Improvement of Biological Effects of Root-Filling Materials for Primary Teeth by Incorporating Sodium Iodide
Abstract
:1. Introduction
2. Results
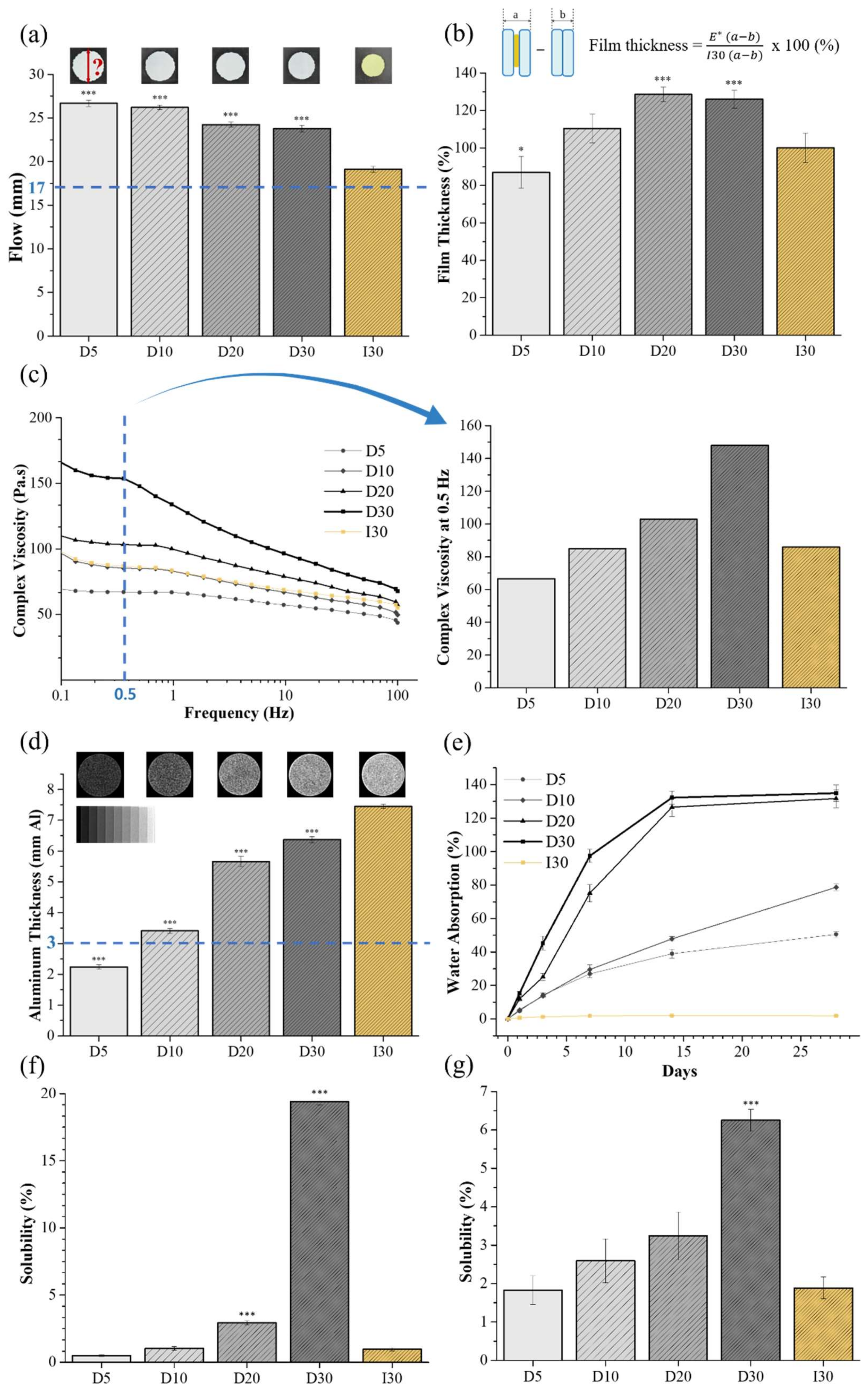
2.1. Physicochemical Characteristics
2.2. Biological Characteristics
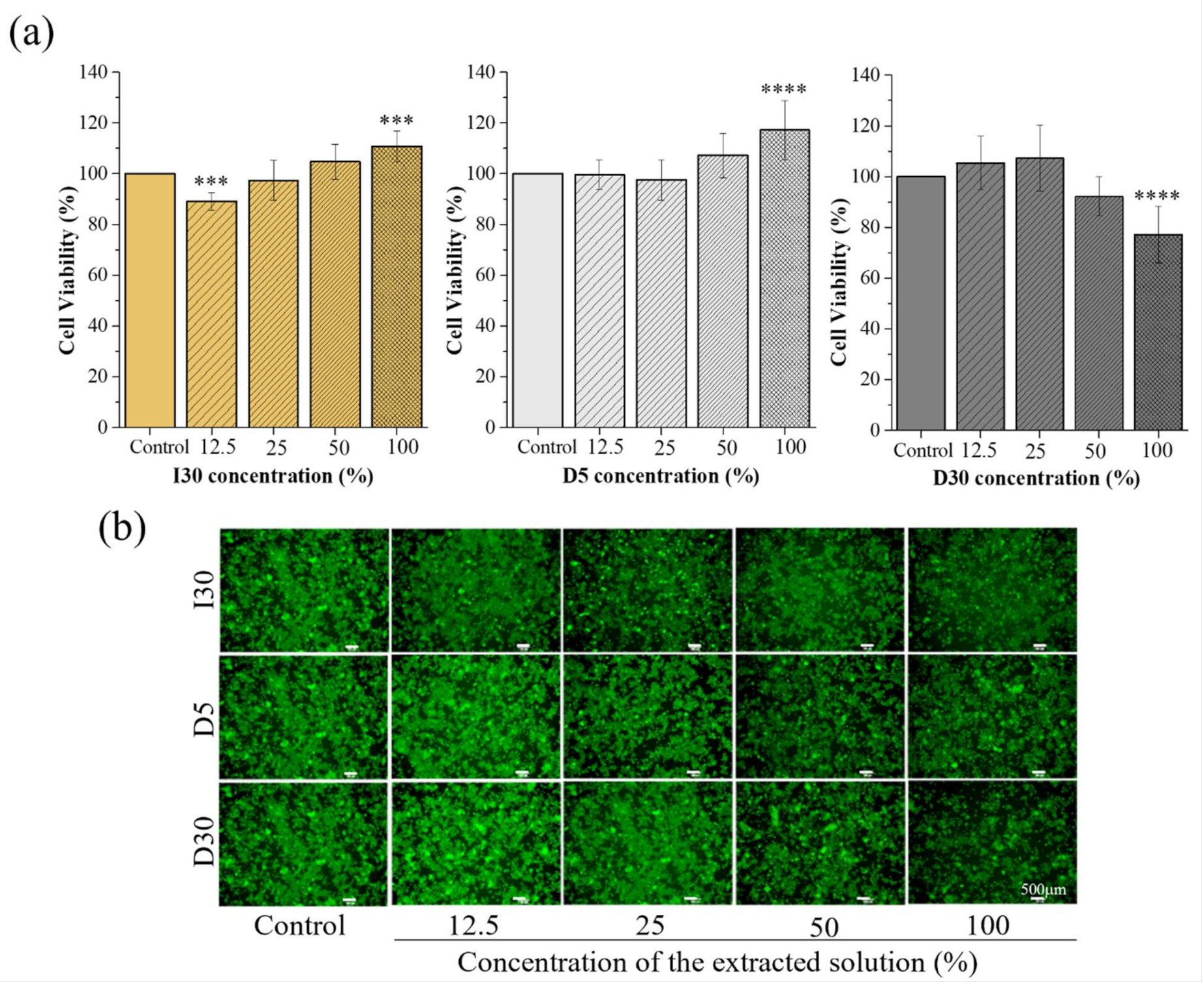
2.2.1. Cytotoxicity Test
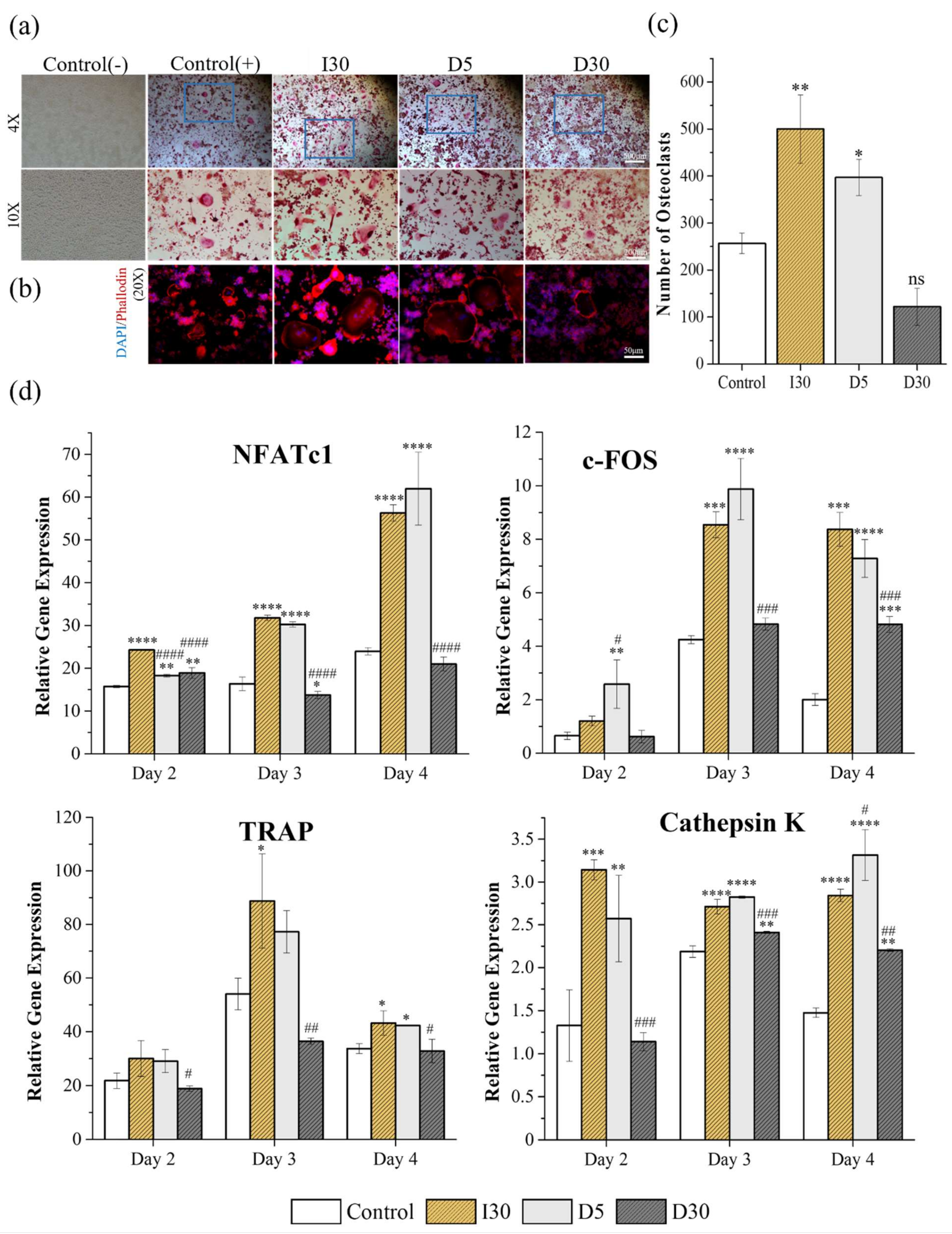
2.2.2. Tartrate-Resistant Acid Phosphatase (TRAP) Staining and Actin Ring Staining (ARS)
2.2.3. Quantitative Polymerase Chain Reaction (qPCR)
3. Discussion
4. Materials and Methods
4.1. Preparation of Samples
4.2. Physicochemical Characteristics of Samples
4.2.1. Flow
4.2.2. Film Thickness
4.2.3. Viscosity
4.2.4. Radiopacity
4.2.5. Water Absorption
4.2.6. Solubility
Solubility from Specimen
Solubility from Extract
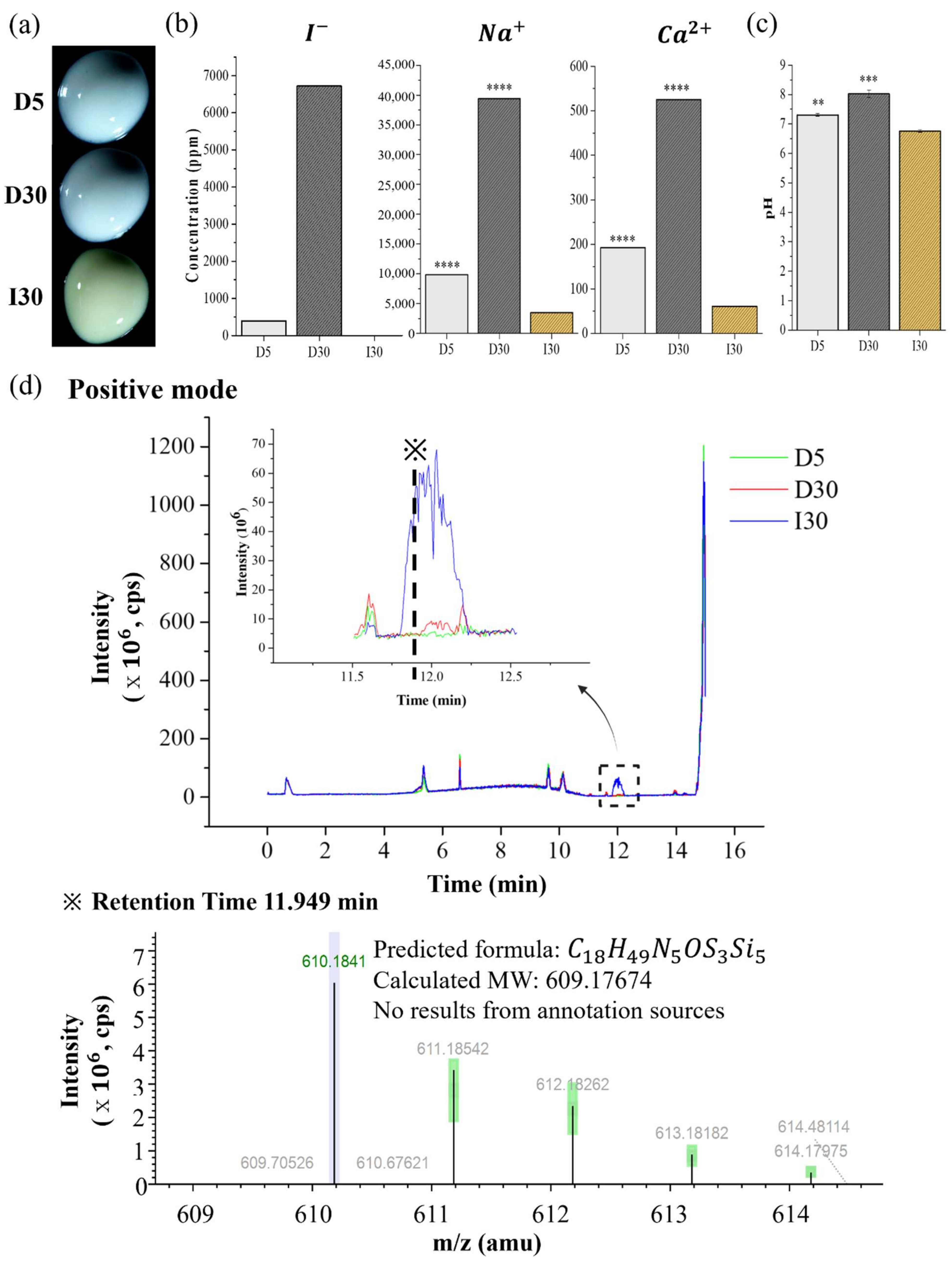
4.2.7. Optical Images of D5, D30, and I30
4.2.8. Extraction Analysis of D5, D30, and I30
4.3. Biological Characteristics of D5, D30, and I30
4.3.1. In Vitro Test
4.3.2. Cytotoxicity Test
4.3.3. Tartrate-Resistant Acid Phosphatase (TRAP) and Actin Ring Staining (ARS)
4.3.4. Quantitative Polymerase Chain Reaction (qPCR)
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waterhouse, P.J.; Whitwortth, J.M. Pediatric endodontics: Endodontic treatment for the primary and young permanent dentition. Cohen’s Pathw. Pulp. 2011, 24, e6-10. [Google Scholar]
- Andrei, M.; Vacaru, R.P.; Coricovac, A.; Ilinca, R. The Effect of Calcium-Silicate Cements on Reparative Dentinogenesis Following Direct Pulp Capping on Animal Models. Molecules 2021, 26, 2725. [Google Scholar] [CrossRef] [PubMed]
- Tchaou, W.S.; Turng, B.F.; Minah, G.E.; Coll, J.A. Inhibition of pure cultures of oral bacteria by root canal filling materials. Pediatr. Dent. 1996, 18, 444–449. [Google Scholar] [PubMed]
