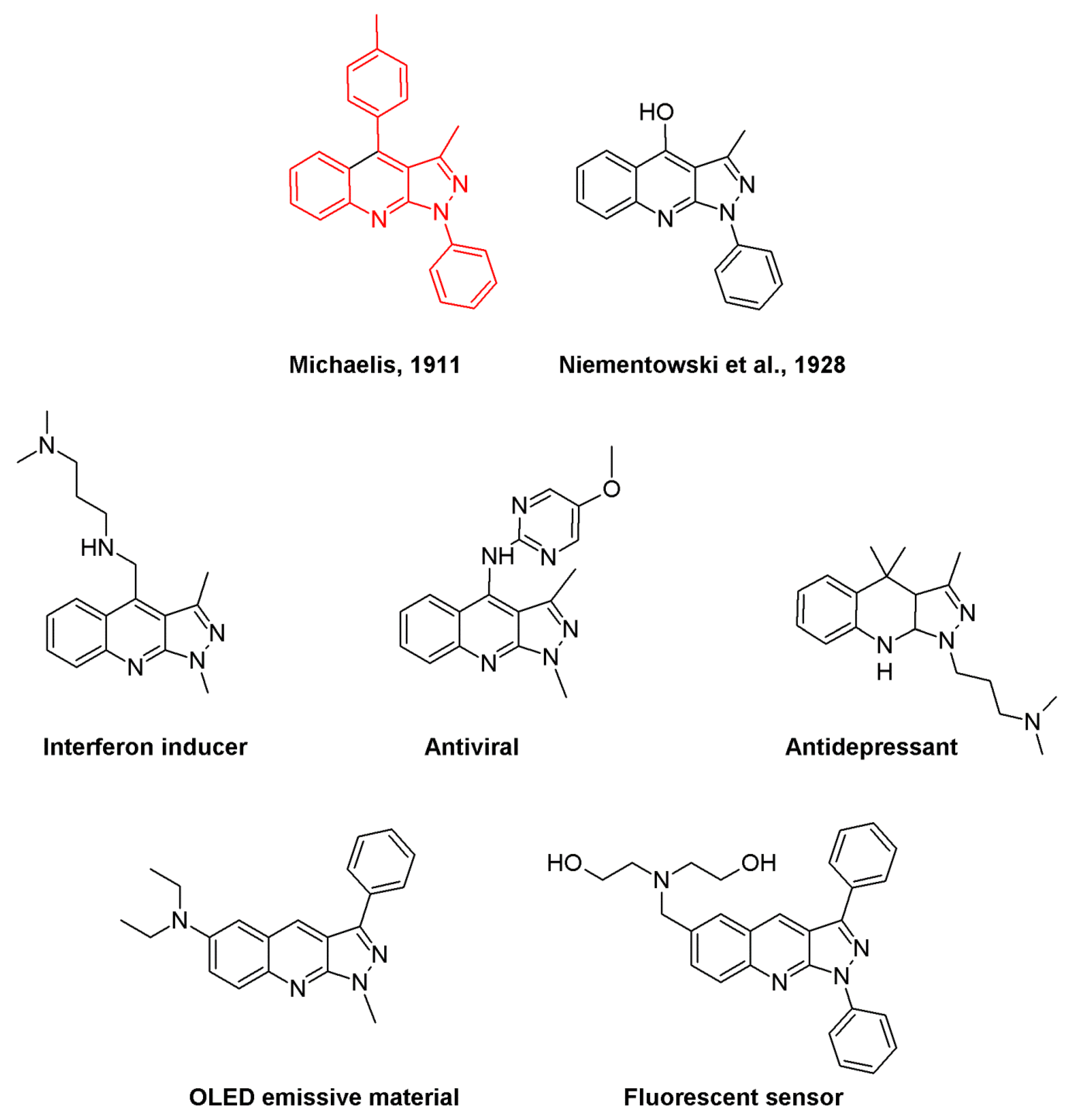
1H-Pyrazolo[3,4-b]quinolines: Synthesis and Properties over 100 Years of Research
Abstract
:1. Introduction
2. The Main Synthesis Method of 1H-Pyrazolo[3,4-b]quinoline
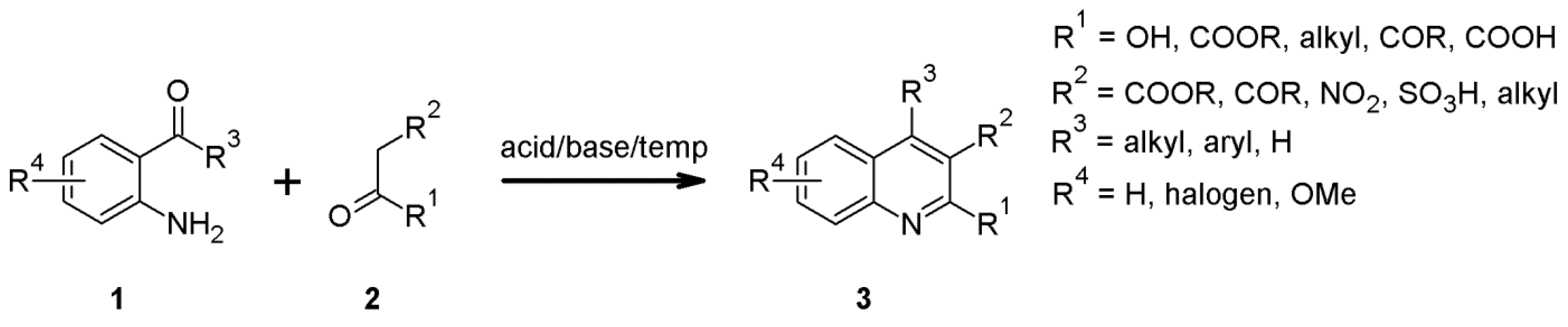
2.1. The Friedländer Synthesis Based on o-Aminoaldehydes, o-Aminoacetophenones and o-Aminobenzophenones
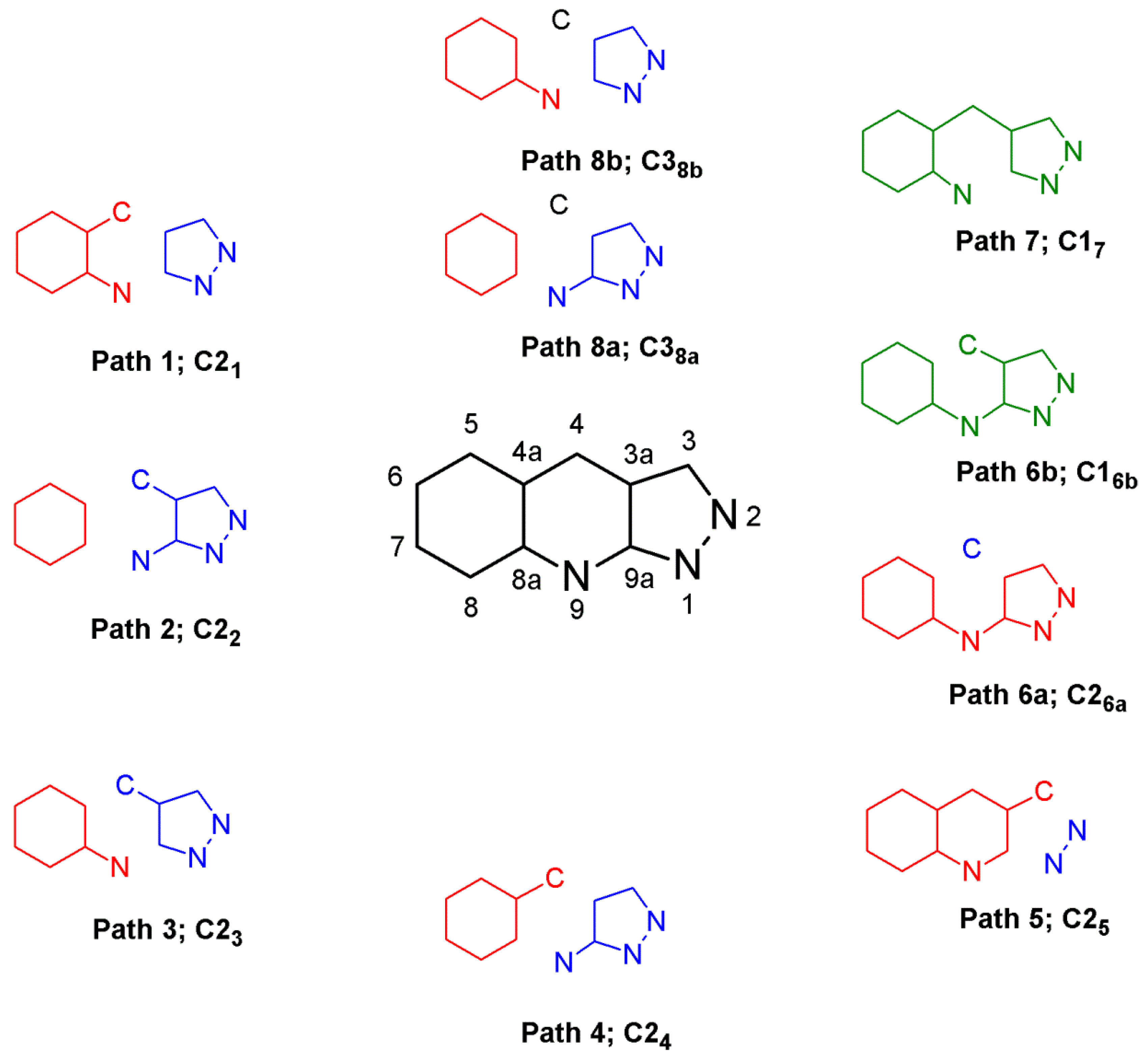
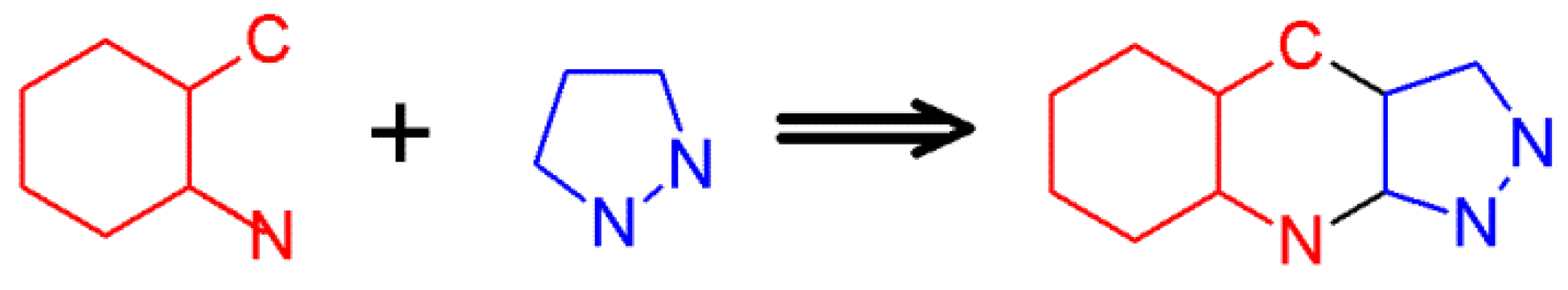
2.2. The Friedländer Synthesis Based on Pyrazole Derivatives
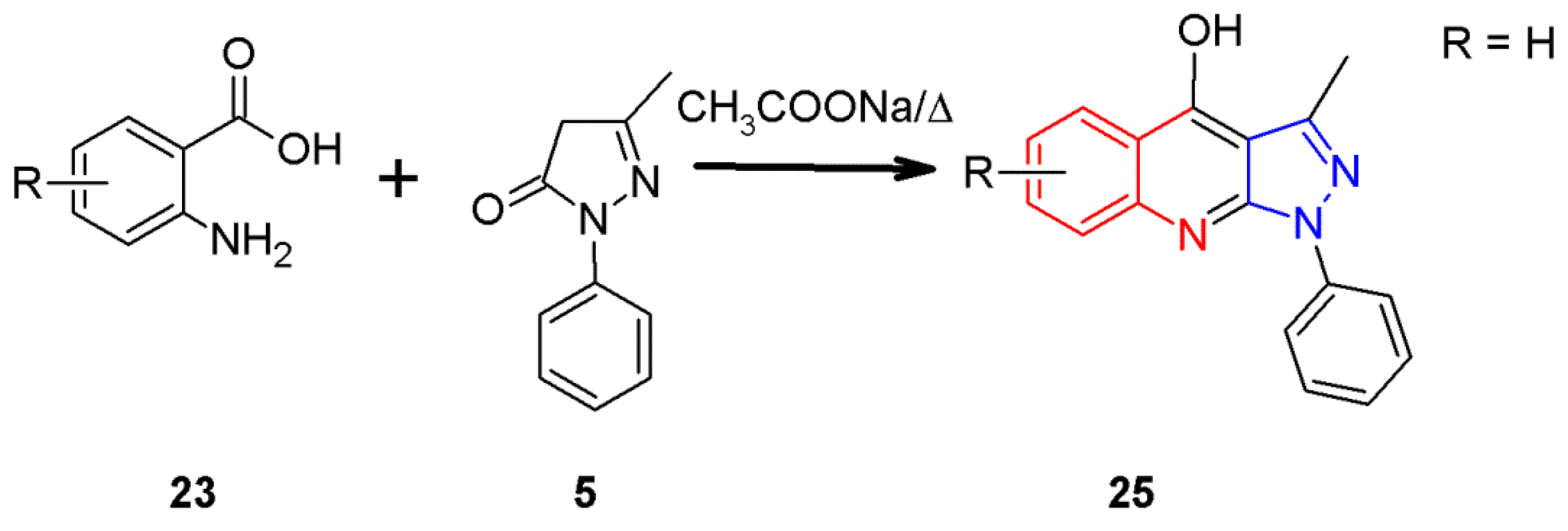
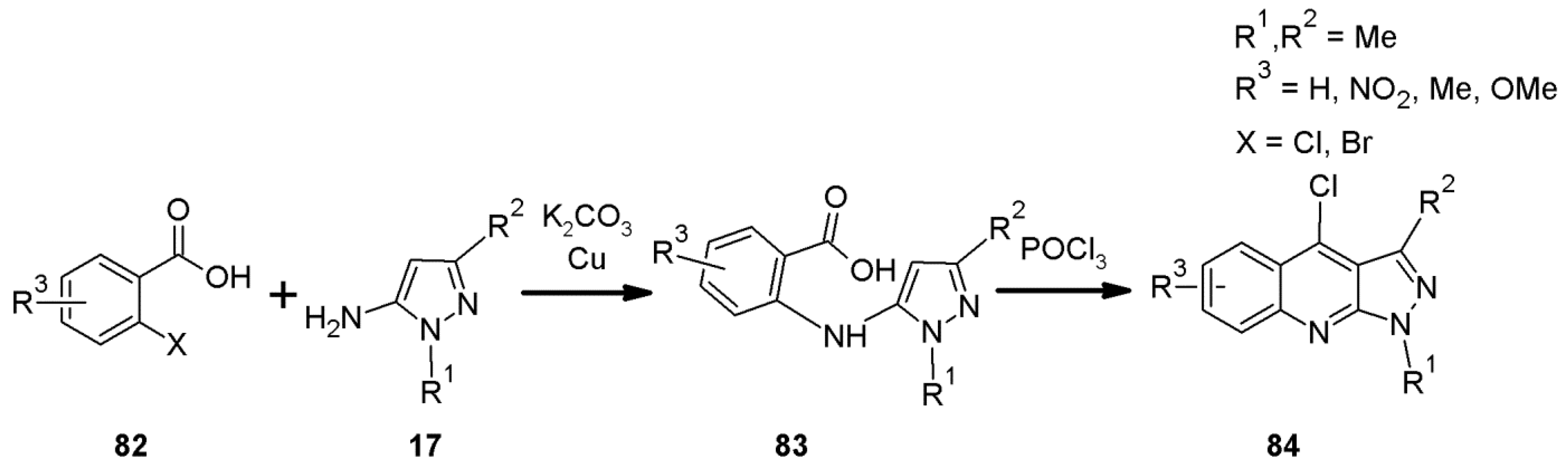
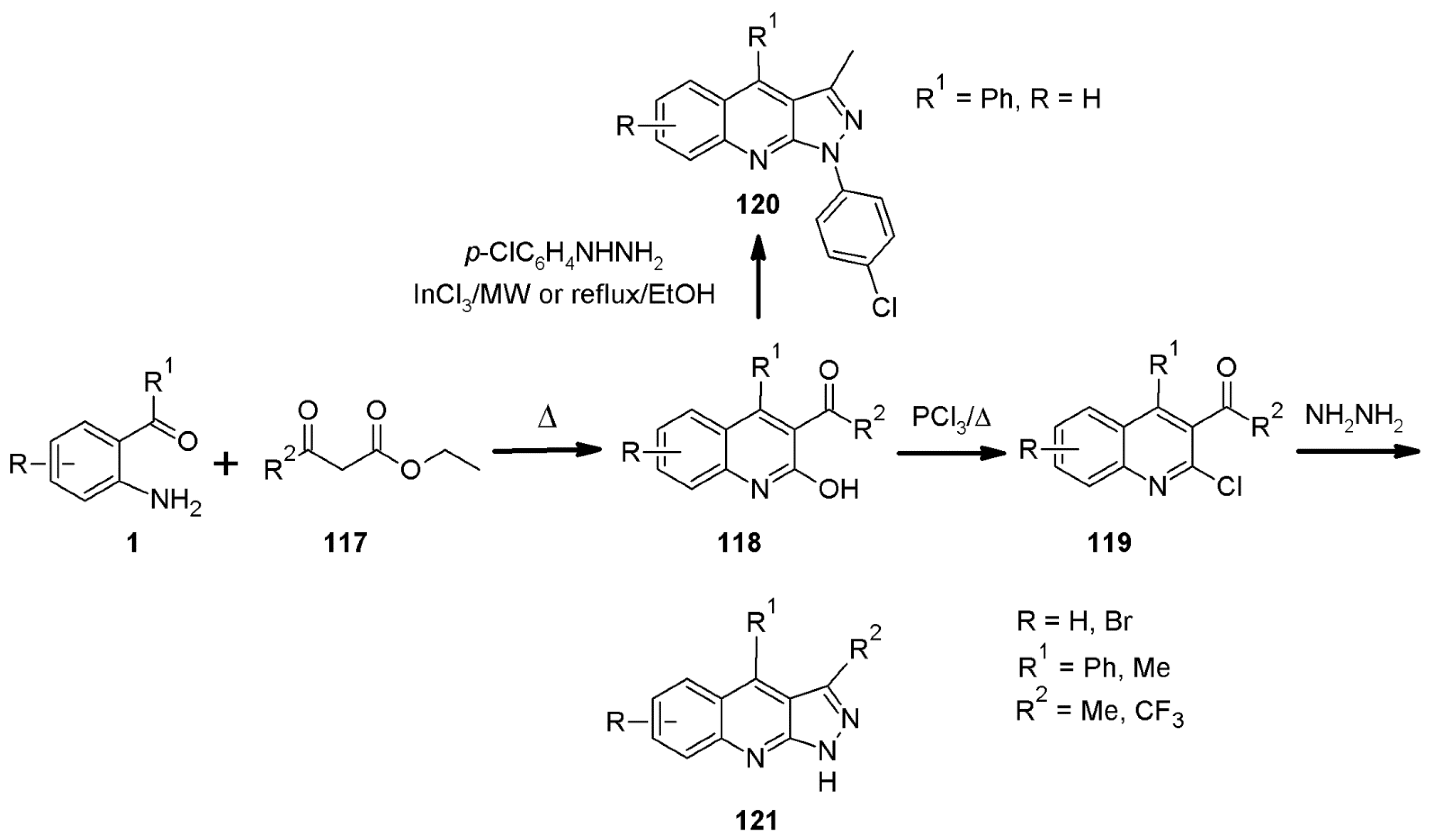
2.3. 1H-Pyrazolo[3,4-b]quinoline Syntheses Based on Anthranilic Acid and Anthranilic Acid Derivatives
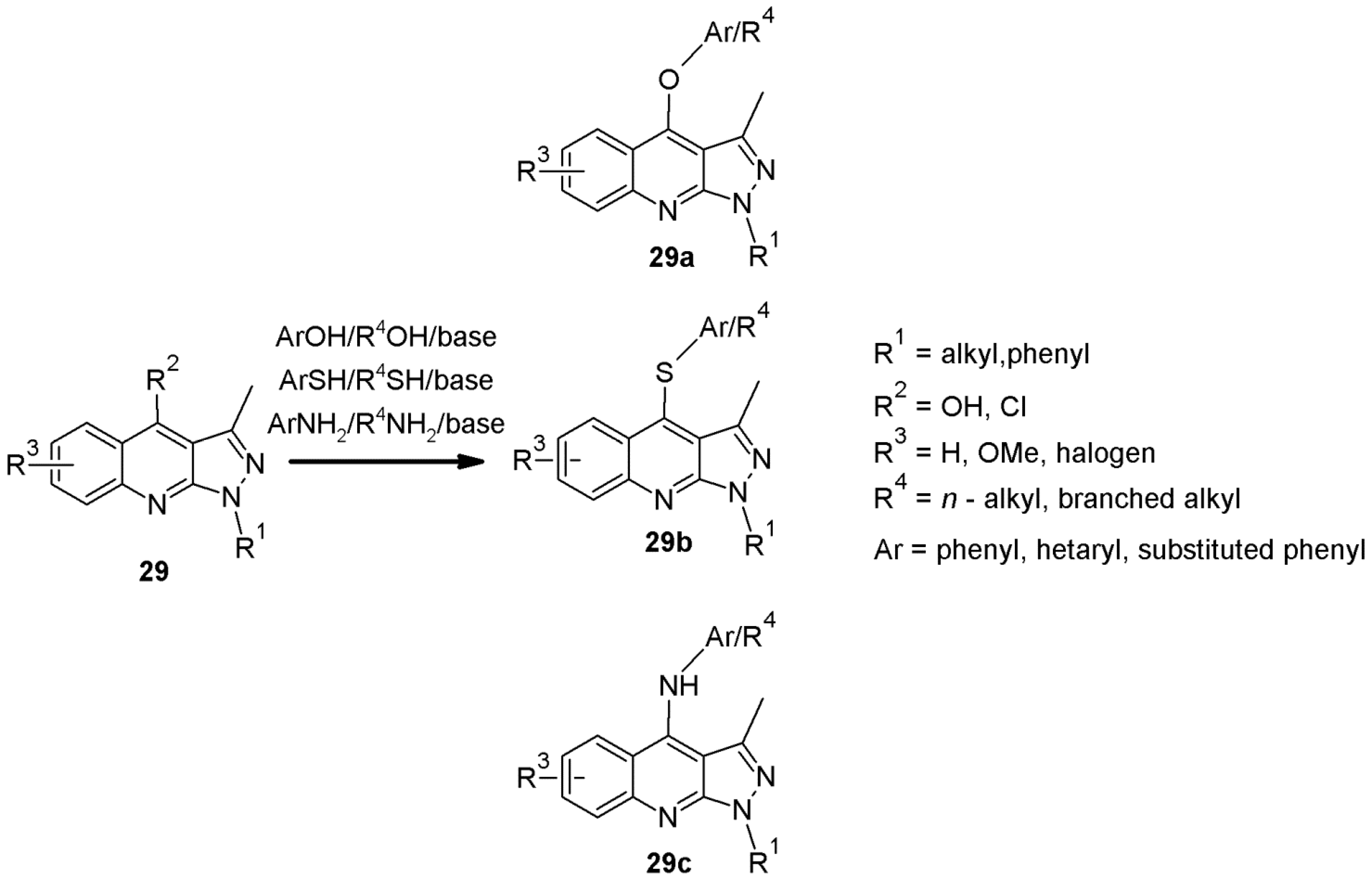
2.4. 1H-Pyrazolo[3,4-b]quinoline Syntheses Based on 4-amino-3-carboxypyrazole Derivatives
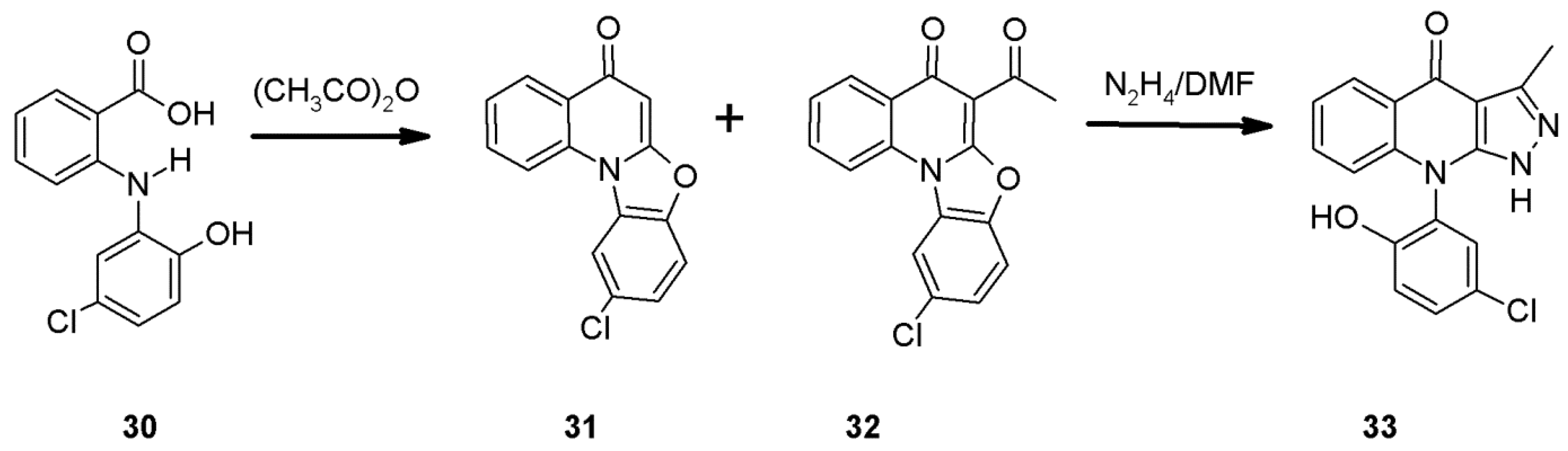
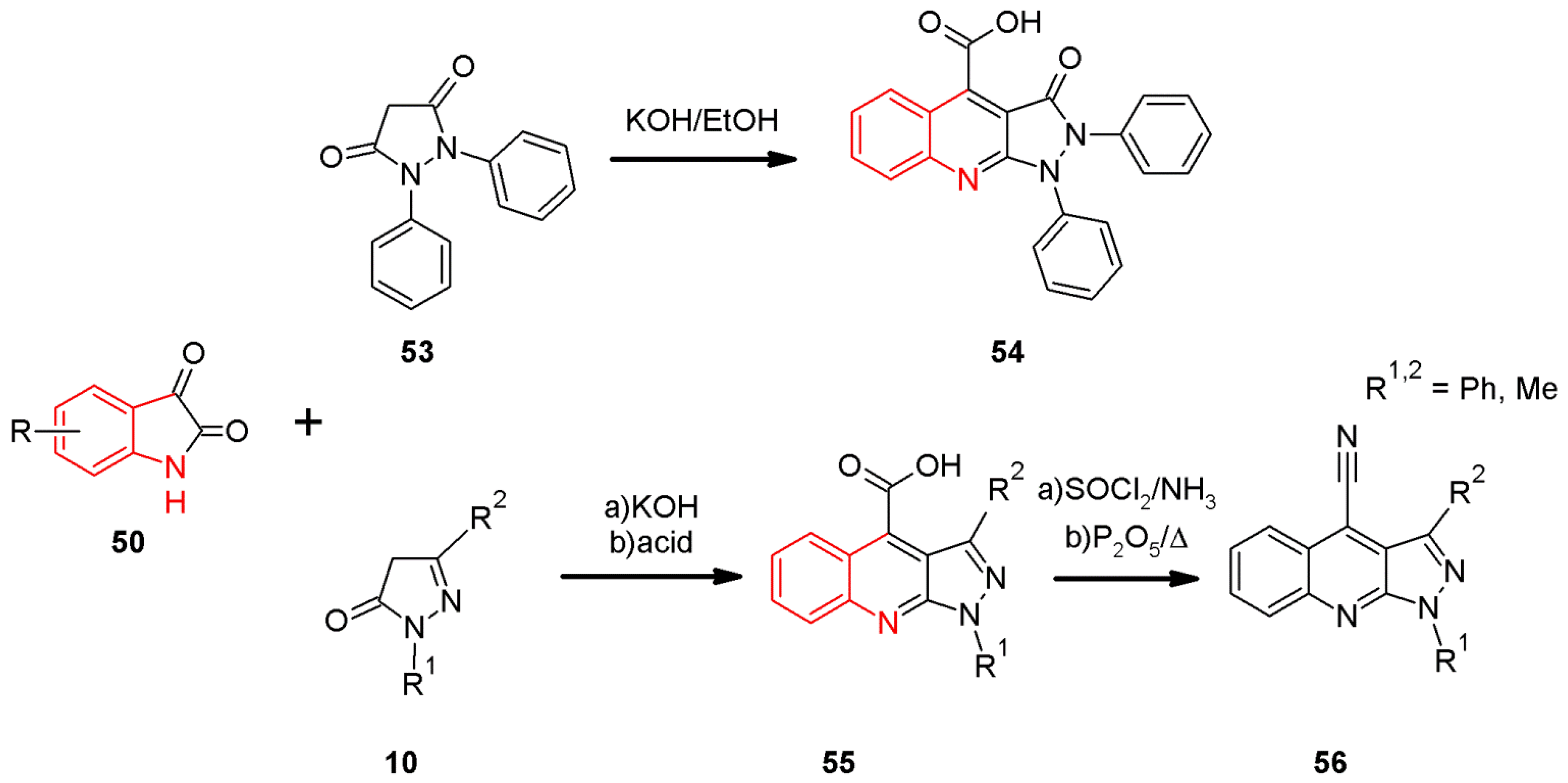
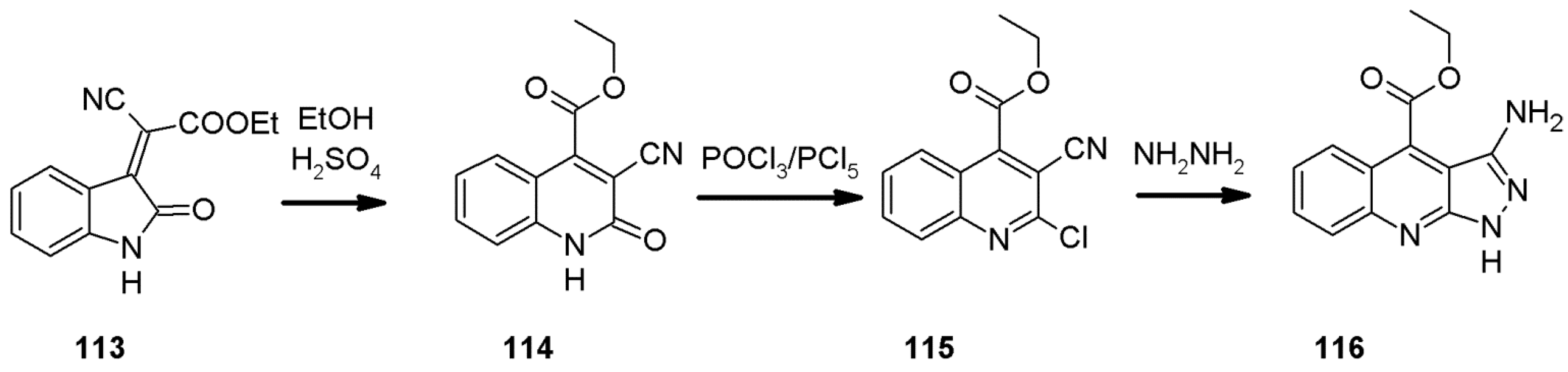
2.5. The Pfitzinger Synthesis of 1H-pyrazolo[3,4-b]quinolines
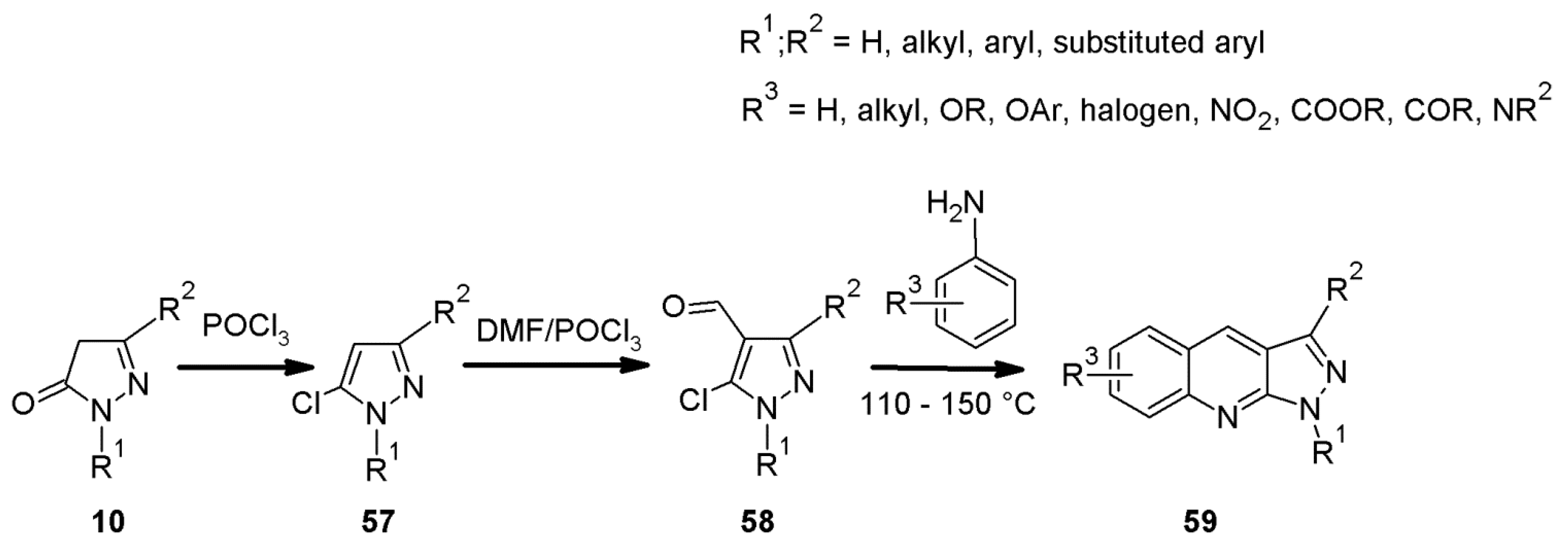
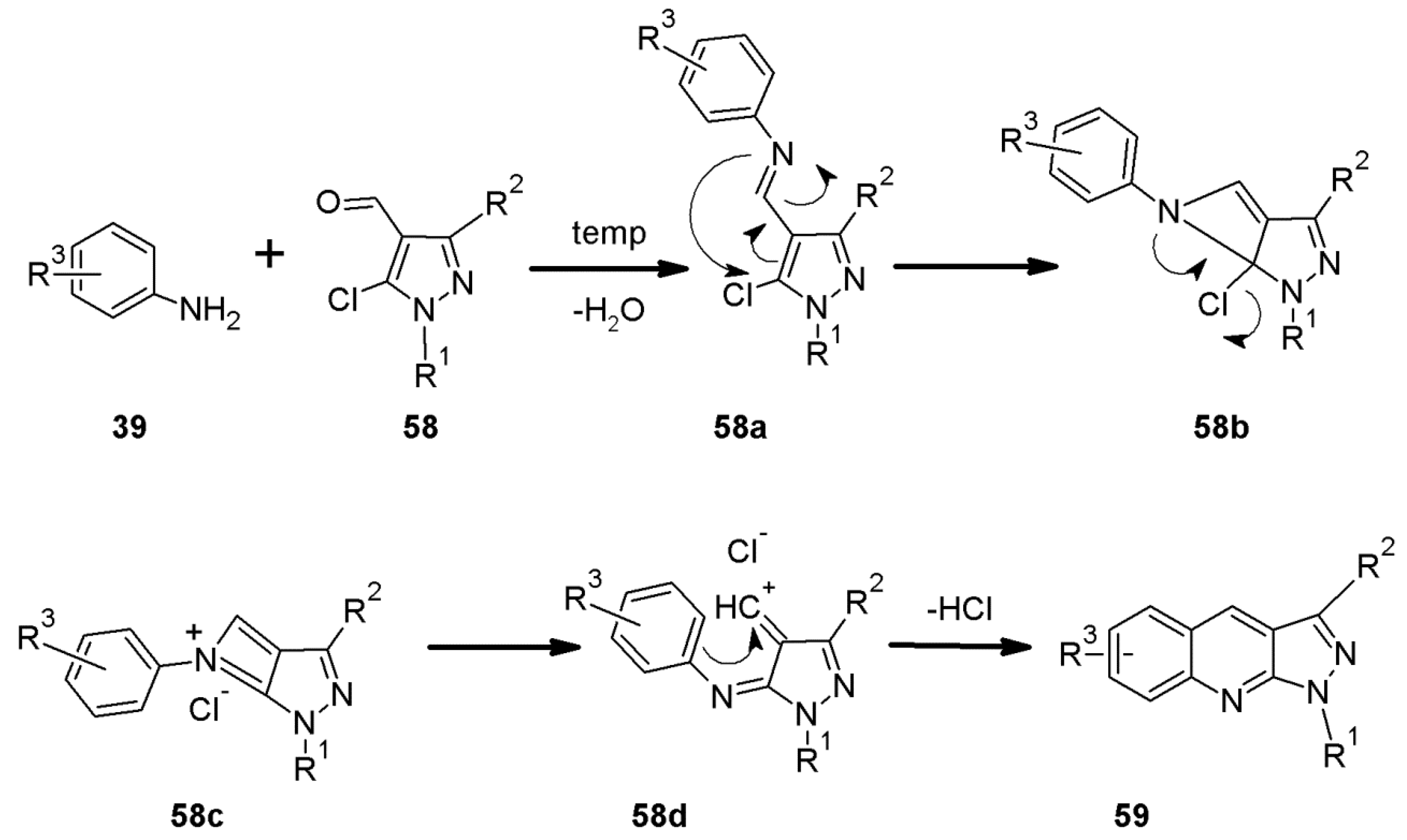
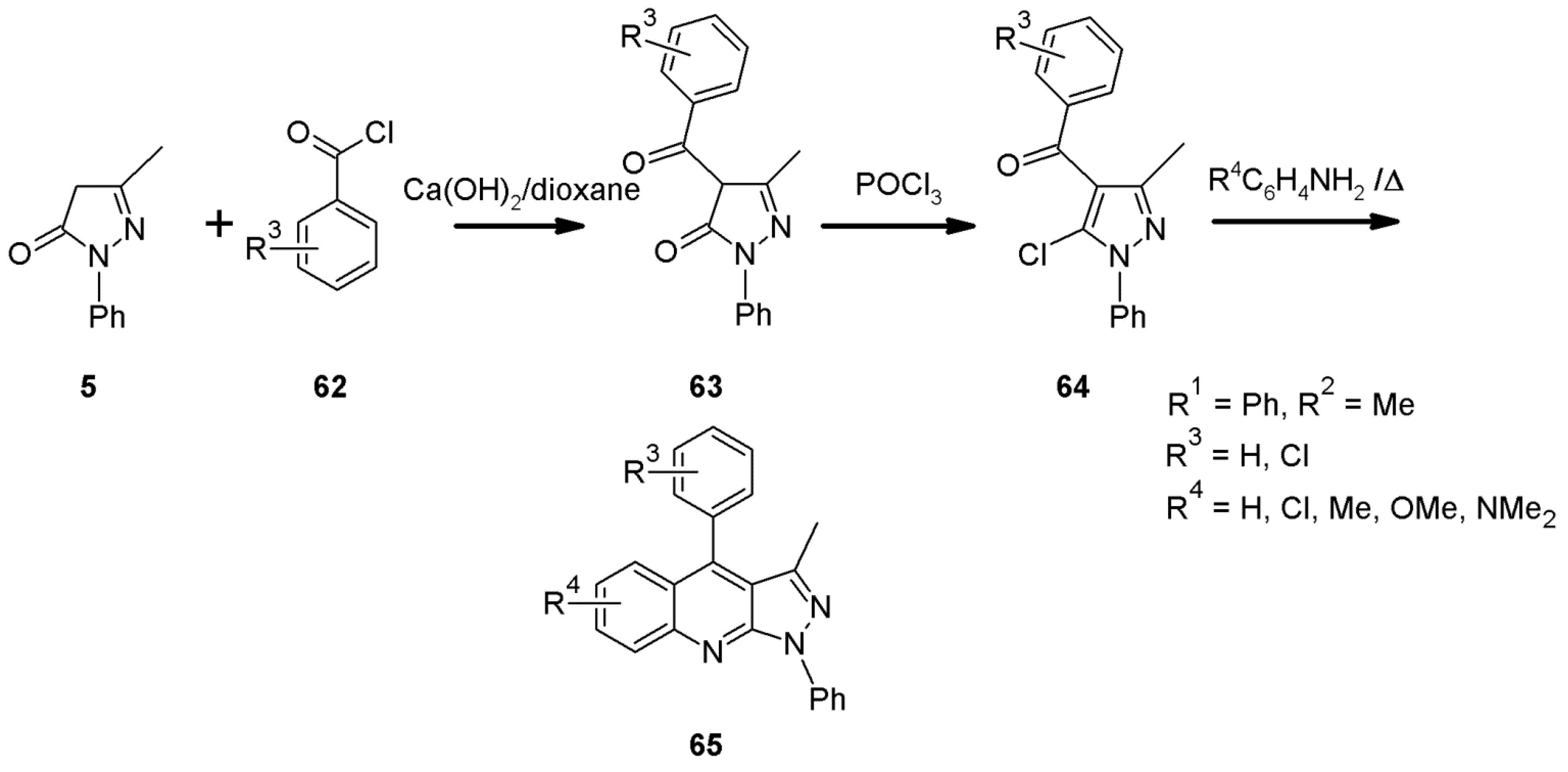
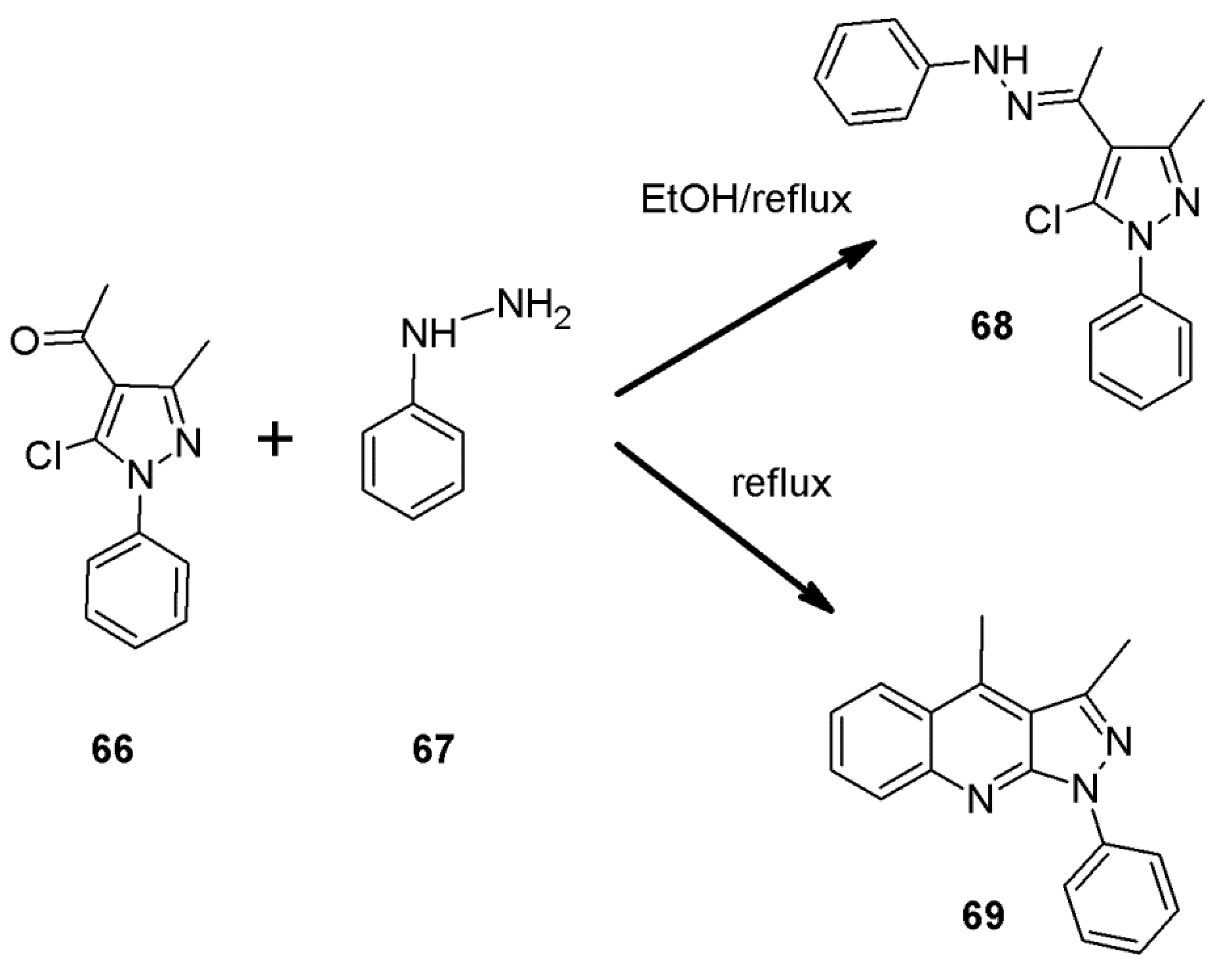
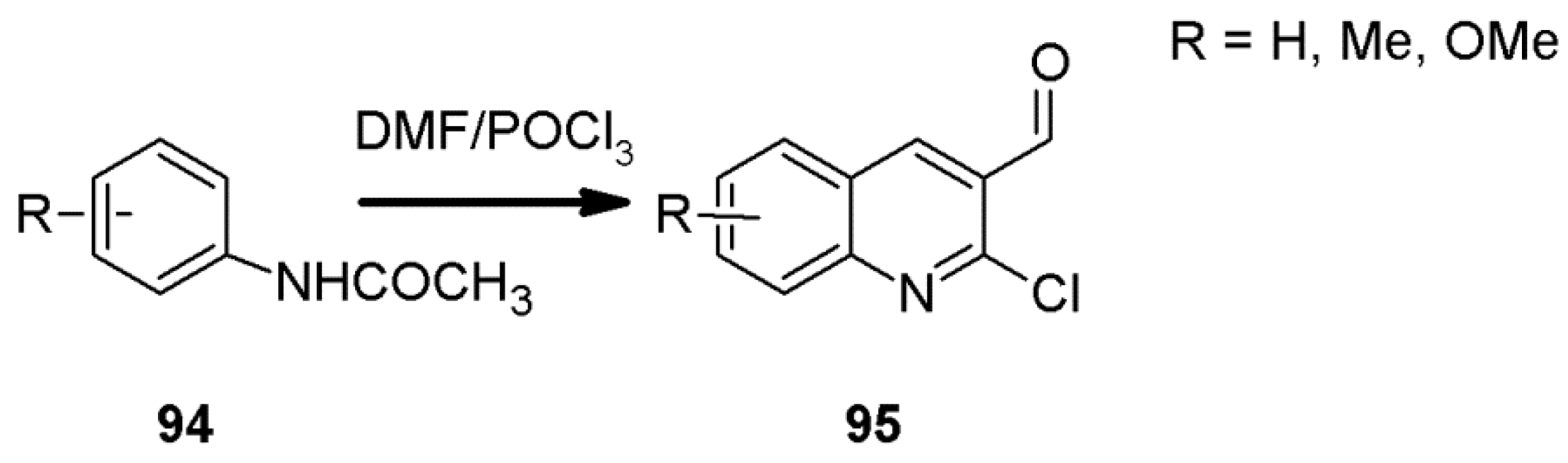
2.6. 1H-Pyrazolo[3,4-b]quinoline Syntheses Based on 5-chloro-4-aroyl/formylpyrazoles and 4-benzylidene-1,3-disubstituted-pyrazol-5-ones
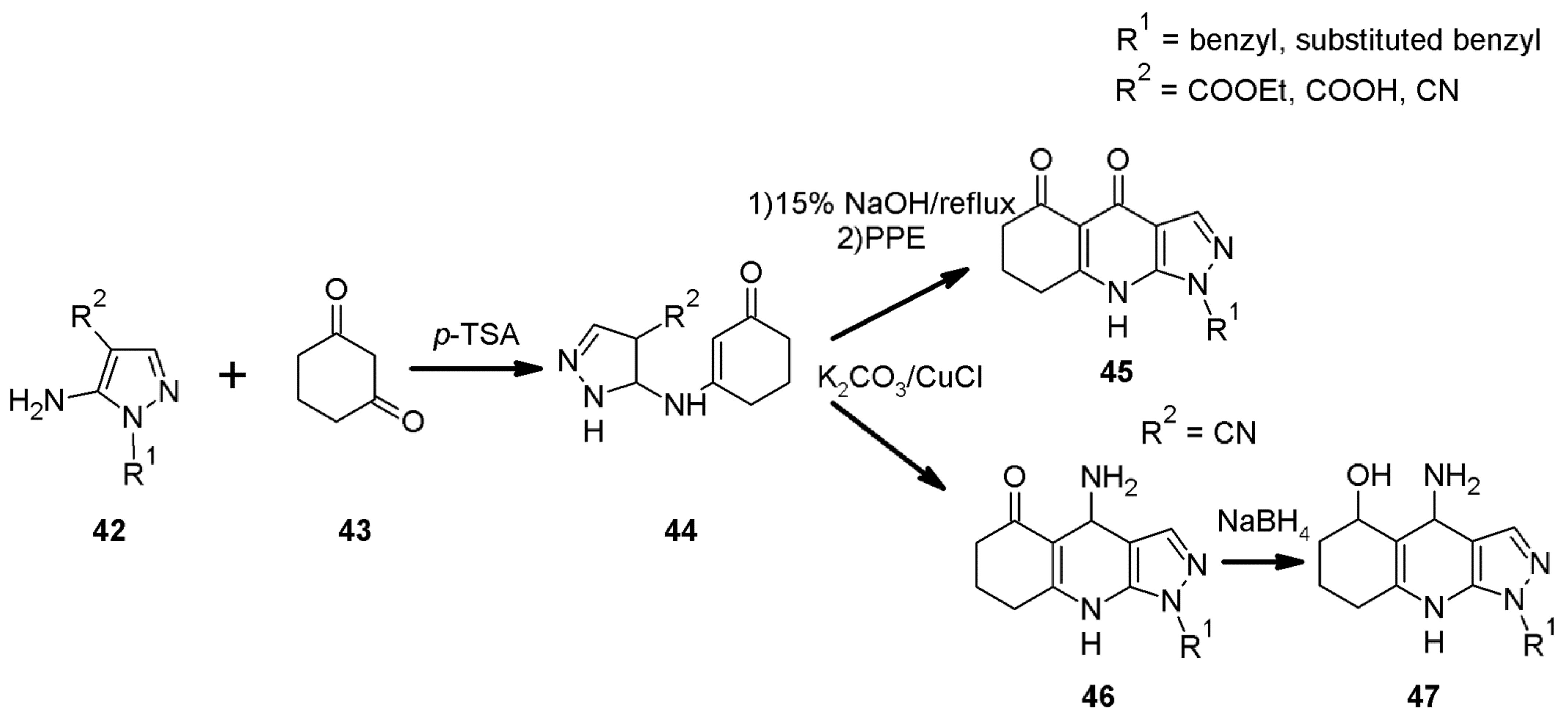
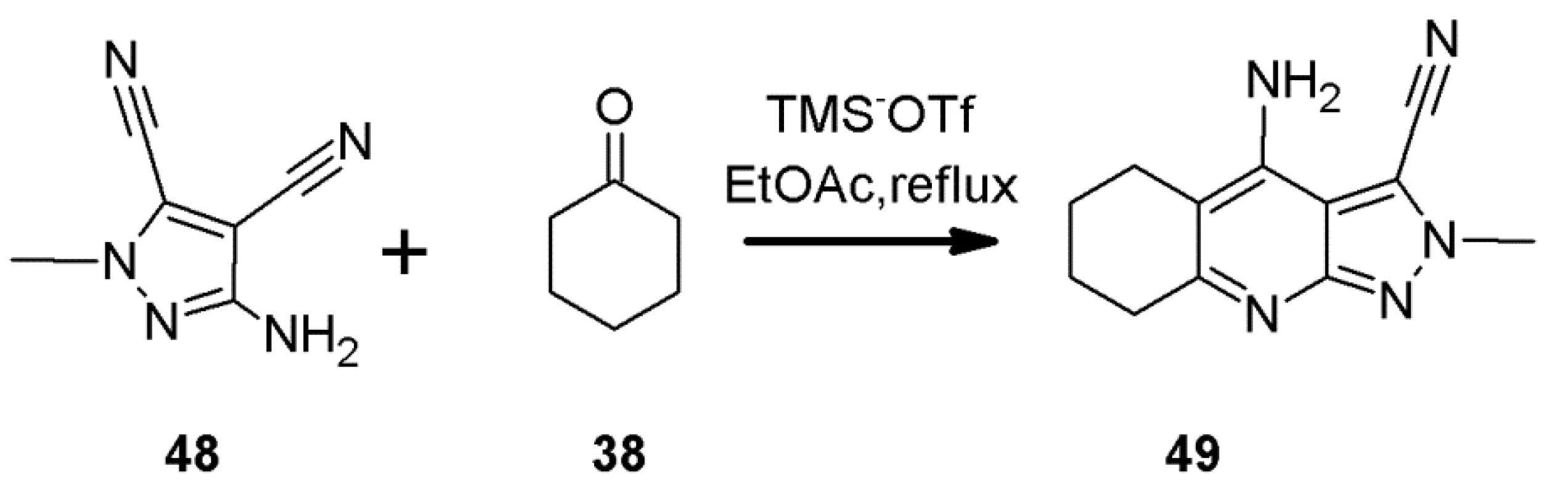
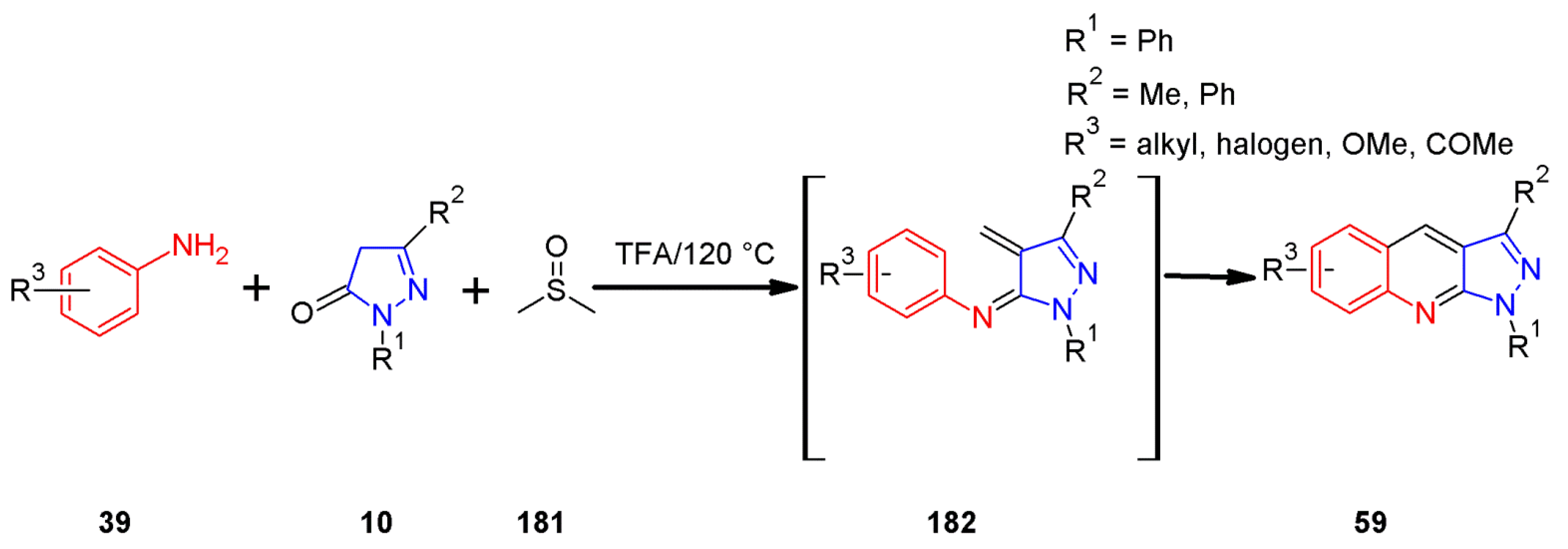
2.7. 1H-Pyrazolo[3,4-b]quinoline Syntheses Based on Aminopyrazoles
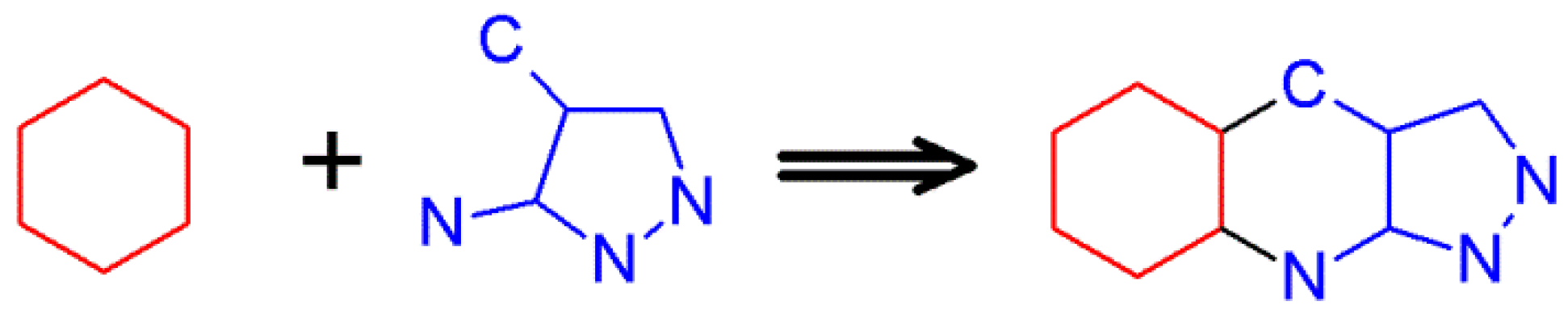
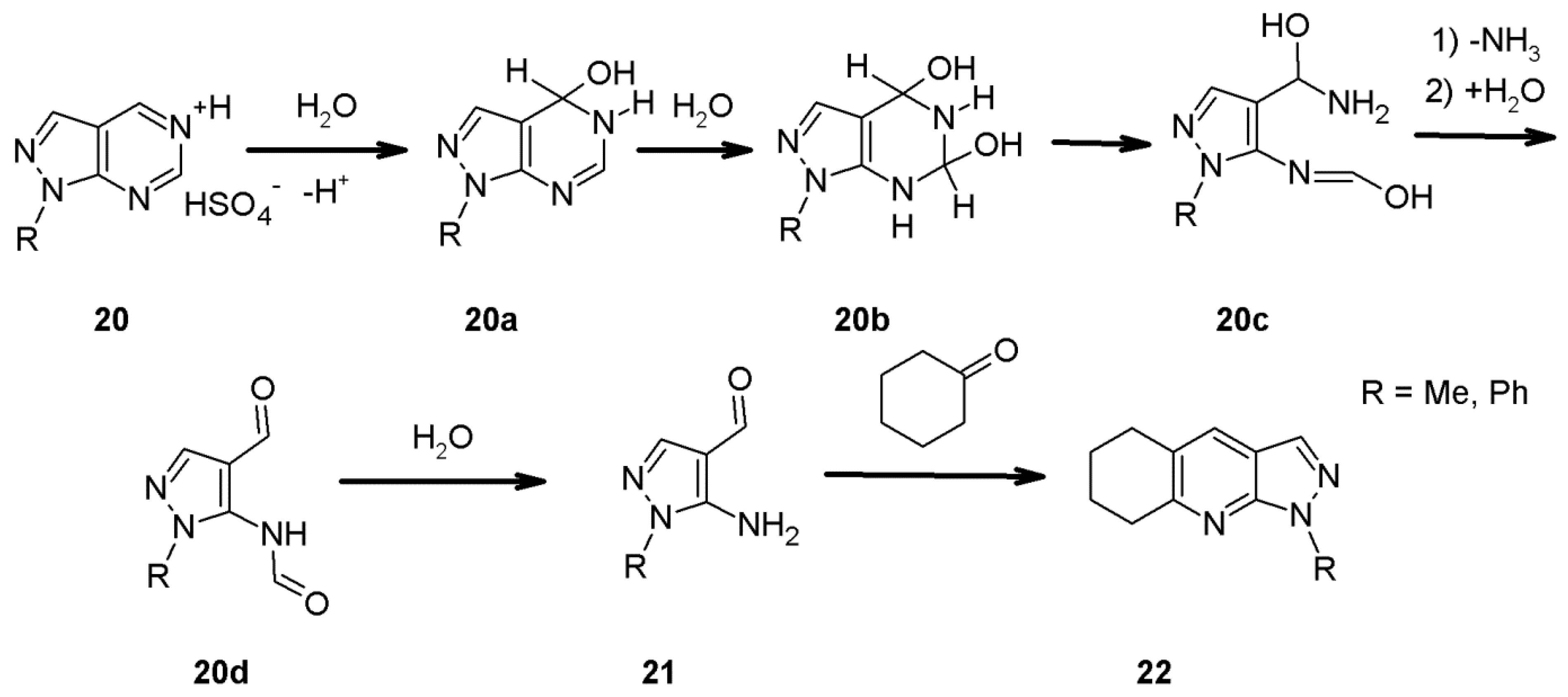
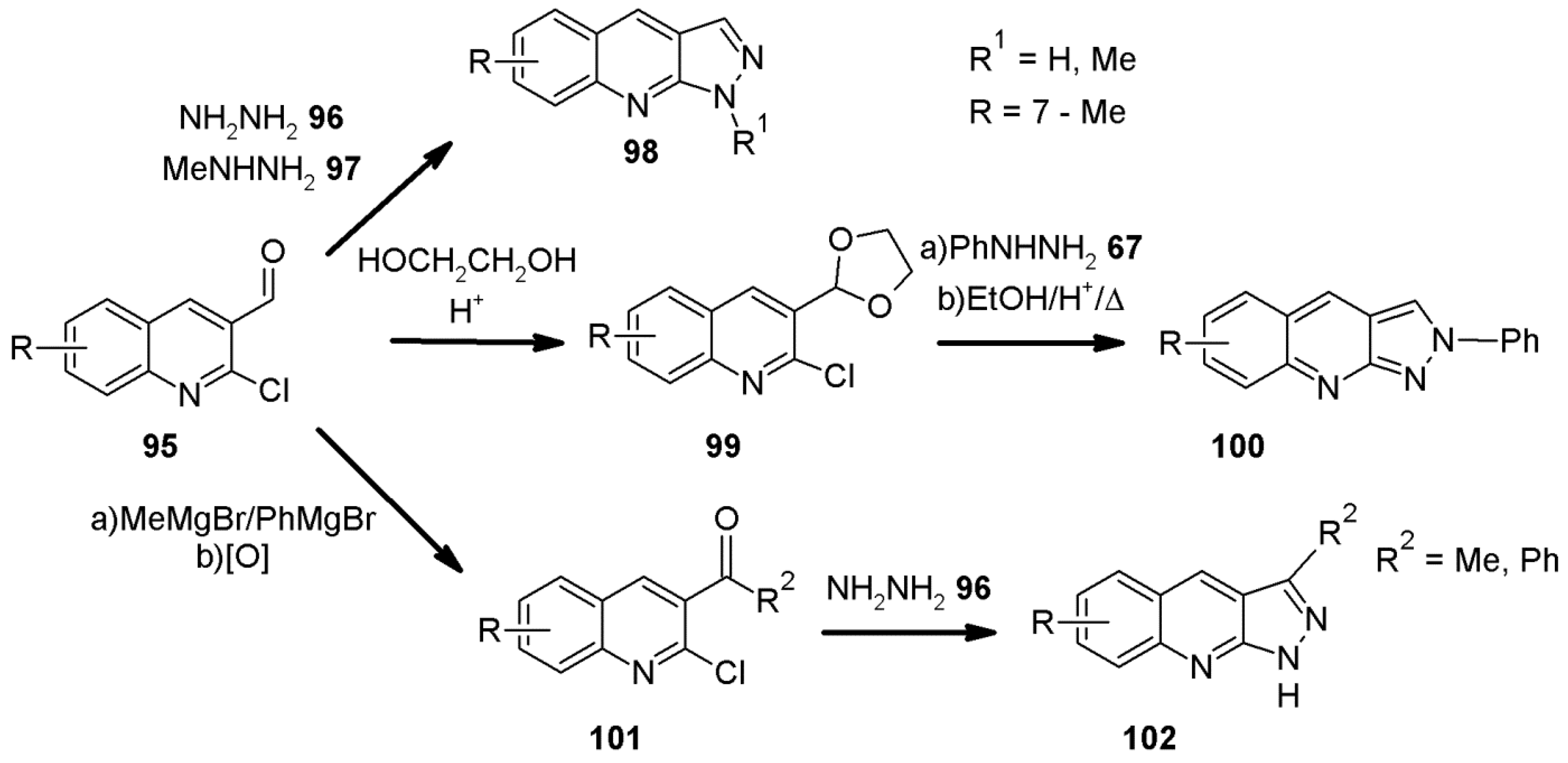
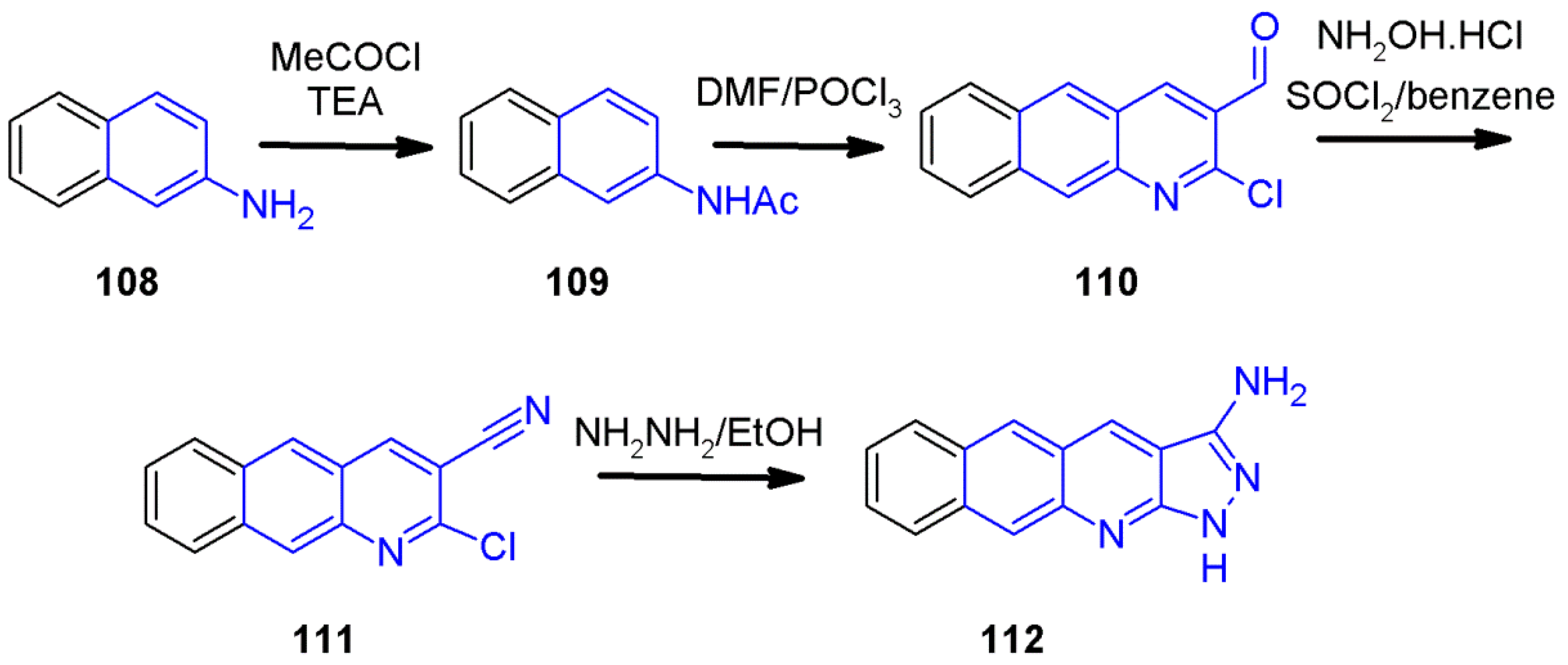
2.8. 1H-Pyrazolo[3,4-b]quinoline Synthes Based on Quinoline Derivatives
2.9. 1H-Pyrazolo[3,4-b]quinoline Syntheses Based on 5-arylaminoaminopyrazoles
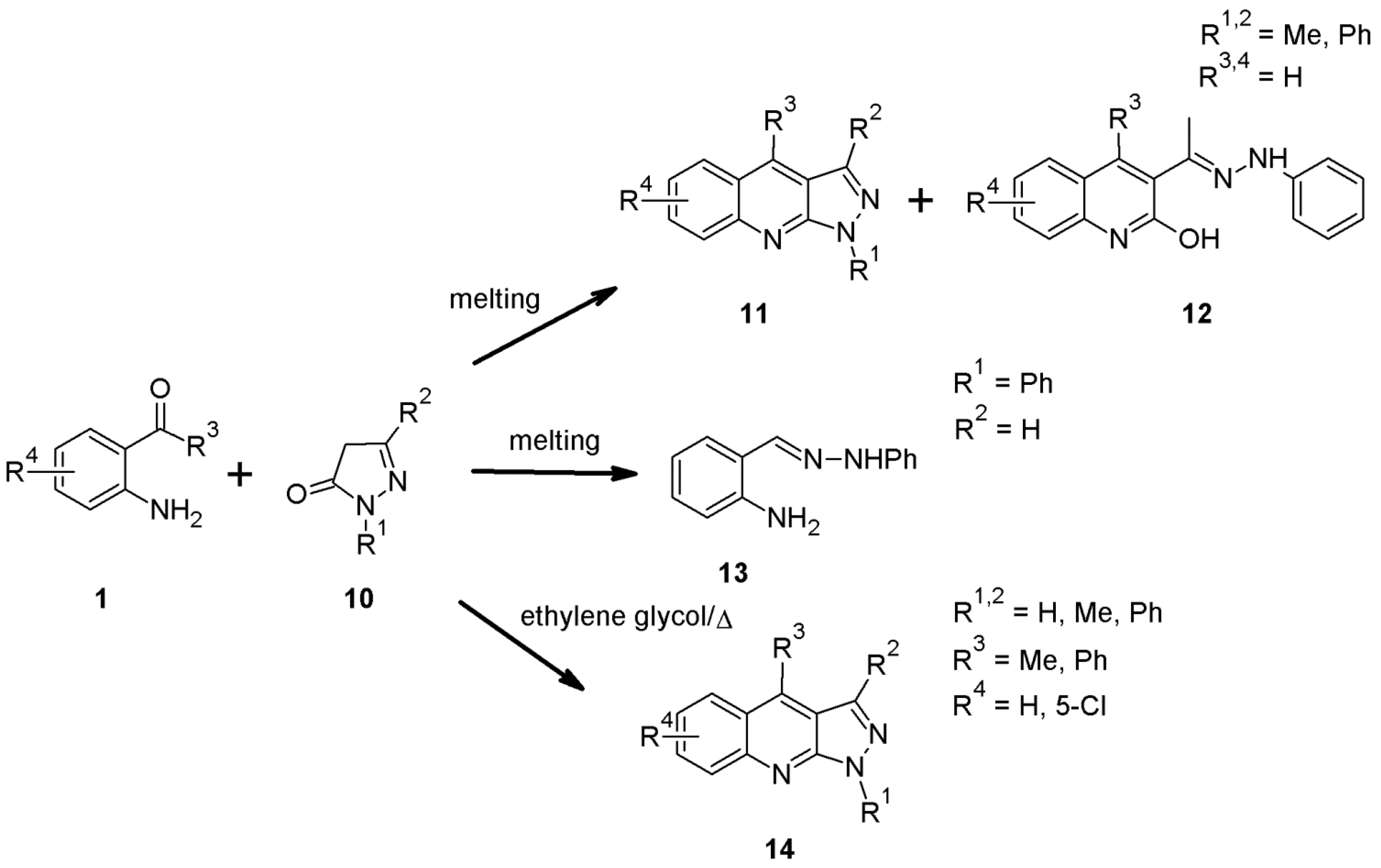
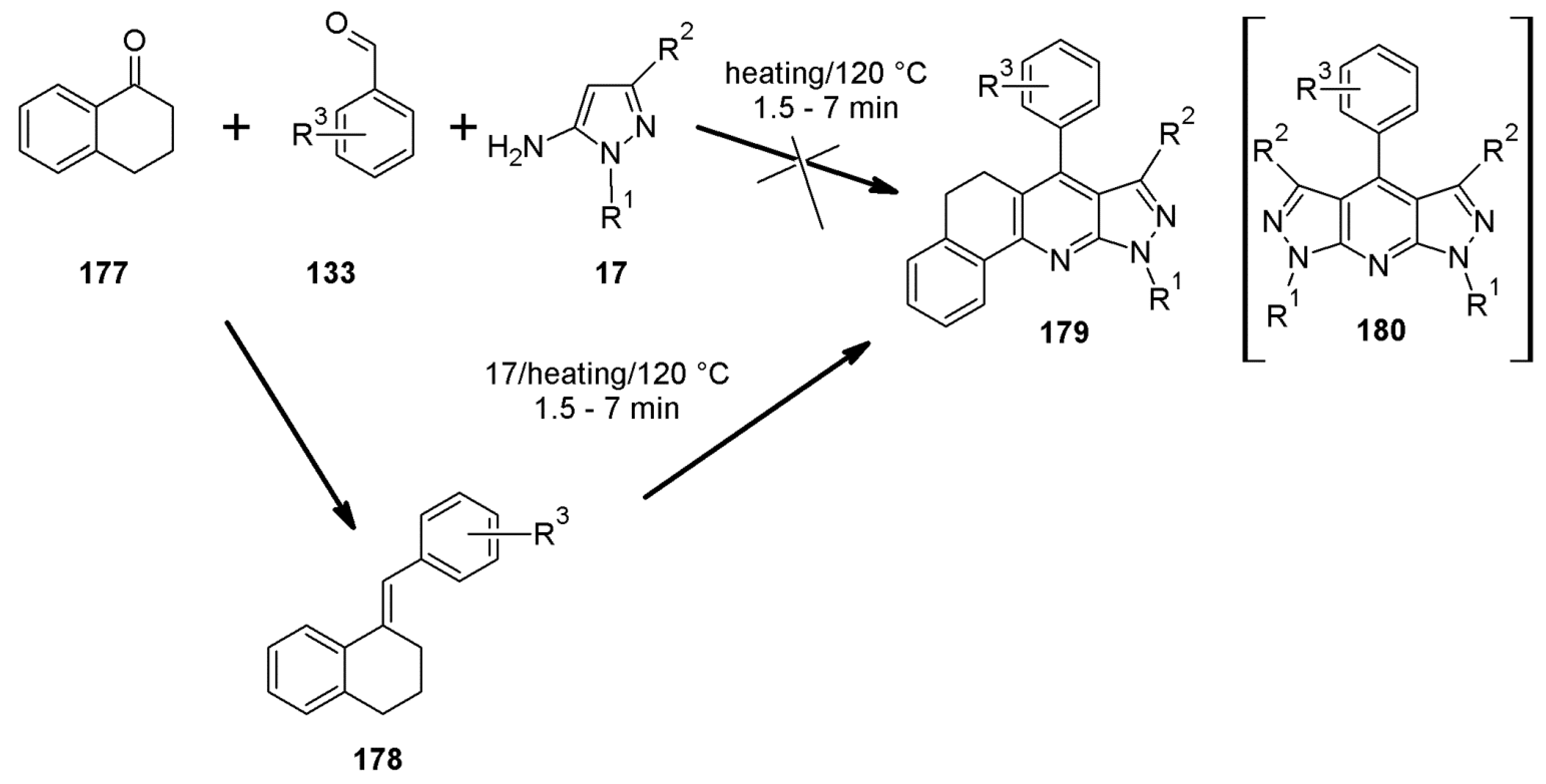
2.10. 1H-Pyrazolo[3,4-b]quinoline Syntheses Based on 4-arylidenepyrazoles
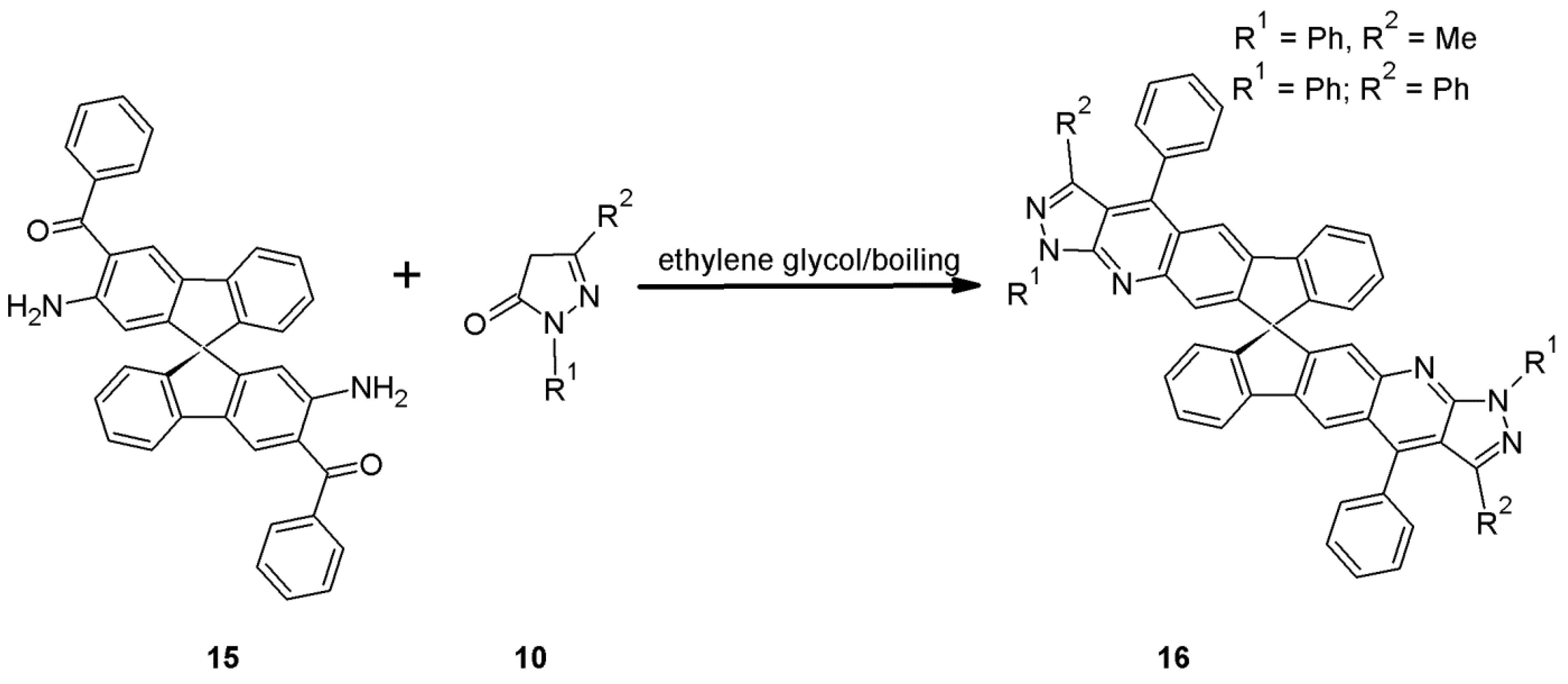
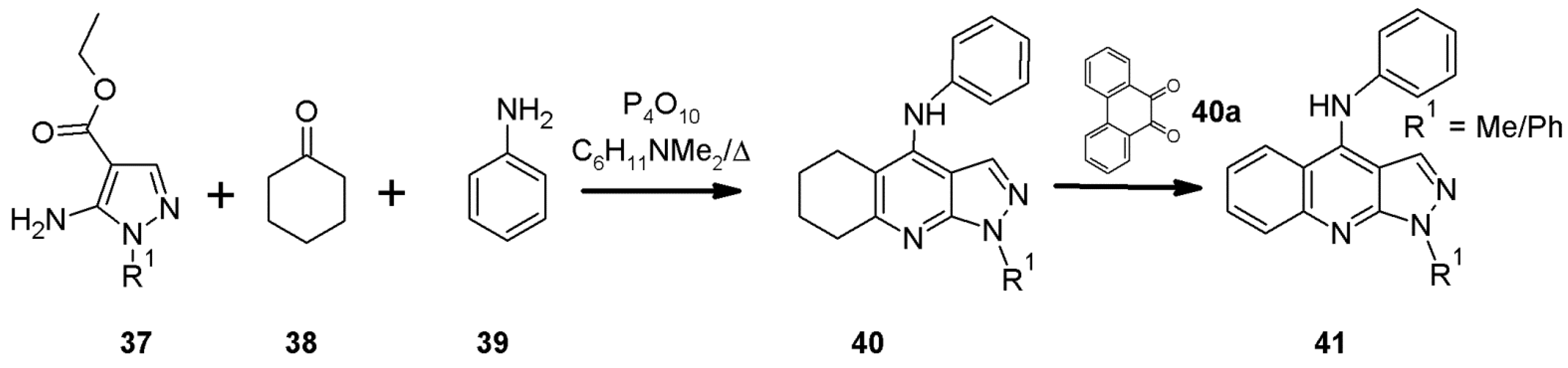
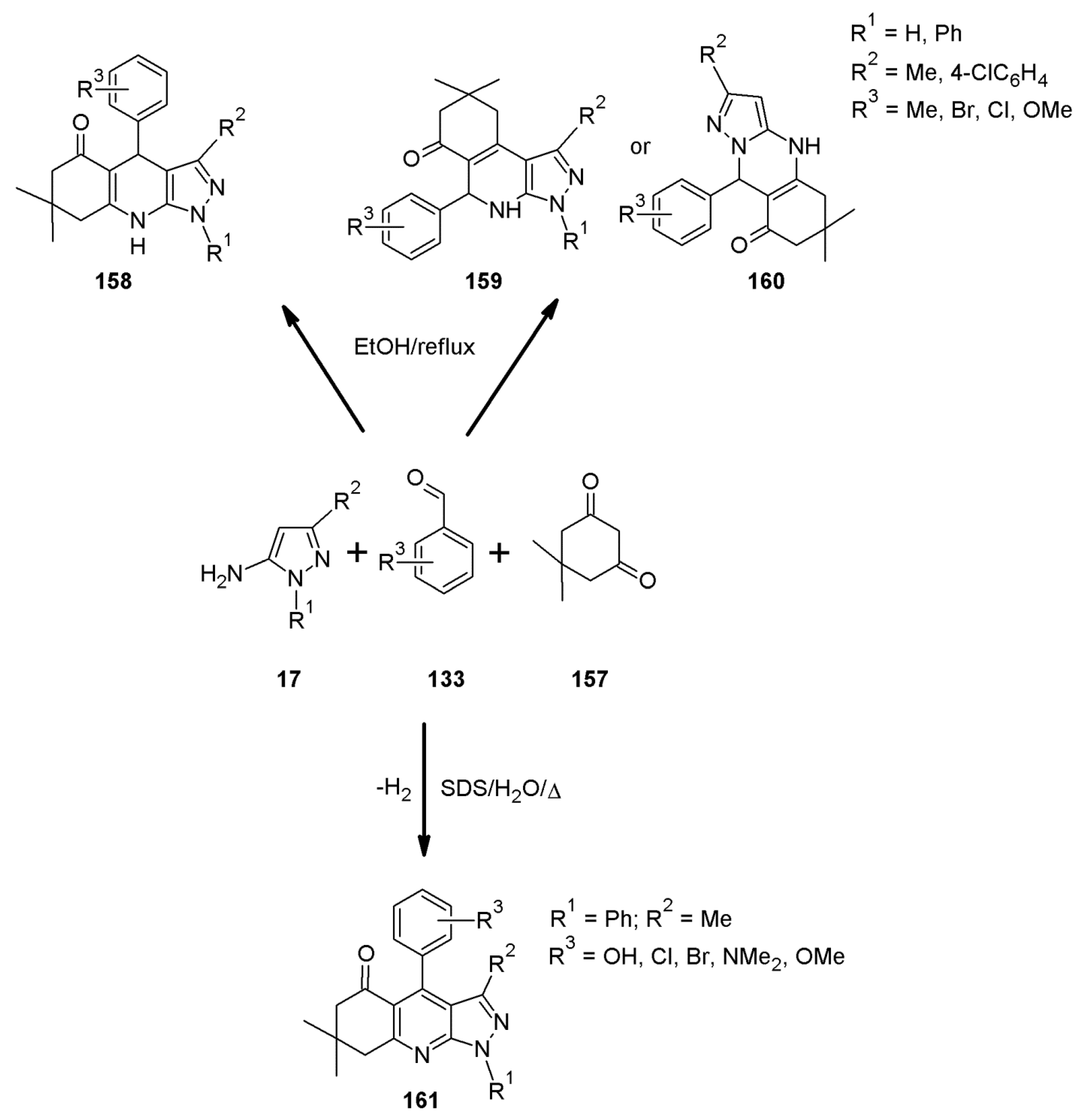
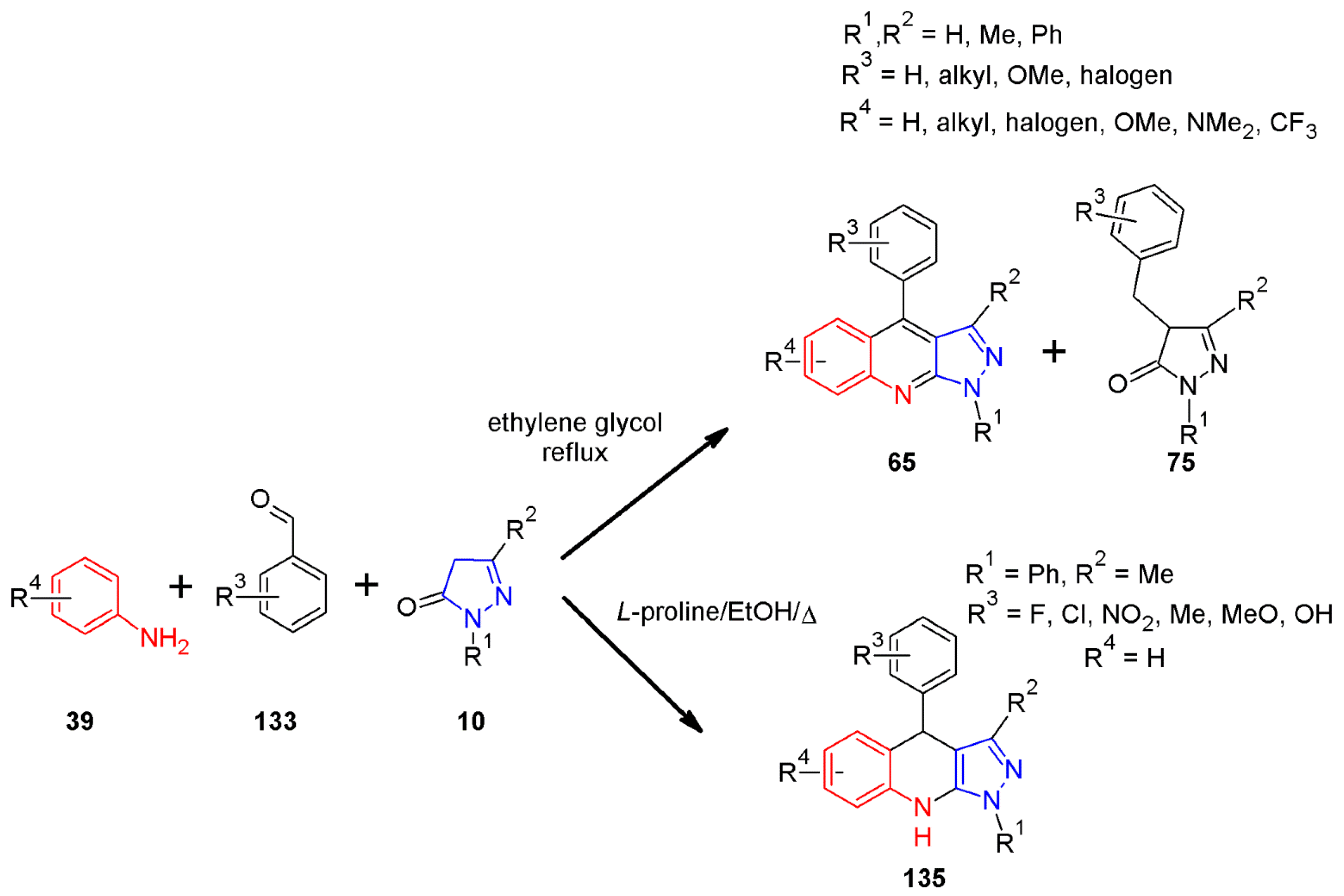
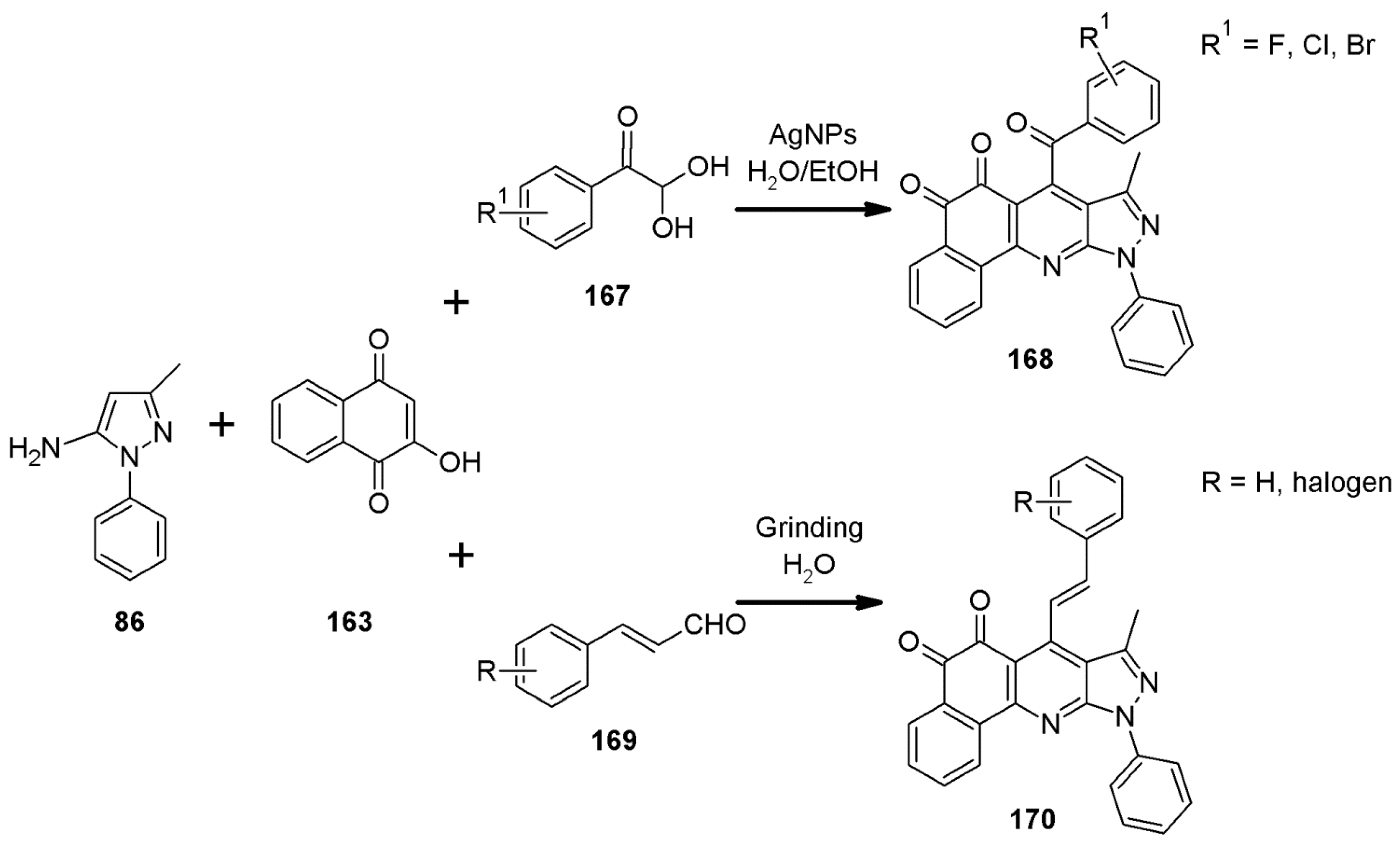
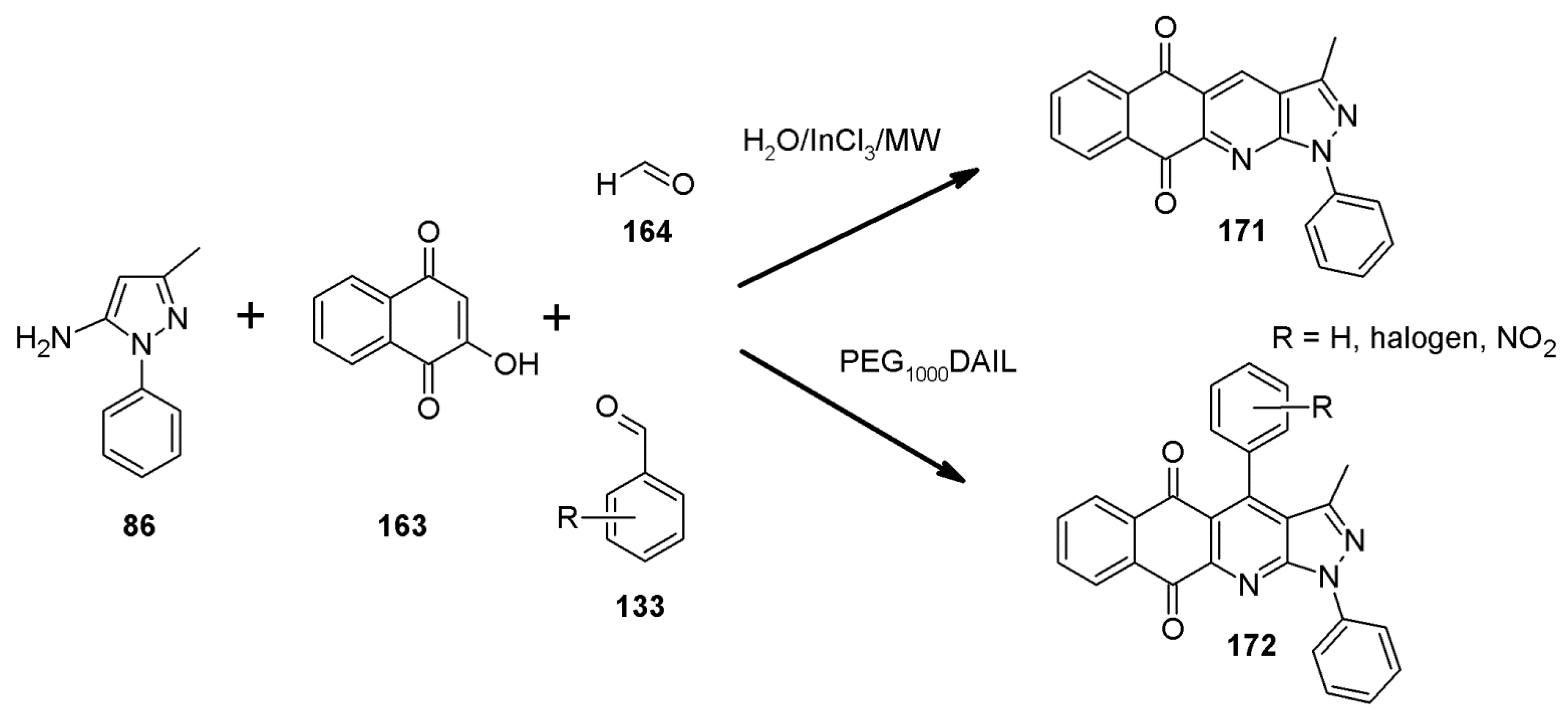
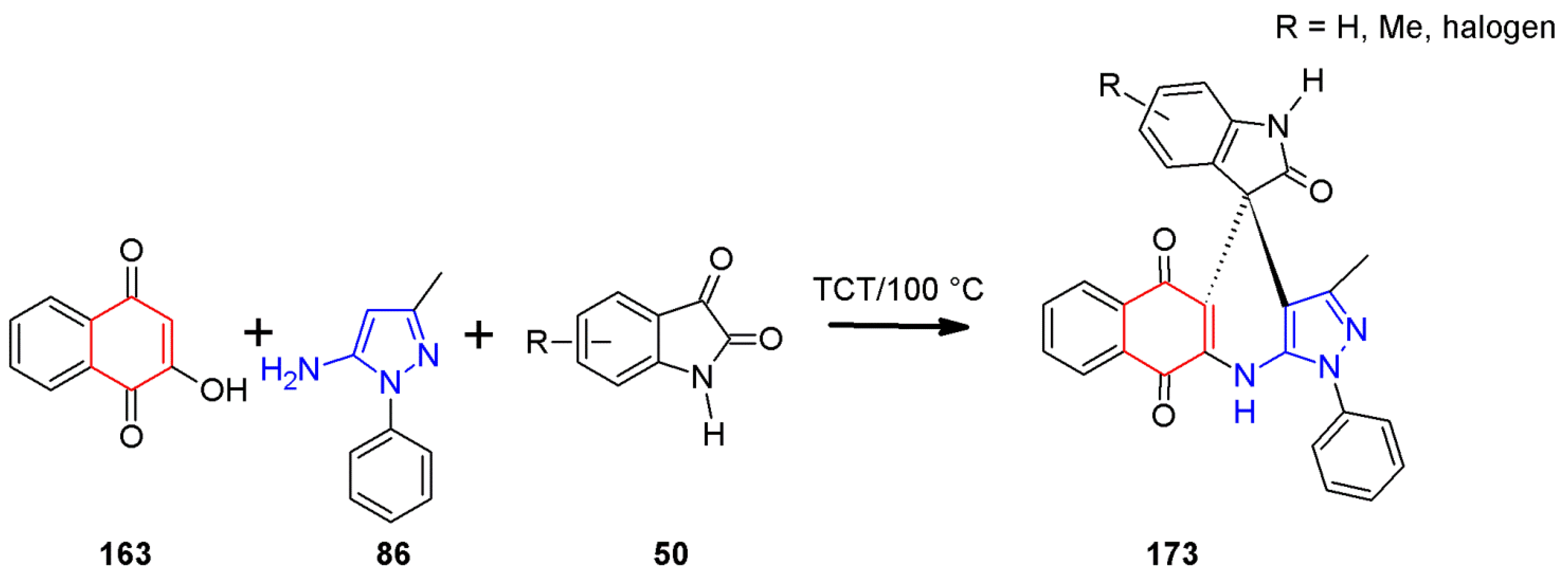
2.11. Multicomponent 1H-Pyrazolo[3,4-b]quinoline Synthesis
3. Photophysical Physical Properties of 1H-Pyrazolo[3,4-b]quinolines and Their Application
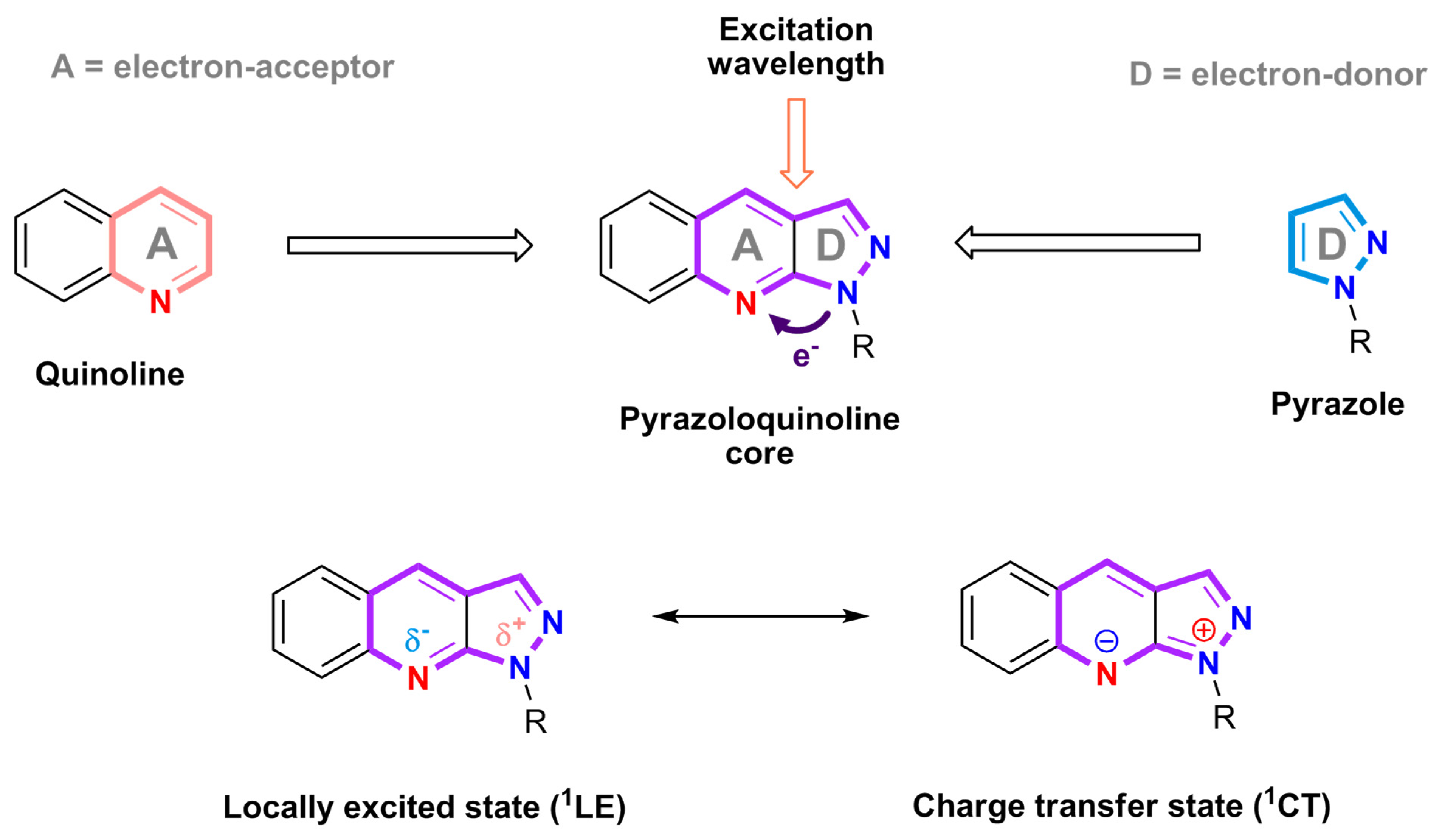
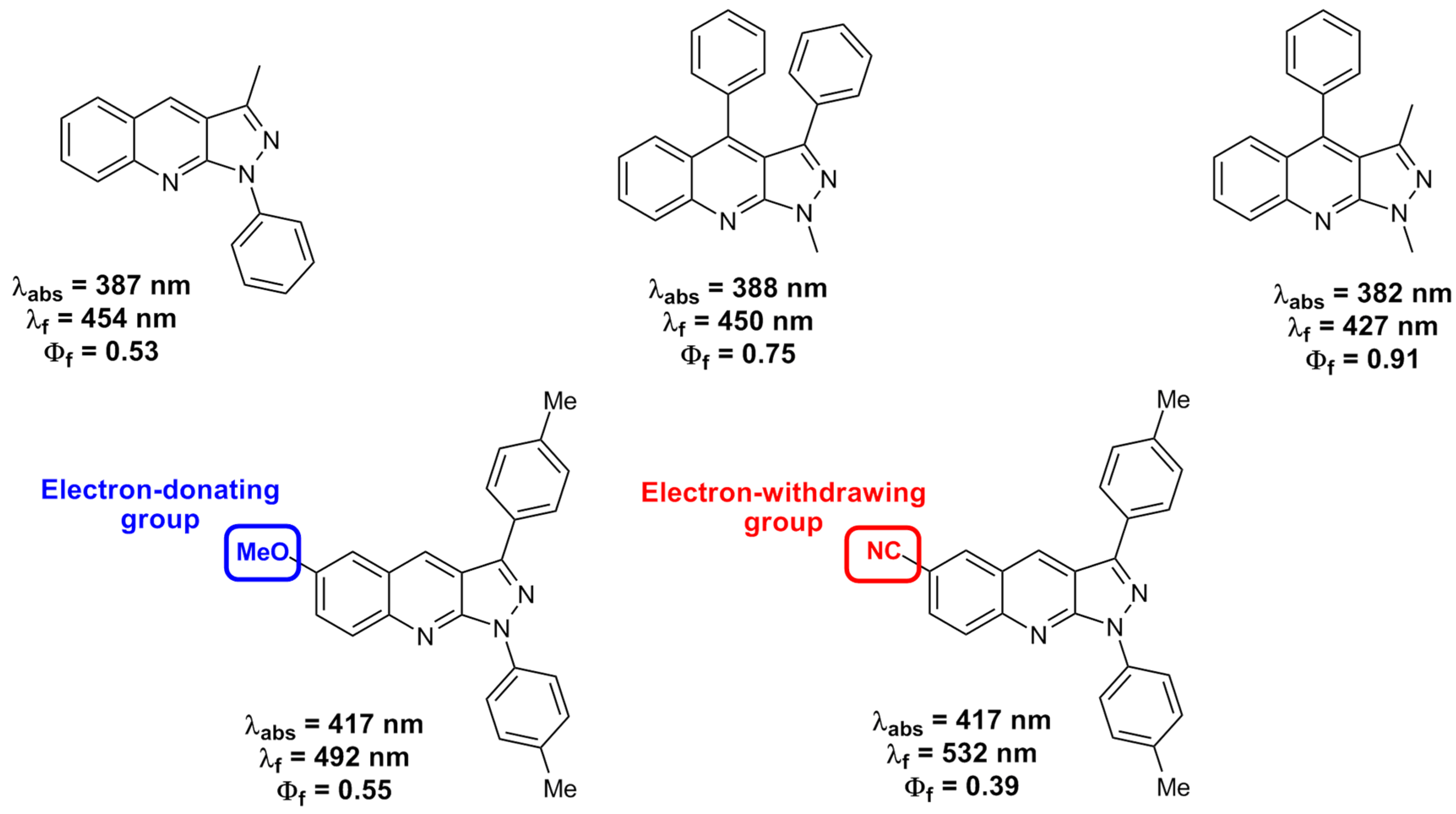
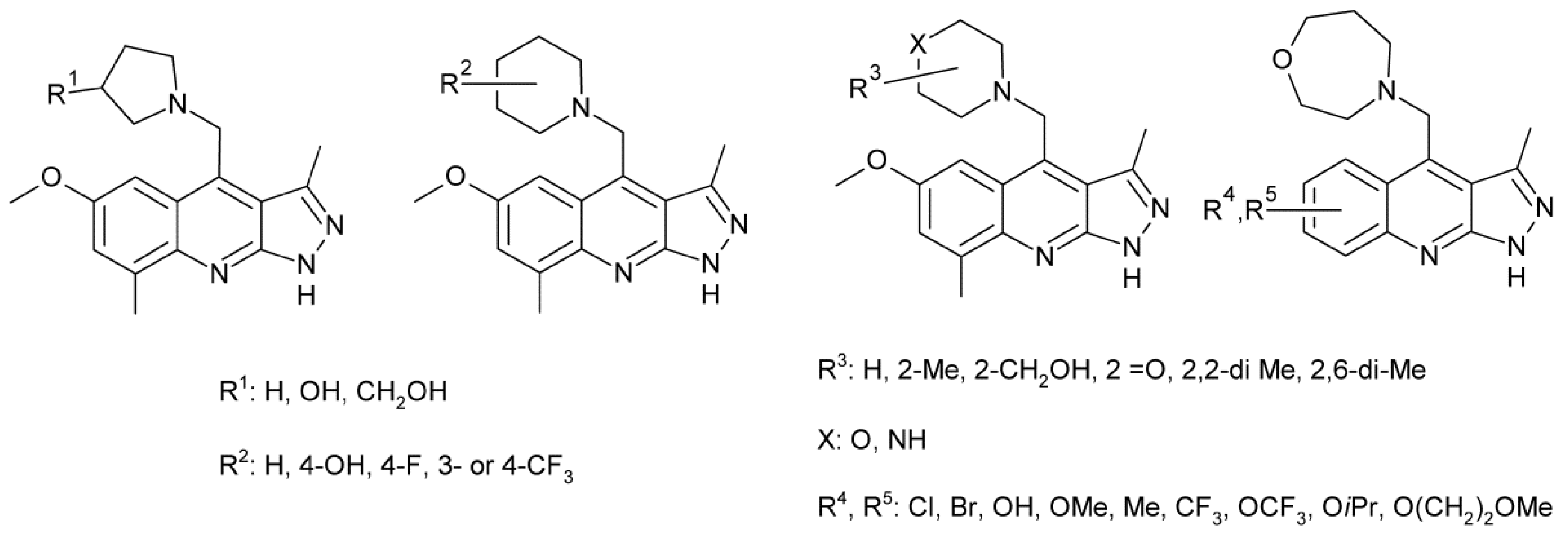
3.1. Structure–Property Relationship
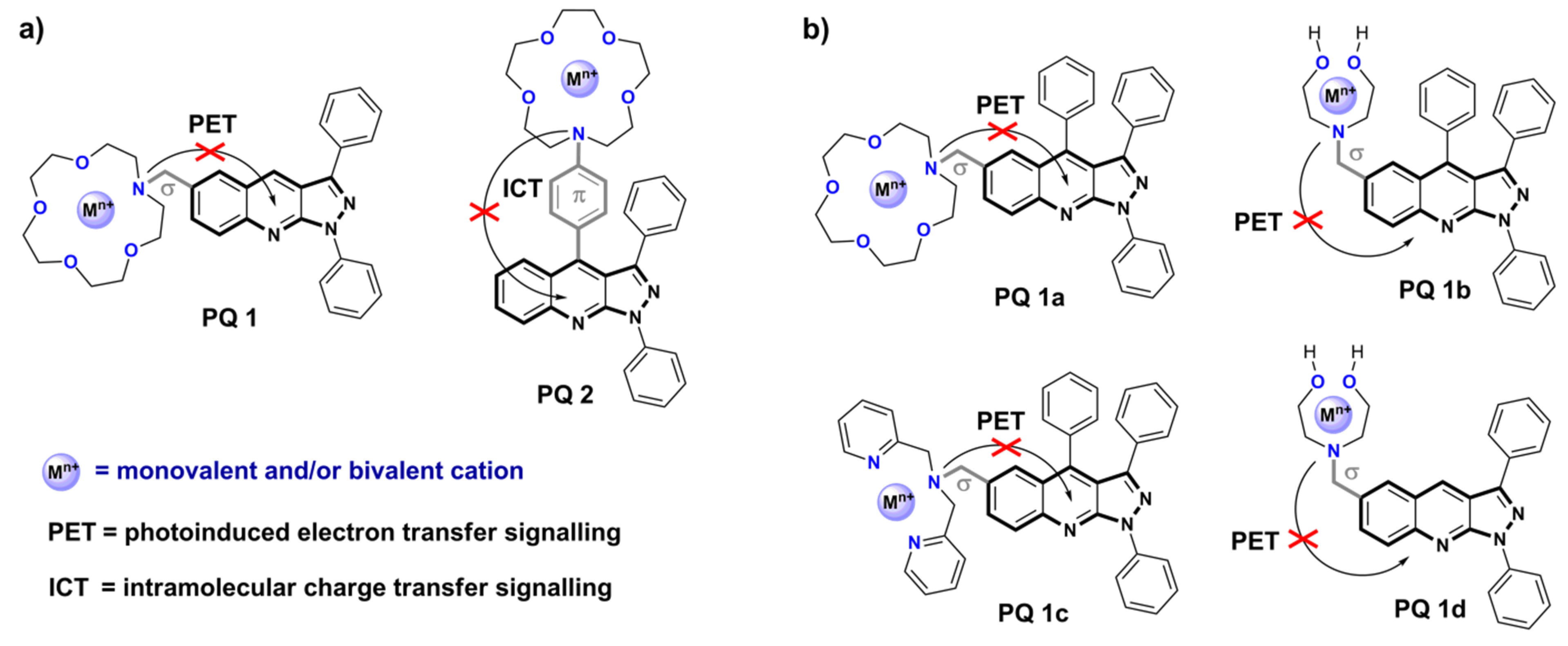
3.2. Application of Pyrazoloquinolines in Fluorescence Sensing
4. Biological Properties of 1H-Pyrazolo[3,4-b]quinolines
4.1. Hypolipemic and Hypocholesteremic Activity
4.2. Interferon-Production-Inducing Activity
4.3. Antiviral Activity
4.4. Antibacterial Activity
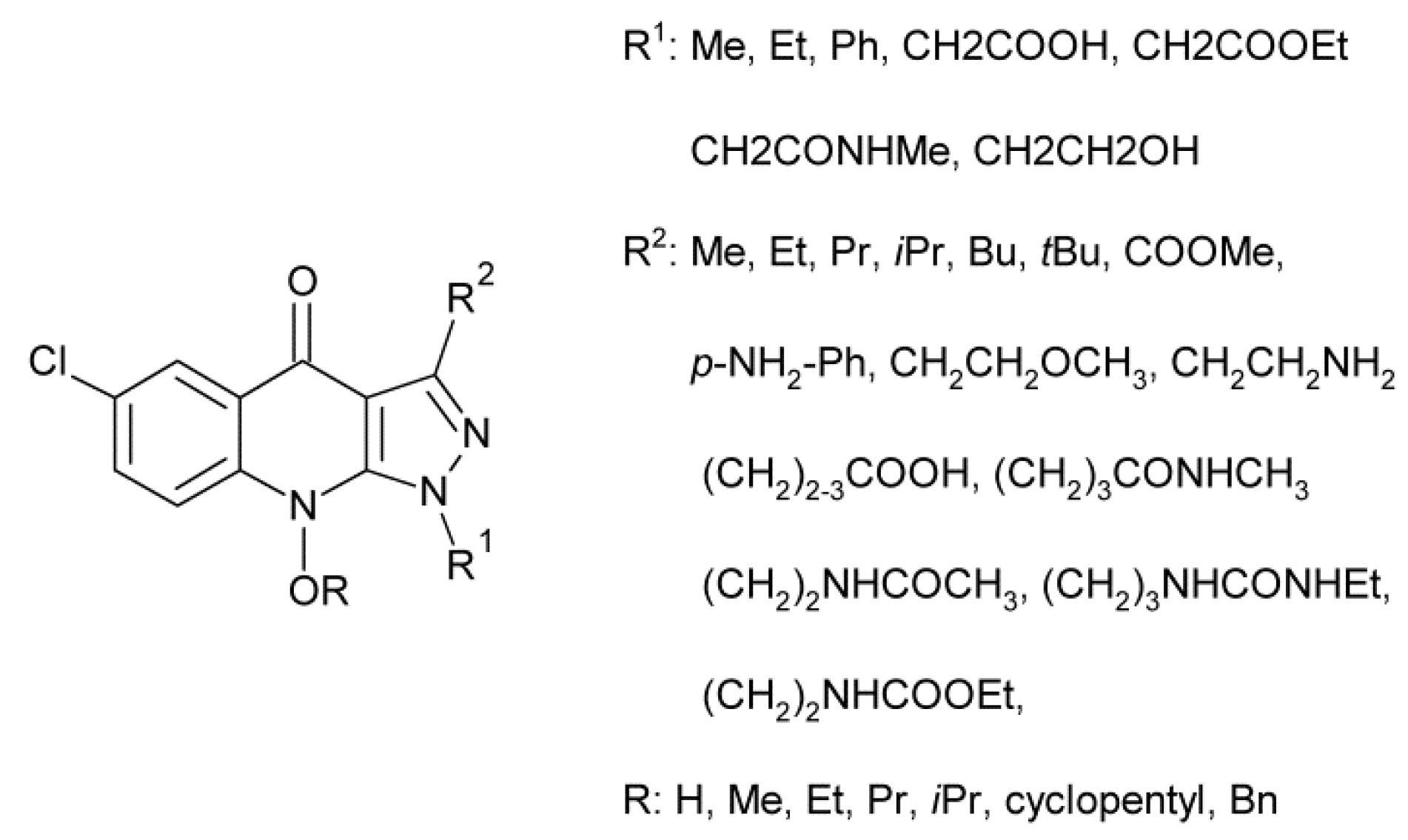
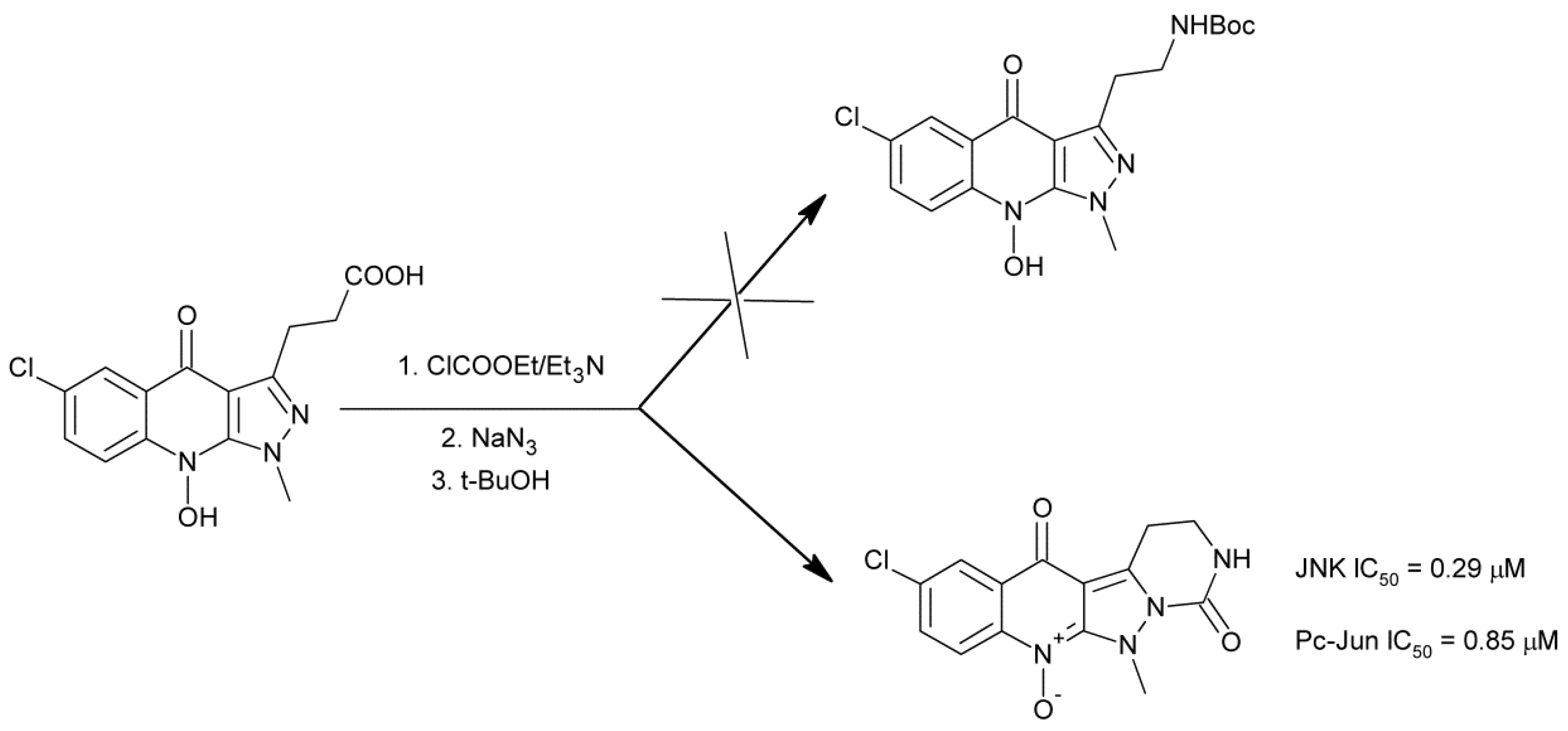
4.5. Anticancer Activity
4.6. Antiparasitic Activity
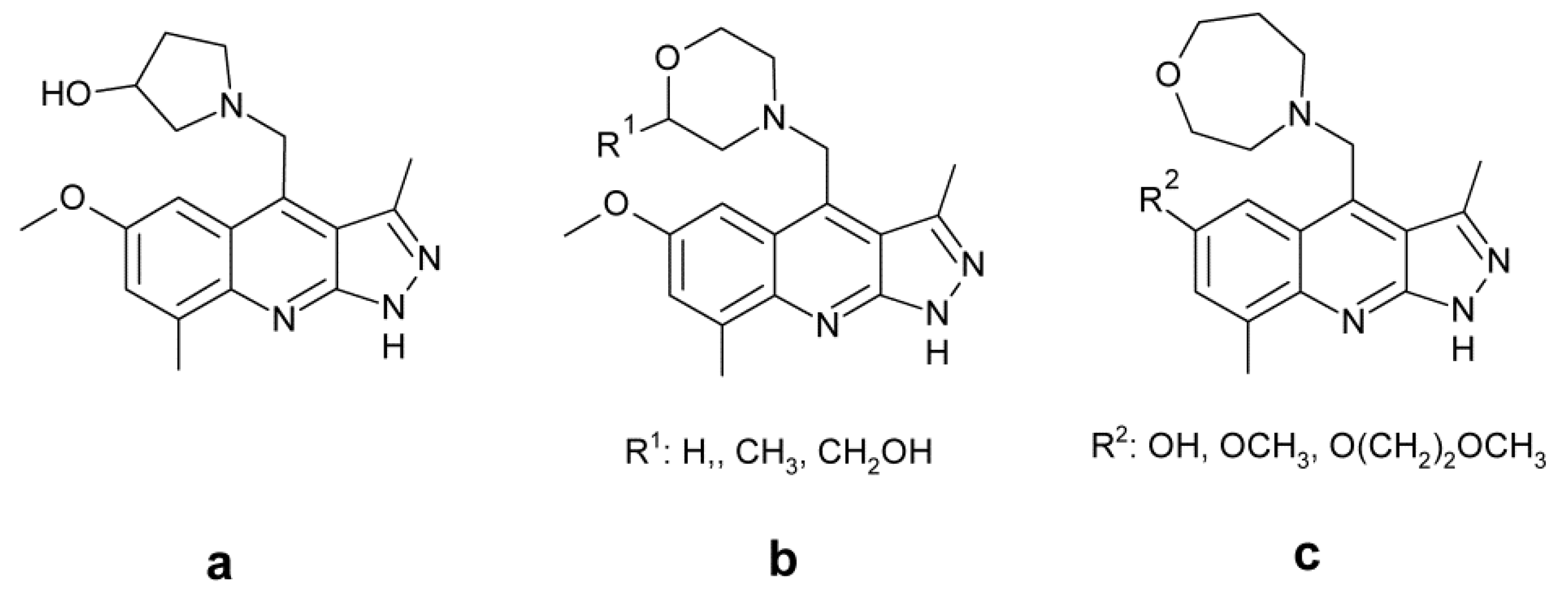
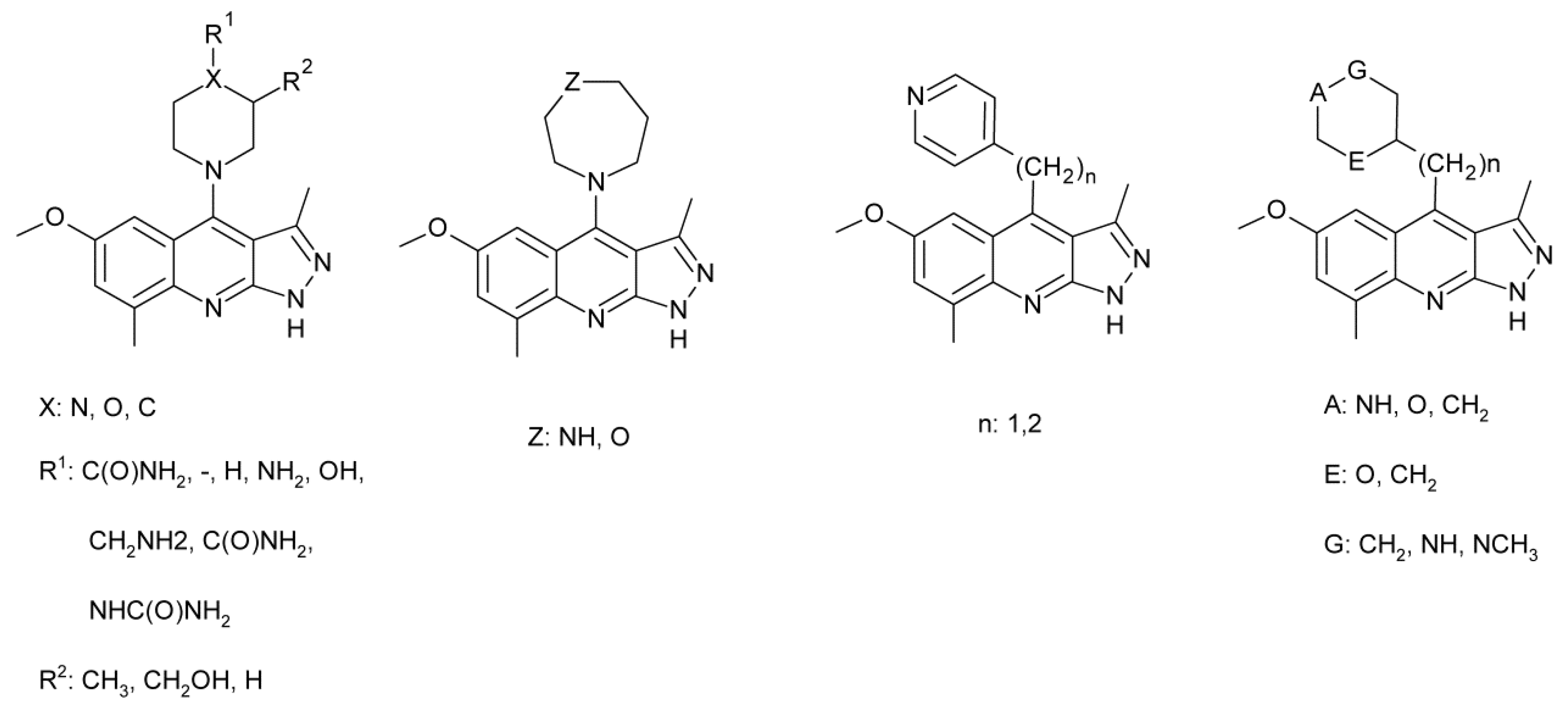
4.7. Treatment of Schizophrenia
4.8. Treatment of Diabetes
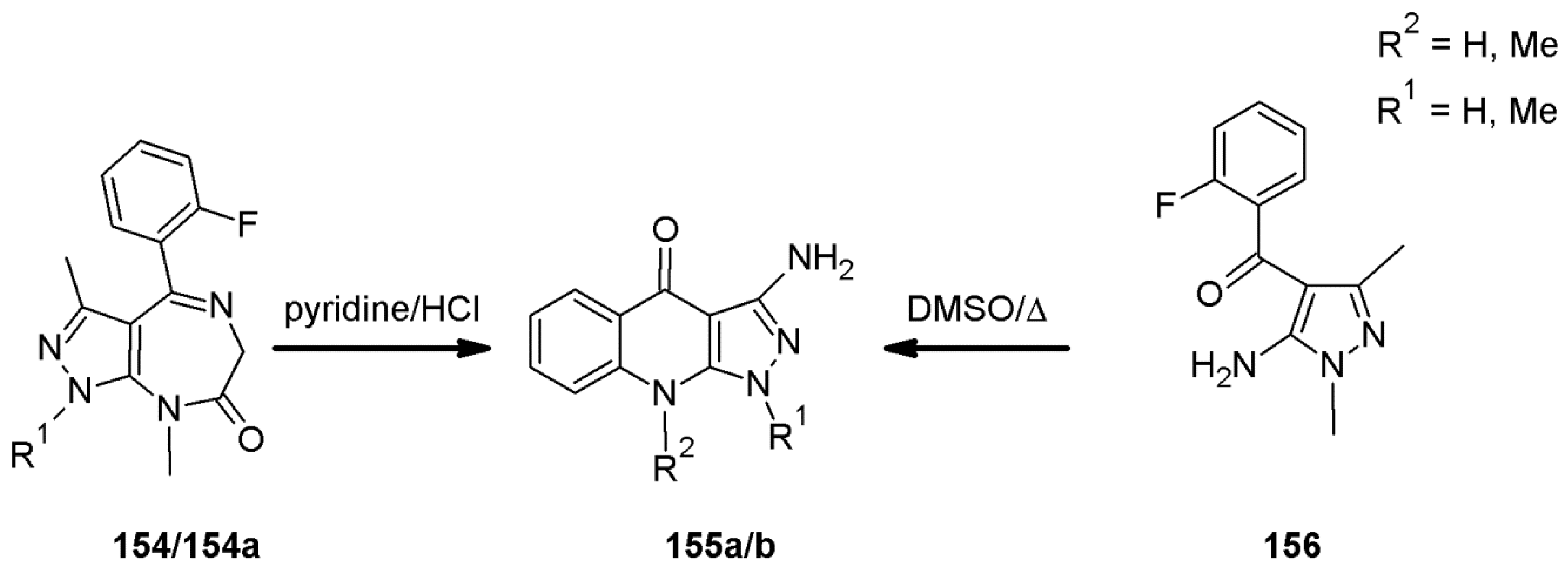
4.9. Benzodiazepine Receptor Inhibitor
4.10. Singlet-Oxygen-Generating Activity for SPA
4.11. Pregnancy Interceptive
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Michaelis, A. Über 5-Aminopyrazole und über Iminopyrine. I. Über substituierte 5-Iminopyrazolone und über 5-Aminopyrazole. Justus Lebigs Ann. Chem. 1911, 385, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Musierowicz, A.; Niementowski, S.; Tomasik, Z. Kondensacja fenylo-1-metylo-3-okso-5[dwuchydropirazolu] i [chloro-2]-fenylo-1-metylo-3-okso-[dwuhydro-4,5-pyrazolu] z o-aminobenzaldehydem. Roczn. Chemii 1928, 8, 325–344. [Google Scholar]
