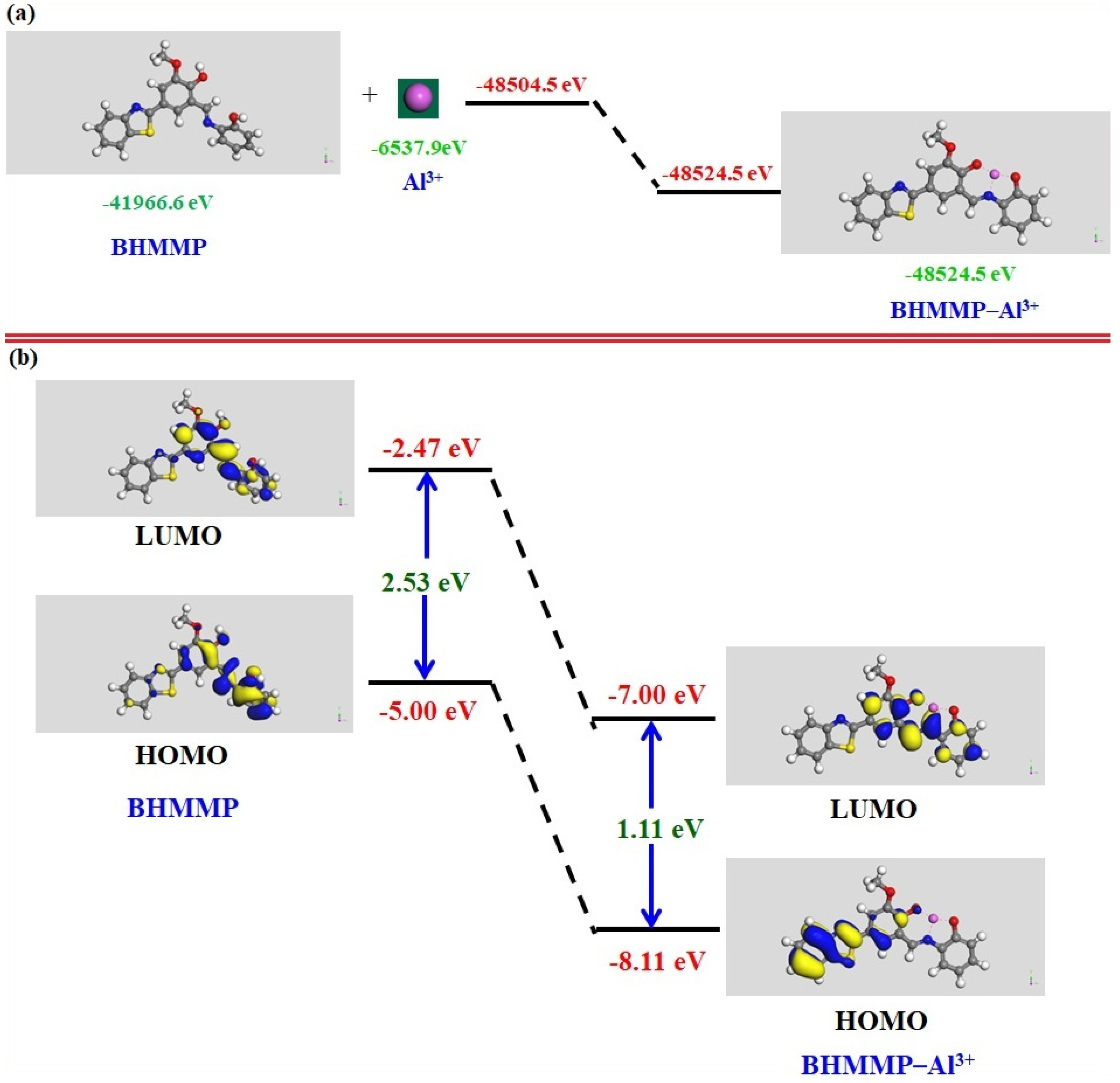
2.3. DFT Study
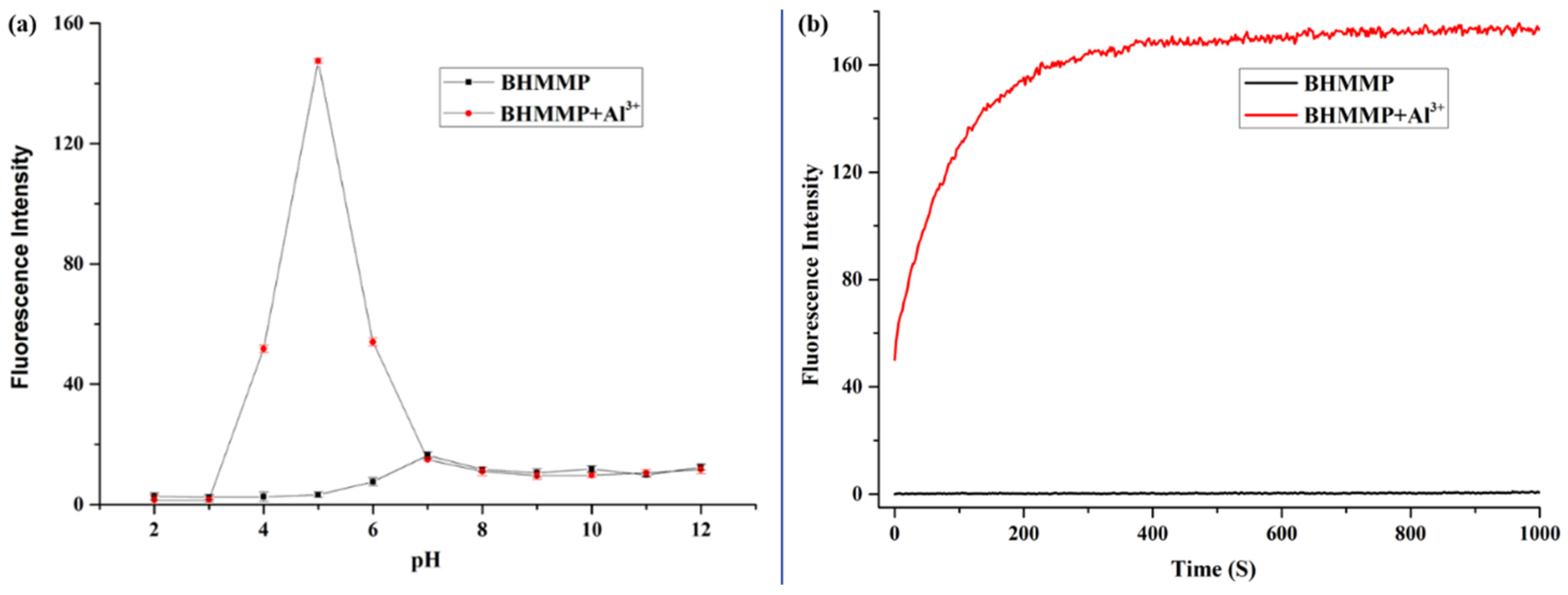
2.4. Effect of pH and Response Time Study
2.5. Reversibility Study
3. Practical Applications
3.1. Quantitative Detection of Al3+ in Actual Water Samples
3.2. Monitoring Al3+ on Test Paper
3.3. Cellular Imaging Experiments
4. Materials and Methods
4.1. Materials and Instruments
4.2. Analytical Procedures for Spectroscopic Experiments
4.3. DFT Investigation
4.4. In Vitro Cytotoxicity Assays
4.5. Cell Imaging in HSC Cells
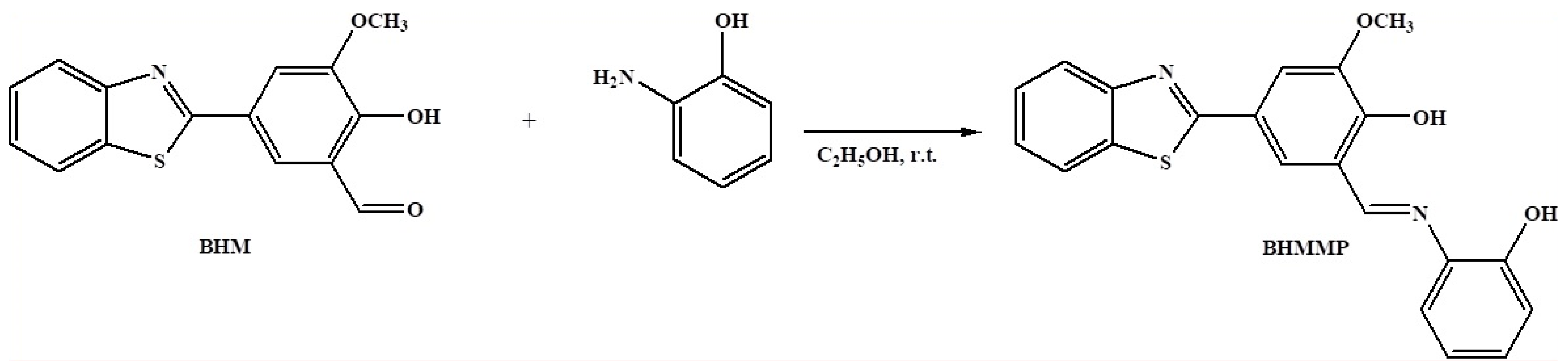
4.6. Synthesis of BHMMP
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhao, G.; Wei, G.; Yan, Z.Q.; Guo, B.Y.; Guang, S.Y.; Wu, R.L.; Xu, H.Y. A multiple fluorescein-based turn-on fluorophore (FHCS) identified for simultaneous determination and living imaging of toxic Al3+ and Zn2+ by improved Stokes shift. Anal. Chim. Acta 2020, 1095, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.J.; Liu, T.T.; Li, N.N.; Zeng, S.; Xing, Z.Y. A novel dual-function probe for recognition of Zn2+ and Al3+ and its application in real samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117786. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, K.S.; Kumara, A.; Hira, S.K.; Dey, S. Recognition of Al3+ through the off-on mechanism as a proficient driving force for the hydrolysis of BODIPY conjugated Schiff base and its application in bio-imaging. Inorg. Chim. Acta 2019, 498, 119157. [Google Scholar] [CrossRef]
- Aydin, D. A novel turn on fluorescent probe for the determination of Al3+ and Zn2+ ions and its cells applications. Talanta 2020, 210, 120615. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.B.; Hao, L.B.; Zang, L.B.; Tang, X.D.; Zhao, Y.Y.; Lu, J.L. A highly selective fluorescent probe for Al3+ based on bis(2-hydroxy-1-naphthaldehyde) oxaloyldihydrazone with aggregation-induced emission enhancement and gel properties. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 224, 117406. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.F.; Bao, Z.Y.; Yu, C.Y.; Qiu, Q.Q.; Wei, M.G.; Xi, W.B.; Qian, Z.S.; Feng, H. A water-soluble molecular probe with aggregation-induced emission for discriminative detection of Al3+ and Pb2+ and imaging in seedling root of Arabidopsis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 223, 117335. [Google Scholar] [CrossRef]
