Nanoemulsified Formulation of Cedrela odorata Essential Oil and Its Larvicidal Effect against Spodoptera frugiperda (J.E. Smith)
Abstract
:1. Introduction
2. Results and discussion
2.1. Essential Oil Extracted from Leaves of Cedrela odorata
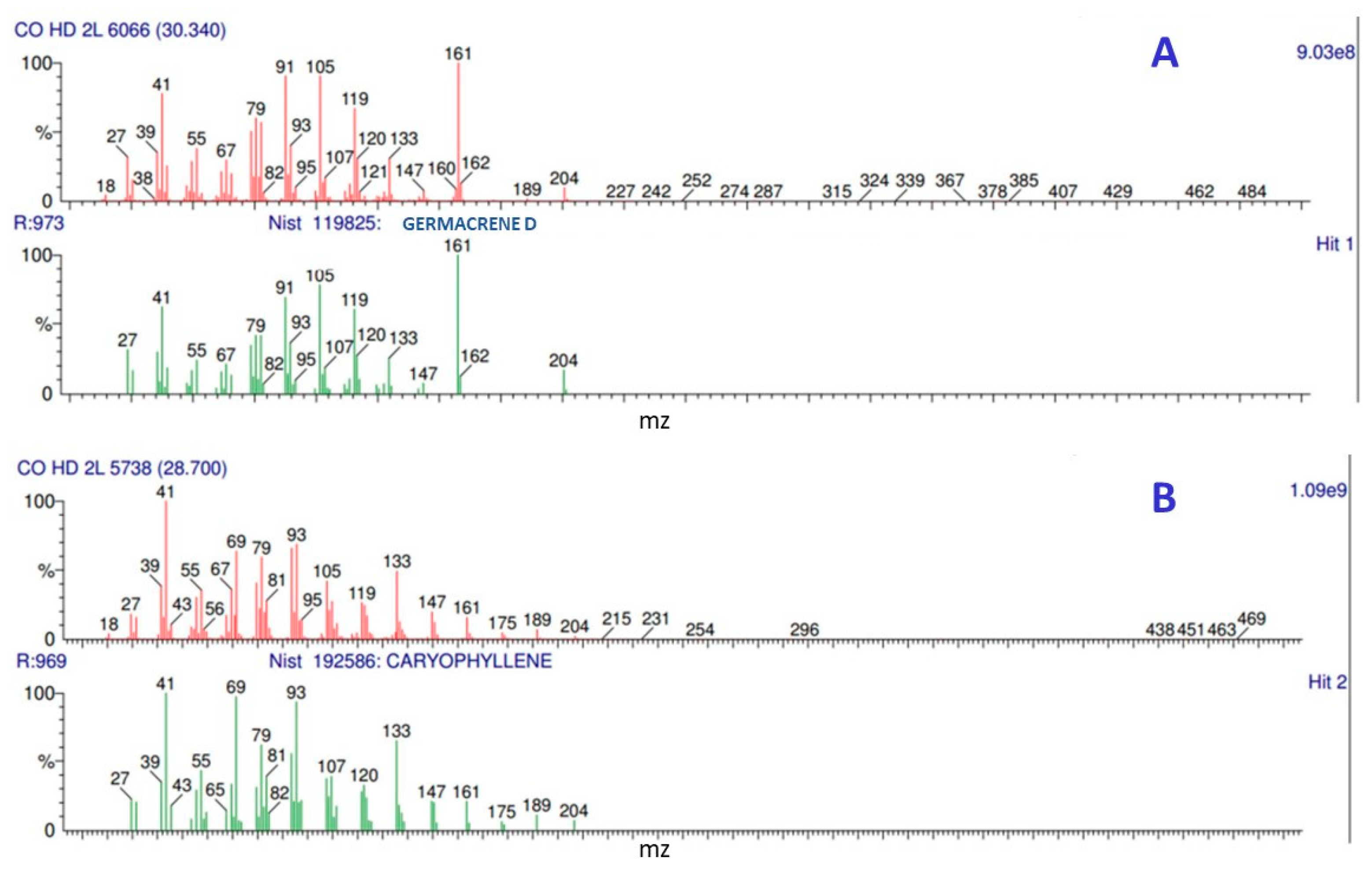
2.2. Composition of the C. odorata Essential Oil
2.3. Nanoemulsification of the Essential oil of Cedrela odorata in Water
2.3.1. Selection of the Surfactant HLB
2.3.2. Non-Ionic Surfactants Selected to Emulsify the Cedrela odorata Essential Oil
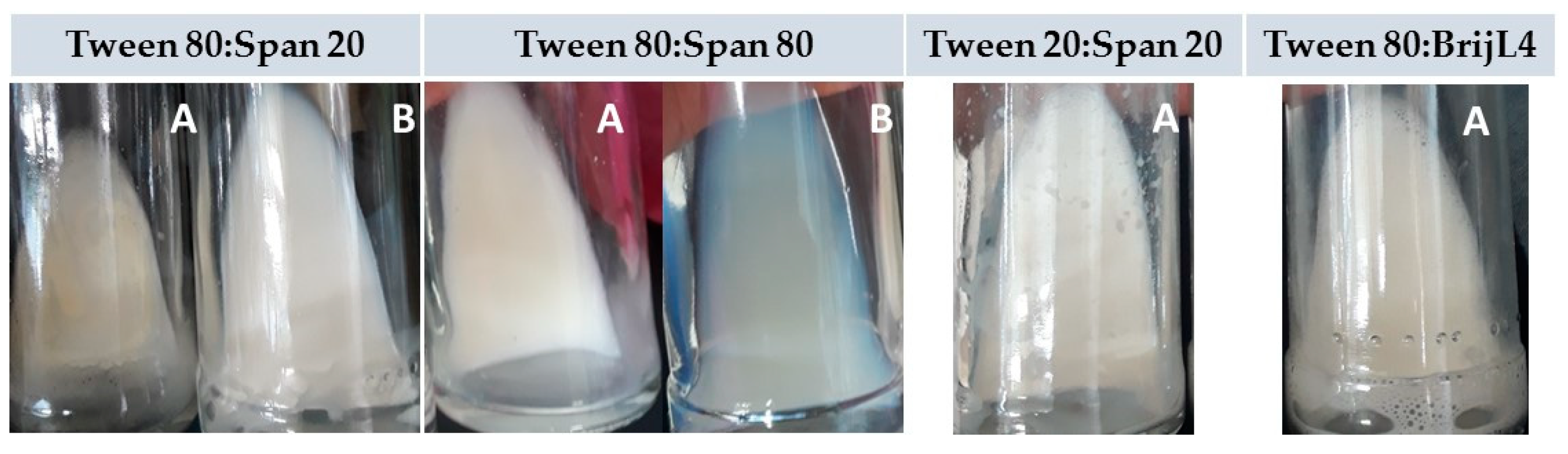
2.3.3. Adjustment of the Ratio among the Cedrela odorata Essential Oil, a Mixture of Surfactants, and Water
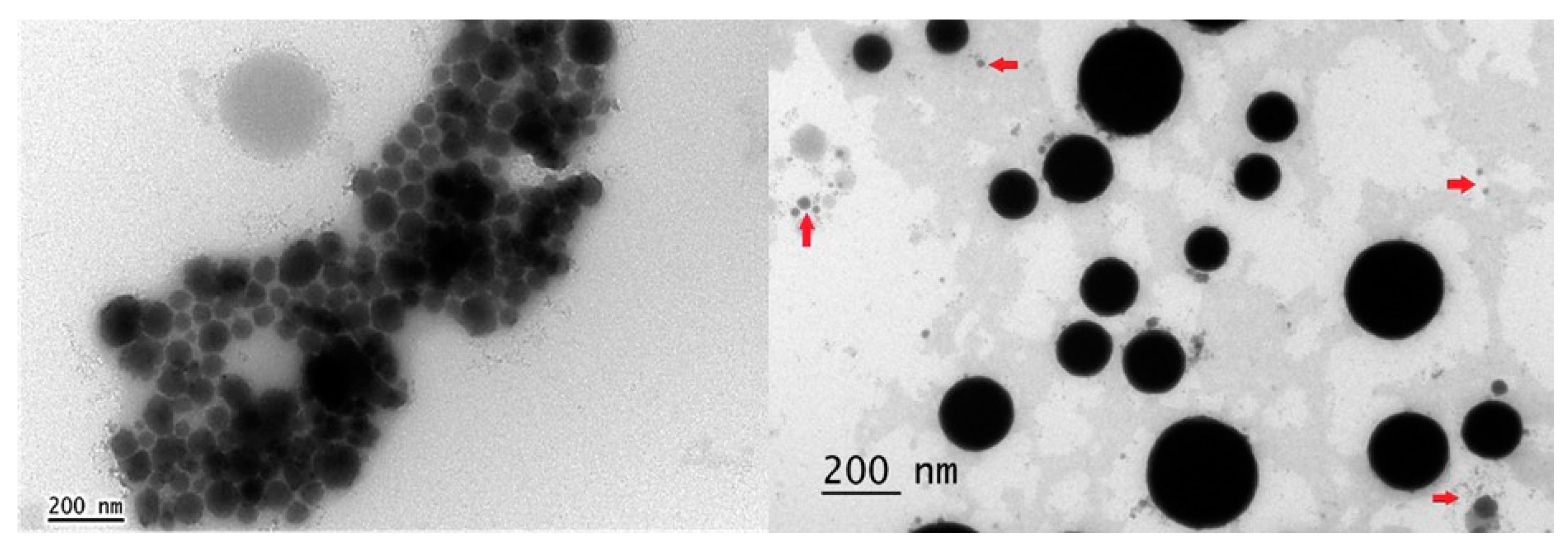
2.4. Nanoemulsion Stability of the Cedrela odorata Essential Oil at 2.5%
2.5. Larvicidal Activity of the Cedrela odorata Essential Oil
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of the Cedrela odorata Essential Oil
3.3. Nanoemulsified Formulation of the C. odorata Essential Oil
3.4. Nanoemulsion Characterization
3.5. Larvicidal Activity of the Cedrela odorata Essential Oil
3.6. Larvicidal Activity of the Cedrela odorata Essential Oil Nanoemulsified Formulation
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- EPPO. EPPO Global Database: Spodoptera frugiperda (LAPHFR). 2022. Available online: https://gd.eppo.int/taxon/LAPHFR/distribution/MX (accessed on 31 March 2022).
