Untargeted Metabolomic Approach to Determine the Regulatory Pathways on Salicylic Acid-Mediated Stress Response in Aphanamixis polystachya Seedlings
Abstract
1. Introduction
2. Results
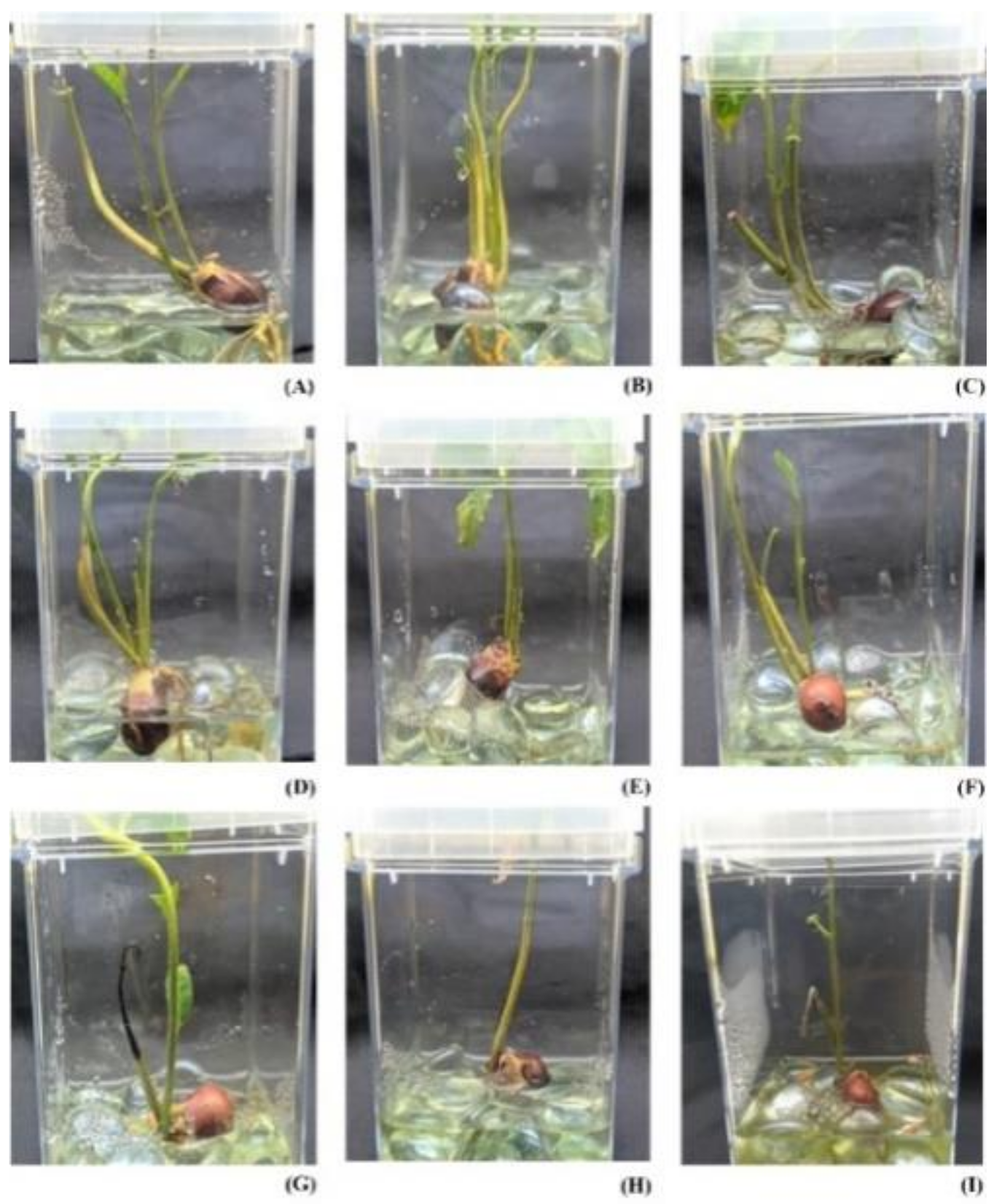
2.1. Effect of Stress on Seedlings
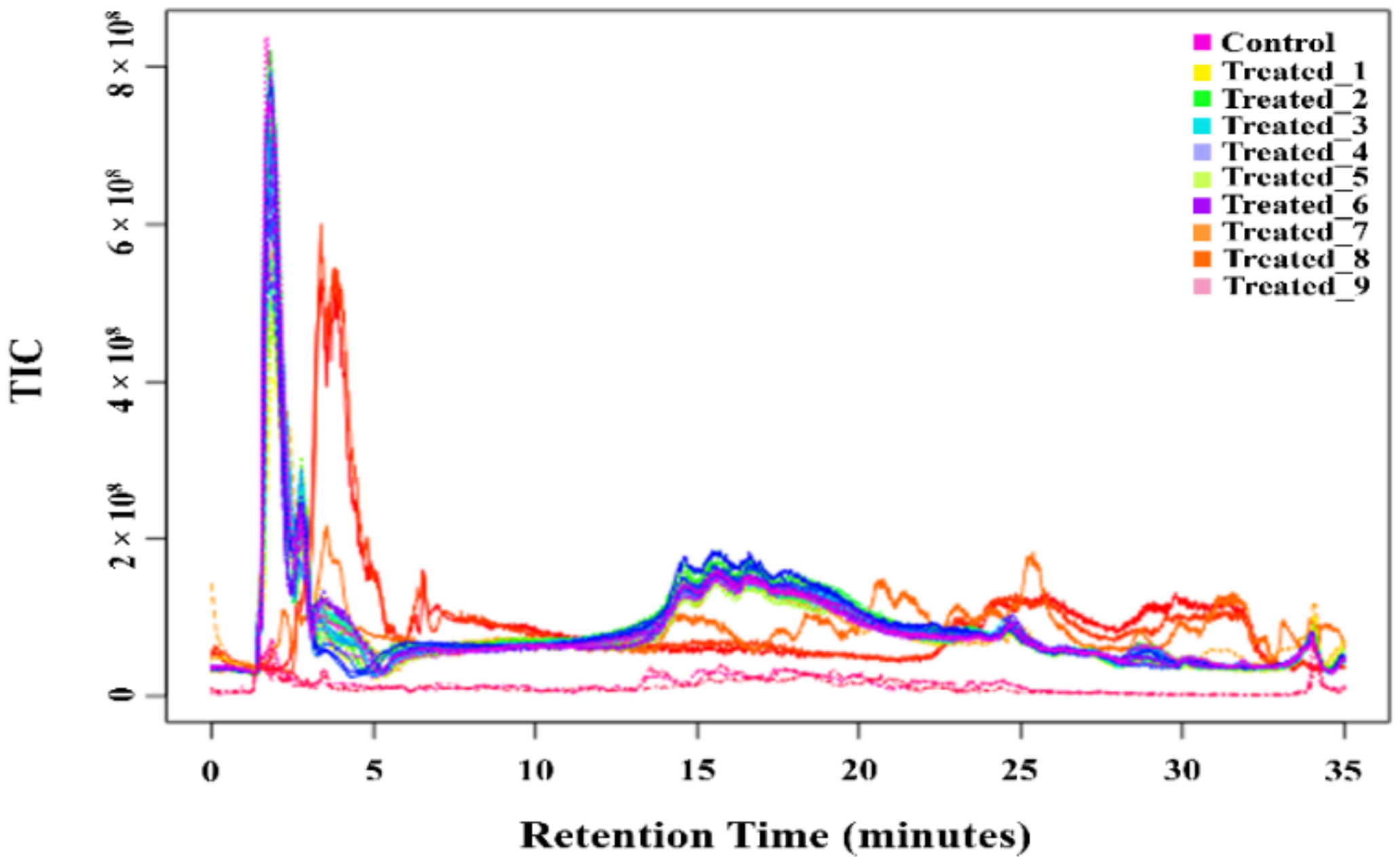
2.2. Determination of Metabolite Fraction in the Stressed Condition
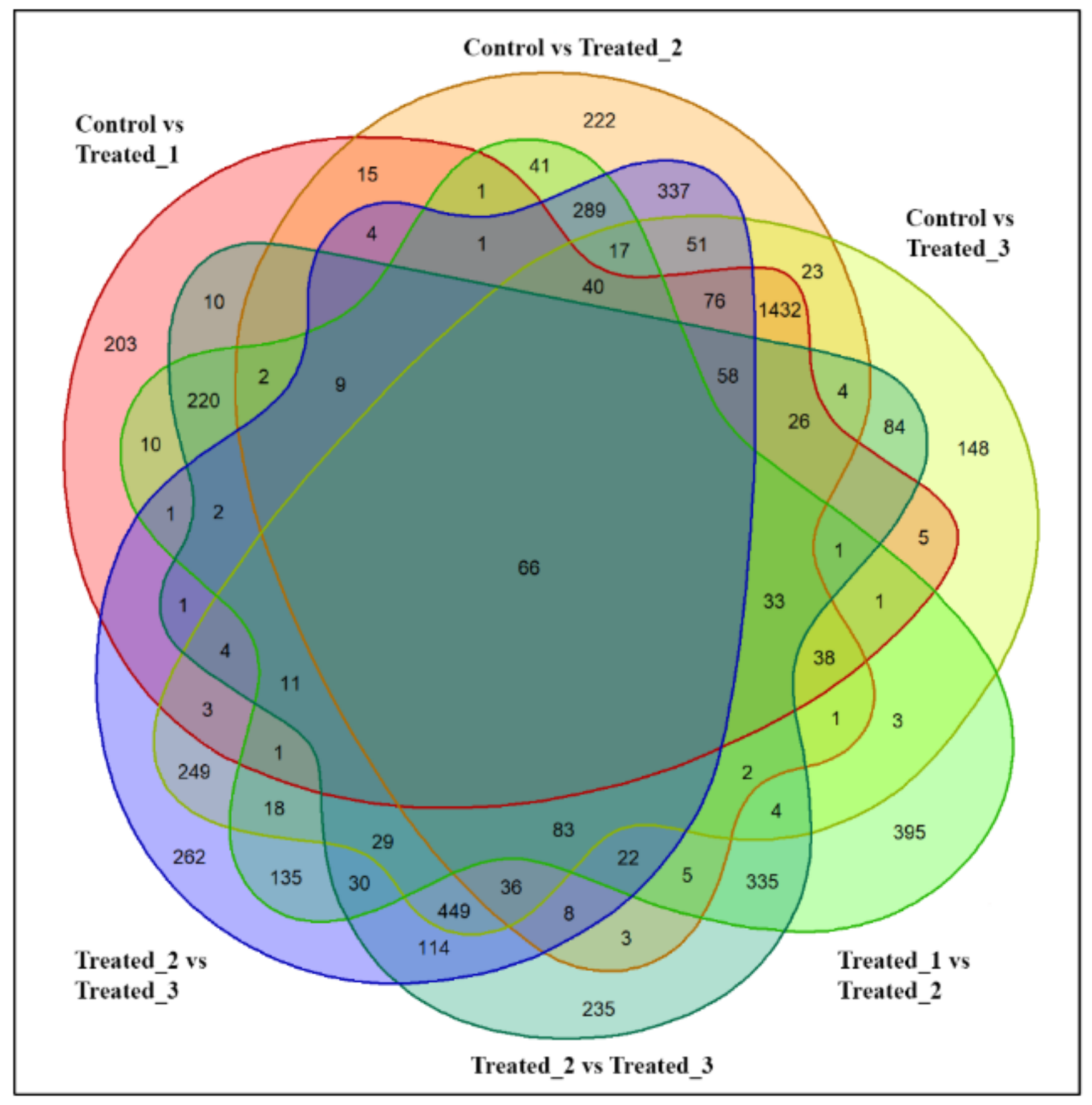
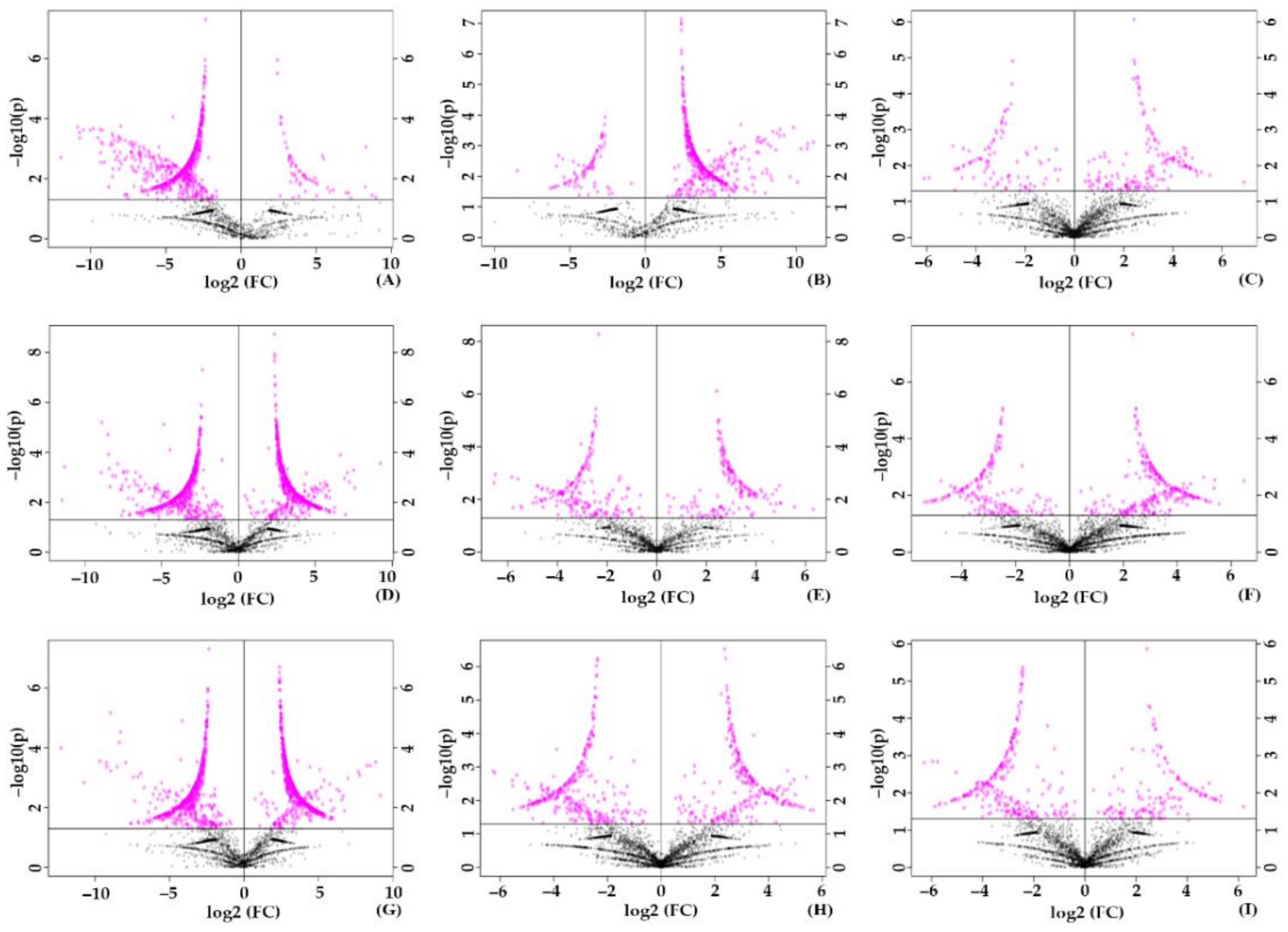
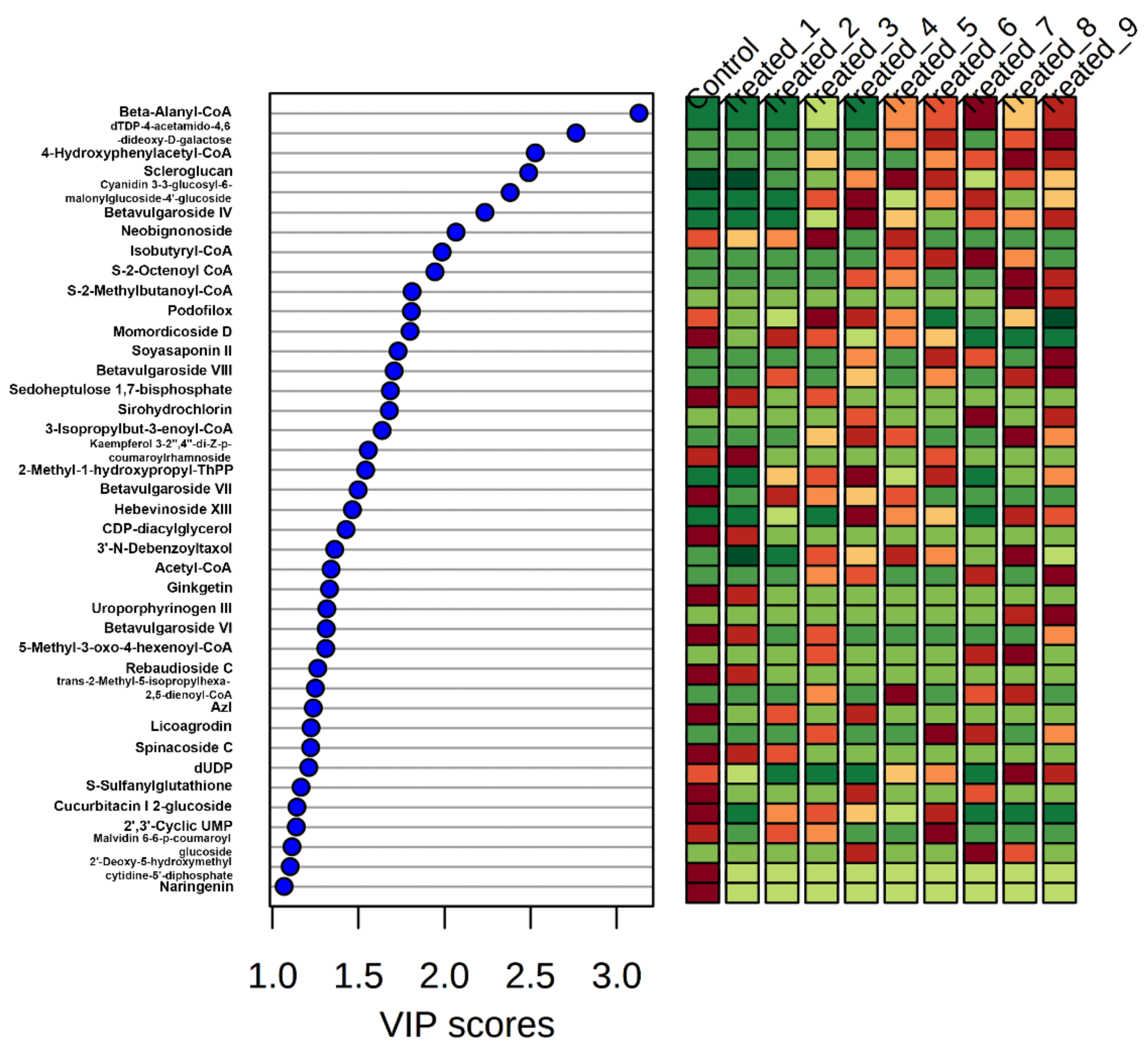
2.3. Differential Production of Metabolites during the Stressed Condition and Identification of Significant Metabolites
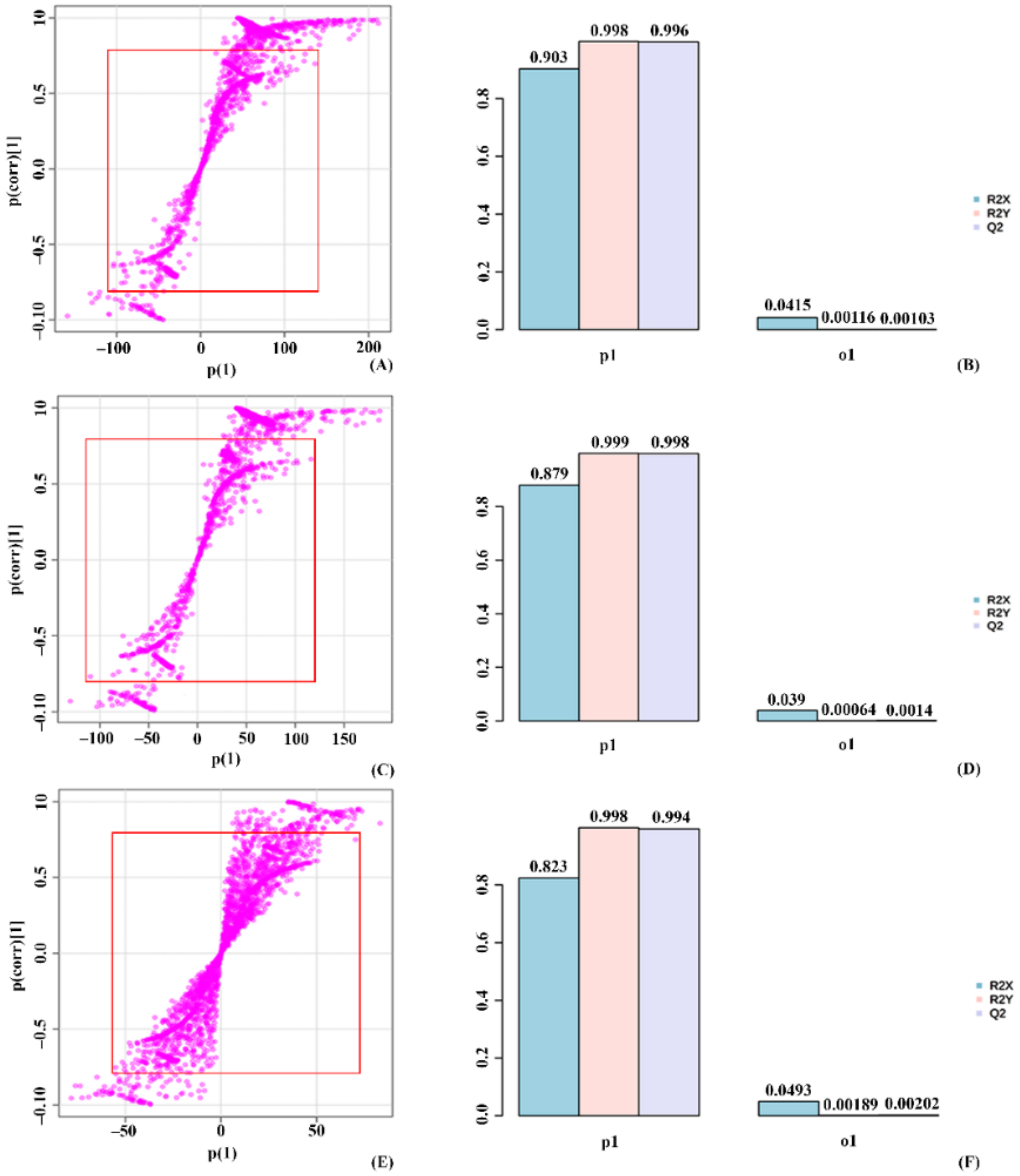
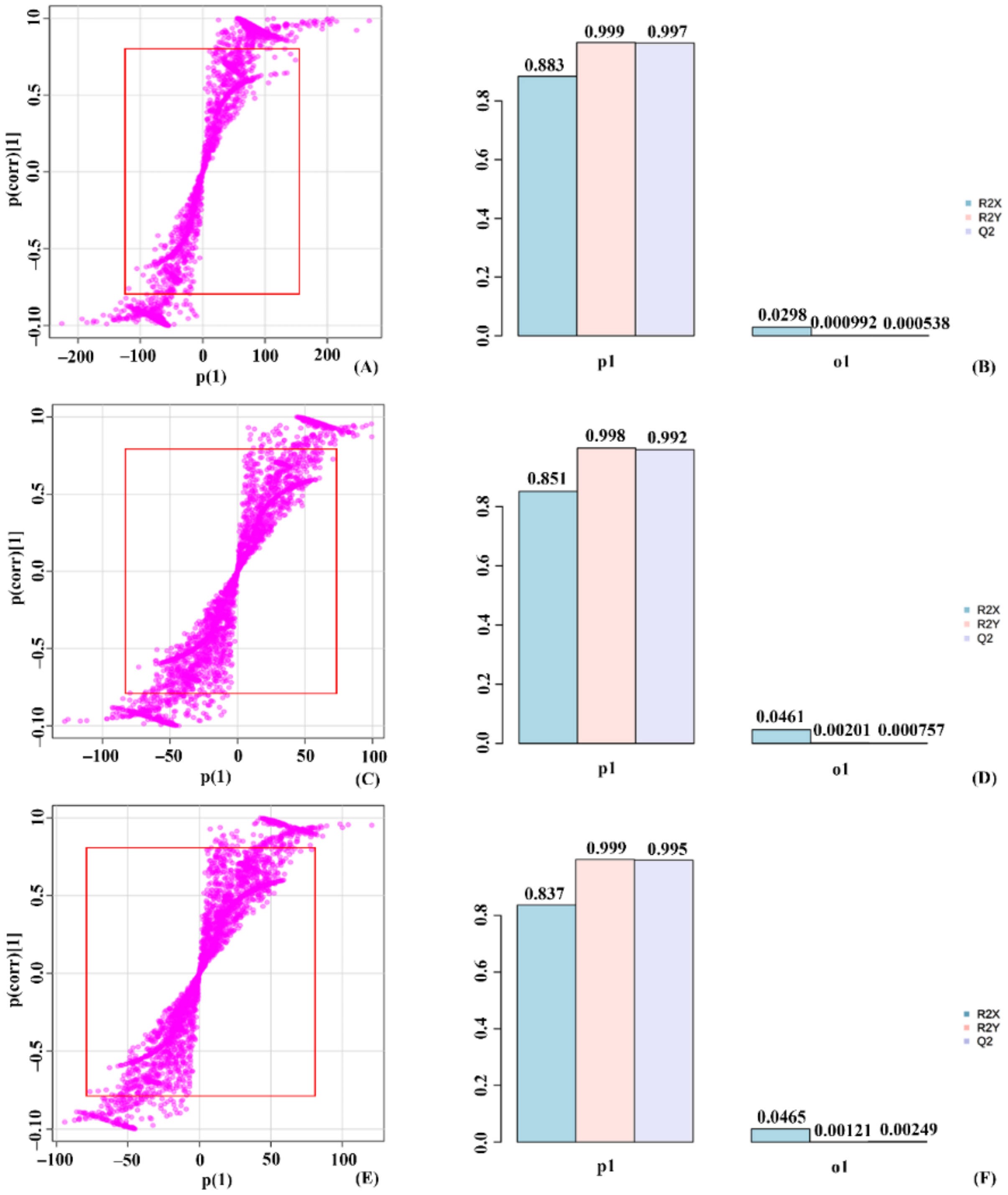
2.4. Orthogonal Partial Least Square Discriminant Analysis (OPLS-DA) on the Validation of Significant Metabolites in Stressed Groups
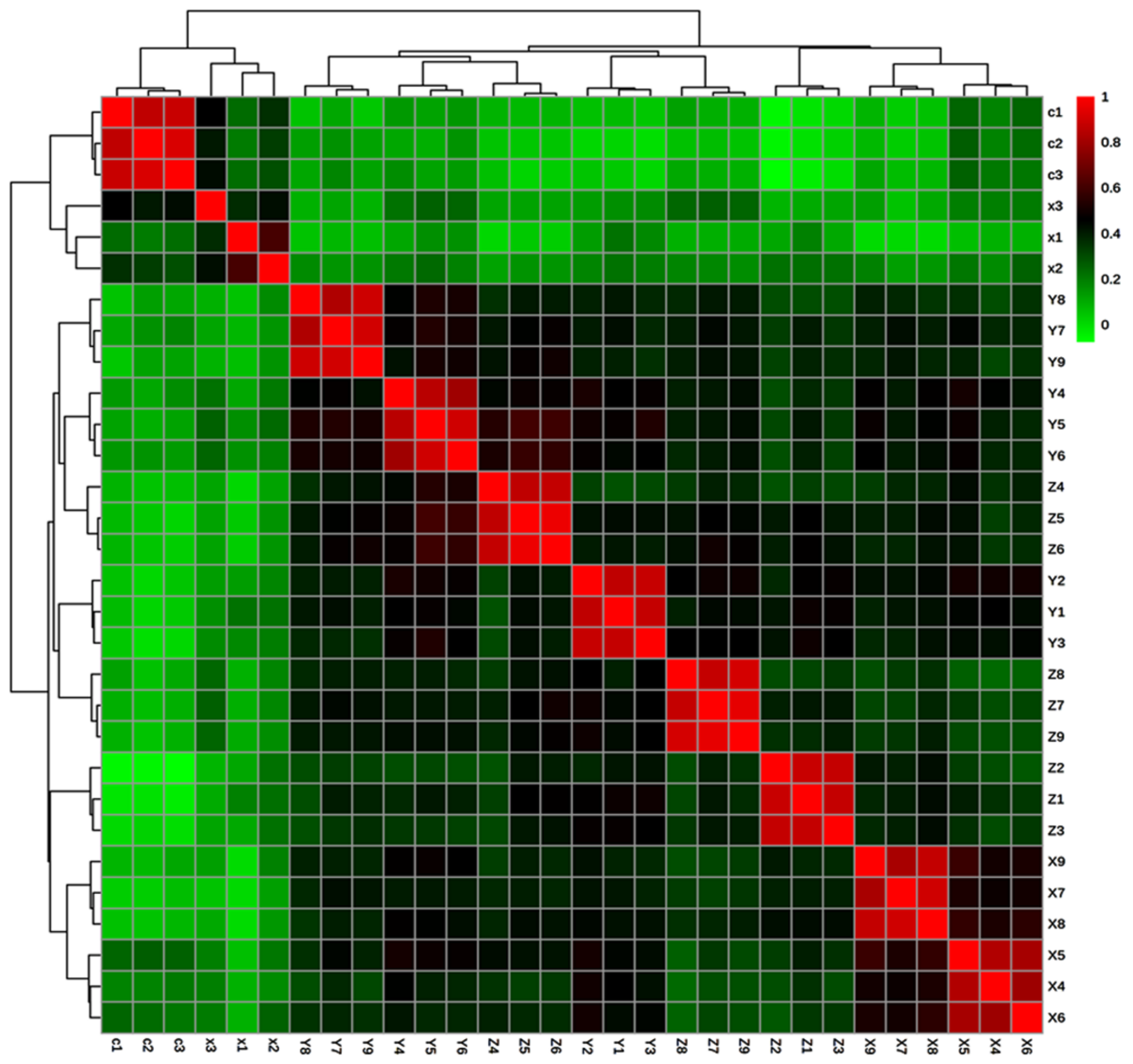
2.5. Correlation of Significantly Active Metabolites in the Stressed Condition
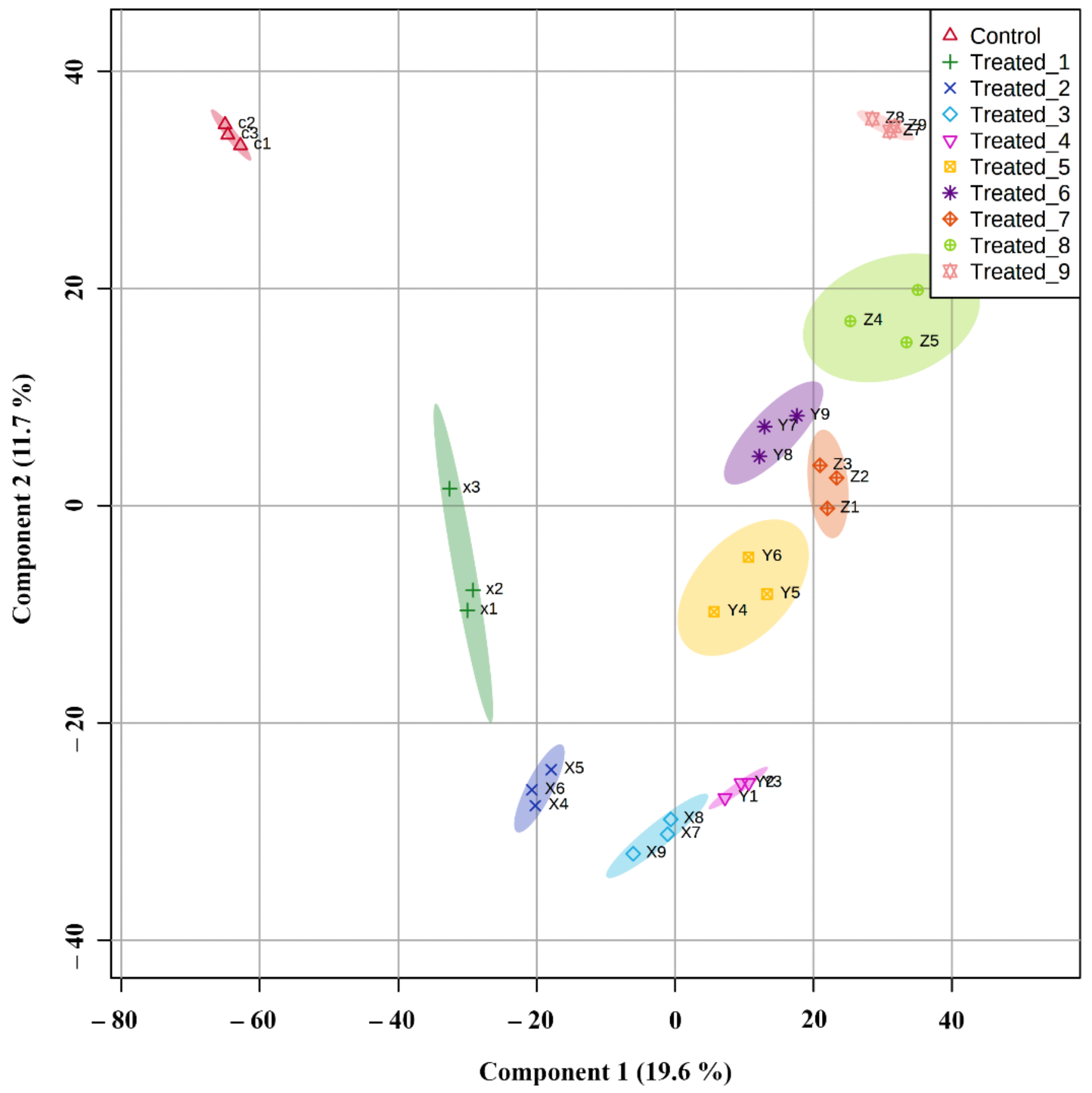
2.6. Principle Component Analysis and Partial Least Square Discriminant Analysis of Significant Metabolites in the Classification of Experimental Groups
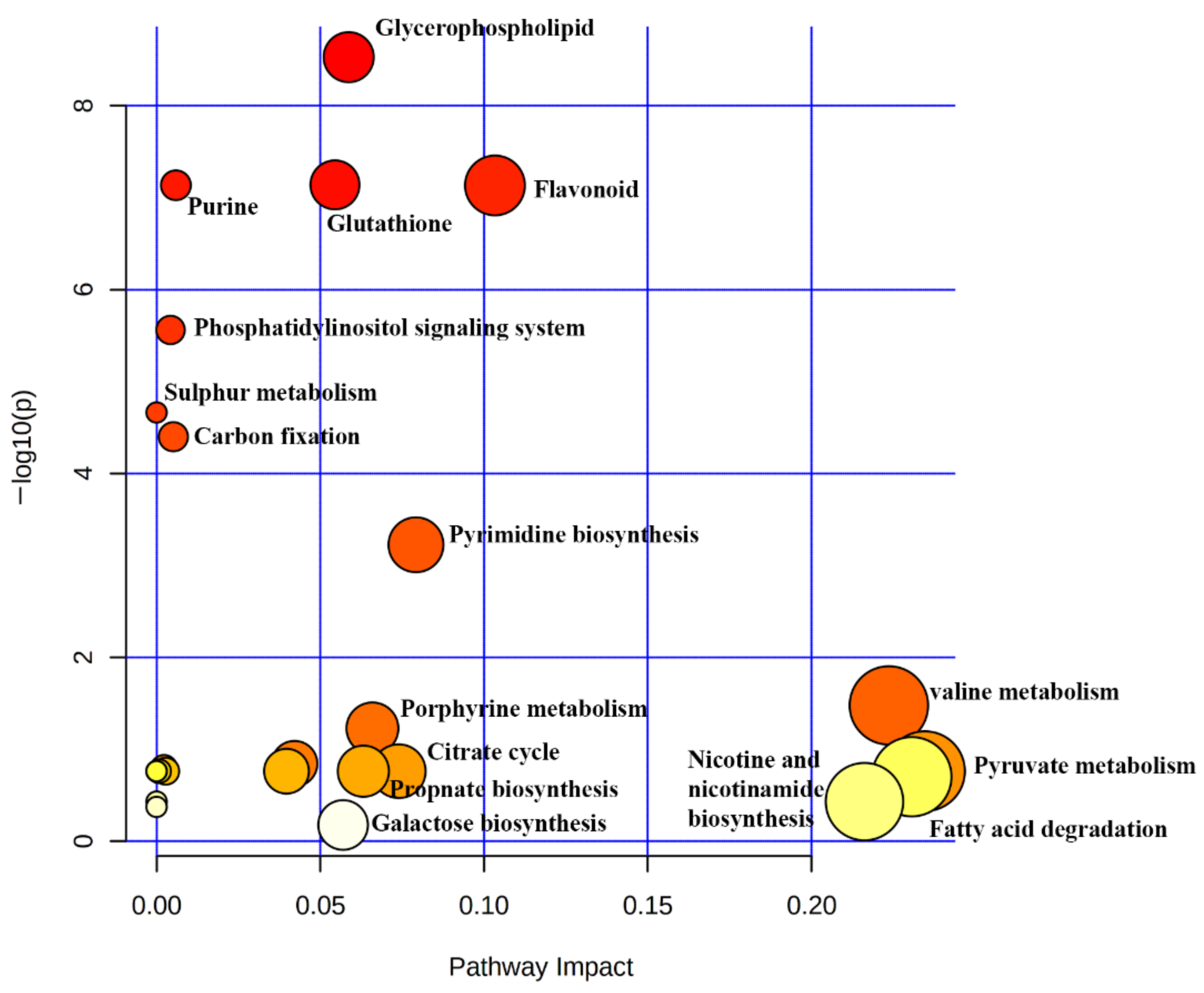
2.7. Determination of Metabolic Pathways Activated during Stressed Conditions
3. Discussion
4. Materials and Methods
4.1. Stress Induction in Seedlings
4.2. Sample Harvesting and Extraction
4.3. Parameters Used for LC-MS Analysis
4.4. Processing of Raw Data using XCMS
4.5. Statistical and Pathway Enrichment Studies using Metaboanalyst
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bino, R.J.; Hall, R.D.; Fiehn, O.; Kopka, J.; Saito, K.; Draper, J.; Nikolau, B.J.; Mendes, P.; Roessner-Tunali, U.; Beale, M.H.; et al. Potential of metabolomics as a functional genomics tool. Trends Plant Sci. 2004, 9, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, K.J. Challenges and prospects of plant proteomics. Plant Physiol. 