- Niu, H.; Leng, Y.F.; Ran, S.M.; Amee, M.; Du, D.Y.; Sun, J.; Chen, K.; Hong, S. Toxicity of soil labile aluminum fractions and aluminum species in soil water extracts on the rhizosphere bacterial community of tall fescue. Ecotox. Environ. Saf. 2019, 187, 109828. [Google Scholar] [CrossRef]
- Chen, F.M.; Ai, H.L.; Wei, M.T.; Qin, C.L.; Feng, Y.; Ran, S.M.; Wei, Z.H.; Niu, H.; Zhu, Q.; Zhu, H.H.; et al. Distribution and phytotoxicity of soil labile aluminum fractions and aluminum species in soil water extracts and their effects on tall fescue. Ecotox. Environ. Saf. 2018, 163, 180–187. [Google Scholar] [CrossRef]
- Wang, W.L.; Jin, L.; Kuang, Y.; Yuan, Z.Z.; Wang, Q.M. Isonicotinoylhydrazide modified 3-acetylcoumarin scaffold as an efficient chemical reversible sensor to detect Al3+ selectively and its application in live cells imaging. Synth. Commun. 2019, 49, 2501–2522. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Gong, X.S.; Li, Z.P.; Zhao, X.; Liu, Z.Q.; Cao, D.X.; Guan, R.F. A simple turn-on ESIPT and PET-based fluorescent probe for detection of Al3+ in real-water sample. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 219, 202–205. [Google Scholar] [CrossRef]
- Mirza, A.; King, A.; Troakes, C.; Exley, C. The Identification of Aluminum in Human Brain Tissue Using Lumogallion and Fluorescence Microscopy. J. Alzheimers Dis. 2016, 54, 1333–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mold, M.; Linhart, C.; Gómez-Ramírez, J.; Villegas-Lanau, A.; Exley, C. Aluminum and Amyloid-β in Familial Alzheimer’s Disease. J. Alzheimers Dis. 2020, 73, 1627–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mclachlan, D.R.; Bergeron, C.; Alexandrov, P.N.; Walsh, W.J.; Pogue, A.I.; Percy, M.E.; Kruck, T.P.A.; Fang, Z.; Sharfman, N.M.; Jaber, V.; et al. Aluminum in Neurological and Neurodegenerative Disease. Mol. Neurobiol. 2019, 56, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.S.; De Souza, C.T.; Leao, D.J.; Correia, F.O.; Almeida, T.S.; Ferreira, S.L.C. Simultaneous Determination of Chromium and Iron in Powdered Milk Using High-Resolution Continuum Source Graphite Furnace Atomic Absorption Spectrometry. Food Anal. Meth. 2020, 13, 284–290. [Google Scholar] [CrossRef]
