Comparison of the Phytochemical Variation of Non-Volatile Metabolites within Mother Tinctures of Arnica montana Prepared from Fresh and Dried Whole Plant Using UHPLC-HRMS Fingerprinting and Chemometric Analysis
Abstract
:1. Introduction
2. Results
2.1. Comparison of Dry Residues
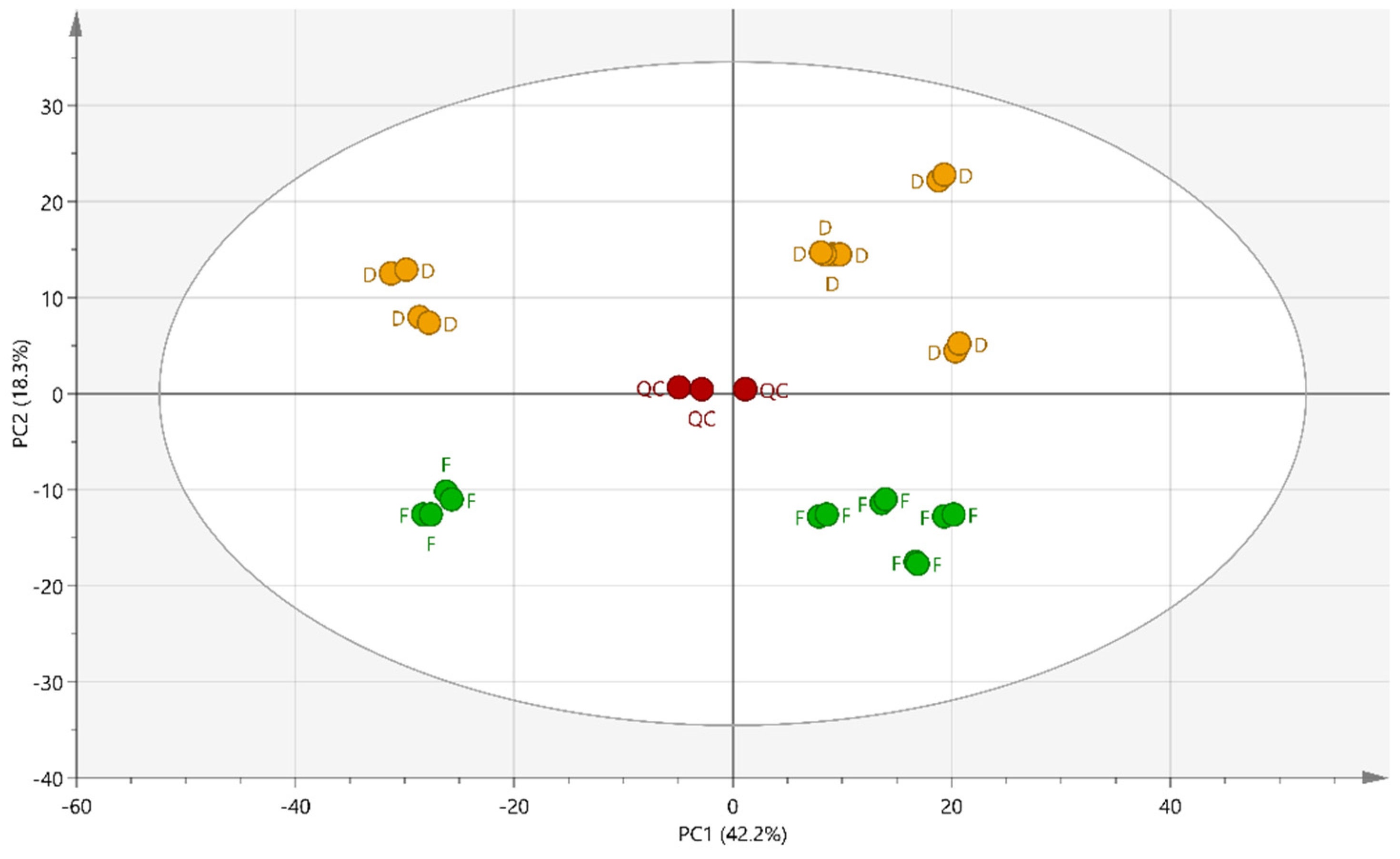
2.2. Multivariate Data Analysis
2.3. Supervised Multivariate Analysis
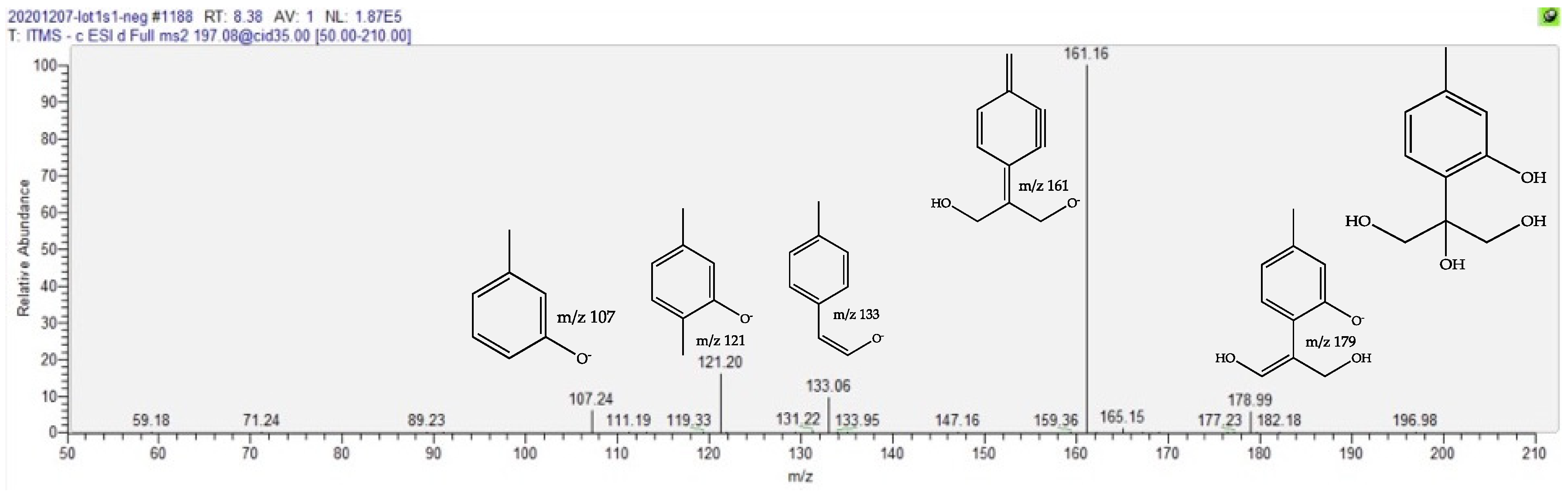
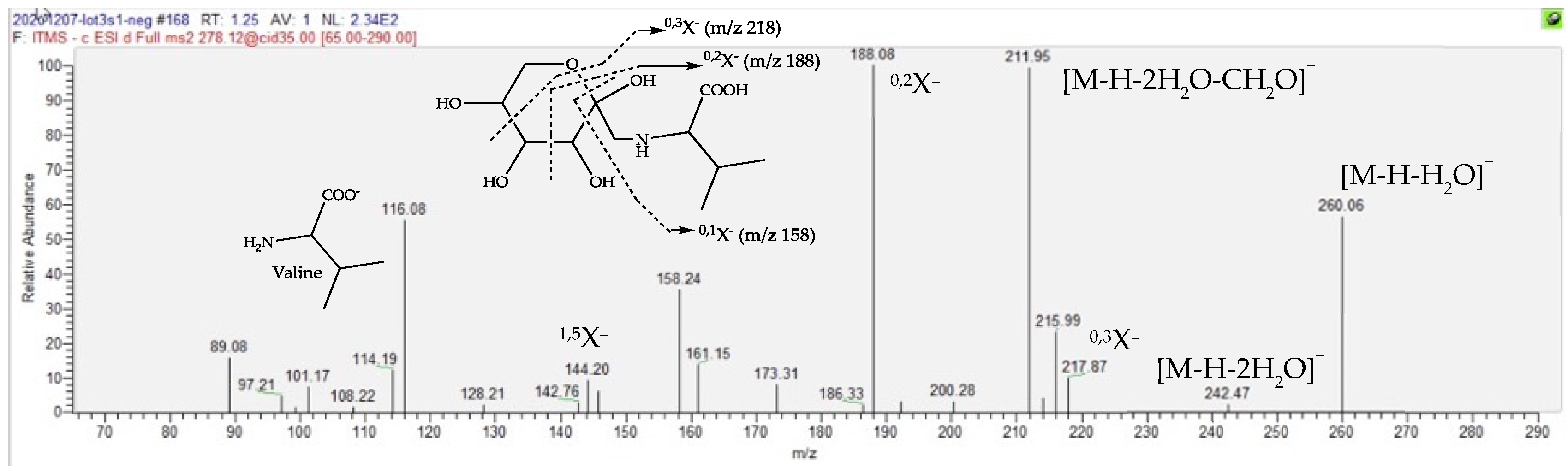
2.4. Dereplication of Discriminating Compounds
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Determination of Dry Residues
4.3. Ultra-High-Performance Liquid Chromatography-Orbitrap Analysis
4.4. Data Processing
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea. Plantaginaceae to Compositae (and Rubiaceae); Cambridge University Press: Cambridge, UK, 1976; Volume 4, ISBN 978-0-521-08717-9. [Google Scholar]
- Perry, N.B.; Burgess, E.J.; Rodríguez Guitián, M.A.; Romero Franco, R.; López Mosquera, E.; Smallfield, B.M.; Joyce, N.I.; Littlejohn, R.P. Sesquiterpene Lactones in Arnica Montana: Helenalin and Dihydrohelenalin Chemotypes in Spain. Planta Med. 2009, 75, 660–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clauser, M.; Aiello, N.; Scartezzini, F.; Innocenti, G.; Dall’Acqua, S. Differences in the Chemical Composition of Arnica Montana Flowers from Wild Populations of North Italy. Nat. Prod. Commun. 2014, 9, 3–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ESCOP Monographs. The Scientific Foundation for Herbal Medicinal Products. Second Edition Completely Revised and Expanded. Arnica Flos Monograph; ESCOP 2003; ESCOP and Georg Thieme Verlag: Stuttgart, Germany, 2003; ISBN 1-901964-07-8.