- Chonat, A.; Rajamani, T.; Rena, E. Obturating Materials in Primary Teeth—A Review. J. Dent. Sci. 2018, 6, 20–25. [Google Scholar]
- Estrela, C.; Sydney, G.B.; Bammann, L.L.; Felippe Júnior, O. Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Braz. Dent. J. 1995, 6, 85–90. [Google Scholar]
- Fava, L.R.; Saunders, W.P. Calcium hydroxide pastes: Classification and clinical indications. Int. Endod. J. 1999, 32, 257–282. [Google Scholar] [CrossRef] [Green Version]
- Estrela, C.; Estrela, C.R.; Hollanda, A.C.; Decurcio Dde, A.; Pécora, J.D. Influence of iodoform on antimicrobial potential of calcium hydroxide. J. Appl. Oral Sci. 2006, 14, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.M.; Chandra, S.; Chandra, S.; Pandey, R.K. Elimination of infection in pulpectomized deciduous teeth: A short-term study using iodoform paste. J. Endod. 1994, 20, 233–235. [Google Scholar] [CrossRef]
- Moskovitz, M.; Tickotsky, N.; Ashkar, H.; Holan, G. Degree of root resorption after root canal treatment with iodoform-containing filling material in primary molars. Quintessence Int. 2012, 43, 361–368. [Google Scholar]
- Moskovitz, M.; Yahav, D.; Tickotsky, N.; Holan, G. Long-term follow up of root canal treated primary molars. Int. J. Paediatr. Dent. 2010, 20, 207–213. [Google Scholar] [CrossRef]
- Petel, R.; Moskovitz, M.; Tickotsky, N.; Halabi, A.; Goldstein, J.; Houri-Haddad, Y. Cytotoxicity and proliferative effects of Iodoform-containing root canal-filling material on RAW 264.7 macrophage and RKO epithelial cell lines. Arch. Oral Biol. 2013, 58, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Walia, T. Pulpectomy in hyperemic pulp and accelerated root resorption in primary teeth: A review with associated case report. J. Indian Soc. Pedod. Prev. Dent. 2014, 32, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Harokopakis-Hajishengallis, E. Physiologic root resorption in primary teeth: Molecular and histological events. J. Oral Sci. 2007, 49, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holan, G. Periodontal breakdown and pathologic root resorption of primary molars following traumatic injuries to the chin: Case report. Pediatr. Dent. 1997, 19, 425–426. [Google Scholar]
- Haralabakis, N.B.; Yiagtzis, S.C.; Toutountzakis, N.M. Premature or delayed exfoliation of deciduous teeth and root resorption and formation. Angle Orthod. 1994, 64, 151–157. [Google Scholar] [CrossRef]
- Peng, L.; Ye, L.; Guo, X.; Tan, H.; Zhou, X.; Wang, C.; Li, R. Evaluation of formocresol versus ferric sulphate primary molar pulpotomy: A systematic review and meta-analysis. Int. Endod. J. 2007, 40, 751–757. [Google Scholar] [CrossRef]
- Tonnar, J.; Lacroix-Desmazes, P. Use of sodium iodide as the precursor to the control agent in ab initio emulsion polymerization. Angew. Chem. Int. Ed. Engl. 2008, 47, 1294–1297. [Google Scholar] [CrossRef]
- Li, Y.; Shan, Z.; Teng, W. The Iodine Status and Prevalence of Thyroid Disorders Among Women of Childbearing Age in China: National Cross-sectional Study. Endocr. Pract. 2021, 27, 1028–1033. [Google Scholar] [CrossRef]
- Zhou, H.M.; Shen, Y.; Zheng, W.; Li, L.; Zheng, Y.F.; Haapasalo, M. Physical properties of 5 root canal sealers. J. Endod. 2013, 39, 1281–1286. [Google Scholar] [CrossRef]
- Kang, T.Y.; Choi, J.W.; Seo, K.J.; Kim, K.M.; Kwon, J.S. Physical, Chemical, Mechanical, and Biological Properties of Four Different Commercial Root-End Filling Materials: A Comparative Study. Materials 2021, 14, 1693. [Google Scholar] [CrossRef]