2001, 126, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Parida, S.K.; Mahto, A.; Das, S.; Mathew, I.E.; Malik, N.; Tyagi, A.K. Expanding frontiers in plant transcriptomics in aid of functional genomics and molecular breeding. Biotechnol. J. 2014, 9, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Mendes, P.; Dixon, R.A. Plant metabolomics: Large-scale phytochemistry in the functional genomics era. Phytochemistry 2003, 62, 817–836. [Google Scholar] [CrossRef]
- Fiehn, O.; Kopka, J.; Dörmann, P.; Altmann, T.; Trethewey, R.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Weckwerth, W. Metabolomics in Systems Biology. Annu. Rev. Plant Biol. 2003, 54, 669–689. [Google Scholar] [CrossRef]
- Wiklund, S.; Johansson, E.; Sjöström, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef]
- Combès, A.; Ndoye, I.; Bance, C.; Bruzaud, J.; Djediat, C.; Dupont, J.; Nay, B.; Prado, S. Chemical Communication between the Endophytic Fungus Paraconiothyrium Variabile and the Phytopathogen Fusarium oxysporum. PLoS ONE 2012, 7, e47313. [Google Scholar] [CrossRef]
- Bowne, J.B.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef]
- Lavergne, F.D.; Broeckling, C.D.; Cockrell, D.M.; Haley, S.D.; Peairs, F.B.; Jahn, C.E.; Heuberger, A.L. GC-MS Metabolomics to Evaluate the Composition of Plant Cuticular Waxes for Four Triticum aestivum Cultivars. Int. J. Mol. Sci. 2018, 19, 249. [Google Scholar] [CrossRef] [PubMed]
- Horváth, E.; Szalai, G.; Janda, T. Induction of Abiotic Stress Tolerance by Salicylic acid Signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Caarls, L.; Pieterse, C.M.J.; Van Wees, S.C.M. How Salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Senaratna, T.; Sivasithamparam, K. Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): Associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regul. 2006, 49, 77–83. [Google Scholar]