- Wan, J.; Huang, C.; Li, C.-Y.; Zhou, H.-X.; Ren, Y.-L.; Li, Z.-Y.; Xing, L.-S.; Zhang, B.; Qiao, X.; Liu, B.; et al. Biology, invasion, and management of the agricultural invader: Fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agric. 2021, 20, 646–663. [Google Scholar] [CrossRef]
- Paredes-Sánchez, F.A.; Rivera, G.; Bocanegra-García, V.; Martínez-Padrón, H.Y.; Berrones-Morales, M.; Niño-García, N.; Herrera-Mayorga, V. Advances in Control Strategies against Spodoptera frugiperda. A Review. Molecules 2021, 26, 5587. [Google Scholar] [CrossRef]
- Niassy, S.; Agbodzavu, M.K.; Kimathi, E.; Mutune, B.; Abdel-Rahman, E.F.M.; Salifu, D.; Hailu, G.; Belayneh, Y.T.; Felege, E.; Tonnang, H.E.Z.; et al. Bioecology of fall armyworm Spodoptera frugiperda (J.E. Smith), its management and potential patterns of seasonal spread in Africa. PLoS ONE 2021, 16, e0249042. [Google Scholar] [CrossRef]
- González-Maldonado, M.B.; Gurrola-Reyes, J.N.; Chaírez-Hernández, I. Biological products for the control of Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev. Colomb. Entomol. 2015, 41, 200–204. [Google Scholar]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Sarkowi, F.N.; Mokhtar, A.S. The Fall Armyworm (faw) Spodoptera Frugiperda: A Review on Biology, Life History, Invasion, Dispersion and Control. Outlooks Pest Manag. 2021, 32, 27–32. [Google Scholar] [CrossRef]
- Van den Berg, H.; Zaim, M.; Yadav, R.S.; Soares, A.; Ameneshewa, B.; Mnzava, A.; Hii, J.; Prasad-Dash, A.; Ejov, M. Global trends in the use of insecticides to control vector-borne diseases. Environ. Health Perspect. 2012, 120, 577–582. [Google Scholar] [CrossRef]
- Blanco, C.A.; Pellegaud, J.G.; Nava-Camberos, U.; Lugo-Barrera, D.; Vega-Aquino, P.; Coello, J.; Terán-Vargas, A.P.; Vargas-Camplis, J. Maize pests in Mexico and challenges for the adoption of integrated pest management programs. J. Integr. Pest Manag. 2014, 5, E1–E9. [Google Scholar] [CrossRef] [Green Version]
- SENASICA 2021. Dirección General de Sanidad Vegetal. Centro Nacional de Referencia Fitosanitaria. Gusano Cogollero Spodoptera frugiperda (J.E. Smith) Lepidoptera: Noctuidae. Ficha Técnica. Available online: https://www.gob.mx/cms/uploads/attachment/file/635234/Gusano_cogollero_en_ma_z_y_arroz.pdf (accessed on 31 March 2022).
- Gutiérrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-Evolved Resistance of the Fall Armyworm (Lepidoptera: Noctuidae) to Synthetic Insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef]
- Rioba, N.B.; Stevenson, P.C. Opportunities and Scope for Botanical Extracts and Products for the Management of Fall Armyworm (Spodoptera frugiperda) for Smallholders in Africa. Plants 2020, 9, 207. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Gooderham, N.J.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Davidsen, J.M.; Harman, C.L.; Taylor, S.V. Updated procedure for the safety evaluation of natural flavor complexes used as ingredients in food. Food Chem. Toxicol. 2018, 113, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, J.; de Albuquerque, R.D.D.G. Nanoemulsions of Essential Oils: New Tool for Control of Vector-Borne Diseases and In Vitro Effects on Some Parasitic Agents. Medicines 2019, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Werrie, P.Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.L. Phytotoxicity of Essential Oils: Opportunities and Constraints for the Development of Biopesticides. A Review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef] [PubMed]
- Belmain, S.R.; Amoah, B.A.; Nyirenda, S.P.; Kamanula, J.F.; Stevenson, P.C. Highly Variable Insect Control Efficacy of Tephrosia vogelii Chemotypes. J. Agric. Food Chem. 2012, 60, 10055–10063. [Google Scholar] [CrossRef] [PubMed]
- Green, B.; Welch, K.; Panter, K.; Lee, S. Plant Toxins That Affect Nicotinic Acetylcholine Receptors: A Review. Chem. Res. Toxicol. 2013, 26, 1129–1138. [Google Scholar] [CrossRef]
- Barlow, S.E.; Wright, G.A.; Barberis, C.M.M.; Farrell, I.W.; Marr, E.C.; Brankin, A.; Pavlik, B.M.; Stevenson, P.C. Distasteful nectar deters floral robbery. Curr. Biol. 2017, 27, 2552–2558.e3. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef] [Green Version]
- FDA. Essential Oils, Oleoresins (Solvent-Free), and Natural Extractives (Including Distillates). Substances Generally Recognized as Safe; 21 Foods and Drugs Administration (CITE: 21CFR582.20); Department of Health and Human Services: Silver Spring, MD, USA, 2022.