- European Medicines Agency (EMA). Community Herbal Monograph on Arnica Montana. L. Flos. 2014. Available online: Https://www.Ema.Europa.Eu/En/Documents/Herbal-Monograph/Final-Community-Herbal-Monograph-Arnica-Montana-l-Flos_en.Pdf (accessed on 10 April 2022).
- Willuhn, G. Arnicae Flos. In Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis; Wichtl, M., Ed.; Medpharma: Stuttgart, Germany, 2004; pp. 54–59. [Google Scholar]
- Kimel, K.; Godlewska, S.; Krauze-Baranowska, M.; Pobłocka-Olech, L. HPLC-DAD-ESI/MS Analysis of Arnica TM Constituents. Acta Pol. Pharm. Drug Res. 2019, 76, 1015–1027. [Google Scholar] [CrossRef]
- De parseval, L. Observations Pratiques de Samuel Hahnemann Et Classification de Ses Recherches Sur Les Proprietes Caracteristiques Des Medicaments; Librairies de l’académie impériale de médecine; J.B. Baillière et Fils: Paris, France, 1857. [Google Scholar]
- Kriplani, P.; Guarve, K.; Baghael, U.S. Arnica montana L.—A Plant of Healing: Review. J. Pharm. Pharmacol. 2017, 69, 925–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Pharmacopeia 10.6. Monographs 1391 (Arnica Flower) and 2371 (Methods of Preparation of Homoeophatic Stocks and Potentisation); EDQM Council of Europe Editions: Strasbourg, France, 2022.
- French Pharmacopeia 11th Edition. Arnica Montana for Homoeopathic Preparations Monograph; Agence Nationale de Sécurité des Médicaments (ANSM): St Denis, France, 2008.
- Demarque, D.; Jouanny, J.; Poitevin, B.; Saint-Jean, Y. Pharmacologie et matière médicale homéopathique, 3rd ed.; CEDH: Paris, France, 2009; ISBN 978-2-915668-39-1. [Google Scholar]
- Wolfender, J.-L.; Marti, G.; Thomas, A.; Bertrand, S. Current Approaches and Challenges for the Metabolite Profiling of Complex Natural Extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef] [PubMed]
- Marti, G.; Joulia, P.; Amiel, A.; Fabre, B.; David, B.; Fabre, N.; Fiorini-Puybaret, C. Comparison of the Phytochemical Composition of Serenoa Repens Extracts by a Multiplexed Metabolomic Approach. Molecules 2019, 24, 2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehl, F.; Marti, G.; Boccard, J.; Debrus, B.; Merle, P.; Delort, E.; Baroux, L.; Raymo, V.; Velazco, M.I.; Sommer, H.; et al. Differentiation of Lemon Essential Oil Based on Volatile and Non-Volatile Fractions with Various Analytical Techniques: A Metabolomic Approach. Food Chem. 2014, 143, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Z.; Harnly, J.M. Identification of Hydroxycinnamoylquinic Acids of Arnica Flowers and Burdock Roots Using a Standardized LC-DAD-ESI/MS Profiling Method. J. Agric. Food Chem. 2008, 56, 10105–10114. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kuhnert, N. Identification and Characterization of Two New Derivatives of Chlorogenic Acids in Arnica (Arnica Montana L.) Flowers by High-Performance Liquid Chromatography/Tandem Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 4033–4039. [Google Scholar] [CrossRef]