- Kováčik, J.; Grúz, J.; Bačkor, M.; Strnad, M.; Repčák, M. Salicylic acid -induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep. 2009, 28, 135–143. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Du, X.; Tang, H.; Shen, C.; Wu, J. Salicylic acid Alleviates the Adverse Effects of Salt Stress in Torreya grandis cv. Merrillii Seedlings by Activating Photosynthesis and Enhancing Antioxidant Systems. PLoS ONE 2014, 9, e109492. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Mokochinski, J.B.; Mazzafera, P.; Sawaya, A.C.H.F.; Mumm, R.; de Vos, R.C.H.; Hall, R.D. Metabolic responses of Eucalyptus species to different temperature regimes. J. Integr. Plant Biol. 2018, 60, 397–411. [Google Scholar] [CrossRef]
- Wang, G.W.; Jin, H.Z.; Zhang, W.D. Constituents from Aphanamixis species and their biological activities. Phytochem. Rev. 2013, 12, 915–942. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, Y.; Li, Y.; Jin, L.; Wei, N.; Wu, H.; Ma, G.; Zheng, Q.; Tian, Y.; Yang, J.; et al. Aphanamixins A–F, Acyclic Diterpenoids from the Stem Bark of Aphanamixis polystachya. Chem. Pharm. Bull. 2014, 62, 494–498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fang, F.-H.; Huang, W.-J.; Zhou, S.-Y.; Han, Z.-Z.; Li, M.-Y.; Liu, L.-F.; Wu, X.-Z.; Yao, X.-J.; Li, Y.; Yuan, C.-S. Aphapolins A and B: Two Nemoralisin Diterpenoids Isolated from Aphanamixis polystachya (Wall.) R. Parker. Eur. J. Org. Chem. 2017, 2017, 4429–4433. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.S.; Gu, Y.C.; Kong, L.Y. Aphagranols D-H: Five new limonoids from the fruits of Aphanamixis grandifolia. Helv. Chim. Acta 2014, 97, 1354–1364. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.S.; Wei, D.D.; Gu, Y.C.; Wang, X.B.; Kong, L.Y. Bioactive terpenoids from the fruits of Aphanamixis grandifolia. J. Nat. Prod. 2013, 76, 1191–1195. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, Y.M.; Luo, J.G.; Luo, J.; Kong, L.Y. Anti-inflammatory diterpene dimers from the root barks of Aphanamixis grandifolia. Org. Biomol. Chem. 2015, 13, 7452–7458. [Google Scholar] [CrossRef]