- Koćwa, A. Reactions in the pyrazolone series. II. Reactions of arylimine derivatives of 1-phenyl-5-methyl-3-pyrazolone with carbanilide or phenyl isocyanate, α-naphthyl isocyanate, and thiocarbanilide or phenyl isothiocyanate. Bull. Intern. Acad. Polon. Sci. 1936, 382–389. [Google Scholar]
- Koćwa, A. Reactions in the pyrazolone series. III. Reactions of arylimine derivatives of 1-phenyl-3-methyl-5-pyrazolone with carbanilide or phenyl isocyanate, α-naphthyl isocyanate, or with thiocarbanilide or phenyl isothiocyanate. Bull. Intern. Acad. Polon. Sci. 1936, 390–402. [Google Scholar]
- Rettig, W.; Rurack, K.; Danel, A.; Rotkiewicz, K.; Grabka, D.; Spieles, M. 1,3-Diphenyl-1H-pyrazolo[3,4-b]quinoline: A versatile fluorophore for the design of brightly emissive molecular sensors. Org. Lett. 2002, 4, 4647–4650. [Google Scholar] [CrossRef]
- Danel, A.; Gondek, E.; Kityk, I. 1H-pyrazolo[3,4-b]quinoline and 1H-pyrazolo[3,4-b]quinoxaline derivatives as promising materials for optoelectronic applications. Opt. Mater. 2009, 32, 267–273. [Google Scholar] [CrossRef]
- Reimann, E. Chinoline. In Houben-Weyl Methods of Organic Chemistry, 4th ed.; Hetarenes II-Part 1; Büchel, K.H., Falbe, J., Hageman, H., Hanack, M., Klamann, D., Krecher, R., Kropf, H., Regitz, M., Schaumann, E., Eds.; Georg Thieme Verlag Stutgart: New York, NY, USA, 1991; Volume E7a, pp. 290–493. [Google Scholar]
- Friedländer, P. Ueber o-Amidobenzaldehyd. Chem. Ber. 1882, 15, 2572–2575. [Google Scholar] [CrossRef] [Green Version]
- Marco-Contelles, J.; Perez-Mayoral, E.; Samadi, A.; Carreiras, M.C.; Soriano, E. Recent advances in Friedlander reaction. Chem. Rev. 2009, 109, 2652–2671. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Yan, S.-J. The Friedlander synthesis of quinolines. Org. React. 1982, 28, 37–201. [Google Scholar] [CrossRef]
- Fallah-Mehrjarch, M. Friedlander synthesis of poly-substituted quinolones: A mini review. Mini-Rev. Org. Chem. 2017, 14, 187–196. [Google Scholar] [CrossRef]
- Ramann, G.A.; Cowen, B.J. Recent advances in metal-free quinoline synthesis. Molecules 2016, 21, 986. [Google Scholar] [CrossRef] [PubMed]
- Nainwal, L.M.; Tasneem, S.; Akhtar, W.; Verma, G.; Faraz Khan, M.; Parvez, S.; Shaquiquzzaman, M.; Akhter, M.; Amumtaz Alam, M. Green recipes to quinoline: A review. Eur. J. Med. Chem. 2019, 164, 121–170. [Google Scholar] [CrossRef] [PubMed]
- Abramovitch, R.; Tomasik, P.; Tomasik, D. Friedländer Condensation of 1H-Pyrazolin-5-ones with o-Aminobenzaldehydes. Synthesis of 1H-Pyrazolo[3,4-b]quinolines. J. Heterocycl. Chem. 1983, 20, 1539–1543. [Google Scholar] [CrossRef]
- Kucybała, Z.; Przyjazna, B.; Linden, L.-A.; Paczkowski, J. Developmnet of new dyeing photoinitiators for free radical polymerization based on 1H-pyrazolo[3,4-b]quinoline skeleton. IV. Polym. Bull. 2000, 45, 327–334. [Google Scholar] [CrossRef]
- Danel, A. Synthesis of Pyrazolo[3,4-b]quinolines as Luminophores for Electroluminecent Devices. Ph.D. Thesis, Jagiellonian University, Krakow, Poland, 1996. [Google Scholar]
- Sabitha, G.; Babu, R.S.; Reddy, S.B.V.; Yadav, J.S. Microwave Assisted Friedländer Condensation Catalyzed by Clay. Synth. Commun. 1999, 29, 4403–4408. [Google Scholar] [CrossRef]
- Qu, Y.-K.; Zheng, Q.; Fan, J.; Liao, L.-S.; Jiang, Z.-Q. Spiro compounds for organic-light emitting diodes. Acc. Mater. Res. 2021, 2, 1261–1271. [Google Scholar] [CrossRef]
- Salbeck, J.; Pudzich, R.; Fuhrmann, T. Spiro compounds for organic electroluminescence and related applications. Adv. Polym. Sci. 2006, 107, 1011–1065. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wu, F.-I.; Shu, C.-F.; Chien, C.-H.; Tao, Y.-T. Spirobifluorene-based pyrazoloquinolines: Efficient blue electroluminescent materials. J. Mater. Chem. 2004, 14, 1585–1589. [Google Scholar] [CrossRef]
- Caluve, P. Heteroannulations with o–aminoaldehydes. Tetrahedron 1980, 36, 2359–2407. [Google Scholar] [CrossRef]