- de Athayde, A.E.; de Araujo, C.E.S.; Sandjo, L.P.; Biavatti, M.W. Metabolomic Analysis among Ten Traditional “Arnica” (Asteraceae) from Brazil. J. Ethnopharmacol. 2021, 265, 113149. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, K.A. Sphingolipid Long Chain Bases. Lipids 1970, 5, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Pruett, S.T.; Bushnev, A.; Hagedorn, K.; Adiga, M.; Haynes, C.A.; Sullards, M.C.; Liotta, D.C.; Merrill, A.H. Biodiversity of Sphingoid Bases (“sphingosines”) and Related Amino Alcohols. J. Lipid Res. 2008, 49, 1621–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperling, P.; Heinz, E. Plant Sphingolipids: Structural Diversity, Biosynthesis, First Genes and Functions. Biochim. Biophys. Acta 2003, 1632, 1–15. [Google Scholar] [CrossRef]
- Kos, O.; Lindenmeyer, M.T.; Tubaro, A.; Sosa, S.; Merfort, I. New Sesquiterpene Lactones from Arnica Tincture Prepared from Fresh Flowerheads of Arnica Montana. Planta Med. 2005, 71, 1044–1052. [Google Scholar] [CrossRef]
- Maldonado, E.; Marquez, C.L.; Ortega, A. A Thymol Derivative from Calea Nelsonii. Phytochemistry 1992, 31, 2527–2528. [Google Scholar] [CrossRef]
- Monache, G.D.; Monache, F.D.; Becerra, J.; Silva, M.; Menichini, F. Thymol Derivatives from Eupatorium Glechonophyllum. Phytochemistry 1984, 23, 1947–1950. [Google Scholar] [CrossRef]
- Tori, M.; Ohara, Y.; Nakashima, K.; Sono, M. Thymol Derivatives from Eupatorium Fortunei. J. Nat. Prod. 2001, 64, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Paßreiter, C.M. Co-Occurrence of 2-Pyrrolidineacetic Acid with the Pyrrolizidines Tussilaginic Acid and Isotussilaginic Acid and Their 1-Epimers in Arnica Species and Tussilago Farfara. Phytochemistry 1992, 31, 4135–4137. [Google Scholar] [CrossRef]
- Xing, H.; Mossine, V.V.; Yaylayan, V. Diagnostic MS/MS Fragmentation Patterns for the Discrimination between Schiff Bases and Their Amadori or Heyns Rearrangement Products. Carbohydr. Res. 2020, 491, 107985. [Google Scholar] [CrossRef] [PubMed]
- Davidek, T.; Kraehenbuehl, K.; Devaud, S.; Robert, F.; Blank, I. Analysis of Amadori Compounds by High-Performance Cation Exchange Chromatography Coupled to Tandem Mass Spectrometry. Anal. Chem. 2005, 77, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Altena, J.H.; van den Ouweland, G.A.M.; Teunis, C.J.; Tjan, S.B. Analysis of the 220-MHz, P.M.R. Spectra of Some Products of the Amadori and Heyns Rearrangements. Carbohydr. Res. 1981, 92, 37–49. [Google Scholar] [CrossRef]
- Meitinger, M.; Hartmann, S.; Schieberle, P. Development of Stable Isotope Dilution Assays for the Quantitation of Amadori Compounds in Foods. J. Agric. Food Chem. 2014, 62, 5020–5027. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. The Comparison of the Contents of Sugar, Amadori, and Heyns Compounds in Fresh and Black Garlic. J. Food Sci. 2016, 81, C1662–C1668. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, S.; Ho, C.-T. Key Aspects of Amadori Rearrangement Products as Future Food Additives. Molecules 2021, 26, 4314. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MS(n). J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Dictionary of Natural Products on USB 30:1; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2021; ISBN 978-0-412-49150-4.