- Kharouf, N.; Arntz, Y.; Eid, A.; Zghal, J.; Sauro, S.; Haikel, Y.; Mancino, D. Physicochemical and Antibacterial Properties of Novel, Premixed Calcium Silicate-Based Sealer Compared to Powder-Liquid Bioceramic Sealer. J. Clin. Med. 2020, 9, 3096. [Google Scholar] [CrossRef] [PubMed]
- Vitti, R.P.; Prati, C.; Sinhoreti, M.A.; Zanchi, C.H.; Souza, E. Chemical-physical properties of experimental root canal sealers based on butyl ethylene glycol disalicylate and MTA. Dent. Mater. 2013, 29, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.P.D.S.; Moura-Netto, C.; Oliveira, N.M.D.; Bresolin, C.R. Physicochemical properties and filling capacity of an experimental iodoform-based paste in primary teeth. Braz. Oral Res. 2020, 34, e089. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, A.; Sword, J.; Nishitani, Y.; Yoshiyama, M.; Agee, K.; Tay, F.R.; Pashley, D.H. Water sorption and solubility of methacrylate resin-based root canal sealers. J. Endod. 2007, 33, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Hungaro Duarte, M.A.; Minotti, P.G.; Rodrigues, C.T.; Zapata, R.O.; Bramante, C.M.; Tanomaru Filho, M.; Vivan, R.R.; Gomes de Moraes, I.; Bombarda de Andrade, F. Effect of different radiopacifying agents on the physicochemical properties of white Portland cement and white mineral trioxide aggregate. J. Endod. 2012, 38, 394–397. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.A.; Leonardo, M.R.; da Silva, R.S.; Assed, S.; Guimarães, L.F. Calcium hydroxide root canal sealers: Evaluation of pH, calcium ion concentration and conductivity. Int. Endod. J. 1997, 30, 205–209. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef]
- Huang, T.H.; Ding, S.J.; Kao, C.T. Biocompatibility of various formula root filling materials for primary teeth. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 486–490. [Google Scholar] [CrossRef]