- Häufel, J.; Breitmaier, E. Synthesis of Pyrazolo Heteroaromatic Compounds by Means of 5-Amino-3-methyl-1-phenylpyrazole-4-carbaldehyde. Angew. Chem. Int. Ed. 1974, 13, 604. [Google Scholar] [CrossRef]
- Jachak, N.M.; Avhale, A.B.; Medhane, V.J.; Toche, R.B. A convenient route for the synthesis of pyrazolo[3,4-d]pyrimidine, pyrazolo[3,4-b][1,6]naphtiridine and pyrazolo[3,4-b]quinoline derivatives. J. Heterocycl. Chem. 2006, 43, 1169–1175. [Google Scholar] [CrossRef]
- Higashino, T.; Iwai, Y.; Hayashi, E. Studies on Pyrazolo[3,4-d]pyrimidine Derivatives. IV. On 1-methyl- and 1-phenyl-1H-pyrazolo[3,4-d]pyrimidine 5-oxide. Chem. Pharm. Bull. 1976, 24, 3120–3134. [Google Scholar] [CrossRef] [Green Version]
- Niementowski, S. Synthesen der Chinolinderivate. Chem. Ber. 1894, 27, 1394–1403. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, T.N. Quinoline derivatives. J. Indian Chem. Soc. 1937, 14, 123–126. [Google Scholar]
- Menzel, K.H.; Putter, R.; Wolfrum, G. Synthesen und reaktionen neuer ortho-kondensierter pyrazoloverbindungen. Angew. Chem. 1962, 21, 839–847. [Google Scholar] [CrossRef]
- Danel, A.; Tomasik, P.; Kappe, T.; Gheath, A.H. Attempted Niementowski condensation of anthranilic acid and its ester with 3-methyl-1-phenylpyrazolin-5-one. Polish J. Chem. 1996, 70, 302–309. [Google Scholar]
- Stein, R.G.; Biel, J.H.; Singh, T. Antimalarials. 4-Substituted 1H-pyrazolo[3,4-b]quinolones. J. Med. Chem. 1970, 13, 153–155. [Google Scholar] [CrossRef]
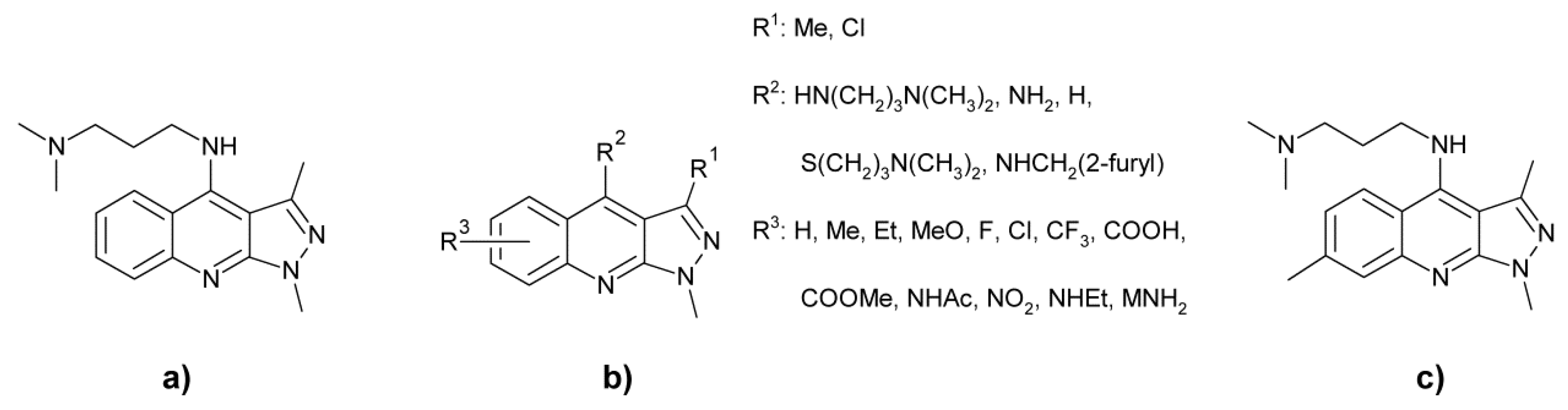
- Crenshaw, R.R.; Luke, G.M.; Siminoff, P. Interferon inducing activities of derivatives of 1,3-dimethyl-4-(3-dimethylaminopropyloamino)-1H-pyrazolo[3,4-b]quinoline and realted compounds. J. Med. Chem. 1976, 19, 262–275. [Google Scholar] [CrossRef]
- Kim, D.H. Further studies on the reaction of N-(2-hydroxyphenyl)anthranilic acid wit acetic anhydride. J. Heterocycl. Chem. 1981, 18, 1389–1392. [Google Scholar] [CrossRef]
- Gal, M.; Feher, Ö.; Tihanyi, E.; Horvath, G.; Jerkovitch, G.; Argay, G.; Kálman, A. The ring closure and rearrangement of 1-(2-amino)-benzoyl-1-methylhydrazones of β-dicarbonyl compounds: On the formation and crystal structure of 3a,9a-dihydro-1,3,3a,9a-tetramethyl-4H-pyrazolo[3,4-b]quinolin-4-one. Tetrahedron Lett. 1980, 21, 1567–1570. [Google Scholar] [CrossRef]
- Nielsen, S.; Pedersen, E.B. Synthesis of 4-arylamino-5,6,7,8-tetrahydro-1H-pyrazolo[3,4-b]quinolones and the corresponding N-Mannich bases. Liebigs Ann. Chem. 1986, 1986, 1728–1735. [Google Scholar] [CrossRef]
- Nielsen, S.; Pedersen, E.B. Synthesis of 4-arylamino-5,6,7,8-tetrahydro-1H-pyrazolo[3,4-b]quinolones and 5,6,7,8-tetrahydrogenated analogs. Chem. Scr. 1986, 26, 331–336. [Google Scholar]
- Gatta, F.; Pomponi, M.; Maurizio, M. Synthesis of 7,8-dihydro-6H-pyrazolo[3,4-b]quinolin-5-ones and related derivatives. J. Heterocycl. Chem. 1991, 28, 1301–1307. [Google Scholar] [CrossRef]
- Campbell, J.B.; Firor, J.W. Synthesis of tricyclic aminopyridines by a cadmium promoted cyclization. J. Org. Chem. 1995, 60, 5243–5249. [Google Scholar] [CrossRef]
- Deeb, A.; El-Mobayed, M.; Essawy, A.N.; El-Hamid, A.A.; Mohamid, A.; El-Hamid, A. Heterocyclic synthesis from 3-amino-4-cyanompyrazole. Collect. Czech. Chem. Commun. 1990, 55, 728–733. [Google Scholar] [CrossRef]
- Silva, D.; Chioua, M.; Samadi, A.; Carreeiras, C.M.; Jimeno, M.-L.; Mendes, E.; De los Rios, C.; Romero, A.; Villarroya, M.; Lopez, M.G.; et al. Synthesis and pharmacological assessment of diversity substituted pyrazolo[3,4-b]quinoline, and benzo[b]pyrazolo[4,3-g][1,8]naphtyridine. Eur. J. Med. Chem. 2011, 46, 4676–4681. [Google Scholar] [CrossRef] [Green Version]
- Pfitzinger, W. Chinolinderivate aus isatinsaure. J. Prakt. Chem. 1886, 33, 100. [Google Scholar] [CrossRef] [Green Version]