- Weremczuk-Jezyna, I.; Kisiel, W.; Wysokińska, H. Thymol Derivatives from Hairy Roots of Arnica Montana. Plant Cell Rep. 2006, 25, 993–996. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Wysokińska, H.; Kalemba, D. Constituents of the Essential Oil from Hairy Roots and Plant Roots of Arnica montana L. J. Essent. Oil Res. 2011, 23, 91–97. [Google Scholar] [CrossRef]
- Liang, H.; Bao, F.; Dong, X.; Tan, R.; Zhang, C.; Lu, Q.; Cheng, Y. Antibacterial Thymol Derivatives Isolated from Centipeda Minima. Molecules 2007, 12, 1606–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Xie, H.; Xiao, H.; Wei, X. Phenolic Constituents from the Roots of Mikania Micrantha and Their Allelopathic Effects. J. Agric. Food Chem. 2013, 61, 7309–7314. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass Spectrometry in the Structural Analysis of Flavonoids. J. Mass Spectrom. JMS 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Maillard, L.C. Action of Amino Acids on Sugars. Formation of Melanoidins in a Methodical Way. C. R. Acad. Sci. 1912, 154, 66–68. [Google Scholar]
- Wang, J.; Lu, Y.-M.; Liu, B.-Z.; He, H.-Y. Electrospray Positive Ionization Tandem Mass Spectrometry of Amadori Compounds. J. Mass Spectrom. JMS 2008, 43, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Domon, B.; Costello, C. A Systematic Nomenclature for Carbohydrate Fragmentations in FAB-MS/MS Spectra of Glycoconjugates. Glycoconj. J. 2005, 5, 397–409. [Google Scholar] [CrossRef]
- Asadi, M.; Nejad Ebrahimi, S.; Hatami, M.; Hadian, J. Changes in Secondary Metabolite Contents of Arnica Chamissonis Less. in Response to Different Harvest Time, Flower Developmental Stages and Drying Methods. J. Med. Plants 2020, 19, 69–88. [Google Scholar] [CrossRef]
- Park, C.H.; Choi, J.S.; Yokozawa, T. Increase in the Hydroxyl Radical-Scavenging Activity of Panax Ginseng and Ginsenosides by Heat-Processing. Drug Discov. Ther. 2018, 12, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijewickreme, A.N.; Krejpcio, Z.; Kitts, D.D. Hydroxyl Scavenging Activity of Glucose, Fructose, and Ribose-Lysine Model Maillard Products. J. Food Sci. 1999, 64, 457–461. [Google Scholar] [CrossRef]
- Chen, X.-M.; Kitts, D.D. Antioxidant Activity and Chemical Properties of Crude and Fractionated Maillard Reaction Products Derived from Four Sugar-Amino Acid Maillard Reaction Model Systems. Ann. N. Y. Acad. Sci. 2008, 1126, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Hauser, C.; Müller, U.; Sauer, T.; Augner, K.; Pischetsrieder, M. Maillard Reaction Products as Antimicrobial Components for Packaging Films. Food Chem. 2014, 145, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Hernandez-Hernandez, O.; Moreno, F.J.; Sanz, Y. Neoglycoconjugates of Caseinomacropeptide and Galactooligosaccharides Modify Adhesion of Intestinal Pathogens and Inflammatory Response(s) of Intestinal (Caco-2) Cells. Food Res. Int. 2013, 1, 1096–1102. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, O.; Sanz, M.L.; Kolida, S.; Rastall, R.A.; Moreno, F.J. In Vitro Fermentation by Human Gut Bacteria of Proteolytically Digested Caseinomacropeptide Nonenzymatically Glycosylated with Prebiotic Carbohydrates. J. Agric. Food Chem. 2011, 59, 11949–11955. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Meng, J.; Lu, R.-R. Improvement of ACE Inhibitory Activity of Casein Hydrolysate by Maillard Reaction with Xylose. J. Sci. Food Agric. 2015, 95, 66–71. [Google Scholar] [CrossRef]