- Pilownic, K.J.; Gomes, A.P.N.; Wang, Z.J.; Almeida, L.H.S.; Romano, A.R.; Shen, Y.; Felix, A.O.C.; Haapasalo, M.; Pappen, F.G. Physicochemical and Biological Evaluation of Endodontic Filling Materials for Primary Teeth. Braz. Dent. J. 2017, 28, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Tanabe, N.; Shoji, M.; Suzuki, N.; Katono, T. Nicotine and lipopolysaccharide stimulate the formation of osteoclast-like cells by increasing macrophage colony-stimulating factor and prostaglandin E2 production by osteoblasts. Life Sci. 2006, 78, 1733–1740. [Google Scholar] [CrossRef]
- Minkin, C. Bone acid phosphatase: Tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif. Tissue Int. 1982, 34, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kim, D.S.; Jang, H.; Kim, H.R.; Kang, H.W. Bioprinting of three-dimensional dentin-pulp complex with local differentiation of human dental pulp stem cells. J. Tissue Eng. 2019, 10, 2041731419845849. [Google Scholar] [CrossRef] [PubMed]
- Lakkakorpi, P.T.; Väänänen, H.K. Cytoskeletal changes in osteoclasts during the resorption cycle. Microsc. Res. Tech. 1996, 33, 171–181. [Google Scholar] [CrossRef]
- Lakkakorpi, P.; Tuukkanen, J.; Hentunen, T.; Järvelin, K.; Väänänen, K. Organization of osteoclast microfilaments during the attachment to bone surface in vitro. J. Bone Miner. Res. 1989, 4, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Jacenko, O. c-fos and bone loss: A proto-oncogene regulates osteoclast lineage determination. Bioessays 1995, 17, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, K.; Tanabe, N.; Suzuki, N.; Kawato, T.; Takeichi, O.; Tsuzukibashi, O. Receptor activator of NF-kappaB ligand induces the expression of carbonic anhydrase II, cathepsin K, and matrix metalloproteinase-9 in osteoclast precursor RAW264.7 cells. Life Sci. 2007, 80, 1311–1318. [Google Scholar] [CrossRef]