- Shvekgheimer, M.G.-A. The Pfitzinger reaction. Chem. Heterocycl. Compd. 2004, 40, 257–294. [Google Scholar] [CrossRef]
- Musante, C.; Fabbrini, L. Contribution to the knowledge of malonyldiphenylhydrazine and its derivatives. Gazz. Chim. Ital. 1954, 84, 595–605. [Google Scholar]
- Holla, D.C.; Seshadri, S. A new synthesis of pyrazolo[3,4-b]quinoline derivatives. Bull. Chem. Soc. Jpn. 1984, 57, 2984–2986. [Google Scholar] [CrossRef]
- Brack, A. Uber kondensierte Pyrazolopyridine. Liebigs Ann. Chem. 1965, 681, 105–110. [Google Scholar] [CrossRef]
- Brack, A. 1H-Pyrazolo[3,4-b]quinolines. DE1186867, 11 May 1962. [Google Scholar]
- Butler, D.E.; De Wald, H.A. New general methods for the substitution of 5-chloropyrazoles. The synthesis of 1,3-dialkyl-5-chloropyrazol-4-yl aryl ketones and new 1,3-dialkyl-2-pyrazolin-5-ones. J. Org. Chem. 1971, 36, 2542–2547. [Google Scholar] [CrossRef]
- Obakachi, V.A.; Kushwaha, N.D.; Kushwata, B.; Mahlalela, M.C.; Shinde, S.R.; Kehinde, I.; Karpoormath, R. Design and synthesis of pyrazolone-based compounds as potent blockers of SARS-CoV-2 viral entry into the host cells. J. Mol. Struct. 2021, 1241, 130665. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Wang, H.; Lin, H.; Wang, J.; Jiang, Y.; Jiang, H.; Zhu, S.; Wang, Z.; Hao, J. Synthesis, electrochemical, photohysical, and electroluminescent properties of organic dyes containing pyrazolo[3,4-b]quinoline chromophore. Dyes Pigments 2015, 121, 138–146. [Google Scholar] [CrossRef]
- Porai-Koshits, B.A.; Kvitko, I.Y.; Shutkova, E.A. Synthesis and transformations of chloropyrazolealdehydes. Pharm. Chem. J. 1970, 4, 138–143. [Google Scholar] [CrossRef]
- Hennig, L.; Müller, T.; Grosche, M. Syntheses von Pyrazolo[3,4-b]chinolinen aus 4-Aroyl-5-chloropyrazolen. J. Prakt. Chem. 1990, 332, 693–698. [Google Scholar] [CrossRef]
- El-Shehry, M.; El-Telbani, E.M.; Swellem, R. Synthesis of polyfunctional substituted pyrazoles. J. Chem. Res. 2009, 10, 625–629. [Google Scholar] [CrossRef]
- Gonzales, E.; Elguero, J. Action des hydrazines sur les acetyl-4-chloro-5(ou 3)methyl-3 (ou 5) pyrazoles: Formation d’une pyrazolo[3,4-b]quinoleine, d’hydrazzones et de cetazines. J. Heterocycl. Chem. 1986, 23, 999–1001. [Google Scholar] [CrossRef]
- Crenshaw, R.R.; Luke, G.M.; Whitehead, D.F. Unexpected formation of a 10H-pyrido[2,3-h]pyrazolo[3,4-b]quinoline derivative, A new ring system. J. Heterocycl. Chem. 1976, 13, 155–156. [Google Scholar] [CrossRef]
- Chaczatrian, K.; Chaczatrian, G.; Danel, A.; Tomasik, P. The synyhesis of 4-aryl-1H-pyrazolo[3,4-b]quinolines by cyclization of 4-arylidenepyrazolin-5-ones with anilines. ARKIVOC 2001, VI, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Hamama, S.W.; El-Gohary, H.G.; Soliman, M.; Zoorob, H.H. A versatile synthesis, PM-3-Semiempirical, antibacterial, and antitumor evaluation of some bioactive pyrazoles. J. Heterocycl. Chem. 2012, 49, 543–554. [Google Scholar] [CrossRef]
- Szlachcic, P.; Kucharek, M.; Jarosz, B.; Danel, A.; Stadnicka, K. Facile and regioselective synthesis of substituted 1H-pyrazolo[3,4-b]quinolones from 2-fluorobenzaldehydes and 1H-pyrazol-5-amines. J. Heterocycl. Chem. 2016, 54, 1729–1745. [Google Scholar] [CrossRef]
- Abramov, M.A.; Ceulemans, E.; Jackers, C.; Van der Auweraer, M.; Dehaen, W. Reactions of 5-amino-1,2-azoles with aromatic and heterocyclic o-chloroaldehydes:[1+1] versus [2+1] cyclocondensation. Tetrahedron 2001, 57, 9123–9129. [Google Scholar] [CrossRef]
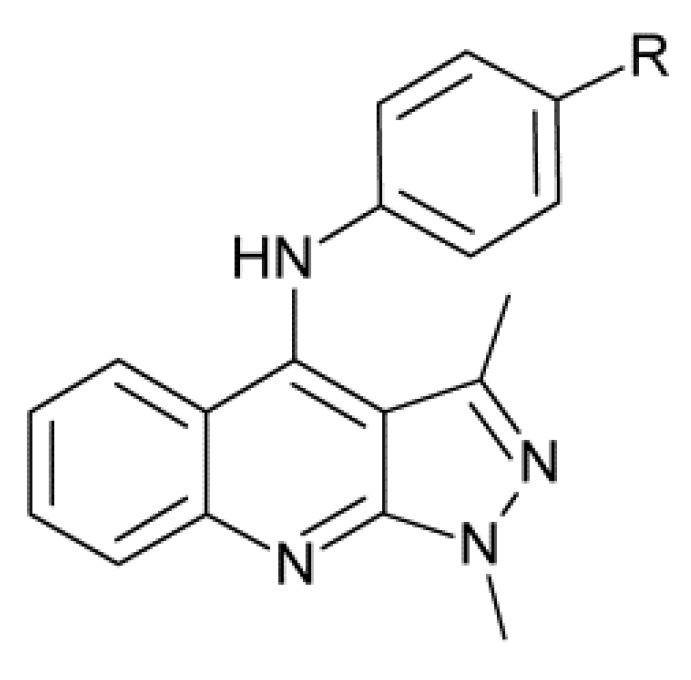
- Zhang, H.-Z.; Classen, G.; Crogran-Grundy, C.; Tsneg, B.; Drewe, J.; Cai, S.X. Discovery and structure—Activity relationship of N-phenyl-1H-pyrazolo[3,4-b]quinolin amines as a new series of potent apoptosis inducers. Bioorg. Med. Lett. 2008, 16, 222–231. [Google Scholar] [CrossRef]
- Pamukcu, R.; Piazza, A. Method for Inhibiting Neoplastic Cells by Exposure to Substituted N-cycloalkylmethyl-1-H-pyrazolo[3,4-b]quinoline-4-amines. U.S. Patent 5,942,520, 24 August 1999. [Google Scholar]