- Guerra-Hernandez, E.; Leon Gomez, C.; Garcia-Villanova, B.; Corzo Sanchez, N.; Romera Gomez, J.M. Effect of Storage on Non-Enzymatic Browning of Liquid Infant Milk Formulae. J. Sci. Food Agric. 2002, 82, 587–592. [Google Scholar] [CrossRef]
- Brownlee, M. Advanced Protein Glycosylation in Diabetes and Aging. Annu. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, L.; Labat-Robert, J. Role of the Maillard Reaction in Aging and Age-Related Diseases. Studies at the Cellular-Molecular Level. Clin. Chem. Lab. Med. 2014, 52, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Colaco, C.A.L.S.; Harrington, C.R. Inhibitors of the Maillard Reaction. CNS Drugs 1996, 6, 167–177. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Soladoye, O.P.; Aluko, R.E. Maillard Reaction Products Derived from Food Protein-Derived Peptides: Insights into Flavor and Bioactivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 3429–3442. [Google Scholar] [CrossRef]
- Floris, B.; Galloni, P.; Conte, V.; Sabuzi, F. Tailored Functionalization of Natural Phenols to Improve Biological Activity. Biomolecules 2021, 11, 1325. [Google Scholar] [CrossRef]
- Nichterlein, K. Arnica Montana (Mountain Arnica): In Vitro Culture and the Production of Sesquiterpene Lactones and Other Secondary Metabolites. In Medicinal and Aromatic Plants VIII; Bajaj, Y.P.S., Ed.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1995; p. 53. ISBN 978-3-662-08612-4. [Google Scholar]
- Jang, E.J.; Shin, Y.; Park, H.J.; Kim, D.; Jung, C.; Hong, J.-Y.; Kim, S.; Lee, S.K. Anti-Melanogenic Activity of Phytosphingosine via the Modulation of the Microphthalmia-Associated Transcription Factor Signaling Pathway. J. Dermatol. Sci. 2017, 87, 19–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uche, L.E.; Gooris, G.S.; Beddoes, C.M.; Bouwstra, J.A. New Insight into Phase Behavior and Permeability of Skin Lipid Models Based on Sphingosine and Phytosphingosine Ceramides. Biochim. Biophys. Acta BBA Biomembr. 2019, 1861, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Školová, B.; Kováčik, A.; Tesař, O.; Opálka, L.; Vávrová, K. Phytosphingosine, Sphingosine and Dihydrosphingosine Ceramides in Model Skin Lipid Membranes: Permeability and Biophysics. Biochim. Biophys. Acta Biomembr. 2017, 1859, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Pavicic, T.; Wollenweber, U.; Farwick, M.; Korting, H.C. Anti-Microbial and -Inflammatory Activity and Efficacy of Phytosphingosine: An in Vitro and in Vivo Study Addressing Acne Vulgaris. Int. J. Cosmet. Sci. 2007, 29, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.B.; Kim, C.D.; Kim, B.J.; Kim, M.