- Panhwar, A.H.; Tuzen, M.; Kazi, T.G. Deep eutectic solvent based advance microextraction method for determination of aluminum in water and food samples: Multivariate study. Talanta 2018, 178, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Lee, W.B.; Kim, D.; Lee, Y.; Nam, S.H. An alternative analytical method for determining arsenic species in rice by using ion chromatography and inductively coupled plasma-mass spectrometry. Food Chem. 2019, 27, 353–358. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Luo, J.J.; Shen, X.Y.; Li, C.Y.; Wang, X.; Nie, B.; Fang, H.F. Quantitative Determining of Ultra-Trace Aluminum Ion in Environmental Samples by Liquid Phase Microextraction Assisted Anodic Stripping Voltammetry. Sensors 2018, 18, 1503. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Q.; Da, L.G.; Yang, L.; Chu, S.Y.; Yang, F.; Yu, S.M.; Jiang, C.L. Colorimetric Fluorescent Paper Strip with Smartphone Platform for Quantitative Detection of Cadmium Ions in Real Samples. J. Hazard. Mater. 2020, 392, 122506. [Google Scholar] [CrossRef]
- Li, M.S.; Xing, Y.L.; Zou, Y.X.; Chen, G.; You, J.M.; Yu, F.B. Imaging of the mutual regulation between zinc cation and nitrosyl via two-photon fluorescent probes in cells and in vivo. Sens. Actuators B Chem. 2020, 309, 127772. [Google Scholar] [CrossRef]
- Zeng, R.F.; Lan, J.S.; Wu, T.; Liu, L.; Liu, Y.; Ho, R.J.Y.; Ding, Y.; Zhang, T. A novel mitochondria-targetted near-infrared fluorescent probe for selective and colorimetric detection of sulfite and its application in vitro and vivo. Food Chem. 2020, 318, 126358. [Google Scholar] [CrossRef]
- Ling, C.; Cui, M.Y.; Chen, J.R.; Xia, L.L.; Deng, D.W.; Gu, Y.Q.; Wang, P. A novel highly selective fluorescent probe with new chalcone fluorophore for monitoring and imaging endogenous peroxynitrite in living cells and drug-damaged liver tissue. Talanta 2020, 215, 120934. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.L.; Wang, W.X.; Liu, J.; Li, Y.F.; Li, C.Y. A novel hepatocyte-targeting ratiometric fluorescent probe for imaging hydrogen peroxide in zebrafish. Sens. Actuators B Chem. 2020, 313, 128054. [Google Scholar] [CrossRef]
- Li, N.N.; Ma, Y.Q.; Sun, X.J.; Lia, M.Q.; Zeng, S.; Xing, Z.Y.; Li, J.L. A dual-function probe based on naphthalene for fluorescent turn-on recognition of Cu2+ and colorimetric detection of Fe3+ in neat H2O. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 210, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Pang, X.X.; Wang, Z.Q.; Chai, Q.; Ye, F. A highly sensitive and selective fluorescent probe for determination of Cu (II) and application in live cell imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 208, 198–205. [Google Scholar] [CrossRef]