- Shekarro, K.; Kaishap, P.P.; Saddanapu, A.A.; Gogoi, S.; Boruah, R.C. Microwave—Assisted palladium mediated efficient synthesis of pyrazolo[3,4-b]pyridines, pyrazolo[3,4-b]quinolines, pyrazolo[1,5-a]pyrimidines and pyrazolo[1,5-a]quinazolines. RSC Adv. 2014, 4, 24001. [Google Scholar] [CrossRef]
- De Wald, H. 9-Substituted-4,9-dihydro-1,3,4,4-tetraalkyl-1H-pyrazolo[3,4-b]quinolines. U.S. Patent 3,790,576, 5 February 1974. [Google Scholar]
- Meth-Cohn, O.; Narine, B.; Tarnowski, B. A versatile new synthesis of quinolones and related fused pyridines, Part 5. The synthesis of 2-chloroquinolne-3- carbaldehydes. J. Chem. Soc. Perkin Trans. 1 1981, 1520–1530. [Google Scholar] [CrossRef]
- Hayes, R.; Meth-Cohn, O. A versatile new synthesis of quinolones and realted fused pyridines. Part 10. Routes to quinolines with fused azacycles. Tetrahedron Lett. 1982, 23, 1613–1615. [Google Scholar] [CrossRef]
- Marson, C.M. Reactions of carbonyl compounds with(monohalo) methyleniminium salts (Vilsmeier reagents). Tetrahedron 1992, 48, 3659–3726. [Google Scholar] [CrossRef]
- Meth-Cohn, O. The synthesis of pyridines, quinolones and other realted systems by the Vilsmeier and the reversed Vilsmeier method. HeteroCycles 1993, 35, 539–557. [Google Scholar] [CrossRef]
- Bhat, B.N.; Bhaduri, A.P. Syntheses of substituted pyrazolo[3,4-b]quinolines, 3,4-dihydro-4-oxopyrimido[4′,5′:4,5]thieno[2,3-b]quinoline and pyrido[1′,2′:1,2]pyrimido[4,5-b]quinoline. J. Heterocycl. Chem. 1986, 23, 925–928. [Google Scholar] [CrossRef]
- Rajendran, S.P.; Manomani, M.; Vijayalakshmi, S. Synthesis of pyrazolo[3,4-b]quinolines and their 1-phenyl derivatives. Org. Prep. Proceures Int. 2009, 26, 383–385. [Google Scholar] [CrossRef]
- Kidwai, M.; Negi, N. Synthesis of some novel substituted quinolones as potent analgesic agents. Monatsh. Chem. 1997, 128, 85–89. [Google Scholar] [CrossRef]
- Mogilaiah, K.; Sudhakar, G.R.; Reddy, N.V. Microwave assisted synthesis of pyrazolo[3,4-b]quinoline containing 1,8-naphtyridine moiety. Indian J. Chem. 2003, 42B, 1753–1755. [Google Scholar]
- Selvi, S.T.; Nadaraj, V.; Mohan, S.; Sasi, R.; Hema, M. Solvent free synthesis and evaluation of antimicrobial activity of pyrimido[4,5-b]- and pyrazolo[3,4-b]quinolines. Bioorg. Med. Lett. 2006, 14, 3896–3903. [Google Scholar] [CrossRef]
- Mali, J.R.; Pratap, U.R.; Jawale, D.V.; Mane, R.A. Water mediated one-pot synthetic route for pyrazolo[3,4-b]quinolines. Tetrahedron Lett. 2010, 51, 3980–3982. [Google Scholar] [CrossRef]
- Paul, S.; Gupta, M.; Gupta, R.; Loupy, A. Microwave assisted solvent free synthesis of pyrazolo[3,4-b]quinolones and pyrazolo[3,4-c]pyrzoles using p-TsOH. Tetrahedron Lett. 2001, 42, 3827–3829. [Google Scholar] [CrossRef]
- El-Bordany, E.A.; Aziz, A.A.; Abou-Elmagd, W.S.I.; Hashem, A.I. Synthesis and spectroscopic characterization of some novel pyrazoloquinoline, pyrazolotetrazine and thiazolidinone derivatives. J. Heterocycl. Chem. 2018, 55, 291–296. [Google Scholar] [CrossRef]
- Munir, R.; Javid, N.; Zia-ur-Rehman, M.; Zaheer, M.; Huma, R.; Roohi, A.; Athar, M.M. Synthesis of novel N-acylhydrazones and their C-N/N-N bond conformational characterization by NMR spectroscopy. Molecules 2021, 26, 4908. [Google Scholar] [CrossRef]
- Teixeira, F.C.; Lucas, C.; Curto, M.J.M.; Andre, V.; Duarte, T.M.; Teixeira, A.P.S. Synthesis of novel pyrazolo[3,4-b]quinoline—Bisphosphonic acids and unexpected intramolecular cyclization and phosphonylation reaction. Org. Biomol. Chem. 2021, 19, 2533–2545. [Google Scholar] [CrossRef]
- Chavan, A.S.; Kharat, A.S.; Bhosle, M.; Dhumal, S.T.; Mane, R.A. Water mediated and Baker yeast accelerated novel synthestic prtocols for tetrahydrobenzo[a]xanthene -11-ones and pyrazolo[3,4-b]quinolones. Synth. Commun. 2021, 51, 1963–1973. [Google Scholar] [CrossRef]
