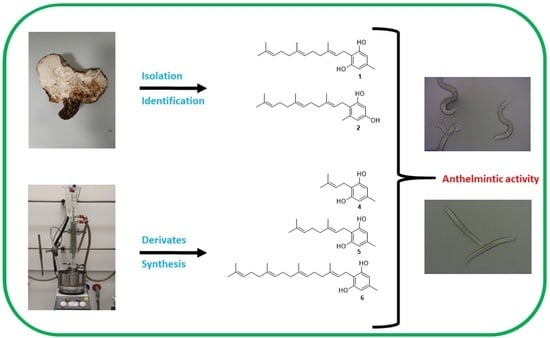
Albatrellus confluens (Alb. & Schwein.) Kotl. & Pouz.: Natural Fungal Compounds and Synthetic Derivatives with In Vitro Anthelmintic Activities and Antiproliferative Effects against Two Human Cancer Cell Lines
Abstract
:1. Introduction
2. Results
A. confluens: Anthelmintic Activity of Crude Extract, Fractions and Metabolites
3. Discussion
4. Materials and Methods
4.1. General
4.2. Fungal Material
4.3. Extract Preparations and Preliminary Anthelmintic Screening
4.4. Isolation of Compounds 1 and 2
4.5. Synthesis of 1, 4–6
4.6. In Vitro Anthelmintic Bioassay
4.6.1. Caenorhabditis elegans
4.6.2. Parasitic Helminths
4.7. Cytotoxic Effects on Human Cancer Cell Lines
4.7.1. Cell Culture
4.7.2. Cytoxicity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moser, W.; Schindler, C.; Keiser, J. Drug combinations against soil transmitted helminth infections. Adv. Parasitol. 2019, 103, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Spangenberg, T. Alternatives to Praziquantel for the prevention and control of schistosomiasis. ACS Infect. Dis. 2021, 7, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Sarkodie, S.A.; Owusu, P.A. Global assessment of environment, health and economic impact of the novel coronavirus (COVID-19). Environ. Dev. Sustain. 2021, 23, 5005–5015. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S.J.; Ziumbe, K.; Negussu, N.; Crowley, S.; Hammami, M. Neglected tropical disease control in a world with COVID-19: An opportunity and a necessity for innovation. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, J.P.; Utzinger, J.; Fontes, G.; da Rocha, E.M.M.; Ehrenberg, N.; Zhou, X.-N.; Steinmann, P. Efforts to mitigate the economic impact of the COVID-19 pandemic: Potential entry points for neglected tropical diseases. Infect. Dis. Poverty 2021, 10, 4–13. [Google Scholar] [CrossRef]
- Mooney, D.; Richards, K.G.; Danaher, M.; Grant, J.; Gill, L.; Mellander, P.E.; Coxon, C.E. An analysis of the spatio-temporal occurrence of anthelmintic veterinary drug residues in groundwater. Sci. Total Environ. 2021, 769, 144804. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cogollo, L.C.; Rodríguez-Vivas, R.I.; del Socorro Basto-Estrella, G.; Reyes-Novelo, E.; Martinez-Morales, I.; Ojeda-Chi, M.M.; Favila, M.E. Toxicity and adverse effects of macrocyclic lactones on dung beetles: A review. Rev. Mex. Biodivers. 2018, 89, 1293–1314. [Google Scholar]
- Wit, J.; Dilks, C.M.; Andersen, E.C. Complementary approaches with free-living and parasitic nematodes to understanding anthelmintic resistance. Trends Parasitol. 2021, 37, 240–250. [Google Scholar] [CrossRef]
- Sharpton, T.J.; Combrink, L.; Arnold, H.K.; Gaulke, C.A.; Kent, M. Harnessing the gut microbiome in the fight against anthelminthic drug resistance. Curr. Opin. Microbiol. 2020, 53, 26–34. [Google Scholar] [CrossRef]
- Tinkler, S.H. Preventive chemotherapy and anthelmintic resistance of soil transmitted helminths—Can we learn nothing from veterinary medicine? One Health 2020, 9, 100106. [Google Scholar] [CrossRef]