- Nogueira-Barradas, T.; de Holanda e Silva, K.G. Nanoemulsions of essential oils to improve solubility, stability and permeability: A review. Environ. Chem. Lett. 2021, 19, 1153–1171. [Google Scholar] [CrossRef]
- Seixas, P.T.L.; Demuner, A.J.; Alvarenga, E.S.; Barbosa, L.C.A.; Marques, A.; Farias, E.D.S.; Picanço, M.C. Bioactivity of essential oils from Artemisia against Diaphania. Sci. Agric. 2018, 75, 519–525. [Google Scholar] [CrossRef]
- He, Y.; Zhao, B.; Yu, Y. Effect, comparison and analysis of pesticide electrostatic spraying and traditional spraying. Bulg. Chem. Commun. 2016, 48, 340–344. [Google Scholar]
- De Oliveira, J.L.; Campos, E.V.R.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Corrias, F.; Melis, A.; Atzei, A.; Marceddu, S.; Dedola, F.; Sirigu, A.; Pireddu, R.; Lai, F.; Angioni, A. Zoxamide accumulation and retention evaluation after nanosuspension technology application in tomato plant. Pest Manag. Sci. 2021, 77, 3508–3518. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, I.F.; Hussein, M.Z. Synthesis and Technology of Nanoemulsion-Based Pesticide Formulation. Nanomaterials 2020, 10, 1608. [Google Scholar] [CrossRef]
- Motl, O.; Trka, A. Composition of the Essential Oil from Cedrela odorata L. J. Soc. Cosmet. Chem. 1973, 24, 747–751. [Google Scholar]
- Brunke, E.J.; Hammerschmidt, F.J.; Koster, F.H. Essential Oil of Cedrela odorata L. (Meliaceae) from Brazil-Revised List of Constituents. In Progress in Essential Oil Research, Proceedings of the International Symposium on Essential Oils, Holzminden/Neuhaus, Federal Republic of Germany, 18–21 September 1985; Brunke, E.J., Ed.; Walter de Gruyter & Co.: Berlin, Germany; Boston, MA, USA, 2019; pp. 117–122. [Google Scholar] [CrossRef]
- Hardt, I.H.; Rieck, A.; Fricke, C.; Konig, W.A. Enantiomeric Composition of Sesquiterpene Hydrocarbons of the Essential Oil of Cedrela odorata L. Flavour Frag. J. 1995, 10, 165–171. [Google Scholar] [CrossRef]
- Asekun, O.T.; Ekundayo, O. Constituents of the leaf essential oil of Cedrela odorata L. from Nigeria. Flavour Fragr. J. 1999, 14, 390–392. [Google Scholar] [CrossRef]
- De Leo, M.; Milella, L.; Braca, A.; De Tommasi, N. Cedrela and Toona genera: A rich source of bioactive limonoids and triterpenoids. Phytochem. Rev. 2018, 17, 751–783. [Google Scholar] [CrossRef]
- CABI. Centre for Agricultural Bioscience International. 2019. Cedrela odorata (Spanish Cedar). Available online: https://www.cabi.org/isc/datasheet/11975 (accessed on 31 March 2022).