- Kerry, M.; Boyd, G.W.; Mackay, S.P.; Meth-Conh, O.; Platt, L. The synthesis of benzo[h]quinolones as topomerase inhibitors. J. Chem. Soc. Perkin Trans. 1 1999, 2315–2321. [Google Scholar] [CrossRef]
- Parekh, N.M.; Sahoo, S.K.; Maheiria, K.C. Qunatum chemical studies and dyeing performance of some novel benzoquinoline based heterocyclic monoazo dyes on polyester fiber. Dye. Pigment. 2012, 95, 142–148. [Google Scholar] [CrossRef]
- Parekh, N.M.; Maheira, K.C. Synthesis of heterocyclic monoazao dyes derived from 1H-benzo[g]pyrazolo[3,4-b]quinoline-3-ylamine: Their antimicrobial activity and their dyeing performance on various fibers. Res. Chem. Intermed. 2012, 38, 885–901. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Lee, C.; Moore, J.; Mittal, R.; Suswam, E.A.; Abbot, K.L.; Pondugula, S.R.; Manne, U.; Narayanan, N.K.; Trivedi, P.; et al. IND-2, a pyrimido[1″,2″:1,5]pyrazolo[3,4-b]quinoline derivative circumvents multi-drug resistance and causes apoptosis in colon cancer cells. Bioorg. Med. Chem. 2015, 23, 602–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Otomasu, H. Synthesis of 1H-pyrazolo[3,4-b]pyridine and related compounds. Yakugaku Zasshi 1976, 96, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Arasakumar, T.; Mathusalini, S.; Lakshmi, K.; Mohan, S.P.; Ata, A.; Lin, C.-H. An object oriented synthetic approach toward angular and linear fused pyrazoloquinolines of biological importance with InCl3 catalyst. Synth. Commun. 2016, 46, 232–241. [Google Scholar] [CrossRef]
- Vostrova, L.N.; Gerenga, S.A.; Kiridenk, A.M.; Grenaderowa, M.V.; Kharchenko, S.L.; Abramovitch, A.E.; Dimitrieva, T.N. 2-Heterylquinoline derivatives. Ukr. Chim. Zh. 1992, 58, 578–781. [Google Scholar]
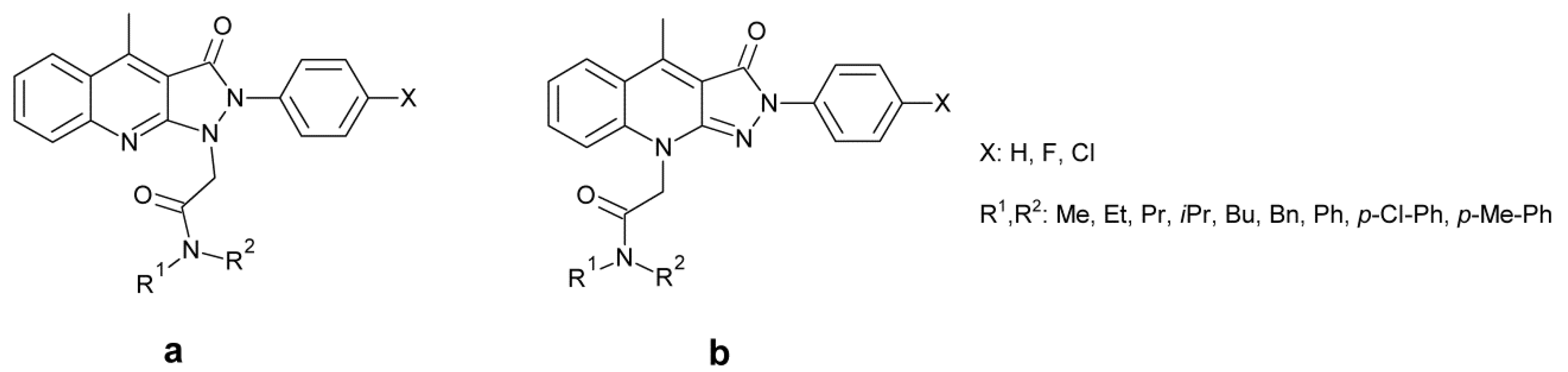
- Jintender Dev, G.; Poornachandra, Y.; Reddy, K.R.; Kumar, R.N.; Ravikumar, N.; Swaroop, K.; Ranjithreddy, P.; Kumar, G.S.; Nanubolu, J.B.; Ganesh, K.; et al. Synthesis of novel pyrazolo[3,4-b]quinolinyl acteamide analogs, their evaluation for antimicrobial and anticancer activities, validation by molecular modelling and COMFA analysis. Eur. J. Med. Chem. 2017, 130, 223–239. [Google Scholar] [CrossRef]
- Hassan, M.M.; Othman, E.; Abass, M. Substituted quinolinones. 18. 3-Acetl-4-methylthiquinolin-2(1H)-one as useful synthon intermediate for synthesis of some new quinolinones. Res. Chem. Intermed. 2013, 39, 1209–1225. [Google Scholar] [CrossRef]
- Abass, M.; Hassanin, H.M.; Allimony, H.; Hassan, H. Substituted quinolinones.27. Regioselctive synthesis of pyrazolo-, oxazolo-, and trazepinoquinoline derivatives. Chem. Heterocycl. Compd. 2015, 51, 1023–1029. [Google Scholar] [CrossRef]
- Mahat, P.K.; Venkatesh, C.; Kumar, U.K.S.; Ila, H.; Junjappa, H. Reaction of α-oxoketene-N,S-arylaminoacetals with Vilsmeier Reagents: An efficient route to highly functionalized quinolones and their benzo/hetero-fused analogues. J. Org. Chem. 2003, 68, 3966–3975. [Google Scholar] [CrossRef] [PubMed]
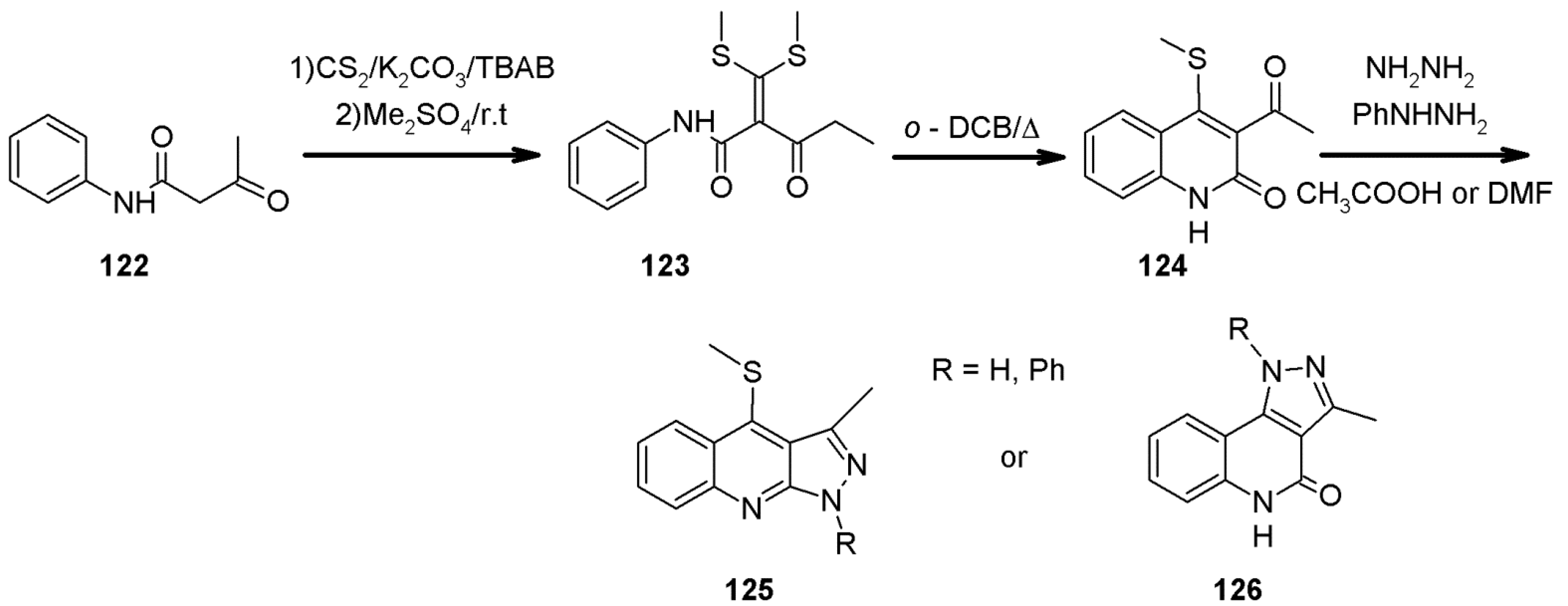
- Danel, A.; Chaczatrian, K.; Tomasik, P. Microwave-assisted, facile route to 1H-pyrazolo[3,4-b]quinolines. ARKIVOC 2000, I, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Kucharek, M.; Danel, A. Palladium catalysed amino group arylation of 1,3-disubstituted-1H-pyrazolo-5-amine based on Buchwald-Hartwig reaction. Chem. Heterocycl. Compd. 2021, 57, 633–639. [Google Scholar] [CrossRef]
- Purnaprajna, V.; Seshadri, S. Studies in the Vilsmeier—Haack reaction: Part XII. Synthesis of the 2-phenylpyrazolo[3,4-b]quinoline system. Indian J. Chem. Sect B 1976, 14B, 971–972. [Google Scholar] [CrossRef]
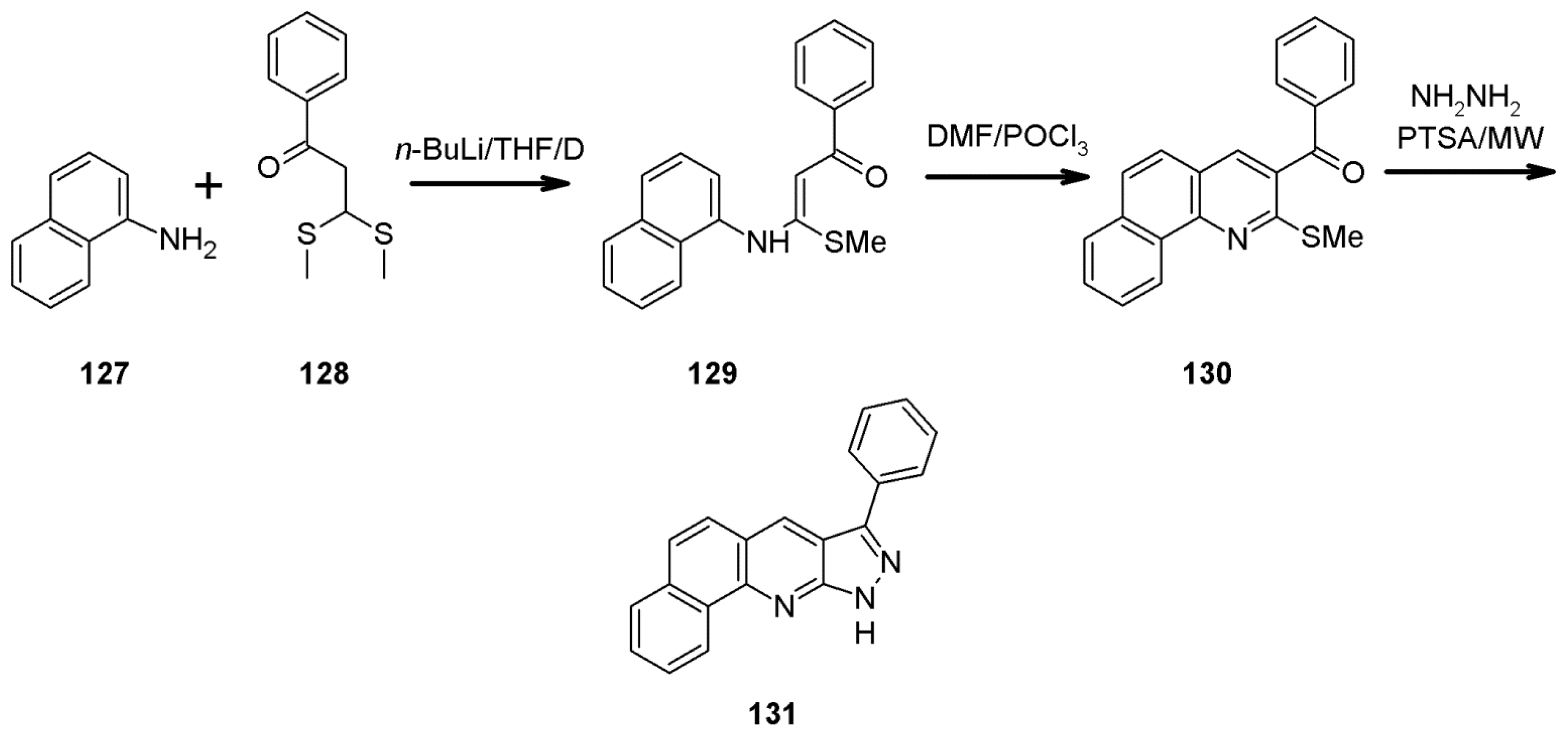
- Kucharek, M.; Danel, A.; Gut, A. Synthesis of 1,3,6-trisubstituted -1H-pyrazolo[3,4-b]quinolines based on intramolecular condensation of 5-N-aryl-1H-pyrazole-5-amine derivatives. Chem. Heterocycl. Compd. 2021, submitted.