- Arguello, F.; Alexander, M.; Sterry, J.A.; Tudor, G.; Smith, E.M.; Kalavar, N.T.; Greene, J.F.; Koss, W.; Morgan, C.D.; Stinson, S.F.; et al. Flavopiridol induces apoptosis of normal lymphoid cells, causes immunosuppression, and has potent antitumor activity in vivo against human leukemia and lymphoma xenografts. Blood 1998, 91, 2482–2490. [Google Scholar]
- Rabi, T.; Ramachandran, C.; Fonseca, H.B.; Nair, R.P.K.; Alamo, A.; Melnick, S.J.; Escalon, E. Novel drug amooranin induces apoptosis through caspase activity in human breast carcinoma cell lines. Breast Cancer Res. Treat. 2003, 80, 321–330. [Google Scholar] [CrossRef]
- Rabi, T.; Catapano, C.V. Aphanin, a triterpenoid from Amoora rohituka inhibits K-Ras mutant activity and STAT3 in pancreatic carcinoma cells. Tumor Biol. 2016, 37, 12455–12464. [Google Scholar] [CrossRef]
- Alvarez, S.I.P.; Spollansky, T.C.; Giulietti, A.M. The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enzym. Microb. Technol. 2000, 26, 252–258. [Google Scholar] [CrossRef]
- De Vos, M.; Van Oosten, R.; Van Poecke, R.M.P.; Van Pelt, J.A.; Pozo, M.J.; Mueller, M.J.; Buchala, A.J.; Métraux, J.; Van Loon, L.C.; Dicke, M.; et al. Signal Signature and Transcriptome Changes of Arabidopsis During Pathogen and Insect Attack. Am. Phytopathol. Soc. 2005, 18, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wan, G.; Liang, Z. Accumulation of Salicylic acid -induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J. Biotechnol. 2010, 148, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Mccue, P.; Zheng, Z.; Pinkham, J.L.; Shetty, K. A model for enhanced pea seedling vigour following low pH and Salicylic acid treatments. Process Biochem. 2000, 35, 603–613. [Google Scholar] [CrossRef]