- Nogueira, T.S.R.; Passos, M.D.S.; Nascimento, L.P.S.; Arantes, M.B.D.S.; Monteiro, N.O.; Boeno, S.I.D.S.; Junior, A.D.C.; Azevedo, O.D.A.; Terra, W.D.S.; Vieira, M.G.C.; et al. Chemical Compounds and Biologic Activities: A Review of Cedrela Genus. Molecules 2020, 25, 5401. [Google Scholar] [CrossRef]
- Céspedes, C.L.; Calderón, J.S.; Lina, L.; Aranda, E. Growth inhibitory effects on fall armyworm Spodoptera frugiperda of some limonoids isolated from Cedrela spp. (Meliaceae). J. Agric. Food Chem. 2000, 48, 1903–1908. [Google Scholar] [CrossRef]
- Jiménez-Durán, A.; Barrera-Cortés, J.; Lina-García, L.P.; Santillan, R.; Soto-Hernández, R.M.; Ramos-Valdivia, A.C.; Ponce-Noyola, T.; Ríos-Leal, E. Biological Activity of Phytochemicals from Agricultural Wastes and Weeds on Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Sustainability 2021, 13, 13896. [Google Scholar] [CrossRef]
- Roel, A.R.; Dourado, D.M.; Matias, R.; Porto, K.R.A.; Bednaski, A.V.; da Costa, R.B. The effect of sub-lethal doses of Azadirachta indica (Meliaceae) oil on the midgut of Spodoptera frugiperda (Lepidoptera, Noctuidae). Rev. Bras. Entomol. 2010, 54, 505–510. [Google Scholar] [CrossRef]
- Islam, M.; Al-Amin, M.D.; Siddiqi, M.M.A.; Akter, S.; Haque, M.M.; Sultana, N.; Chowdhury, A.S. Isolation of quercetin-3-O-beta-D-glucopyranoside from the leaves of Azadirachta Indica and antimicrobial and cytotoxic screening of the crude extracts. Dhaka Univ. J. Biol. Sci. 2012, 60, 11–14. [Google Scholar] [CrossRef] [Green Version]
- Tavares, W.; Barreto, M.; Seca, A. Aqueous and Ethanolic Plant Extracts as Bio-Insecticides-Establishing a Bridge between Raw Scientific Data and Practical Reality. Plants 2021, 10, 920. [Google Scholar] [CrossRef]
- Fathi, E.; Sefidkon, F. Influence of drying and extraction methods on yield and chemical composition of the essential oil of Eucalyptus sargentii. J. Agric. Sci. Technol. 2012, 14, 1035–1042. [Google Scholar]
- Milica, A.; Mirjana, C.; Jovana, S. Effect of weather conditions, location and fertilization on coriander fruit essential oil quality. J. Essent. Oil-Bear. Plants 2016, 19, 1208–1215. [Google Scholar] [CrossRef]
- Maia, B.H.L.N.S.; de Paula, J.R.; Sant’Ana, J.; Silva, M.F.D.G.F.D.; Fernandes, J.B.; Vieira, P.C.; Costa, M.D.S.S.; Ohashi, O.S.; Silva, J.N.M. Essential oils of Toona and Cedrela species (Meliaceae): Taxonomic and ecological implications. J. Braz. Chem. Soc. 2000, 11, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Martins, A.P.; Salgueiro, L.R.; Da Cunha, A.P.; Vila, R.; Cañigueral, S.; Tomi, F.; Casanova, J. Chemical composition of the bark oil of Cedrela odorata from S. Tome and Principe. J. Essent. Oil Res. 2003, 15, 422–424. [Google Scholar] [CrossRef]
- Noge, K.; Becerra, J.X. Germacrene D, A Common Sesquiterpene in the Genus Bursera (Burseraceae). Molecules 2009, 14, 5289–5297. [Google Scholar] [CrossRef]
- Duarte, A.R.; Naves, R.R.; Santos, S.C.; Seraphin, J.C.; Ferri, P.H. Genetic and Environmental Influence on Essential Oil Composition of Eugenia dysenterica. J. Braz. Chem. Soc. 2010, 21, 1459–1467. [Google Scholar] [CrossRef] [Green Version]
- Ogunwande, I.A.; Ekundayo, O.; Olawore, N.O.; Adeleke, K.A. Constituents of the Essential Oils of the Leaves and Stem Bark of Cedrela mexicana L. Grown in Nigeria. J. Essent. Oil Res. 2005, 17, 289–291. [Google Scholar] [CrossRef]
- Vargas Suarez, A.; Satyal, P.; Setzer, W.N. Volatile Components of the Wood of Spanish Cedar, Cedrela odorata, from Costa Rica. Am. J. Essent. Oils Nat. Prod. 2018, 6, 27–30. [Google Scholar]
- Villanueva, H.E.; Tuten, J.A.; Haber, W.A.; Setzer, W.N. Chemical composition and antimicrobial activity of the bark essential oil of Cedrela odorata from Monteverde, Costa Rica. Der Pharma Chem. 2009, 1, 14–18. [Google Scholar]
- Muellner, A.N.; Pennington, T.D.; Chase, M.W. Molecular phylogenetics of Neotropical Cedreleae (mahogany family, Meliaceae) based on nuclear and plastid DNA sequences reveal multiple origins of “Cedrela odorata”. Mol. Phylogenet. Evol. 2009, 52, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Franco-Vega, A.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Estimation of mass transfer coefficients of the extraction process of essential oil from orange peel using microwave assisted extraction. J. Food Eng. 2016, 170, 136–143. [Google Scholar] [CrossRef]