- Ye, F.; Liang, X.M.; Xu, K.X.; Pang, X.X.; Chai, Q.; Fu, Y. A novel dithiourea- appended naphthalimide “on-off” fluorescent probe for detecting Hg2+ and Ag+ and its application in cell imaging. Talanta 2019, 200, 494–502. [Google Scholar] [CrossRef]
- Wu, N.; Zhao, L.X.; Jiang, C.Y.; Li, P.; Liu, Y.; Fu, Y.; Ye, F. A naked-eye visible colorimetric and fluorescent chemosensor for rapid detection of fluoride anions: Implication for toxic fluorine-containing pesticides detection. J. Mol. Liq. 2020, 302, 112549. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Li, L.; Liang, X.; Li, S.; Fu, Y. A dual thiourea-appended perylenebisimide “turn-on” fluorescent chemosensor with high selectivity and sensitivity for Hg2+ in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 241, 118678. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yang, L.; Li, P.; Li, S.J.; Li, L.; Pang, X.X.; Ye, F.; Fu, Y. A novel colorimetric and “turn-off” fluorescent probe based on catalyzed hydrolysis reaction for detection of Cu2+ in real water and in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 227, 117540. [Google Scholar] [CrossRef]
- Ye, F.; Wu, N.; Li, P.; Liu, Y.L.; Li, S.J.; Fu, Y. A lysosome-targetable fluorescent probe for imaging trivalent cations Fe3+, Al3+ and Cr3+ in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 222, 117242. [Google Scholar] [CrossRef]
- Dhineshkumar, E.; Iyappan, M.; Anbuselvan, C. A novel dual chemosensor for selective heavy metal ions Al3+, Cr3+ and its applicable cytotoxic activity, HepG2 living cell images and theoretical studies. J. Mol. Struct. 2020, 1210, 128033. [Google Scholar] [CrossRef]
- Ko, M.; Kurapati, S.; Jo, Y.; Cho, B.; Cho, D. Tuned Al3+ selectivity and π-extended properties of di-2-picolylamine-substituted quinoline-based tolan. Tetrahedron Lett. 2020, 61, 151808. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Wang, J.; Jing, X.M.; Wang, S.J.; Xiao, L.W.; Li, L.L. A fluorescent light-up probe for selective detection of Al3+ and its application in living cell imaging. Inorg. Chim. Acta 2020, 500, 119231. [Google Scholar] [CrossRef]