- Singh, P.K.; Shahi, S.K.; Singh, A.P. Effects of Salt Stress on Physico-Chemical Changes in Maize (Zea mays L.) Plants in Response To Salicylic acid. Indian J. Plant Sci. 2015, 4, 69–77. [Google Scholar]
- Latif, F.; Ullah, F.; Mehmood, S.; Khattak, A.; Khan, A.U.; Khan, S.; Husain, I. Effects of Salicylic acid on growth and accumulation of phenolics in Zea mays L. under drought stress. Acta Agric. Scand. Sect. B Soil Plant Sci. 2016, 66, 325–332. [Google Scholar]
- Mirzajani, Z.; Hadavi, E.; Kashi, A. Changes in the essential oil content and selected traits of sweet basil (Ocimum basilicum L.) as induced by foliar sprays of citric acid and Salicylic acid. Ind. Crop. Prod. 2015, 76, 269–274. [Google Scholar] [CrossRef]
- Rodrigues, K.C.S.; Fett-neto, A.G. Oleoresin yield of Pinus elliottii in a subtropical climate: Seasonal variation and effect of auxin and Salicylic acid -based stimulant paste. Ind. Crop. Prod. 2009, 30, 316–320. [Google Scholar] [CrossRef]
- Elyasi, R.; Majdi, M.; Bahramnejad, B.; Mirzaghaderi, G. Spatial modulation and abiotic elicitors responses of the biosynthesis related genes of mono / triterpenes in black cumin (Nigella sativa). Ind. Crop. Prod. 2016, 79, 240–247. [Google Scholar] [CrossRef]
- Wada, K.C.; Yamada, M.; Shiraya, T.; Takeno, K. Salicylic acid and the flowering gene FLOWERING LOCUS T homolog are involved in poor-nutrition stress-induced flowering of Pharbitis nil. J. Plant Physiol. 2010, 167, 447–452. [Google Scholar] [CrossRef]
- Sayyari, M.; Ghavami, M.; Ghanbari, F.; Kordi, S. Assessment of Salicylic acid impacts on growth rate and some physiological parameters of lettuce plants under drought stress conditions. Int. J. Agric. Crop Sci. 2013, 5, 1951–1957. [Google Scholar]
- Westerhuis, J.A.; van Velzen, E.J.J.; Hoefsloot, H.C.J.; Smilde, A.K. Multivariate paired data analysis: Multilevel PLSDA versus OPLSDA. Metabolomics 2010, 6, 119–128. [Google Scholar] [CrossRef]