- Shrewsbury, R.P. Applied Pharmaceutics in Contemporary Compounding; Morton Publishing Company: Englewood, CO, USA, 2015; pp. 165–177. [Google Scholar]
- Meher, J.G.; Yadav, N.P.; Sahu, J.J.; Sinha, P. Determination of required hydrophilic-lipophilic balance of citronella oil and development of stable cream formulation. Drug Dev. Ind. Pharm. 2013, 39, 1540–1546. [Google Scholar] [CrossRef]
- Griffin, W.C. Classification of surface-active agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311–326. [Google Scholar]
- Bachynsky, M.O.; Shah, N.H.; Patel, C.I.; Malick, A.W. Factors affecting the efficiency of a self-emulsifying oral delivery system. Drug Dev. Ind. Pharm. 1997, 23, 809–816. [Google Scholar] [CrossRef]
- Matsaridou, I.; Barmpalexis, P.; Salis, A.; Nikolakakis, I. The influence of surfactant HLB and oil/surfactant ratio on the formation and properties of self-emulsifying pellets and microemulsion reconstitution. AAPS PharmSciTech 2012, 13, 1319–1330. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Mazarei, Z.; Rafati, H. Nanoemulsification of Satureja khuzestanica essential oil and pure carvacrol; comparison of physicochemical properties and antimicrobial activity against food pathogens. LWT—Food Sci. Technol. 2019, 100, 328–334. [Google Scholar] [CrossRef]
- Shahavi, M.H.; Hosseini, M.; Jahanshahi, M.; Meyer, R.L.; Darzi, G.N. Evaluation of critical parameters for preparation of stable clove oil nanoemulsion. Arab. J. Chem. 2019, 12, 3225–3230. [Google Scholar] [CrossRef] [Green Version]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Fabrication of vitamin E-enriched nanoemulsions: Factors affecting particle size using spontaneous emulsification. J. Colloid Interface Sci. 2013, 391, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.; Pandey, I.P. Study of some physiochemical factors determining emulsion stability with mixed emulsifiers. J. Ind. Res. Technol. 2013, 3, 12–16. [Google Scholar]
- Cho, Y.-H.; Kim, S.; Bae, E.K.; Mok, C.K.; Park, J. Formulation of a Cosurfactant-Free O/W Microemulsion Using Nonionic Surfactant Mixtures. J. Food Sci. 2008, 73, 115–121. [Google Scholar] [CrossRef]
- Fernandez, A.; Salager, J.L.; Scorzza, C. Surfactante, I. Generalidades II. Materias Primas; Cuaderno FIRP # 301PP; Universidad de Los Andes (ULA): Merida, Venezuela, 2004; pp. 3–6. [Google Scholar]
- Liu, L.; Niu, J.; Wu, J.-Y. Formulation of highly stable PCM nano-emulsions with reduced supercooling for thermal energy storage using surfactant mixtures. Sol. Energy Mater. Sol. Cells 2021, 223, 110983. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Formation of Flavor Oil Microemulsions, Nanoemulsions and Emulsions: Influence of Composition and Preparation Method. J. Agric. Food Chem. 2011, 59, 5026–5035. [Google Scholar] [CrossRef]
- Delmas, T.; Piraux, H.; Couffin, A.C.; Texier, I.; Vinet, F.; Poulin, P.; Cates, M.E.; Bibette, J. How to Prepare and Stabilize Very Small Nanoemulsions. Langmuir 2011, 27, 1683–1692. [Google Scholar] [CrossRef]
- Bernardi, D.S.; Pereira, T.A.; Maciel, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; Rocha-Filho, P.A. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: In vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 44. [Google Scholar] [CrossRef]
- Díaz-Blancas, V.; Medina, D.I.; Padilla-Ortega, E.; Bortolini-Zavala, R.; Olvera-Romero, M.; Luna-Bárcenas, G. Nanoemulsion Formulations of Fungicide Tebuconazole for Agricultural Applications. Molecules 2016, 21, 1271. [Google Scholar] [CrossRef] [Green Version]
- Gündel, S.D.S.; de Souza, M.E.; Quatrin, P.M.; Klein, B.; Wagner, R.; Gündel, A.; Vaucher, R.D.A.; Santos, R.C.V.; Ourique, A.F. Nanoemulsions containing Cymbopogon flexuosus essential oil: Development, characterization, stability study and evaluation of antimicrobial and antibiofilm activities. Microb. Pathog. 2018, 118, 268–276. [Google Scholar] [CrossRef]