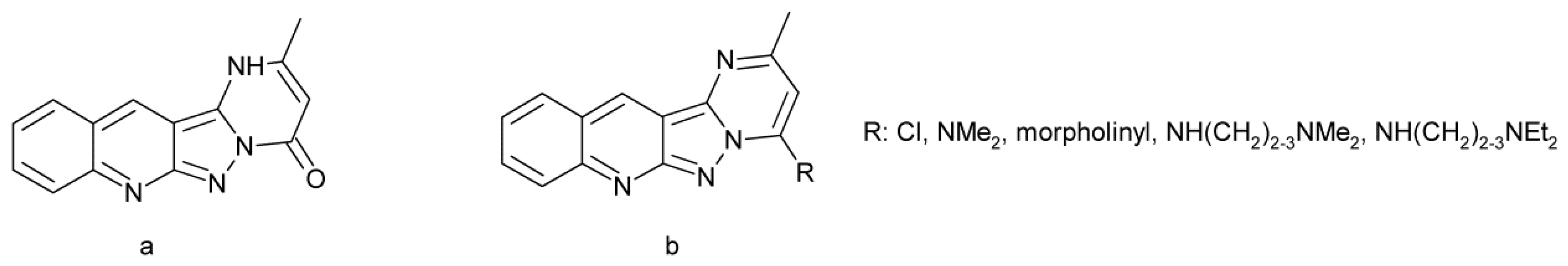
- Yang, S.-W.; Smotryski, J.; McElroy, W.T.; Tan, Z.; Ho, G.; Tulshian, D.; Greeniee, W.J.; Guzzi, M.; Zhang, X.; Mullins, D.; et al. Discovery of orally active pyrazoloquinolines as potent PDE10 inhibitors for the management of schizophrenia. Bioorg. Med. Lett. 2012, 22, 235–239. [Google Scholar] [CrossRef]
- Ho, G.D.; Yang, S.-W.; Smotryski, J.; Bercovici, A.; Nechuta, T.; Smith, E.M.; McElroy, W.; Tan, Z.; Tulshian, D.; McKittrick, B.; et al. The discovery of potent, selective, and orally active pyrazoloquinolines as potent PDE10A inhibitors for the treatment of Schozophrenia. Bioorg. Med. Lett. 2012, 22, 1019–1022. [Google Scholar] [CrossRef]
- McElroy, W.T.; Tan, Z.; Basu, K.; Yang, S.-W.; Smotryski, J.; Ho, G.D.; Tulshian, D.; Greenlee, W.J.; Mullins, D.; Guzzi, M.; et al. Pyrazoloquinolines as PDE10A inhibitors: Discover of a tool compound. Bioorg. Med. Lett. 2012, 22, 1335–1339. [Google Scholar] [CrossRef]
- Coutts, R.T.; Edwards, J.B. The preparation of 9-hydroxypyrazolo[3,4-b]quinolines. Can. J. Chem. 1966, 44, 2009–20014. [Google Scholar] [CrossRef]
- Coutts, R.T.; El-Hawari, A.-M. Derivatives of 2-pyrazolin-5-one.V. Preparation and properties of 1′,2′-dihydrospiro[[2]pyrazoline-4,3′(4′H)-quinoline]-5-one derivatives and related compounds. Can. J. Chem. 1977, 55, 2856–2866. [Google Scholar] [CrossRef]
- Kametani, T.; Yamanaka, T.; Ogasawara, K. Nitrenes. Part IV. Synthesis of oxazolo[5,4-b]quinoline through a nitrene intermediate. J. Chem. Soc. C 1969, 385–387. [Google Scholar] [CrossRef]
- Nishiwaki, T.; Fukuhara, G.; Takahashi, T. Studies on heterocyclic chemistry. Part XVI. Reaction of 4-o-nitrobenylidene -Δ2-pyrazoli-5-ones with trialkyl phosphites: Synthesis of phosphorus-substituted pyrazolo[3,4-b]quinoline derivatives. J. Chem. Soc. Perkin Trans. I 1973, 15, 1606–1609. [Google Scholar] [CrossRef]
- Danel, A.; Tomasik, P. Condensation of 2-nitrobenzaldehyde with pyrazolin-5-ones. Structure of 4-(2-nitrobenzylidene)pyrazolin-5-ones and their reductive cyclization. Polish J. Chem. 1995, 69, 1013–1017. [Google Scholar] [CrossRef]
- DeWald, H. The synthesis of 4-(o-fluorohenyl)-6,8-dihydro-3,8-dimethyl-pyrazolo[3,4-e][1,4]diazepin-7(H)one, A metabolite of Zolazepam. J. Heterocycl. Chem. 1974, 11, 1061–1062. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, S.; Wu, S.-S.; Gao, Y.; Tu, S.-J. A diversity oriented synthesis of pyrazolo[4,3-f]quinoline derivatives with potential biactivities via microwave—Assisted multicomponent reactions. Mol. Divers. 2011, 15, 497–505. [Google Scholar] [CrossRef]
- De Andrade, A.; Dos Santos, G.C.; Da Silva-Filho, L.C. Synthesis of quinoline derivatives by multicomponent reaction using niobium pentachloride as Lewis Acid. J. Heterocycl. Chem. 2015, 52, 273–277. [Google Scholar] [CrossRef]
- Jiang, B.; Rajale, T.; Wever, W.; Tu, S.-J.; Li, G. Multicomponent reactions for the synthesis of heterocycles. Chem. Asian. J. 2010, 5, 2318–2335. [Google Scholar] [CrossRef]
- Chebanov, V.A.; Gura, K.A.; Desenko, S.M. Aminoazoles as key reagents in multicomponent heterocyclizations. In Synthesis of Heterocycles via Multicomponent Reactions I: Topics in Heterocyclic Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; Volume 23, pp. 41–84. [Google Scholar] [CrossRef]
- Quiroga, J.; Insuasty, B.; Hormanza, A.; Saitz, C.; Jullian, C. Syntheses of 4-Aryl-4,7,8,9-tetrahydro-6H-pyrazolo[3,4-b]quinolin-5-ones. J. Heterocycl. Chem. 1998, 35, 575–578. [Google Scholar] [CrossRef]
- Quiroga, J.; Mejia, D.; Insuasty, B.; Abonia, R.; Nogueras, M.; Sanchez, A.; Cobo, J.; Low, N.J. Regioselective synthesis of 4,7,8,9-tetrahydro-2H-pyrazolo[3,4-b]quinolin-5(6H)-ones. Mechanism and structural analysis. Tetrahedron 2001, 57, 6947–6953. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Kshiar, B.; Myrboh, B. L-Proline as an efficient enatioinduction organocatalyst in the solvent-free synthesis of pyrazolo[3,4-b]quinoline derivatives via one-pot multicomponent reaction. RSC Adv. 2016, 6, 95944. [Google Scholar] [CrossRef]
- Khumalo, M.R.; Maddila, S.N.; Maddila, S.; Jonnalagadda, S.B. A multicomponent, facile and catalyst-free microwave-assisted protocol for the synthesis of pyrazolo[3,4-b]quinolines under green conditions. RSC Adv. 2019, 9, 30768. [Google Scholar] [CrossRef] [Green Version]
- Devi, L.; Nagraraju, K.; Maddila, S.; Jonnalagadda, S.B. A green, efficient protocol for the catalyst free synthesis of tetrahydro-1H-pyrazolo[3,4-b]quinolin-5(4H)-ones supported by ultrasonicirradiation. Chem. Data Collect. 2020, 30, 100566. [Google Scholar] [CrossRef]
- Zahedifar, M.; Shojaei, R.; Sheibani, H. Convenient regioselctive reaction in presence of H3PW12O40: Synthesis and characterization of pyrazolo[3,4-b]quinoline-3,5-diones. Res. Chem. Intermed. 2018, 44, 873–882. [Google Scholar] [CrossRef]
- Zahedifar, M.; Pouramiri, B.; Razavi, R. Triethanoloamine lactate-supprted nanomagnetic cellulose: A green and efficient catalyst dor the synthesis of pyrazolo[3,4-b]quinolines and theoretical study. Res. Chem. Intermed. 2020, 46, 2749–2765. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Shi, D.-Q. Three-component one-pot synthesis of pyrazolo[3,4-b]quinolin-5(6H)-one derivatives in aqueous media. J. Heterocycl. Chem. 2012, 49, 212–216. [Google Scholar] [CrossRef]
- Kamakar, K.; Murthy, N.S.; Ramesh, K.; Satish, G.; Nanubolu, J.B.; Nageswar, Y.V.D. Polyethlene glycol (PEG-400): An efficient and recyclable reaction medium for the synthesis of pyrazolo[3,4-b]quinoline. Tetrahedron Lett. 2012, 53, 2897–2903. [Google Scholar] [CrossRef]
- Tomasik, P.; Chaczatrian, K.; Chaczatrian, G.; Danel, A. Facile synthesis of 4-aryl-1H-pyrazolo[3,4-b]quinolines. Polish J. Chem. 2003, 77, 1141–1147. [Google Scholar]
- Hu, Y.; Liu, Y.-F.; Zhang, J.; Zhang, Y.-Z.; Bi, F.-L. Synthesis of 1,3,4-triphenyl-1H-pyrazolo[3,4-b]quinoline derivatives under microwave irradiation. Ziran Kexueban 2012, 42, 241–244. [Google Scholar]
- Hedge, H.; Shetty, N.S. Facile one-pot multicomponent synthesis of 1H-pyrazolo[3,4-b]quinolones using L-proline as a catalyst. Chem. Heterocycl. Compd. 2017, 53, 883–886. [Google Scholar] [CrossRef]
- Jaing, B.; Zhang, G.; Ma, N.; Shi, F.; Tu, S.-J.; Kaur, P.; Li, G. A new rapid multicomponent domino reaction for the foramtion of functinalized benzo[h]pyrazolo[3,4-b]quinolines. Org. Biomol. Chem. 2011, 9, 3834–3838. [Google Scholar] [CrossRef]
- Rajesh, S.M.; Bala, B.D.; Perumal, S.; Menedez, J.C. L-Proline—Catalysed sequential four component “on water” protocol for the synthesis od structurally complex heterocyclic ortho-quinones. Green Chem. 2011, 13, 3248–3254. [Google Scholar] [CrossRef]
- Quiroga, J.; Diaz, Y.; Bueno, J.; Insuasty, B.; Abonia, R.; Ortiz, A.; Nogueras, M.; Cobo, J. Microwave induced three-component synthesis and antimicrobacerial activity of benzopyrazolo[3,4-b]quinolindiones. Eur. J. Med. Chem. 2014, 74, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Khalafy, J.; Arlan, F.M.; Marjani, A.P.; Sarchami, V. One-pot, three-component synthesis of polyfunctionalized benzo[h]pyrazolo[3,4-b][1,6]naphtyridine and benzo[g]pyrazolo[3,4-b]quinoline derivatives in the presence of silver nanoparticles (AgNPs). J. Heterocycl. Chem. 2020, 57, 3961–3969. [Google Scholar] [CrossRef]
- Yadaw, R.; Parvin, T. Multicomponent synthesis of styryl linked benzo[h]pyrazolo[3,4-b]quinoline-5,6(10H)-diones by liquid assisted grinding. New. J. Chem. 2021, 45, 10388–10395. [Google Scholar] [CrossRef]
- Polo, E.; Ferrer-Perutz, K.; Trilleras, J.; Quiroga, J.; Gutierrez, M. Microwave—Assisted one pot synthesis in water carbonylpyrazolo[3,4-b]pyridine derivatives catalysed by InCl3 and sonochemical assisted condensation with aldehydes to obtain new chalcone derivatives containing the pyrazolpyridinic moiety. RSC Adv. 2017, 7, 50044–50055. [Google Scholar] [CrossRef] [Green Version]
- Khurana, J.M.; Chaudhary, A.; Nand, B.; Lumb, A. Aqua mediated indium(II) chloride catalysed synthesis of fused pyrimidines and pyrazoles. Tetrahedron Lett. 2012, 53, 3018–3022. [Google Scholar] [CrossRef]
- Wu, L.-Q.; Dong, R.-Y.; Yang, C.-G.; Yan, F.-L. Synthesis of 4-aryl-3-methyl-1-phenyl-1H-benzo[h]pyrazolo[3,4-b]quinoline-5,10-diones using diammonium hydrogen phosphate as an efficient and versatile catalyst in water. J. Chin. Chem. Soc. 2010, 57, 19–23. [Google Scholar] [CrossRef]
- Re, Y.-M.; Jin, S.; Yan, H.-J.; Zhang, Z. PEG1000-Based dicationic acidic ionic liquid catalysed one-pot synthesis of 4-aryl-3-methyl-1-phenyl-1H-benzo[h]pyrazolo[3,4-b]quinoline-5,10-diones via multicomponent reactions. Catalysis 2015, 5, 1649–1656. [Google Scholar] [CrossRef] [Green Version]
- Karamthulla, S.; Pal, S.; Parvin, T.; Choudhury, L.H. L-Proline catalysed multicomponent reactions: Facile access to 2H-benzo[g]pyrazolo[3,4-b]quinoline-5,10(4H,11H)-dione derivatives. RSC Adv. 2014, 4, 15319–15324. [Google Scholar] [CrossRef]
- Yin, A.; Yang, L.; Wu, L. Synthesis of spiro[pyrazolo[3,4-b]pyridine-4,3′-indoline] and spiro[benzo[h]pyrazolo[3,4-b]quinoline-4,3′-indoline]derivatives using wet cyanuric chloride under solvet free condition. J. Chem. Sci. 2013, 125, 601–606. [Google Scholar] [CrossRef]
- Dabiri, M.; Tisseh, Z.N.; Nobahar, M.; Bazgir, A. Organic reaction in water: A highly efficient and environmentally friendly synthesis of spiro compouns catalysed by L-proline. Helv. Chim. Acta 2011, 94, 824–830. [Google Scholar] [CrossRef]
- Zhu, G.; Gao, L.; Yu, Q.; Qin, Y.; Xi, J.; Rong, L. An efficient synthesis of 1′,7′,8′,9′-tetrahydrospiro[indoline-3,4′-pyrazolo[3,4-b]quinoline]2,5′(6′H)-dione derivatives in aqueous medium. J. Hetereocycl. Chem. 2018, 55, 871–878. [Google Scholar] [CrossRef]
- Baradarani, M.M.; Saatluo, B.; Ghabeli, S.; Shokri, Z.; Shahbazi, M.; Joule, J.A. Synthesis of hexahydrospiro[pyrazolo3,4-b]quinoline-4,1′-pyrrolo[3,2,1-ij]quinoline-2′,5(1H,4′H)-diones] from 5,6 dihydro-4H-pyrrolo[3,2,1-ij]quinoline-1,2-dione using Fe3O4@Cu(OH)x as Nanaocatalyst. J. Heterocycl. Chem. 2019, 56, 1999–2007. [Google Scholar] [CrossRef]
- Quiroga, J.; Portilla, J.; Serrano, H.; Abonia, R.; Insuasty, B.; Nogueras, M.; Cobo, J. Regioslective synthesis of fused benzopyrazolo[3,4-b]quinolines under solvent-free conditions. Tetrahedron Lett. 2007, 48, 1987–1990. [Google Scholar] [CrossRef]
- Manickam, S.; Balijapalli, U.; Sathiyanarayan, K.I. SnCl2-cataluzed synthesis of dihydro-5H-benzo[f]pyrazolo[3,4-b]quinoline and dihydroindeno[2,1-b]pyrazolo[4,3-e]pyridine with high fluorescence and their photophysical properties. New. J. Chem. 2018, 42, 860–871. [Google Scholar] [CrossRef]
- Yadaw, P.; Awasthi, A.; Gokulnath, S.; Tiwari, D.K. DMSO as a methine source in TFA-mediated one pot tanden regioselctive synthesis of 3 substiuted -1-aryl-1H-pyrazolo[3,4-b]quinolines from anilines and pyrazolones. J. Org. Chem. 2021, 86, 2658–2666. [Google Scholar] [CrossRef]
- Pietrzycki, W.; Danel, A.; Tomasik, P. Quantum chemical ci-1 analysis of uv absorption spectra of 1h-pyrazolo[3,4-b]quinoline system. Bull. Soc. Chim. Belges 1994, 103, 725–741. [Google Scholar] [CrossRef]
- Gondek, E.; Koścień, E.; Sanetra, J.; Danel, A.; Wisła, A.; Kityk, A.V. Optical absorption of 1H-pyrazolo[3,4-b]quinoline and its derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 3101–3106. [Google Scholar] [CrossRef]
- Gondek, E.; Kityk, I.V.; Sanetra, J.; Szlachcic, P.; Armatys, P.; Wisla, A.; Danel, A. New-synthesized pyrazoloquinoline as promising luminescent materials. Opics Laser Technol. 2006, 38, 487–492. [Google Scholar] [CrossRef]
- Koścień, E.; Gondek, E.; Kityk, A.V. Optical absorption of 1-phenyl-3-methyl-1H-pyrazolo[3,4-b]quinoline derivatives: Molecular dynamics modeling. Opt. Commun. 2007, 280, 95–102. [Google Scholar] [CrossRef]
- Koścień, E.; Gondek, E.; Pokładko, M.; Jarosz, B.; Vlokh, R.O.; Kityk, A.V. Photoluminescence of 1,3-dimethyl pyrazoloquinoline derivatives. Mater. Chem. Phys. 2009, 114, 860–867. [Google Scholar] [CrossRef]
- Koścień, E.; Gondek, E.; Jarosz, B.; Danel, A.; Nizioł, J.; Kityk, A.V. Photoluminescence of 1-phenyl-3-methyl pyrazoloquinoline derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; He, K.; Li, Y.; Tan, H.; Zhu, M.; Wang, Y.; Liu, Y.; Zhu, W.; Wu, H. A novel near-infrared-emitting cyclometalated platinum (II) complex with donor–acceptor–acceptor chromophores. Dyes Pigment. 2014, 107, 146–152. [Google Scholar] [CrossRef]
- Slodek, A.; Matussek, M.; Filapek, M.; Szafraniec-Gorol, G.; Szlapa, A.; Grudzka-Flak, I.; Szczurek, M.; Malecki, J.G.; Maron, A.; Schab-Balcerzak, E.; et al. Small Donor-Acceptor Molecules Based on a Quinoline-Fluorene System with Promising Photovoltaic Properties. Eur. J. Org. Chem. 2016, 2016, 2500–2508. [Google Scholar] [CrossRef]
- Yan, Z.; Guang, S.; Xu, H.; Liu, X.-Y. Quinoline-based azo derivative assembly: Optical limiting property and enhancement mechanism. Dyes Pigment. 2013, 99, 720–726. [Google Scholar] [CrossRef]
- dos Santos, G.C.; Servilha, R.O.; de Oliveira, E.F.; Lavarda, F.C.; Ximenes, V.F.; da Silva-Filho, L.C. Theoretical-Experimental Photophysical Investigations of the Solvent Effect on the Properties of Green- and Blue-Light-Emitting Quinoline Derivatives. J. Fluoresc. 2017, 27, 1709–1720. [Google Scholar] [CrossRef]
- Goel, A.; Kumar, V.; Singh, S.P.; Sharma, A.; Prakash, S.; Singh, C.; Anand, R.S. Non-aggregating solvatochromic bipolar benzo[f]quinolines and benzo[a]acridines for organic electronics. J. Mater. Chem. 2012, 22, 14880. [Google Scholar] [CrossRef]
- EL-Shabaan, M.M.; Gaml, E.A. Fluorescent quinoline carboxylate derivative: Study on the optical properties and photo diode application. Phys. B Condens. Matter 2022, 626, 413578. [Google Scholar] [CrossRef]
- Onozawa-Komatsuzaki, N.; Funaki, T.; Kasuga, K.; Nakazawa, Y.; Sayama, K.; Sugihara, H. Synthesis and Electrochemical Properties of 2,6-Bis(quinoline-2-yl)pyridyl Ruthenium Complexes as Near-Infrared Sensitizers for Dye-Sensitized Solar Cells. Jpn. J. Appl. Phys. 2012, 51, 10NE11. [Google Scholar] [CrossRef]
- Lanke, S.K.; Sekar, N. Pyrazole based NLOphores: Synthesis, photophysical, DFT, TDDFT studies. Dye. Pigment. 2016, 127, 116–127. [Google Scholar] [CrossRef]
- Barberá, J.; Clays, K.; Giménez, R.; Houbrechts, S.; Persoons, A.; Serrano, J.L. Versatile optical materials: Fluorescence, non-linear optical and mesogenic properties of selected 2-pyrazoline derivatives. J. Mater. Chem. 1998, 8, 1725–1730. [Google Scholar] [CrossRef]
- Willy, B.; Müller, T.J.J. Rapid One-Pot, Four-Step Synthesis of Highly Fluorescent 1,3,4,5-Tetrasubstituted Pyrazoles. Org. Lett. 2011, 13, 2082–2085. [Google Scholar] [CrossRef] [PubMed]
- Götzinger, A.C.; Theßeling, F.A.; Hoppe, C.; Müller, T.J.J. One-Pot Coupling–Coupling–Cyclocondensation Synthesis of Fluorescent Pyrazoles. J. Org. Chem. 2016, 81, 10328–10338. [Google Scholar] [CrossRef]
- Castillo, J.-C.; Tigreros, A.; Portilla, J. 3-Formylpyrazolo[1,5-a]pyrimidines as Key Intermediates for the Preparation of Functional Fluorophores. J. Org. Chem. 2018, 83, 10887–10897. [Google Scholar] [CrossRef] [PubMed]
- Taydakov, I.V.; Akkuzina, A.A.; Avetisov, R.I.; Khomyakov, A.V.; Saifutyarov, R.R.; Avetissov, I.C. Effective electroluminescent materials for OLED applications based on lanthanide 1.3-diketonates bearing pyrazole moiety. J. Lumin. 2016, 177, 31–39. [Google Scholar] [CrossRef]
- Lewińska, G.; Khachatryan, K.; Danel, K.S.; Danel, Z.; Sanetra, J.; Marszałek, K.W. Investigations of the Optical and Thermal Properties of the Pyrazoloquinoline Derivatives and Their Application for OLED Design. Polymers 2020, 12, 2707. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Romero, I.; Portilla, J. Synthesis of Fluorescent 1,7-Dipyridyl-bis-pyrazolo[3,4-b:4′,3′-e]pyridines: Design of Reversible Chemosensors for Nanomolar Detection of Cu 2+. ACS Omega 2019, 4, 6757–6768. [Google Scholar] [CrossRef] [Green Version]
- Orrego-Hernández, J.; Lizarazo, C.; Cobo, J.; Portilla, J. Pyrazolo-fused 4-azafluorenones as key reagents for the synthesis of fluorescent dicyanovinylidene-substituted derivatives. RSC Adv. 2019, 9, 27318–27323. [Google Scholar] [CrossRef] [Green Version]
- Kanwal, I.; Rasool, N.; Zaidi, S.H.M.; Zakaria, Z.A.; Bilal, M.; Hashmi, M.A.; Mubarik, A.; Ahmad, G.; Shah, S.A.A. Synthesis of Functionalized Thiophene Based Pyrazole Amides via Various Catalytic Approaches: Structural Features through Computational Applications and Nonlinear Optical Properties. Molecules 2022, 27, 360. [Google Scholar] [CrossRef]
- Bhalekar, S.; Bhagwat, A.; Sekar, N. 3 Fluorescent styryl chromophores with rigid (pyrazole) donor and rigid (benzothiophenedioxide) acceptor—Complete density functional theory (DFT), TDDFT and nonlinear optical study. In Computational Chemistry; De Gruyter: Berlin, Germany, 2021; pp. 33–60. [Google Scholar]
- Makowska-Janusik, M.; Gondek, E.; Kityk, I.V.; Wisła, J.; Sanetra, J.; Danel, A. Second-order optical effects in several pyrazolo-quinoline derivatives. Chem. Phys. 2004, 306, 265–271. [Google Scholar] [CrossRef]
- Niziol, J.; Danel, A.; Boiteux, G.; Davenas, J.; Jarosz, B.; Wisla, A.; Seytre, G. Optical properties of new pyrazolo[3,4-b] quinoline and its composites. Synth. Met. 2002, 127, 175–180. [Google Scholar] [CrossRef]
- Gąsiorski, P.; Danel, K.S.; Matusiewicz, M.; Uchacz, T.; Vlokh, R.; Kityk, A.V. Synthesis and Spectroscopic Study of Several Novel Annulated Azulene and Azafluoranthene Based Derivatives. J. Fluoresc. 2011, 21, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Bhattacharyya, S.P. Intramolecular Charge Transfer: Theory and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; ISBN 978-3-527-34156-6. [Google Scholar]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef] [PubMed]
- Gondek, E.; Danel, A.; NizioŁ, J.; Armatys, P.; Kityk, I.V.; Szlachcic, P.; Karelus, M.; Uchacz, T.; Chwast, J.; Lakshminarayana, G. Some spirobiindane based 1H-pyrazolo[3,4-b] quinoline chromophore as novel chromophore for light-emitting diodes. J. Lumin. 2010, 130, 2093–2099. [Google Scholar] [CrossRef]
- Kolbus, A.; Grabka, D.; Danel, A.; Szary, K. Spectral properties of 1H-pyrazolo[3,4-b]quinoline substituted with N,N-diethylamine moiety. Opt. Mater. 2016, 57, 102–106. [Google Scholar] [CrossRef]
- Rettig, W. Photoinduced charge separation via twisted intramolecular charge transfer states. In Electron Transfer I. Topics in Current Chemistry; Springer-Verlag GmbH: Berlin, Heidelberg, Germany, 1994; pp. 253–299. [Google Scholar]
- Grabowski, Z.R.; Rotkiewicz, K.; Siemiarczuk, A. Dual fluorescence of donor-acceptor molecules and the Twisted Intramolecular Charge Transfer (ICT) states. J. Lumin. 1979, 18–19, 420–424. [Google Scholar] [CrossRef]
- Michl, J. Electronic structure of aromatic π-electron systems as reflected in their MCD spectra. Pure Appl. Chem. 1980, 52, 1549–1563. [Google Scholar] [CrossRef] [Green Version]
- Miura, T.; Urano, Y.; Tanaka, K.; Nagano, T.; Ohkubo, K.; Fukuzumi, S. Rational Design Principle for Modulating Fluorescence Properties of Fluorescein-Based Probes by Photoinduced Electron Transfer. J. Am. Chem. Soc. 2003, 125, 8666–8671. [Google Scholar] [CrossRef]
- Fox, M.A. Photoinduced Electron Transfer. Photochem. Photobiol. 1990, 52, 617–627. [Google Scholar] [CrossRef]
- Rurack, K.; Resch-Genger, U. Rigidization, preorientation and electronic decoupling—the ‘magic triangle’ for the design of highly efficient fluorescent sensors and switches. Chem. Soc. Rev. 2002, 31, 116–127. [Google Scholar] [CrossRef]
- Mu, L.; He, Z.; Kong, X.; Liang, C.; Wang, Y.; Danel, A.; Kulig, E.; Milburn, G.H.W. Towards Color Stable Blue Primary for Displays: Suppress Field-Dependent Color Change in a Multilayered Electroluminescent Device. J. Disp. Technol. 2011, 7, 96–104. [Google Scholar] [CrossRef]
- Gąsiorski, P.; Gondek, E.; Danel, K.S.; Pokladko-Kowar, M.; Armatys, P.; Kityk, A.V. Green electroluminescence from azafluoranthene derivatives in polymer based electroluminescent devices. Opt. Mater. 2013, 35, 681–684. [Google Scholar] [CrossRef]
- Jaglarz, J.; Kępińska, M.; Sanetra, J. Thermo-optical properties of 1H[3,4-b] quinoline films used in electroluminescent devices. Opt. Mater. 2014, 36, 1275–1280. [Google Scholar] [CrossRef]
- Tao, Y.T.; Balasubramaniam, E.; Danel, A.; Wisla, A.; Tomasik, P. Pyrazoloquinoline derivatives as efficient blue electroluminescent materials. J. Mater. Chem. 2001, 11, 768–772. [Google Scholar] [CrossRef]