- Westerhuis, J.A.; Hoefsloot, H.C.J.; Smit, S.; Vis, D.J.; Smilde, A.K.; Velzen, E.J.J.; Duijnhoven, J.P.M.; Dorsten, F.A. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- Zambon, A.; Righi, V.; Parenti, F.; Libertini, E.; Rossi, M.C.; Mucci, A. Nucleoside 2′,3′-Cyclic Monophosphates in Aphanizomenon flos-aquae Detected through Nuclear Magnetic Resonance and Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 12780–12785. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Lawson, T.; Zakhleniuk, O.V.; Lloyd, J.C.; Raines, C.A.; Fryer, M. Increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol. 2005, 138, 1174, Erratum in Plant Physiol. 2005, 138, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, M.; Zhang, S. Sedoheptulose-1,7-bisphosphatase is involved in methyl jasmonate-and dark-induced leaf senescence in tomato plants. Int. J. Mol. Sci. 2018, 19, 3673. [Google Scholar] [CrossRef] [PubMed]
- Hilliou, F.; van der Fits, L.; Memelink, J. Chapter Thirteen Molecular regulation of monoterpenoid indole alkaloid biosynthesis. In Recent Advances in Phytochemistry; Elsevier: Amsterdam, The Netherlands, 2001; pp. 275–295. [Google Scholar]
- Sottomayor, M.; Lopes Cardoso, I.; Pereira, L.G.; Ros Barceló, A. Peroxidase and the biosynthesis of terpenoid indole alkaloids in the medicinal plant Catharanthus roseus (L.) G. Don. Phytochem. Rev. 2004, 3, 159–171. [Google Scholar] [CrossRef]
- Casique-Arroyo, G.; Martínez-Gallardo, N.; De La Vara, L.G.; Délano-Frier, J.P. Betacyanin biosynthetic genes and enzymes are differentially induced by (a)biotic stress in Amaranthus hypochondriacus. PLoS ONE 2014, 9, 19–22. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Fini, A.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Tattini, M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal. Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef]