- Crellen, T.; Walker, M.; Lamberton, P.H.; Kabatereine, N.B.; Tukahebwa, E.M.; Cotton, J.A.; Webster, J.P. Reduced efficacy of praziquantel against Schistosoma mansoni is associated with multiple rounds of mass drug administration. Clin. Infect. Dis. 2016, 63, 1151–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, M.; Freitas, L.T.; Halder, J.B.; Brack, M.; Keiser, J.; King, C.H.; Levecke, B.; Lim, Y.A.; Pieri, O.; Sow, D.; et al. Improving anthelmintic treatment for schistosomiasis and soil transmitted helminthiases through sharing and reuse of individual participant data [version 1; peer review: Awaiting peer review]. Wellcome Open Res. 2022, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L.; Lücking, R. Chapter 4, Fungal diversity revisited: 2.2 to 3.8 million species. In The Fungal Kingdom; Heitman, J., Howlett, B.J., Eds.; ASM Press: Washington, DC, USA, 2017; pp. 79–95. [Google Scholar]
- Hawksworth, D.L. Mushrooms: The extent of the unexplored potential. Int. J. Med. Mushrooms 2001, 3, 1–5. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef]
- Zeb, M.; Lee, C.H. Medicinal properties and bioactive compounds from wild mushrooms native to North America. Molecules 2021, 26, 251. [Google Scholar] [CrossRef]
- Comans-Perez, R.J.; Sanchez, J.E.; Al-Ani, L.K.T.; Gonzalez-Cortazar, M.; Castaneda-Ramirez, G.S.; Mendoza de Gives, P.; Sanchez-Garcia, A.D.; Millan-Orozco, J.; Aguilar-Marcelino, L. Biological control of sheep nematode Haemonchus contortus using edible mushrooms. Biol. Control 2020, 152, 104420. [Google Scholar] [CrossRef]
- Sasaki, T.; Takagi, M.; Yaguchi, T.; Miyadoh, S.; Okada, T.; Koyama, M. A new anthelmintic cyclodepsipeptide, PF1022A. J. Antibiot. 1992, 45, 692–697. [Google Scholar] [CrossRef] [Green Version]
- Harder, A.; Holden-Dye, L.; Walker, R.; Wunderlich, F. Mechanisms of action of emodepside. Parasitol. Res. 2005, 97, S1–S10. [Google Scholar] [CrossRef]
- Assmus, F.; Hoglund, R.M.; Monnot, F.; Specht, S.; Scandale, I.; Tarning, J. Drug development for the treatment of onchocerciasis: Population pharmacokinetic and adverse events modeling of emodepside. PLoS Negl. Trop. Dis. 2022, 16, e0010219. [Google Scholar] [CrossRef]
- Thorn, R.G.; Barron, G.L. Carnivorous mushrooms. Science 1984, 224, 76–78. [Google Scholar] [CrossRef] [Green Version]
- Heydari, R.; Pourjam, E.; Goltapeh, E.M. Antagonistic effect of some species of Pleurotus on the root-knot nematode, Meloidogyne javanica in vitro. Plant Pathol. J. 2006, 5, 173–177. [Google Scholar] [CrossRef]
- Pineda-Alegria, J.A.; Sanchez-Vazquez, J.E.; Gonzalez-Cortazar, M.; Zamilpa, A.; Lopez-Arellano, M.E.; Cuevas-Padilla, E.J.; Mendoza-de-Gives, P.; Aguilar-Marcelino, L. The edible mushroom Pleurotus djamor produces metabolites with lethal activity against the parasitic nematode Haemonchus contortus. J. Med. Food 2017, 20, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Sreedevi, R.; Pradeeb, B. V Anthelmintic and antibacterial activity of red pigment from Aspergillus terreus. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 249–257. [Google Scholar]
- Trappe, J.M.; Claridge, A.W. Hypogeous fungi: Evolution of reproductive and dispersal strategies through interactions with animals and mycorrhizal plants. In The Fungal Community, 3rd ed.; Dighton, J., White, J.F., Oudemans, P., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2005; pp. 613–623. [Google Scholar]
- Spiteller, P. Chemical defence strategies of Higher fungi. Chem. Eur. J. 2008, 14, 9100–9110. [Google Scholar] [CrossRef]