- Dal Mas, J.; Zermiani, T.; Thiesen, L.C.; Silveira, J.L.; Silva, K.A.; Souza, M.M.; Malheiros, A.; Bresolin, T.M.; Lucinda-Silva, R.M. Nanoemulsion as a carrier to improve the topical anti-inflammatory activity of stem bark extract of Rapanea ferruginea. Int. J. Nanomed. 2016, 11, 4495–4507. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.R.; Santiago, R.R.; de Souza, T.P.; Egito, E.S.; Oliveira, E.E.; Soares, L.A. Development and evaluation of emulsions from Carapa guianensis (Andiroba) oil. AAPS PharmSciTech 2010, 11, 1383–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badawy, M.E.; Abdelgaleil, S.A.; Mahmoud, N.F.; Marei, A.E.S.M. Preparation and characterizations of essential oil and monoterpene nanoemulsions and acaricidal activity against two-spotted spider mite (Tetranychus urticae Koch). Int. J. Acarol. 2018, 44, 330–340. [Google Scholar] [CrossRef]
- Khan, B.A.; Akhtar, N.; Khan, H.M.S.; Waseem, K.; Mahmood, T.; Rasul, A.; Igbal, M.; Khan, H. Basics of pharmaceutical emulsions: A review. Afr. J. Pharm. Pharmacol. 2011, 5, 2715–2725. [Google Scholar] [CrossRef]
- Sharma, S.; Loach, N.; Gupta, S.; Mohan, L. Phyto-nanoemulsion: An Emerging Nano-Insecticidal Formulation. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100331. [Google Scholar] [CrossRef]
- Negrini, M.; Fidelis, E.G.; Schurt, D.A.; Silva, F.D.S.; Pereira, R.S.; Bizzo, H.R. Insecticidal activity of essential oils in controlling fall armyworm, Spodoptera frugiperda. Arq. Inst. Biol. 2019, 86, e1112018. [Google Scholar] [CrossRef] [Green Version]
- Zavala-Gómez, C.E.; Zamora-Avella, D.; Rodríguez-Chávez, J.L.; Zavala-Sánchez, M.Á.; Campos-Guillén, J.; Bah, M.M.; García-Serrano, L.A.; Cárdenas-Ortega, N.C.; Sosa-Domínguez, A.; Ramos-López, M.A. Bioactivity of 1,8-Cineole and Essential Oil of Salvia keerlii (Lamiaceae) Against Spodoptera frugiperda. Southwest. Entomol. 2021, 46, 385–396. [Google Scholar] [CrossRef]
- Zorga, J.; Kunicka-Styczynska, A.; Gruska, R.; Smigielski, K. Ultrasound-Assisted Hydrodistillation of Essential Oil from Celery Seeds (Apium graveolens L.) and Its Biological and Aroma Profiles. Molecules 2020, 25, 5322. [Google Scholar] [CrossRef]
- Kiran, S.R.; Reddy, A.S.; Devi, P.S.; Reddy, K.J. Insecticidal, antifeedant and oviposition deterrent effects of the essential oil and individual compounds from leaves of Chloroxylon swietenia DC. Pest Manag. Sci. 2006, 62, 1116–1121. [Google Scholar] [CrossRef]
- Cárdenas-Ortega, N.C.; González-Chávez, M.M.; Figueroa-Brito, R.; Flores-Macías, A.; Romo-Asunción, D.; Martínez-González, D.E.; Pérez-Moreno, V.; Ramos-López, M.A. Composition of the essential oil of Salvia ballotiflora (Lamiaceae) and its insecticidal activity. Molecules 2015, 20, 8048–8059. [Google Scholar] [CrossRef] [Green Version]
- Benelli, G.; Govindarajan, M.; AlSalhi, M.S.; Devanesan, S.; Maggi, F. High toxicity of camphene and γ-elemene from Wedelia prostrata essential oil against larvae of Spodoptera litura (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. Int. 2018, 25, 10383–10391. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.M.; Haddi, K.; Ribeiro, B.M.; Corrêia, R.F.T.; Tomé, H.V.V.; Santos-Amaya, O.; Pereira, E.J.G.; Guedes, R.N.C.; Santos, G.R.; Oliveira, E.E.; et al. Essential oil of Siparuna guianensis as an alternative tool for improved lepidopteran control and resistance management practices. Sci. Rep. 2018, 8, 7215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papachristos, D.P.; Stamopoulos, D.C. Fumigant toxicity of three essential oils on the eggs of Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae). J. Stored Prod. Res. 2004, 40, 517–525. [Google Scholar] [CrossRef]
- Oladimeji, A.O.; Babatunde, O.; Musa, R.T.; M’civer, F.A.; Lawal, A.T.; Ogunwande, I.A. GC-MS analysis and cytotoxic activity of essential oils from the leaves of Abrus precatorius L. Gaertn. Asian Pac. J. Trop. Dis. 2016, 6, 372–375. [Google Scholar] [CrossRef]
- Quert Álvarez, R.; Miranda Martínez, M.; Leyva Córdova, B.; García Corrales, H.; Gelabert Ayón, F. Rendimiento de aceite esencial en Pinus caribaea MorElet según el secado al sol ya la sombra. III. Rev. Cuba. De Farm. 2001, 35, 47–50. [Google Scholar]