- Cheruiyot, E.K.; Mumera, L.M.; Ng’etich, W.K.; Hassanali, A.; Wachira, F.; Wanyoko, J.K. Shoot epicatechin and epigallocatechin contents respond to water stress in tea [Camellia sinensis (L.) O. Kuntze]. Biosci. Biotechnol. Biochem. 2008, 72, 1219–1226. [Google Scholar] [CrossRef][Green Version]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Otegui, M.S.; Grotewold, E. Abiotic stresses induce different localizations of anthocyanins in Arabidopsis. Plant Signal. Behav. 2015, 10, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Li, P.; Wang, J.; Rehman, N.U.; Zhao, J. Isoflavone malonyltransferases GmiMaT1 and GmiMaT3 differently modify isoflavone glucosides in soybean (Glycine max) under various stresses. Front. Plant Sci. 2017, 8, 735. [Google Scholar] [CrossRef]
- Plengmuankhae, W.; Tantitadapitak, C. Low temperature and water dehydration increase the levels of asiaticoside and madecassoside in Centella asiatica (L.) Urban. S. Afr. J. Bot. 2015, 97, 196–203. [Google Scholar] [CrossRef]
- Nasrollahi, V.; Mirzaie-asl, A.; Piri, K.; Nazeri, S.; Mehrabi, R. The effect of drought stress on the expression of key genes involved in the biosynthesis of triterpenoid saponins in liquorice (Glycyrrhiza glabra). Phytochemistry 2014, 103, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Mudge, K.W.; Lee, J.W. Effect of water stress on ginsenoside production and growth of American ginseng. Horttechnology 2006, 16, 517–522. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, Y.J.; Jang, M.G.; Joo, S.C.; Kwon, W.S.; Kim, S.Y.; Jung, S.K.; Yang, D.C. Investigation of ginsenosides in different tissues after elicitor treatment in panax ginseng. J. Ginseng Res. 2014, 38, 270–277. [Google Scholar] [CrossRef]
- Farh, M.E.A.; Kim, Y.J.; Abbai, R.; Singh, P.; Jung, K.H.; Kim, Y.J.; Yang, D.C. Pathogenesis strategies and regulation of ginsenosides by two species of Ilyonectria in Panax ginseng: Power of speciation. J. Ginseng Res. 2020, 44, 332–340. [Google Scholar] [CrossRef]