- Danel, K.S.; Gondek, E.; Pokladko, M.; Kityk, I.V. 4-(p-N,N-Diarylphenyl)substituted 1H-pyrazolo[3,4-b]quinolines as novel luminophors for organic light emitting diode—synthesis, electroluminescent devices. J. Mater. Sci. Mater. Electron. 2009, 20, 171–176. [Google Scholar] [CrossRef]
- Mac, M.; Uchacz, T.; Danel, A.; Musiolik, H. Applications of Fluorescent Sensor Based on 1H-pyrazolo[3,4-b]quinoline in Analytical Chemistry. J. Fluoresc. 2013, 23, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Mac, M.; Uchacz, T.; Wróbel, T.; Danel, A.; Kulig, E. New Fluorescent Sensors Based on 1H-pyrazolo[3,4-b]quinoline Skeleton. J. Fluoresc. 2010, 20, 525–532. [Google Scholar] [CrossRef]
- Mac, M.; Uchacz, T.; Danel, A.; Danel, K.; Kolek, P.; Kulig, E. A New Fluorescent Sensor Based on 1H-pyrazolo[3,4-b]quinoline Skeleton. Part 2. J. Fluoresc. 2011, 21, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Uchacz, T.; Szlachcic, P.; Wojtasik, K.; Mac, M.; Stadnicka, K. Amino derivatives of 1,3-diphenyl-1H-pyrazolo[3,4-b]quinoline—Photophysics and implementation of molecular logic switches. Dyes Pigment. 2016, 124, 277–292. [Google Scholar] [CrossRef]
- Paisley, N.R.; Tonge, C.M.; Hudson, Z.M. Stimuli-Responsive Thermally Activated Delayed Fluorescence in Polymer Nanoparticles and Thin Films: Applications in Chemical Sensing and Imaging. Front. Chem. 2020, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Gerasimova, M.A.; Tomilin, F.N.; Malyar, E.Y.; Varganov, S.A.; Fedorov, D.G.; Ovchinnikov, S.G.; Slyusareva, E.A. Fluorescence and photoinduced proton transfer in the protolytic forms of fluorescein: Experimental and computational study. Dyes Pigment. 2020, 173, 107851. [Google Scholar] [CrossRef]
- Meng, X.; You, L.; Li, S.; Sun, Q.; Luo, X.; He, H.; Wang, J.; Zhao, F. An ICT-based fluorescence enhancement probe for detection of Sn 2+ in cancer cells. RSC Adv. 2020, 10, 37735–37742. [Google Scholar] [CrossRef]
- Sasaki, S.; Drummen, G.P.C.; Konishi, G. Recent advances in twisted intramolecular charge transfer (TICT) fluorescence and related phenomena in materials chemistry. J. Mater. Chem. C 2016, 4, 2731–2743. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Guo, Z.; Chi, W.; Fu, W.; Abedi, S.A.A.; Liu, X.; Tian, H.; Zhu, W.-H. Fluorescence umpolung enables light-up sensing of N-acetyltransferases and nerve agents. Nat. Commun. 2021, 12, 3869. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, P.A. Introductory Heterocyclic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 978-1-119-41759-0. [Google Scholar]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containg Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Graeve, R.E.; Pociask, J.R.; Stein, R.G. Dialkylamino Alkylamino Pyrazolo (3,4b) quinolines. U.S. Patent 3600393A, 17 August 1971. [Google Scholar]
- De Andrea, M.; Ravera, R.; Gioia, D.; Gariglio, M.; Landolfo, S. The interferon system: An overview. Eur. J. Paediatr. Neurol. 2002, 6 (Suppl. A), A41–A46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagheri, A.; Moezzi, S.M.I.; Mosaddeghi, P.; Parashkouhi, S.N.; Hoseini, S.M.F.; Badakhshan, F.; Negahdaripour, M. Interferon-inducer antivirals: Potential candidates to combat COVID-19. Int. Immunopharmacol. 2021, 91, art.107245. [Google Scholar] [CrossRef]
- Siminoff, P.; Bernard, A.M.; Hursky, V.S.; Price, K.E. BL-20803, a New, Low-Molecular-Weight Interferon Inducer. Antimicrob. Agents Chemother. 1973, 3, 742–743. [Google Scholar] [CrossRef] [Green Version]
- Siminoff, P. Target Cells for Interferon Induction by BL-20803. J. Infect. Dis. 1976, 133, A37–A42. [Google Scholar] [CrossRef]
- Siminof, P.; Crenshaw, R.R. Simulation of Interferon Production in Mice and in Mouse Spleen Leukocytes by Analogues of BL-20803. Antimicrob. Agents Chemother. 1977, 11, 571–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, E.R.; Hamilton, J.R.; Overall, J.C.; Glasgow, L.A. Antiviral Activity of BL-3849A, a Low-Molecular-Weight Oral Interferon Inducer. Antimicrob. Agents Chemother. 1976, 10, 691–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rádl, S.; Zikán, V.; Šmejkal, F. Připrava některých 4-anilino-1,3-dimetyl-1H-pyrazolo(3,4-b)chinolinů s protivirovým účinkem. Ceskoslov. Farm. 1984, 33, 429–432. [Google Scholar]
- Rádl, S.; Zikán, V.; Šmejkal, F. Syntheses of 1, 2, and 9-methyl derivatives of 4,9-dihydro-3-methyl-4-oxo-1H(2H)-pyrazolo[3,4-b]quinoline and their antiviral activity. Coll. Czechoslov. Chem. Commun. 1985, 50, 1057–1063. [Google Scholar] [CrossRef]
- Rádl, S.; Zikán, V.; Šmejkal, F. Připrava některých 4-fenoxy- a 4-fenylotioderivátů 1H-pyrazolo/3,4-b/chinolinu s protivirovým účinkem. Ceskoslov. Farm. 1985, 34, 119–122. [Google Scholar]
- Rádl, S.; Roubinek, F.; Šmejkal, F.; Zikán, V. Připrava některých 1-substituivaných 4-dialkylaminoalkylamino-3-metyl-1H-pyrazolo/3,4-b/chinolinů s protivirovým účinkem. Ceskoslov. Farm. 1985, 34, 190–193. [Google Scholar]
- Rádl, S.; Zikán, V.; Šmejkal, F. Připrava 4-(hydroxyanilino)-a4-(alkoxyanilino)derivátů 1,3-dimetyl-1H-pyrazolo/3,4-b/chinolinu s potenciálním protivirovým účinkem. Ceskoslov. Farm. 1985, 34, 383–385. [Google Scholar]
- Rádl, S.; Roubinek, F.; Zikán, V.; Šmejkal, F. Připrava některých 1-substituivaných 4-alkylamin-3-metyl-1H-pyrazolo(3,4-b)chinolinů s potenciálním protivirovým účinkem. Ceskoslov. Farm. 1986, 34, 105–109. [Google Scholar]
- Rádl, S.; Zikán, V. Syntheses of 1, 2, and 9-methyl derivatives of 4,9-dihydro-6-methoxy-3-methyl-4-oxo-1H(2H)-pyrazolo[3,4-b]quinoline and 4,9-dihydro-6-hydroxy-3-methyl-4-oxo-1H(2H)-pyrazolo[3,4-b]quinoline and their antiviral activity. Coll. Czechoslov. Chem. Commun. 1987, 52, 788–792. [Google Scholar] [CrossRef]
- Rádl, S.; Zikán, V. Synthesis and biological activity of some basic-substituted 4,9-dihydro-3-methyl-4-oxo-1H(2H)-pyrazolo[3,4-b]quinolines. Coll. Czechoslov. Chem. Commun. 1988, 53, 1812–1819. [Google Scholar] [CrossRef]
- Zikán, V.; Rádl, S.; Šmejkal, F.; Zelená, D. Substituované 4-anilino-1,3-dimethyl-1H-pyrazolo/3,4-b/chinoliny a Způsoby Jejich Přípravy. CS Patent 233445B1, 14 March 1985. [Google Scholar]
- Zikán, V.; Rádl, S.; Šmejkal, F.; Zelená, D. Substituované 4,9-dihydro-3-methyl-4-oxo-1H-pyrazolo(3,4-b)chinoliny a Spůsob Jejich Výroby. CS Patent 235167B1, 15 May 1985. [Google Scholar]
- Zikán, V.; Rádl, S. Substituované 4-fenoxy-3-methyl-1H-pyrazolo(3,4-b)chinoliny a Způsoby Jejich Přípravy. CS Patent 235176B1, 15 May 1985. [Google Scholar]
- Zikán, V.; Rádl, S.; Šmejkal, F.; Zelená, D. 4-Karboxyalkylamino-1,3-dimethyl-1H-pyrazolo(3,4-b)chinoliny a Způsob Jejich Přípravy. CS Patent 235177B1, 15 May 1985. [Google Scholar]
- Zikán, V.; Rádl, S. 4-Chlor-6-methoxy-3-methyl-1H-pyrazolo(3,4-b)chinolin a Jeho N1-methylderivát. CS Patent 252340B1, 13 August 1987. [Google Scholar]
- Zikán, V.; Rádl, S. O,N-substituované 4,9-dihydro-6-hydroxy-1,3-dimethyl-4-oxo-1H-pyrazolo(3,4-b)chinoliny. CS Patent 252341B1, 13 August 1987. [Google Scholar]
- Zikán, V.; Rádl, S.; Šmejkal, F. 4,9-dihydro-6-methoxy-3-methyl-4-oxo-1H-pyrazolo(3,4-b)chinolin. CS Patent 252621B1, 17 September 1987. [Google Scholar]
- Chintha, C.; Gupta, N.; Ghate, M.; Vyas, V.K. Homology modeling, binding site identification, and docking study of human β-arrestin: An adaptor protein involved in apoptosis. Med. Chem. Res. 2014, 23, 1189–1201. [Google Scholar] [CrossRef]
- Vyas, V.K.; Qureshi, G.; Dayani, H.; Jha, A.; Ghate, M. Pharmacophore-based identification and in vitro validation of apoptosis inducers as anticancer agents. SAR QSAR Enviromental Res. 2020, 31, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Kemnitzer, W.; Kuemmerle, J.; Zhang, H.-Z.; Kasibhatla, S.; Tseng, B.; Drewe, J.; Cai, S.X. Discovery of 3-aryl-5-aryl-1,2,4-oxadiazoles as a new series of apoptosis inducers. 2. Identification of more aqueous soluble analogs as potential anticancer agents. Bioorg. Med. Chem. Lett. 2009, 19, 4410–5515. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.; Kelly, J.M.; Chackalamannil, S. 4-Substituted Pyrazoloquinoline Derivatives. U.S. Patent 5506236A, 9 April 1996. [Google Scholar]
- Albin, R.; Chase, R.; Risano, C.; Lieberman, M.; Ferrari, E.; Skelton, A.; Buontempo, P.; Cox, S.; DeMartino, J.; Wright-Minogue, J.; et al. SCH 43478 and analogs: In vitro activity and in vivo efficacy of novel agents for herpesvirus type 2. Antivirial Res. 1997, 35, 139–146. [Google Scholar] [CrossRef]
- Bell, M.R.; Ackerman, J.H. Pyrazolo[3,4-b]quinolines and Their Use as Antiviral Agents. U.S. Patent 4920128A, 24 April 1990. [Google Scholar]
- Bekhit, A.A.; El-Sayed, O.A.; Aboul-Enein, H.Y.; Siddiqui, Y.M.; Al-Ahdal, M.N. Synthesis of Aldehydo-sugar Derivatives of Pyrazoloquinoline as Inhibitors of Herpes Simplex Virus Type 1 Replication. J. Enzym. Inhib. Med. Chem. 2004, 19, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Bekhit, A.A.; El-Sayed, O.A.; Aboul-Enein, H.Y.; Siddiqui, Y.M.; Al-Ahdal, M.N. Evaluation of Some Pyrazoloquinolines as Inhibitors of Herpes Simplex Virus Type 1 Replication. Arch. Pharm. 2005, 338, 74–77. [Google Scholar] [CrossRef]
- Arif, J.M.; Kunhi, M.; Subramanian, M.P.; Bekhit, A.A.; El-Sayed, O.A.; Al-Hussein, K.; Aboul-Enein, H.Y.; Al-Khodairy, F.M. Evaluation of Cytotoxic Potential of Newly Synhtesized Antivirial Aminopyrazoloquinoline Derivatives. Int. J. Biomedical Sci. 2007, 3, 194–198. [Google Scholar]
- Arif, J.M.; Kunhi, M.; Subramanian, M.P.; Bekhit, A.A.; El-Sayed, O.A.; Al-Hussein, K.; Aboul-Enein, H.Y.; Al-Khodairy, F.M. Cytotoxic and genotoxic potentials of newly synthesized antivirial aminopyrazoloquinoline derivatives. Med. Chem. Res. 2008, 17, 297–304. [Google Scholar] [CrossRef]
- Shuck-Lee, D.; Chen, F.F.; Willard, R.; Raman, S.; Ptak, R.; Hammarskjold, M.-L.; Rekosh, D. Heterocyclic Compounds That Inhibit Rev-RRE Function and Human Immunodeficiency Virus Type 1 Replication. Antimicrob. Agents Chemother. 2008, 52, 3169–3179. [Google Scholar] [CrossRef] [Green Version]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistnat and pandrug-resistant bacteria: An international expert propos al for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, O.A.; Aboul-Enein, H.Y. Synthesis and Antimicrobial Activity of Novel Pyrazolo[3,4-b]quinoline Derivatives. Arch. Pharm. 2001, 334, 117–120. [Google Scholar] [CrossRef]
- Lapa, G.B.; Bekker, O.B.; Mirchink, E.P.; Danilenko, V.N.; Preobrazhenskaya, M.N. Regioselective acylation of congeners of 3-amino-1H-pyrazolo[3,4-b]quinolines, their activity on bacterial serine/threonine protein kinases and in vitro antibacterial (including antimycobacterial) activity. J. Enzym. Inhib. Med. Chem. 2012, 28, 1088–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Garattini, S.; Bertele’, V. Effiacy, safety, and cost of new anticancer drugs. BMJ 2002, 325, 269–271. [Google Scholar] [CrossRef] [Green Version]
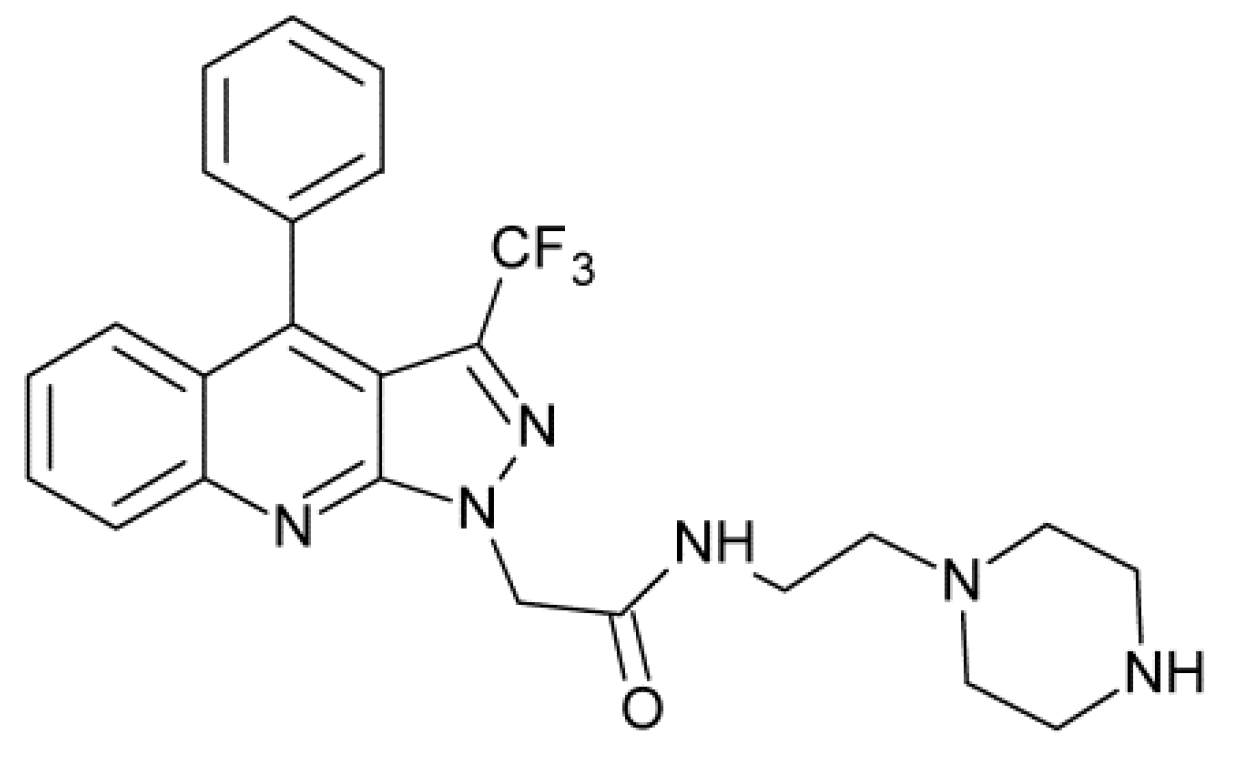
- Karthikeyan, C.; Amawi, H.; Viana, A.G.; Sanglard, L.; Hussein, N.; Saddler, M.; Ashby, C.R., Jr.; Moorthy, N.S.H.N.; Trivedi, P.; Tiwari, A.K. 1H-Pyrazolo[3,4-b]quinoline-3-amine derivatives inhibit growth of colon cancer cells via apoptosis and sub G1 cell cycle arrest. Bioorg. Med. Chem. Lett. 2018, 28, 2244–2249. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Malla, R.; Ashby, C.R., Jr.; Amawi, H.; Abbott, K.L.; Moore, J.; Chen, J.; Balch, C.; Lee, C.; Flannery, P.C.; et al. Pyrimido[1″,2″:1,5]pyrazolo[3,4-b]quinolines: Novel compounds that reverse ABCG2-mediated resistance in cancer cells. Cancer Lett. 2016, 376, 118–126. [Google Scholar] [CrossRef]
- Goodsell, D.S. The Molecular Perspective: The ras Oncogene. The Oncologist 1999, 4, 263–264. [Google Scholar] [CrossRef] [Green Version]
- Downward, J. Targeting RAS signaling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef]
- Kumar, C.C.; Prorock-Rogers, C.; Kelly, J.; Dong, Z.; Lin, J.-J.; Armstrong, L.; Kung, H.-F.; Weber, M.J.; Afonso, A. SCH 51344 Inhibits ras Transformation by a Novel Mechanism. Cancer Res. 1995, 55, 5106–5117. [Google Scholar]
- Walsh, A.B.; Dhanasekaran, M.; Bar-Sagi, D.; Kumar, C.C. SCH 51344-induced reversal of RAS-transformation is accompanied by the specific inhibition of the RAS and RAC-dependent cell morphology pathway. Oncogene 1997, 15, 2553–2560. [Google Scholar] [CrossRef]
- Kumar, C.C.; Ohashi, K.; Nagata, K.; Walsh, A.; Bar-Sagi, D.; Mizuno, K. SCH 51344, An Inhibitor of RAS/RAC-Mediated Cell Morphology Pathway. Ann. N. Y. Acad. Sci. 1999, 886, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.C. Inhibition of RAS-transformation by SCH51344. Gan Kagaku Ryoho (Jpn. J. Cancer Chemother.) 1997, 24, 1503–1511. [Google Scholar]
- Gelman, I.H.; Xi, Q.; Kumar, C.C. Reexpression of the Major PKC Substrate, SSeCKS, Correlates with the Tumor-Suppressive Effects of SCH51344 on Rat-6/src and Rat-6/ras Fibroblasts but Not on Rat-6/raf Fibroblasts. Ann. N. Y. Acad. Sci. 1999, 886, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.V.M.; Salah, E.; Radic, B.; Gridling, M.; Elkins, J.M.; Stukalov, A.; Jemth, A.-S.; Göktürk, C.; Sanjiv, K.; Strömberg, K.; et al. Stereosceific targeting of MTH1 by (S)-crizotinib as an anticancer strategy. Nature 2014, 508, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Huber, K.; Superti-Furga, G. Aminoheteroaryl Compounds as MTH1 Inhibitors. WO Patent 2014/033136A1, 6 March 2014. [Google Scholar]
- Ursu, A.; Waldmann, H. Hide and seek: Identification and confirmation of small molecule protein targets. Bioorg. Med. Chem. Lett. 2015, 25, 3079–3086. [Google Scholar] [CrossRef]
- SCH 51344, Cat. No. 5280. Available online: https://www.tocris.com/products/sch-51344_5280 (accessed on 1 March 2022).
- Nieborowska-Sikorska, M.; Maifrede, S.; Dasgupta, Y.; Sullivan, K.; Flis, S.; Le, B.V.; Solecka, M.; Belyaeva, E.A.; Kubovcakova, L.; Nawrocki, M.; et al. Ruxolitinib-induced defects in DNA repair cause sensitivity to PARP inhibitors in myeloproliferative neoplasms. Blood 2017, 130, 2848–2859. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, T.; Kawatani, M.; Muroi, M.; Kondoh, Y.; Futamura, Y.; Aono, H.; Tanaka, M.; Honda, K.; Osada, H. Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival. Sci. Rep. 2016, 6, art.26521. [Google Scholar] [CrossRef] [Green Version]
- Papeo, G. MutT Homolog 1 (MTH1): The Silencing of a Target. J. Med. Chem. 2016, 59, 2343–2345. [Google Scholar] [CrossRef]
- Zhou, N.; Xu, Y.; Liu, X.; Wang, Y.; Peng, J.; Luo, X.; Zheng, M.; Chen, K.; Jiang, H. Combinatorial Pharmacophore-Based 3D-QSAR Analysis and Virtual Screening of FGFR1 Inhibitors. Int. J. Mol. Sci. 2015, 16, 13407–13425. [Google Scholar] [CrossRef] [Green Version]
- Huber, K.; Superti-Furga, G. Aminoheteroaryl Compounds as MTH1 Inhibitors. U.S. Patent 2016/0015702A1, 21 January 2016. [Google Scholar]
- Bôkel, C.; Weidemann, T. Methods and Compositions for Reducing Interleukin-4 or Interleukin-13 Signaling. U.S. Patent 2013/0084275A1, 4 April 2013. [Google Scholar]
- Wolin, R.; Wang, D.; Kelly, J.; Afonso, A.; James, L.; Kirschmeier, P.; McPhail, A.T. Synthesis and evaluation of pyrazolo[3,4-b]quinoline ribofuranosides and their derivatives as inhibitors of oncogenic Ras. Bioorg. Med. Chem. Lett. 1996, 6, 195–200. [Google Scholar] [CrossRef]
- Wolin, R.L.; Afonso, A.; Kelly, J.M.; Njoroge, F.G. Pyrazoloquinolines. U.S. Patent 5,595,998A, 21 January 1997. [Google Scholar]
- Wolin, R.L.; Afonso, A. Pyrazoloquinolines. U.S. Patent 5597821A, 28 January 1997. [Google Scholar]
- Kaufmann, S.H.; Earnshaw, W.C. Induction of Apoptosis by Cancer Chemotherapy. Exp. Cell Res. 2000, 256, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.; Siddiqui, Y.M.; Bekhit, A.A.; El-Sayed, O.A.; Aboul-Enein, H.Y.; Al-Ahdal, M.N. Effect of Pyrazoloquinoline Derivatives on the Growth of Leishmania donovani Promastigotes. Arch. Pcharm. 2005, 338, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C. Balancing therapeutic safety and efficacy to improve clinical and economic outcomes in schizophrenia: A clinical review. Am. J. Manag. Care 2014, 20 (Suppl. 8), 160–165. [Google Scholar]
- Lally, J.; MacCabe, J.H. Antipsychotic medication in schizophrenia: A review. Br. Med Bull. 2015, 114, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Gao, Q.; Guo, H.; Li, D.; Wang, J.; Gao, W.; Han, C.; Li, Y.; Yang, L. Inhibition mechanism exploration of quinoline derivatives as PDE10A inhibitors by in silico analysis. Mol. BioSyst. 2013, 9, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Kjellgren, E.R.; Glue, O.E.S.; Reinholdt, P.; Meyer, J.E.; Kongsted, J.; Poongavanam, V. A comparative study of binding affinities for 6,7-dimethoxy-4-pyrrolidylquinozalines as phosphodiesterase 10A inhibitors using the linear interaction energy method. J. Mol. Graph. Model. 2015, 61, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yung, J.H.M.; Giacca, A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9, 706. [Google Scholar] [CrossRef] [Green Version]
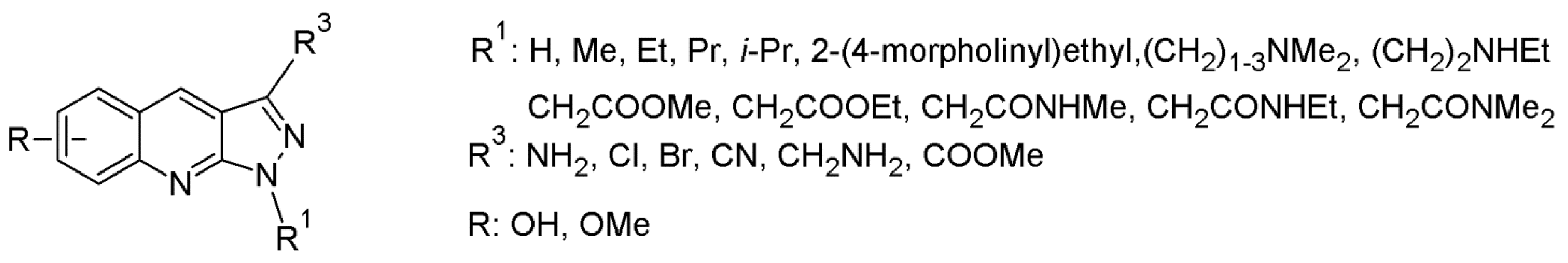
- Liu, M.; Xin, Z.; Clampit, J.E.; Wang, S.; Gum, R.J.; Haasch, D.L.; Trevillyan, J.M.; Abad-Zapatero, C.; Fry, E.H.; Sham, H.L.; et al. Synthesis and SAR of 1,9-dihydro-9-hydroxypyrazolo[3,4-b]quinoline-4-ones as novel, selective c-Jun N-terminal kinase inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 2590–2594. [Google Scholar] [CrossRef]
- El-Sayed, O.A.; Habib, N.S.; Aboul-Enein, H.Y.; Costa, B.; Lucacchini, A.; Martini, C. Synthesis and Benzodiazepine Receptor Binding Activity of 2,9-Disubstituted Quniolino[2′,3′-5,4](3-pyrazolino)[3,2-b]purin-4-ones. Arch. Pharm. 2002, 335, 207–212. [Google Scholar] [CrossRef]
- Cappelli, A.; Bini, G.; Valenti, S.; Giuliani, G.; Paolino, M.; Anzini, M.; Vomero, S.; Giorgi, G.; Giordani, A.; Stasi, L.P.; et al. Synthesis and Structure—Activity Relationship Studies in Translocator Protein Ligands Based on a Pyrazolo[3,4-b]quinoline Scaffold. J. Med. Chem. 2011, 54, 7165–7175. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Diagnostic and Therapeutic Potential of TSPO Studies Regarding Neurodegenerative Diseases, Psychiatric Disorders, Alcohol Use Disorders, Traumatic Brain Injury, and Stroke: An Update. Cells 2020, 9, 870. [Google Scholar] [CrossRef] [Green Version]
- Sanger, D.J.; Zivkovic, B. Discriminative stimulus effects of alpidem, a new imidazopyridine anxiolytic. Psychopharmacology 1994, 113, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Glickman, J.F.; Schmid, A.; Ferrand, S. Scintillation Proximity Assays in High-Throughput Screening. Assay Drug Dev. Technol. 2008, 6, 433–455. [Google Scholar] [CrossRef] [PubMed]
- Pai, J.J.-K.; Kirkup, M.P.; Frank, E.A.; Pachter, J.A.; Bryant, R.W. Compounds Capable of Generating Singlet Oxygen Represent a Source of Artifactual Data in Scintillation Proximity Assays Measuring Phosphopeptide Binding to SH2 Domains. Anal. Biochem. 1999, 270, 33–40. [Google Scholar] [CrossRef] [PubMed]
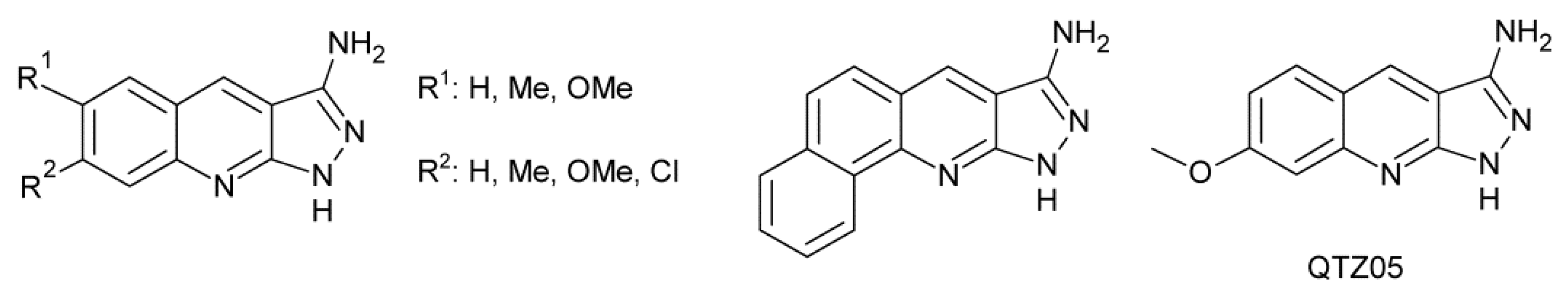
- Mehrotra, P.K.; Shukla, R.; Dwivedi, A.; Srivastava, R.P.; Bhat, N.; Seth, M.; Bhaduri, A.P.; Kamboj, V.P. Pregnancy interceptive efficacy and biological profile of 3-amino-6,7-dimethyl-1H-pyrazolo[3,4-b] quinoline (compound 85/83) in rodents. Contraception 1991, 43, 507–519. [Google Scholar] [CrossRef]














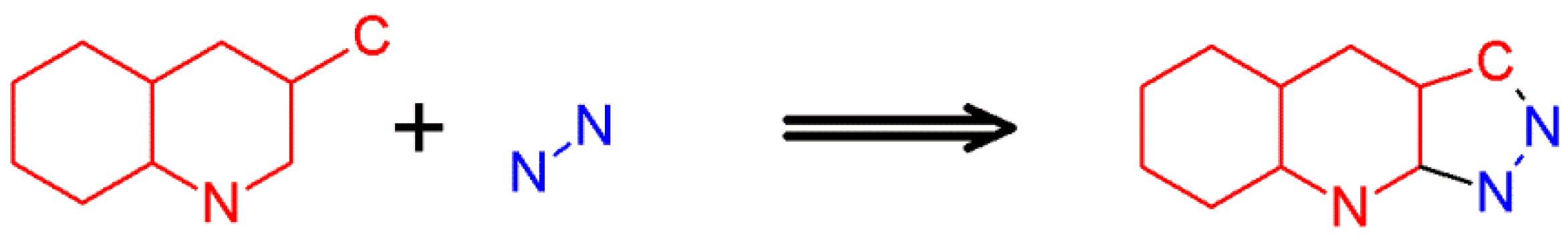
























Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danel, A.; Gondek, E.; Kucharek, M.; Szlachcic, P.; Gut, A. 1H-Pyrazolo[3,4-b]quinolines: Synthesis and Properties over 100 Years of Research. Molecules 2022, 27, 2775. https://doi.org/10.3390/molecules27092775
Danel A, Gondek E, Kucharek M, Szlachcic P, Gut A. 1H-Pyrazolo[3,4-b]quinolines: Synthesis and Properties over 100 Years of Research. Molecules. 2022; 27(9):2775. https://doi.org/10.3390/molecules27092775
Chicago/Turabian StyleDanel, Andrzej, Ewa Gondek, Mateusz Kucharek, Paweł Szlachcic, and Arkadiusz Gut. 2022. "1H-Pyrazolo[3,4-b]quinolines: Synthesis and Properties over 100 Years of Research" Molecules 27, no. 9: 2775. https://doi.org/10.3390/molecules27092775
APA StyleDanel, A., Gondek, E., Kucharek, M., Szlachcic, P., & Gut, A. (2022). 1H-Pyrazolo[3,4-b]quinolines: Synthesis and Properties over 100 Years of Research. Molecules, 27(9), 2775. https://doi.org/10.3390/molecules27092775