- Anke, H.; Sterner, O. Nematicidal metabolites from higher fungi. Curr. Org. Chem. 1997, 1, 361–374. [Google Scholar] [CrossRef]
- Chitwood, D.J. Phytochemical based strategies for nematode control. Annu. Rev. Phytopathol. 2002, 40, 221–249. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Bustos, J.F.; Sleebs, B.E.; Gasser, R.B. An appraisal of natural products active against parasitic nematodes of animals. Parasites Vectors 2019, 12, 306. [Google Scholar] [CrossRef] [Green Version]
- Dube, M.; Saoud, M.; Rennert, R.; Fotso, G.W.; Andrae-Marobela, K.; Imming, P.; Häberli, C.; Keiser, J.; Arnold, N. Anthelmintic activity and cytotoxic effects of compounds isolated from the fruits of Ozoroa insignis Del. (Anacardiaceae). Biomolecules 2021, 11, 1893. [Google Scholar] [CrossRef]
- Ryvarden, L.; Gilbertson, R.L. European Polypores Part I. Fungiflora; Lubrecht & Cramer Ltd.: Oslo, Norway, 1993. [Google Scholar]
- Koch, B.; Steglich, W. Monoterpenoid pigments from Albatrellus fletti (Basidiomycetes). Eur. J. Org. Chem. 2007, 2007, 1631–1635. [Google Scholar] [CrossRef]
- Nukata, M.; Hashimoto, T.; Yamamoto, I.; Iwasaki, N.; Tanaka, M.; Asakawa, Y. Neogrifolin derivatives possessing anti-oxidative activity from the mushroom Albatrellus ovinus. Phytochemistry 2002, 59, 731–737. [Google Scholar] [CrossRef]
- Yaqoob, A.; Li, W.M.; Liu, V.; Wang, C.; Mackedenski, S.; Tackaberry, L.E.; Massicotte, H.B.; Egger, K.N.; Reimer, K.; Lee, C.H. Grifolin, neogrifolin and confluentin from the terricolous polypore Albatrellus flettii suppress KRAS expression in human colon cancer cells. PLoS ONE 2020, 15, e0231948. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, N.G.; Zhang, X.; Magolan, J. Efficient synthesis of cannabigerol, grifolin, and piperogalin via alumina-promoted allylation. J. Nat. Prod. 2020, 83, 2587–2591. [Google Scholar] [CrossRef] [PubMed]
- Besl, H.; Höfle, G.; Jendrny, B.; Jägers, E.; Steglich, W. Pilz pigmente, XXXI. Farnesylphenole aus Albatrellus-Arten (Basidiomycetes). Chem. Ber. 1977, 110, 3770–3776. [Google Scholar] [CrossRef]
- Zhi-Hui, D.; Ze-Jun, D.; Liu, J.-K. Albaconol, A novel prenylated resorcinol (=benzene-1,3-diol) from Basidiomycetes Albatrellus confluens. Helv. Chim. Acta 2001, 84, 259–262. [Google Scholar] [CrossRef]
- Yang, X.-L.; Quin, C.; Wang, F.; Dong, Z.J.; Liu, J.-K. A New meroterpenoid pigment from the Basidiomycete Albatrellus confluens. Chem. Biodivers 2008, 5, 484–489. [Google Scholar] [CrossRef]
- Hashimoto, T.; Quang, D.N.; Nukada, M.; Asakawa, Y. Isolation, synthesis and biological activity of grifolic acid dervivatives from the inedible mushroom Albatrellus dispansus. Heterocycles 2005, 65, 2431–2439. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.-H.; Feng, T.; Liu, J.-K. One new ergostane-type steroid and three new phthalide derivatives from cultures of the basidiomycete Albatrellus confluens. J. Asian Nat. Prod. Res. 2015, 17, 107–113. [Google Scholar] [CrossRef]
- Hellwig, V.; Nopper, R.; Mauler, F.; Freitag, J.; Liu, J.-K.; Ding, Z.-H.; Stadler, M. Activities of prenylphenol derivatives from fruitbodies of Albatrellus spp. on the human and rat vanilloid receptor 1 (VR1) and characterisation of the novel natural product, confluentin. Arch. Der Pharm. 2003, 336, 119–126. [Google Scholar] [CrossRef]