- Kim, Y.C.; Choi, D.; Cha, A.; Lee, Y.G.; Baek, N.I.; Rimal, S.; Sang, J.; Lee, Y.; Lee, S. Critical enzymes for biosynthesis of cucurbitacin derivatives in watermelon and their biological significance. Commun. Biol. 2020, 3, 1–11. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS online: A web-based platform to process untargeted metabolomic data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Maille, G.O.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN A Metabolite Mass Spectral Database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhesh, K.V.; Ashalatha, S.N.; Mahima, K.; Baskar, V.; Thiruvengadam, M. Untargeted Metabolomic Approach to Determine the Regulatory Pathways on Salicylic Acid-Mediated Stress Response in Aphanamixis polystachya Seedlings. Molecules 2022, 27, 2966. https://doi.org/10.3390/molecules27092966
Rakhesh KV, Ashalatha SN, Mahima K, Baskar V, Thiruvengadam M. Untargeted Metabolomic Approach to Determine the Regulatory Pathways on Salicylic Acid-Mediated Stress Response in Aphanamixis polystachya Seedlings. Molecules. 2022; 27(9):2966. https://doi.org/10.3390/molecules27092966
Chicago/Turabian StyleRakhesh, Kanakarajan Vijayakumari, Sunkarankutty Nair Ashalatha, Karthikeyan Mahima, Venkidasamy Baskar, and Muthu Thiruvengadam. 2022. "Untargeted Metabolomic Approach to Determine the Regulatory Pathways on Salicylic Acid-Mediated Stress Response in Aphanamixis polystachya Seedlings" Molecules 27, no. 9: 2966. https://doi.org/10.3390/molecules27092966
APA StyleRakhesh, K. V., Ashalatha, S. N., Mahima, K., Baskar, V., & Thiruvengadam, M. (2022). Untargeted Metabolomic Approach to Determine the Regulatory Pathways on Salicylic Acid-Mediated Stress Response in Aphanamixis polystachya Seedlings. Molecules, 27(9), 2966. https://doi.org/10.3390/molecules27092966