-Y.; Park, C.S.; Yoon, T.-J.; Seo, Y.-J.; Suhr, K.-B.; Park, J.-K.; Lee, J.-H. Anti-Angiogenic Effect of Tetraacetyl-Phytosphingosine. Exp. Dermatol. 2007, 16, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.B.; An, S.; Kim, M.J.; Kim, K.R.; Choi, Y.M.; Ahn, K.J.; An, I.-S.; Cha, H.J. Phytosphingosine-1-Phosphate and Epidermal Growth Factor Synergistically Restore Extracellular Matrix in Human Dermal Fibroblasts in Vitro and in Vivo. Int. J. Mol. Med. 2017, 39, 741–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.P.; Cha, H.J.; Lee, K.S.; Lee, K.K.; Son, J.H.; Kim, K.N.; Lee, D.K.; An, S. Phytosphingosine-1-Phosphate Represses the Hydrogen Peroxide-Induced Activation of c-Jun N-Terminal Kinase in Human Dermal Fibroblasts through the Phosphatidylinositol 3-Kinase/Akt Pathway. Arch. Dermatol. Res. 2012, 304, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Falniowski, A.; Bazos, I.; Hodálová, I.; Lansdown, R.; Petrova, A. Arnica Montana. The IUCN Red List of Threatened Species 2011: E.T162327A5574104. 2011. Available online: Https://Dx.Doi.Org/10.2305/IUCN.UK.2011-1.RLTS.T162327A5574104.En (accessed on 10 April 2022).
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-Independent MS/MS Deconvolution for Comprehensive Metabolome Analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Fraisier-Vannier, O.; Chervin, J.; Cabanac, G.; Puech, V.; Fournier, S.; Durand, V.; Amiel, A.; André, O.; Benamar, O.A.; Dumas, B.; et al. MS-CleanR: A Feature-Filtering Workflow for Untargeted LC–MS Based Metabolomics. Anal. Chem. 2020, 92, 9971–9981. [Google Scholar] [CrossRef] [PubMed]



| Proposed Identification | Fold | Rt (min) | Molecular Formula | Experimental [M-H]− (Error in ppm) | Negative ion MS/MS fragments (RI) | Experimental [M+H]+ (Error in ppm) | Positive Ion MS/MS Fragments (RI) | References |
|---|---|---|---|---|---|---|---|---|
| Compounds increased in MT prepared from fresh plant | ||||||||
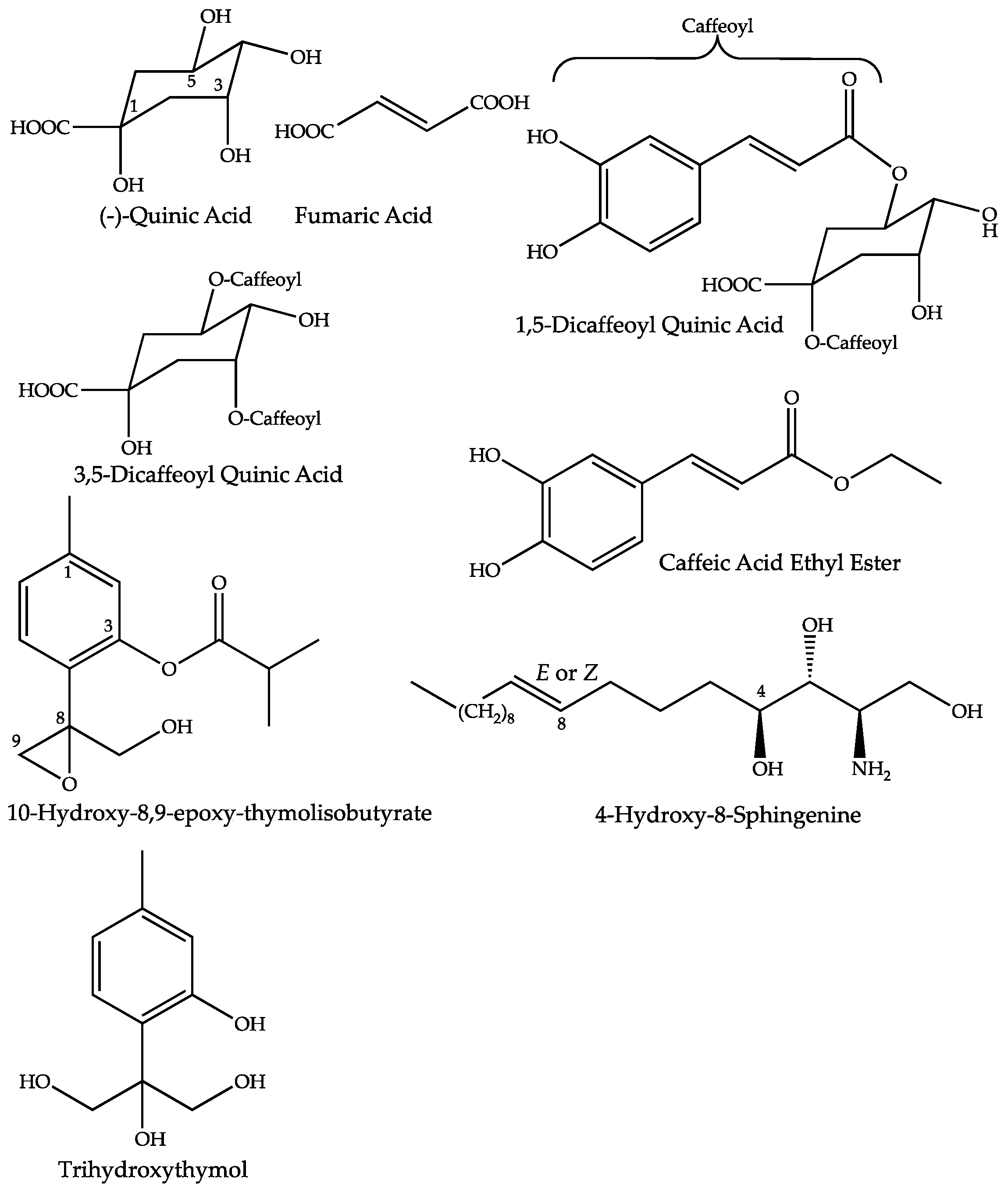
| Quinic Acid | 2.2 | 1.06 | C7H12O6 | 191.0562 (0.36) | 173 (bp), 127, 111, 93, 85 | [7,16,17] | ||
| Fumaric Acid | 1.4 | 1.33 | C4H4O4 | 115.0039 (2.25) | 97 (bp) | [7,16,17] | ||
| 1,5-Dicaffeoyl-Quinic Acid | 1.4 | 25.63 | C25H24O12 | 515.1201 (0.60) | 353 (bp), 335 (10), 191 (18) | [7,16,17] | ||
| 3,5-Dicaffeoyl-Quinic Acid | 1.5 | 25.69 | C25H24O12 | 515.1201 (0.60) | 353 (bp), 335 (10), 203 (1) 191 (18), 179 (4), 173 (1) | [7,16,17] | ||
| Caffeic Acid Ethyl Ester | 2.2 | 31.90 | C11H12O4 | 207.0659 (1.70) | 179 (bp), 135 (20) | [18] | ||
| 4-Hydroxy-8-Sphingenine | 3.1 | 50.89 | C18H37O3N | None | 316.2849 (0.13) | 298 (bp), 280 (75), 262 (5) | [19,20,21] | |
| 10-Hydroxy-8,9-epoxy-thymolisobutyrate | 1.3 | 37.07 | C14H18O4 | 249.1136 (1.59) | 251.1279 (0.75) | 163 (75), 145 (bp) | [22,23,24,25] | |
| Trihydroxy Thymol | 1.6 | 8.44 | C10H14O4 | 197.0823 (1.41) | 179 (10), 161 (bp), 133 (10), 121 (15) | [22,23,24,25] | ||
| Compounds increased in MT prepared from dried plant | ||||||||