- Wang, F.; Luo, D.-Q.; Liu, J.-K. Aurovertin E, a new polyene pyrone from the basidiomycete Albatrellus confluens. J. Antibiot. 2005, 58, 412–415. [Google Scholar] [CrossRef] [Green Version]
- Hirata, Y.; Nahanishi, K. Grifolin, an antibiotic from a basidiomycete. J. Biol. Chem. 1950, 184, 135–144. [Google Scholar] [CrossRef]
- Cardillo, G.; Cricchio, R.; Merlini, L.; Nasini, G. Poliisoprenifoli naturali. Sintesis della grifolina e della ostrutina. Gazz Chim. Ital. 1969, 99, 308–315. [Google Scholar]
- Quang, D.N.; Hashimoto, T.; Asakawa, Y. Inedible mushrooms: A good source of biologically active substances. Chem. Rec. 2006, 6, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Mahiou, V.; Roblot, F.; Hocquemiller, R.; Cavé, A. Piperogalin, a new prenylated diphenol from Peperomia galioides. J. Nat. Prod. 1995, 58, 324–328. [Google Scholar] [CrossRef]
- Ali, H.M.; Abo-Shady, A.; Sharaf Eldeen, H.A.; Soror, H.A.; Shousha, W.G.; Abdel-Barry, O.A.; Saleh, A.M. Structural features, kinetics and SAR study of radical scavenging and antioxidant activities of phenolic and anilinic compounds. Chem. Cent. J. 2013, 7, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baiocchi, T.; Anesko, K.; Mercado, N.; Park, H.; Kin, K.; Strickhouser-Monzon, B.; Robles, P.; Bowman, C.; Wang, H.; Sternberg, P.W.; et al. Signaling by AWC olfactory neurons is necessary for Caenorhabditis elegans’ response to prenol, an odor associated with nematode-infected insects. Genetics 2020, 216, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, A.M.; D’Souza, P.; Agarwal, A.; Bokkolla, R.M.; Balasubramaniam, M. Geraniol, the putative anthelmintic principle of Cymbopogon martinii. Phytother. Res. 2003, 17, 957. [Google Scholar] [CrossRef]
- Otify, A.M.; Serag, A.; Porzel, A.; Wessjohann, L.A.; Farag, M.A. NMR Metabolome-based classification of Cymbopogon species: A prospect for phyto-equivalency of its different accessions using chemometric tools. Food Anal. Methods 2022, in press. [CrossRef]
- Nirmal, S.A.; Girme, A.S.; Bhalke, R.D. Major constituents and anthelmintic activity of volatile oils from leaves and flowers of Cymbopogon martini Roxb. Nat. Prod. Res. 2007, 21, 1217–1220. [Google Scholar] [CrossRef]
- Jaradat, N.; Adwan, L.; K’aibni, S.; Shraim, N.; Zaid, A.N. Chemical composition, anthelmintic, antibacterial and antioxidant effects of Thymus bovei essential oil. BMC Complement Altern Med. 2016, 16, 418. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Rahman, F.H.; Alaniz, N.M.; Saleh, M.A. Nematicidal activity of terpenoids. J. Environ. Sci. Health B 2013, 48, 16–22. [Google Scholar] [CrossRef]
- Navarro-Moll, M.C.; Romero, M.C.; Montilla, M.P.; Valero, A. In vitro and in vivo activity of three sesquiterpenes against L3 larvae of Anisakis type I. Exp. Parasitol. 2011, 127, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Menna-Barreto, R.F.; Laranja, G.A.; Silva, M.C.; Coelho, M.G.; Paes, M.C.; Oliveira, M.M.; de Castro, S.L. Anti-Trypanosoma cruzi activity of Pterodon pubescens seed oil: Geranylgeraniol as the major bioactive component. Parasitol. Res. 2008, 103, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mirza, Z.; Soto, E.R.; Hu, Y.; Nguyen, T.-T.; Koch, D.; Aroian, R.V.; Ostroff, G.R. Anthelmintic activity of yeast particle-encapsulated terpenes. Molecules 2020, 25, 2958. [Google Scholar] [CrossRef] [PubMed]