- Li, Y.Y.; Xu, K.; Si, Y.; Yang, C.P.; Peng, Q.C.; He, J.; Hu, Q.G.; Li, K. An aggregation-induced emission (AIE) fluorescent chemosensor for the detection of Al(III) in aqueous solution. Dyes Pigments 2019, 171, 107682. [Google Scholar] [CrossRef]
- Zeng, S.; Li, S.J.; Sun, X.J.; Li, M.Q.; Ma, Y.Q.; Xing, Z.Y.; Li, J.L. A naphthalene-quinoline based chemosensor for fluorescent “turn-on” and absorbance-ratiometric detection of Al3+ and its application in cells imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 205, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Xing, Z.Y.; Ma, X.Y.; Liu, Y.T.; Zhang, Y. A highly selective colorimetric and fluorescent turn-on chemosensor for Al3+ based on naphthalimide derivative. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 167, 59–65. [Google Scholar] [CrossRef]
- Hou, J.T.; Wang, B.Y.; Wang, S.; Wu, Y.Q.; Liao, Y.X.; Ren, W.X. Detection of hydrazine via a highly selective fluorescent probe: A case study on the reactivity of cyano-substituted C=C bond. Dyes Pigments 2020, 178, 108366. [Google Scholar] [CrossRef]
- Huang, Y.F.; Zhang, Y.B.; Huo, F.J.; Liu, Y.M.; Yin, C.X. Mitochondrial-targeted near-infrared “dual mode” fluorescent dyes with large Stokes shift for detection of hypochlorous acid and its bioimaging in cell and mice. Dyes Pigments 2020, 179, 108387. [Google Scholar] [CrossRef]
- Liu, Y.; Bi, A.Y.; Gao, T.; Cao, X.Z.; Gao, F.; Rong, P.F.; Wang, W.; Zeng, W.B. A novel self-assembled nanoprobe for the detection of aluminum ions in real water samples and living cells. Talanta 2019, 194, 38–45. [Google Scholar] [CrossRef]
- Lu, Z.N.; Wang, L.; Zhang, X.; Zhu, Z.J. A selective fluorescent chemosensor for Cd2+ based on 8-hydroxylquinoline-benzothiazole conjugate and imaging application. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 213, 57–63. [Google Scholar] [CrossRef]
- Shi, Z.C.; Zhao, Z.G. Microwave irradiation synthesis of novel indole triazole Schiff base fluorescent probe for Al3+ ion. Inorg. Chim. Acta 2019, 498, 119135. [Google Scholar] [CrossRef]
- Fu, J.X.; Yao, K.; Chang, Y.X.; Li, B.; Yang, L.; Xu, K.X. A novel colorimetric-fluorescent probe for Al3+ and the resultant complex for F− and its applications in cell imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 222, 117234. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Cheng, J.P.; Li, L.J.; Zhan, Y.F.; Li, W.J.; Chang, Z.D.; Sun, C.Y. A new Schiff base fluorescent-colorimetric probe containing fluorene-naphthalene structure: Multifunction detection. Inorg. Chim. Acta 2019, 498, 119131. [Google Scholar] [CrossRef]