- Marks, S.C., Jr.; Cahill, D.R. Experimental study in the dog of the non-active role of the tooth in the eruptive process. Arch. Oral Biol. 1984, 29, 311–322. [Google Scholar] [CrossRef]
- Cahill, D.R.; Marks, S.C., Jr. Tooth eruption: Evidence for the central role of the dental follicle. J. Oral Pathol. 1980, 9, 189–200. [Google Scholar] [CrossRef]
- Wise, G.E.; King, G.J. Mechanisms of tooth eruption and orthodontic tooth movement. J. Dent. Res. 2008, 87, 414–434. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, I.; Takahashi, N.; Jimi, E.; Udagawa, N.; Suda, T. Regulation of osteoclast function. Mod. Rheumatol. 2012, 22, 167–177. [Google Scholar] [CrossRef]
- Nair, P.N.; Sjögren, U.; Schumacher, E.; Sundqvist, G. Radicular cyst affecting a root-filled human tooth: A long-term post-treatment follow-up. Int. Endod. J. 1993, 26, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Bosshardt, D.D. Multinucleated Giant Cells: Good Guys or Bad Guys? Tissue Eng. Part B Rev. 2018, 24, 53–65. [Google Scholar] [CrossRef]
- Bolan, M.; Rocha, M.J. Histopathologic study of physiological and pathological resorptions in human primary teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 104, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.K.; Moon, S.Y.; Cha, J.H.; Kim, K.W.; Yoo, Y.J. Prostaglandin E(2) is a main mediator in receptor activator of nuclear factor-kappaB ligand-dependent osteoclastogenesis induced by Porphyromonas gingivalis, Treponema denticola, and Treponema socranskii. J. Periodontol. 2005, 76, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Húngaro Duarte, M.A.; de Oliveira El Kadre, G.D.; Vivan, R.R.; Guerreiro Tanomaru, J.M.; Tanomaru Filho, M.; de Moraes, I.G. Radiopacity of portland cement associated with different radiopacifying agents. J. Endod. 2009, 35, 737–740. [Google Scholar] [CrossRef]