- Michiels, J.; Missotten, J.; Dierick, N.; Fremaut, D.; Maene, P.; De Smet, S. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets. J. Sci. Food Agric. 2008, 88, 2371–2381. [Google Scholar] [CrossRef]
- Gobert, G.N.; Tran, M.H.; Moertel, L.; Mulvenna, J.; Jones, M.K.; McManus, D.P.; Loukas, A. Transcriptional changes in Schistosoma mansoni during early schistosomula development and in the presence of erythrocytes. PLoS Negl. Trop. Dis. 2010, 4, e600. [Google Scholar] [CrossRef] [Green Version]
- Sirak, B.; Asres, K.; Hailu, A.; Dube, M.; Arnold, N.; Häberli, C.; Keiser, J.; Imming, P. In Vitro Antileishmanial and antischistosomal activities of anemonin isolated from the fresh leaves of Ranunculus multifidus Forsk. Molecules 2021, 26, 7473. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; El Allam, A.; Zeouk, I.; Taha, D.; Zengin, G.; Goh, B.H.; Catauro, M.; Montesano, D.; El Omari, N. Pharmacological effects of grifolin: Focusing on anticancer mechanisms. Molecules 2022, 27, 284. [Google Scholar] [CrossRef]
- Ye, M.; Liu, J.K.; Lu, Z.X.; Zhao, Y.; Liu, S.F.; Li, L.L.; Tan, M.; Weng, X.X.; Li, W.; Cao, Y. Grifolin, a potential antitumor natural product from the mushroom Albatrellus confluens, inhibits tumor cell growth by inducing apoptosis in vitro. FEBS Lett. 2005, 579, 3437–3443. [Google Scholar] [CrossRef] [Green Version]
- Che, X.; Yan, H.; Sun, H.; Dongol, S.; Wang, Y.; Lv, Q.; Jiang, J. Grifolin induces autophagic cell death by inhibiting the Akt/mTOR/S6K pathway in human ovarian cancer cells. Oncol. Rep. 2016, 36, 1041–1047. [Google Scholar] [CrossRef] [Green Version]
- Stiernagle, T. Maintenance of C. elegans (11 February 2006). In WormBook; The C. elegans Research Community, Ed. Available online: http://www.wormbook.org (accessed on 14 December 2021).
- Thomsen, H.; Reider, K.; Franke, K.; Wessjohann, L.A.; Keiser, J.; Dagne, E.; Arnold, N. Characterization of constituents and anthelmintic properties of Hagenia abyssinica. Sci. Pharm. 2012, 80, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, F.C.; Pasche, V.; Panic, G.; Endriss, Y.; Keiser, J. Life cycle maintenance and drug-sensitivity assays for early drug discovery in Schistosoma mansoni. Nat. Protoc. 2019, 14, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Haeberli, C. Evaluation of commercially available anthelmintics in laboratory models of human intestinal Nematode infections. ACS Infect. Dis. 2021, 7, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Nasr, F.A.; Noman, O.M.; Alyhya, N.A.; Ali, I.; Saoud, M.; Rennert, R.; Dube, M.; Hussain, W.; Green, I.R.; et al. Cichorins D–F: Three New Compounds from Cichorium intybus and Their Biological Effects. Molecules 2020, 25, 4160. [Google Scholar] [CrossRef] [PubMed]
- Grabovyi, G.A.; Mohr, J.T. Total synthesis of grifolin, grifolic acid, LL-Z1272α, LL-Z1272β, and ilicicolinic acid A. Org. Lett. 2016, 18, 5010–5013. [Google Scholar] [CrossRef]
- Zhang, F.L.; Casey, P.J. Protein prenylation: Molecular mechanisms and functional consequences. Ann. Rev. Biochem. 1996, 65, 241–269. [Google Scholar] [CrossRef]