- Wang, J.H.; Feng, L.H.; Chao, J.B.; Wang, Y.; Shuang, S.M. A new ‘turn-on’ and reversible fluorescent sensor for Al3+ detection and live cell imaging. Anal. Methods 2019, 11, 5598–5606. [Google Scholar] [CrossRef]
- Leng, X.; Jia, X.; Qiao, C.F.; Xu, W.F.; Ren, C.T.; Long, Y.; Yang, B.Q. Synthesis, characterization and Al3+ sensing application of a new chromo-fluorogenic chemosensor. J. Mol. Struct. 2019, 1193, 69–75. [Google Scholar] [CrossRef]
- Mondal, A.; Ahmmed, E.; Chakraborty, S.; Sarkar, A.; Lohar, S.; Chattopadhyay, P. Aggregation Induced Emission Enhancement (AIEE) of Naphthalene-Appended Organic Moiety: An Al3+ Ion Selective Turn-On Fluorescent Probe. Chemistryselect 2020, 5, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Berrones-Reyes, J.; Muñoz-Flores, B.M.; Gómez-Treviño, A.; Treto-Suárez, M.A.; Páez-Hernández, D.; Schott, E.; Zarate, X.; Jiménez-Pérez, V.M. Novel fluorescent Schiff bases as Al3+ sensors with high selectivity and sensitivity, and their bioimaging applications. Mater. Chem. Phys. 2019, 233, 89–101. [Google Scholar] [CrossRef]
- Sarma, S.; Bhowmik, A.; Sarma, M.J.; Banu, S.; Phukan, P.; Das, D.K. Condensation product of 2-hydroxy-1-napthaldehyde and 2-aminophenol: Selective fluorescent sensor for Al3+ ion and fabrication of paper strip sensor for Al3+ ion. Inorg. Chim. Acta 2018, 469, 202–208. [Google Scholar] [CrossRef]
- Ren, Y.P.; Han, J.; Wang, Y.; Tang, X.; Wang, L.; Ni, L. An OFF-ON-OFF type fluorescent probe based on a naphthalene derivative for Al3+ and F- ions and its biological application. Luminescence 2018, 33, 15–21. [Google Scholar] [CrossRef]
- Maity, D.; Dey, S.; Roy, P. A two-pocket Schiff-base molecule as a chemosensor for Al3+. New J. Chem. 2017, 41, 10677–10685. [Google Scholar] [CrossRef]
- Alici, O.; Erdemir, S. A cyanobiphenyl containing fluorescence ‘turn on’ sensor for Al3+ ion in CH3CN-water. Sens. Actuators B Chem. 2015, 208, 159–163. [Google Scholar] [CrossRef]
- Sheet, S.K.; Sen, B.; Thounaojam, R.; Aguan, K.; Khatua, S. Highly selective light-up Al3+ sensing by a coumarin based Schiff base probe: Subsequent phosphate sensing DNA binding and live cell imaging. J. Photochem. Photobiol. A-Chem. 2017, 332, 101–111. [Google Scholar] [CrossRef]