- Polydorou, O.; König, A.; Hellwig, E.; Kümmerer, K. Long-term release of monomers from modern dental-composite materials. Eur. J. Oral Sci. 2009, 117, 68–75. [Google Scholar] [CrossRef]
- Deviot, M.; Lachaise, I.; Högg, C.; Durner, J.; Reichl, F.X.; Attal, J.P.; Dursun, E. Bisphenol A release from an orthodontic resin composite: A GC/MS and LC/MS study. Dent. Mater. 2018, 34, 341–354. [Google Scholar] [CrossRef]
- An, Y.H.; Kim, J.A.; Yim, H.G.; Han, W.J.; Park, Y.B.; Jin Park, H.; Young Kim, M.; Jang, J.; Koh, R.H.; Kim, S.H.; et al. Meniscus regeneration with injectable Pluronic/PMMA-reinforced fibrin hydrogels in a rabbit segmental meniscectomy model. J. Tissue Eng. 2021, 12, 20417314211050141. [Google Scholar] [CrossRef]
- Tian, X.; Yuan, X.; Feng, D.; Wu, M.; Yuan, Y.; Ma, C.; Xie, D.; Guo, J.; Liu, C.; Lu, Z. In vivo study of polyurethane and tannin-modified hydroxyapatite composites for calvarial regeneration. J. Tissue Eng. 2020, 11, 2041731420968030. [Google Scholar] [CrossRef]
- Kim, T.H.; Singh, R.K.; Kang, M.S.; Kim, J.H.; Kim, H.W. Inhibition of osteoclastogenesis through siRNA delivery with tunable mesoporous bioactive nanocarriers. Acta Biomater. 2016, 29, 352–364. [Google Scholar] [CrossRef]

| Samples | Flow (mm) | Film Thickness (mm) | Viscosity at 0.5 Hz (Pa∙s) | Radiopacity (mmAl) |
|---|---|---|---|---|
| D5 | 66.86 | |||
| D10 | 83.18 | |||
| D20 | 100.04 | |||
| D30 | 134.00 | |||
| I30 | 83.69 |
| Samples | Water Absorption at 28 Days (g) | Solubility (%) | |
|---|---|---|---|
| Solubility from Specimen | Solubility from Extract | ||
| D5 | |||
| D10 | |||
| D20 | |||
| D30 | |||
| I30 | |||
| Samples | Ion Release (ppm) | pH | ||
|---|---|---|---|---|
| Iodine | Sodium | Calcium | ||
| I30 | 0 | |||