| Methoxy Pyrrolidinone | 2.2 | 1.09 | C5H9O2N | None | 116.0704 (0.90) | 98 (22), 88 (18),84 (bp), 56 (18) | [26] | |
| Fructose Valine conjugate | 151.1 | 1.23 | C11H21O7N | 278.1245 (0.05) | 260 (20), 218 (20), 212 (40), 188 (bp), 158 (15, 116 (30)) | 280.1391 (0.08) | 262 (bp), 244 (2), 216 (2), 130 (2) | [27,28,29,30] |
| Fructose Oxoproline conjugate | 577.2 | 1.25 | C11H17O8N | 290.0880 (1.55) | 272 (20), 254 (10), 200 (bp), 170 (10), 128 (10) | 292.1026 (0.32) | * 256 (90), 238 (bp), 142 (2), 130 (40) | [31] |
| Fructose (Iso)Leucine conjugate | 103.8 | 1.35 | C12H23O7N | 292.1400 (0.33) | 274 (20), 226 (55), 202 (bp), 172 (15), 130 (30) | 294.1548 (0.03) | 276 (bp), 258 (4), 230 (4), 146 (4), 144 (2) | [30,31,32] |
| 1-Methoxyoxaloyl-4,5-DiCaffeoyl Quinic Acid | 1.5 | 25.45 | C28H26O15 | 601.1200 (0.18) | 557 (20), 515 (80), 439 (15), 395 (bp), 377 (18), 299 (10), 233 (5) | [16,17] | ||
| 1,5-DiCaffeoyl-3-Methoxyoxaloyl Quinic Acid | 1.2 | 25.95 | C28H26O15 | 601.1203 (0.68) | 515 (90), 439 (70), 395 (bp), 377 (15), 233 (30), 173 (5) | [16,17] | ||
| 3,5-DiCaffeoyl-4-Methoxyoxaloyl Quinic Acid | 1.5 | 28.54 | C28H26O15 | 601.1203 (0.68) | 515 (40), 439 (38), 395 (bp), 377 (10), 233 (28), 173 (5) | [16,17] | ||
| 1,3-DiCaffeoyl-4-Methoxyoxaloyl Quinic Acid | 1.4 | 30.60 | C28H26O15 | 601.1195 (0.48) | 515 (10), 439 (15), 395 (bp), 233 (5) | [16,17] | ||
| 3,5-DiCaffeoyl-1,4-DiMethoxyoxaloyl Quinic Acid | 8.9 | 29.51 | C31H28O18 | 687.1208 (0.76) | 601 (bp), 599 (25), 557 (30), 437 (40), 275 (25) | [16,17] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duthen, S.; Gadéa, A.; Trempat, P.; Boujedaini, N.; Fabre, N. Comparison of the Phytochemical Variation of Non-Volatile Metabolites within Mother Tinctures of Arnica montana Prepared from Fresh and Dried Whole Plant Using UHPLC-HRMS Fingerprinting and Chemometric Analysis. Molecules 2022, 27, 2737. https://doi.org/10.3390/molecules27092737
Duthen S, Gadéa A, Trempat P, Boujedaini N, Fabre N. Comparison of the Phytochemical Variation of Non-Volatile Metabolites within Mother Tinctures of Arnica montana Prepared from Fresh and Dried Whole Plant Using UHPLC-HRMS Fingerprinting and Chemometric Analysis. Molecules. 2022; 27(9):2737. https://doi.org/10.3390/molecules27092737
Chicago/Turabian StyleDuthen, Simon, Alice Gadéa, Pascal Trempat, Naoual Boujedaini, and Nicolas Fabre. 2022. "Comparison of the Phytochemical Variation of Non-Volatile Metabolites within Mother Tinctures of Arnica montana Prepared from Fresh and Dried Whole Plant Using UHPLC-HRMS Fingerprinting and Chemometric Analysis" Molecules 27, no. 9: 2737. https://doi.org/10.3390/molecules27092737
APA StyleDuthen, S., Gadéa, A., Trempat, P., Boujedaini, N., & Fabre, N. (2022). Comparison of the Phytochemical Variation of Non-Volatile Metabolites within Mother Tinctures of Arnica montana Prepared from Fresh and Dried Whole Plant Using UHPLC-HRMS Fingerprinting and Chemometric Analysis. Molecules, 27(9), 2737. https://doi.org/10.3390/molecules27092737