- Liang, C.S.; Jiang, S.M. Single sensor for multiple analytes in different optical channel: Applying for multi-ion response modulation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 183, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Q.; Wan, X.J.; Dong, Y.S.; Li, W.B.; Wu, L.S.; Pei, H.; Yao, Y.W. Facile synthesis of a water-soluble fluorescence sensor for Al3+ in aqueous solution and on paper substrate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 173, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Sen, B.; Sanjoy, S.K.; Thounaojam, R.; Jamatia, R.; Pal, A.K.; Aguan, K.; Khatua, S. A coumarin based Schiff base probe for selective fluorescence detection of Al3+ and its application in live cell imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Jiang, J.M.; Li, J.F.; Wu, W.P.; Zhang, J.L. Theoretical Design of Near-Infrared Al3+ Fluorescent Probes Based on Salicylaldehyde Acylhydrazone Schiff Base Derivatives. Inorg. Chem. 2019, 58, 12618–12627. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Kumar, S.; Singhal, D.; Gupta, R. Turn-On Fluorescent Sensors for the Selective Detection of Al3+ (and Ga3+) and PPi Ions. Inorg. Chem. 2019, 58, 10364–10376. [Google Scholar] [CrossRef]
- Chandra, R.; Manna, A.K.; Sahu, M.; Rout, K.; Patra, G.K. Simple salicylaldimine-functionalized dipodal bis Schiff base chromogenic and fluorogenic chemosensors for selective and sensitive detection of Al3+ and Cr3+. Inorg. Chim. Acta 2020, 499, 119192. [Google Scholar] [CrossRef]
- Tian, L.M.; Xue, J.; Li, S.L.; Yang, Z.Y. A novel chromone derivative as dual probe for selective sensing of Al(III) by fluorescent and Cu(II) by colorimetric methods in aqueous solution. J. Photochem. Photobiol. A-Chem. 2019, 382, 111955. [Google Scholar] [CrossRef]
- Sun, X.J.; Liu, T.T.; Fu, H.; Li, N.N.; Xing, Z.Y.; Yang, F. A naphthalimide-based fluorescence “off-on-off” chemosensor for relay detection of Al3+ and ClO−. Front. Chem. 2019, 7, 549. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.Q.; Sun, X.J.; Li, M.Q.; Zeng, S.; Xing, Z.Y.; Li, J.L. A quinoline-based schiff base for significant fluorescent “turn-on” and absorbance-ratiometric detection of Al3+. Chem. Pap. 2019, 73, 1469–1479. [Google Scholar] [CrossRef]