| D5 | 393.6 | |||
| D30 | 6719.8 | |||
| Samples | Concentration of Samples | |||
|---|---|---|---|---|
| 12.5% | 25% | 50% | 100% | |
| I30 | 89.10 ± 3.50 *** | 97.32 ± 7.83 | 104.67 ± 7.20 | 110.73 ± 6.04 *** |
| D5 | 99.67 ± 5.79 | 97.55 ± 8.00 | 107.20 ± 8.80 | 117.14 ± 11.60 **** |
| D30 | 105.30 ± 10.56 | 107.20 ± 12.94 | 92.26 ± 7.70 | 77.13 ± 11.20 *** |
| Control | I30 | D5 | D30 | |
|---|---|---|---|---|
| Number of osteoclasts | ||||
| Size of osteoclasts |
| Time | Primer | Relative Gene Expression | |||
|---|---|---|---|---|---|
| Control | I30 | D5 | D30 | ||
| Day 2 | NFATc1 | ||||
| c-Fos | |||||
| TRAP | |||||
| Cathepsin K | |||||
| Day 3 | NFATc1 | ||||
| c-Fos | |||||
| TRAP | |||||
| Cathepsin K | 7 | ||||
| Day 4 | NFATc1 | ||||
| c-Fos | |||||
| TRAP | |||||
| Cathepsin K | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-M.; Vu, H.T.; Shin, S.-J.; Ahn, J.-Y.; Kim, Y.-J.; Song, S.; Han, M.-R.; Lee, J.-H.; Kim, J.-S.; Knowles, J.C.; et al. Improvement of Biological Effects of Root-Filling Materials for Primary Teeth by Incorporating Sodium Iodide. Molecules 2022, 27, 2927. https://doi.org/10.3390/molecules27092927
Choi J-M, Vu HT, Shin S-J, Ahn J-Y, Kim Y-J, Song S, Han M-R, Lee J-H, Kim J-S, Knowles JC, et al. Improvement of Biological Effects of Root-Filling Materials for Primary Teeth by Incorporating Sodium Iodide. Molecules. 2022; 27(9):2927. https://doi.org/10.3390/molecules27092927
Chicago/Turabian StyleChoi, Ji-Myung, Huong Thu Vu, Seong-Jin Shin, Jun-Yong Ahn, You-Jin Kim, Sol Song, Mi-Ran Han, Jun-Haeng Lee, Jong-Soo Kim, Jonathan C. Knowles, and et al. 2022. "Improvement of Biological Effects of Root-Filling Materials for Primary Teeth by Incorporating Sodium Iodide" Molecules 27, no. 9: 2927. https://doi.org/10.3390/molecules27092927