| Natural and Synthetic Compounds | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Anthelmintic activity against C. elegans | 32.0 ± 4.8 | 100 ± 0 * | 0.7 ± 0.9 | 85.6 ± 5.1 * | 95.3 ± 2.8 * | 1.6 ± 2.3 |
| Corresponding alcohols | 9 | 9 | - | 7 | 8 | 10 |
| Anthelmintic activity against C. elegans | 32.1 ± 3.0 | 32.1 ± 3.0 | - | 17.8 ± 1.7 | 99.5 ± 0.7 * | 0 |
| Activity % * | ||||||
|---|---|---|---|---|---|---|
| Organism | 1 | 2 | 4 | 5 | 6 | Reference |
| NTS ** (10 µM) | 36.0 ± 4 | 26.0 ± 2 | 93.3 ± 0 *** | 55.0 ± 5.0 *** | 75.0 ± 5.0 *** | Auranofin 100 |
| NTS ** (1 µM) | nt | nt | 38.9 ± 1.7 | nt | 55.0 ± 1.7 *** | nt |
| S. mansoni (10 µM) | 27.3 ± 2 | 35.1 ± 2 | 29.2 ± 4.2 | 0 | 16.7 ± 0 | Praziquantel 100 |
| N. americanus (10 µM) | 16.5 ± 12.5 | 13.4 ± 8.4 | 12.1 ± 8.9 | 29.6 ± 2.4 | 28.2 ± 7.4 | Levamisole 100 |
| S. ratti (10 µM) | 18.6 ± 7.8 | 18 ± 2 | 1.9 ± 13.1 | 2.9 ± 5.6 | 0 | Levamisole 100 |
| H. polygyrus (10 µM) | 16.4 ± 0.4 | 16 ± 4.3 | 23.4 ± 7.3 | 38.6 ± 4.8 | 36.6 ± 1.9 | Levamisole 100 |
| A. ceylanicum (10 µM) | 2.0 ± 2.5 | 7.5 ± 0 | 24.4 ± 7.8 | 19 ± 14.1 | 17.5 ± 3.9 | Abamectin 100 |
| Activity % * | |||||
|---|---|---|---|---|---|
| Organism | 7 | 8 | 9 | 10 | Reference |
| NTS ** (10 µM) | 33.3 ± 0 | 31.3 ± 2 | 25 ± 0 | 31.3 ± 2 | Auranofin 100 |
| S. mansoni (10 µM) | nt | nt | nt | nt | nt |
| N. americanus (10 µM) | 11.6 ± 2 | 11.3 ± 10 | 21.3 ± 1 | 36.3 ± 1 | Levamisole 100 |
| S. ratti (10 µM) | 13 ± 5 | 39.6 ± 0.4 | 0.2 ± 9 | 0 | Levamisole 100 |
| H. polygyrus (10 µM) | 0 | 1.8 ± 5 | 34.6 ± 5.5 | 0 | Levamisole 100 |
| A. ceylanicum (10µM) | 18.8 ± 2.9 | 13.7 ± 1.7 | 10 ± 6.4 | 23.6 ± 5.9 | Abamectin 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dube, M.; Llanes, D.; Saoud, M.; Rennert, R.; Imming, P.; Häberli, C.; Keiser, J.; Arnold, N. Albatrellus confluens (Alb. & Schwein.) Kotl. & Pouz.: Natural Fungal Compounds and Synthetic Derivatives with In Vitro Anthelmintic Activities and Antiproliferative Effects against Two Human Cancer Cell Lines. Molecules 2022, 27, 2950. https://doi.org/10.3390/molecules27092950
Dube M, Llanes D, Saoud M, Rennert R, Imming P, Häberli C, Keiser J, Arnold N. Albatrellus confluens (Alb. & Schwein.) Kotl. & Pouz.: Natural Fungal Compounds and Synthetic Derivatives with In Vitro Anthelmintic Activities and Antiproliferative Effects against Two Human Cancer Cell Lines. Molecules. 2022; 27(9):2950. https://doi.org/10.3390/molecules27092950
Chicago/Turabian StyleDube, Mthandazo, Dayma Llanes, Mohamad Saoud, Robert Rennert, Peter Imming, Cécile Häberli, Jennifer Keiser, and Norbert Arnold. 2022. "Albatrellus confluens (Alb. & Schwein.) Kotl. & Pouz.: Natural Fungal Compounds and Synthetic Derivatives with In Vitro Anthelmintic Activities and Antiproliferative Effects against Two Human Cancer Cell Lines" Molecules 27, no. 9: 2950. https://doi.org/10.3390/molecules27092950
APA StyleDube, M., Llanes, D., Saoud, M., Rennert, R., Imming, P., Häberli, C., Keiser, J., & Arnold, N. (2022). Albatrellus confluens (Alb. & Schwein.) Kotl. & Pouz.: Natural Fungal Compounds and Synthetic Derivatives with In Vitro Anthelmintic Activities and Antiproliferative Effects against Two Human Cancer Cell Lines. Molecules, 27(9), 2950. https://doi.org/10.3390/molecules27092950