- Wang, J.X.; Xing, Z.Y.; Tian, Z.N.; Wu, D.Q.; Xiang, Y.Y.; Li, J.L. A dual-functional probe for sensing pH change and ratiometric detection of Cu2+. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 235, 118318. [Google Scholar] [CrossRef]
- Liu, T.T.; Li, S.J.; Fu, H.; Tian, Z.N.; Sun, X.J.; Xing, Z.Y. A fluorescence turn-on probe for the recognition of Al3+ and its application in cell image. J. Photochem. Photobiol. A-Chem. 2020, 403, 112865. [Google Scholar] [CrossRef]
- Zeng, S.; Li, S.J.; Sun, X.J.; Liu, T.T.; Xing, Z.Y. A dual-functional chemosensor for fluorescent on-off and ratiometric detection of Cu2+ and Hg2+ and its application in cell imaging. Dyes Pigments 2019, 170, 107642. [Google Scholar] [CrossRef]
- Liu, T.T.; Li, S.J.; Zhang, X.S.; Wang, J.; Deng, Y.X.; Sun, X.J.; Xing, Z.Y.; Wu, R.F. A Facile Probe for Fluorescence Turn-on and Simultaneous Naked-Eyes Discrimination of H2S and biothiols (Cys and GSH) and Its Application. J. Fluoresc. 2022, 32, 175–188. [Google Scholar] [CrossRef]
- Delley, B. An All-Electron Numerical Method for Solving the Local Density Functional for Polyatomic Molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Zeng, S.; Li, S.J.; Sun, X.J.; Li, M.Q.; Xing, Z.Y.; Li, J.L. A benzothiazole-based chemosensor for significant fluorescent turn-on and ratiometric detection of Al3+ and its application in cell imaging. Inorg. Chim. Acta 2019, 486, 654–662. [Google Scholar] [CrossRef]
- Tian, Z.N.; Wu, D.Q.; Sun, X.J.; Liu, T.T.; Xing, Z.Y. A Benzothiazole-Based Fluorescent Probe for Ratiometric Detection of Al3+ and Its Application in Water Samples and Cell Imaging. Int. J. Mol. Sci. 2019, 20, 5993. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Z.; Wang, J.; Huang, J.; Chen, X.; Zong, Z.; Fan, C.; Huang, G. A Significant Fluorescence Turn-On Probe for the Recognition of Al3+ and Its Application. Molecules 2022, 27, 2569. https://doi.org/10.3390/molecules27082569
Xing Z, Wang J, Huang J, Chen X, Zong Z, Fan C, Huang G. A Significant Fluorescence Turn-On Probe for the Recognition of Al3+ and Its Application. Molecules. 2022; 27(8):2569. https://doi.org/10.3390/molecules27082569
Chicago/Turabian StyleXing, Zhiyong, Junli Wang, Junhui Huang, Xiangfeng Chen, Ziao Zong, Chuanbin Fan, and Guimei Huang. 2022. "A Significant Fluorescence Turn-On Probe for the Recognition of Al3+ and Its Application" Molecules 27, no. 8: 2569. https://doi.org/10.3390/molecules27082569