- Navadha, J.; Sah, G.C.; Devendra, M. GC-MS Analysis and Antimicrobial Activity of Essential Oil of Senecio Pedunculatus. J. Appl. Chem. 2013, 6, 49–51. [Google Scholar]
- Gómez-Vega, A.G. Formulación y Caracterización de una Nanoemulsión de Aceite de Parafina Tipo Agua-En-Aceite (W/O). Master’s Thesis, Centro de Investigación en Materiales Avanzados, Nuevo León, Monterrey, México, 2014. [Google Scholar]
- Alcántara-Martínez, E.; Ríos-Leal, E.; García-Rojas, C.M.C.; Barrera-Cortés, J. Evaluación de dos surfactantes producidos por levaduras para su aplicación en la formulación de microemulsiones. Rev. Mex. De Agroecosistemas 2016, 3, 211–219. [Google Scholar]
- ICI Americas. The HLB System: A Time-Saving Guide to Emulsifier Selection, 2nd ed.; Chemmunique, Ed.; ICI Americas: Wilmington, DE, USA, 1980; pp. 3–22. [Google Scholar]
- Isaac, V.L.B.; Cefali, L.C.; Chiari, B.G.; Oliveira, C.C.L.G.; Salgado, H.R.N.; Corrêa, M.A. Protocolo para ensaios físico-químicos de estabilidade de fitocosméticos. Rev. Ciênc. Farm. Básica Apl. 2008, 29, 81–96. [Google Scholar]
- Savardekar, P.; Bajaj, A. Nanoemulsions—A review. Int. J. Pharm. Chem. 2016, 6, 312–322. [Google Scholar]
- Sugumar, S.; Clarke, S.K.; Nirmala, M.J.; Tyagi, B.K.; Mukherjee, A.; Chandrasekaran, N. Nanoemulsion of eucalyptus oil and its larvicidal activity against Culex quinquefasciatus. Bull. Entomol. Res. 2014, 104, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaldi, F.; Hanieh, P.N.; Longhi, C.; Carradori, S.; Secci, D.; Zengin, G.; Ammendolia, M.G.; Mattia, E.; Fevero, E.; Marianecci, C.; et al. Neem oil nanoemulsions: Characterization and antioxidant activity. J. Enzym. Inhib. Med. Chem. 2017, 32, 1265–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orafidiya, L.O.; Oladimeji, F.A. Determination of the required HLB values of some essential oils. Int. J. Pharm. 2002, 237, 241–249. [Google Scholar] [CrossRef]
- Knaak, N.; Wiest, S.L.; Andreis, T.F.; Fiuza, L.M. Toxicity of essential oils to the larvae of Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Biopestic. 2013, 6, 49–53. [Google Scholar]
- Ghotbi, R.S.; Khatibzadeh, M.; Kordbacheh, S. Preparation of Neem seed oil nanoemulsion. In Proceedings of the 5th International Conference on Nanotechnology: Fundamentals and Applications, Prague, Czech Republic, 11–13 August 2014; pp. 1–5. [Google Scholar]




| Leaf Parameter | Dehydrated Leaf | Fresh Leaf |
|---|---|---|
| Color | Greenish-yellow | Greenish-yellow |
| Odor | Dry leaf | Dry leaf |
| Density (g mL−1) | 0.91 ± 0.01 | 0.90 ± 0.018 |
| Refractive index | 1.5 ± 0.01 | 1.5 ± 0.01 |
| Yield (%) | 0.58 ± 0.13 1 | 0.26 ± 0.04 |
| Peak | Retention Time (min) | Compound | FL (%) June 2018 | DL (%) June 2018 | DL (%) September 2018 |
|---|---|---|---|---|---|
| 1 | 27.589 | copaene | 3.75 | 8.17 | 1.83 |
| 2 | 27.955 | β-elemene | 1.49 | 3.25 | 3.23 |
| 3 | 28.315 | cis-caryophyllene | - | 1.92 | - |
| 4 | 28.825 | caryophyllene | 34.12 | 23.93 | 51.79 |
| 5 | 29.050 | alloaromadendrene | 1.12 | - | 3.04 |
| 6 | 29.200 | β-cubebene | 1.29 | - | - |
| 7 | 29.305 | (−)-aristolene | - | 1.78 | - |
| 8 | 29.385 | β-gurjunene | 0.73 | - | 1.65 |
| 9 | 29.550 | α-caryophyllene | 3.32 | 4.59 | 4.68 |
| 10 | 29.835 | γ-elemene | 1.16 | - | - |
| 11 | 30.456 | germacrene D | 36.85 | 25.24 | 9.92 |
| 12 | 30.605 | α-selinene | 0.89 | - | 1.62 |
| 13 | 30.696 | β-germacrene | - | 1.73 | - |
| 14 | 30.895 | (-)β-elemene | 1.44 | - | 1.8 |
| 15 | 30.976 | (−)-isoaromadendrene-(V) | - | 1.95 | - |
| 16 | 31.050 | valencene | - | - | 1.87 |
| 17 | 31.206 | δ-cadinene | 1.34 | 3.62 | 2.77 |
| 18 | 31.256 | cis-α-bisabolene | 7.30 | 8.83 | 3.16 |
| 19 | 32.636 | caryophyllene oxide | 1.23 | 2.72 | 1.97 |
| 20 | 33.356 | cubenol | - | - | 3.01 |
| 21 | 33.687 | (−)-spathulenol | - | 1.00 | - |
| 22 | 39.889 | phytol | 1.20 | 3.95 | 1.44 |
| % Total | 97.23 | 92.68 | 93.78 |
| Tween 80 | Span 20 | HLB | %CI |
|---|---|---|---|
| 6 | 94 | 8.98 | 50 |
| 22 | 78 | 10.01 | 4 |
| 29 | 71 | 10.46 | 0 |
| 34 | 66 | 10.78 | 0 |
| 38 | 62 | 11.03 | 10 |
| 45 | 55 | 11.48 | 20 |
| 54 | 46 | 12.06 | 24 |
| 67 | 33 | 12.89 | 24 |
| Temperature | Time (Days) | 0 | 1 | 7 | 15 | 30 | 90 |
|---|---|---|---|---|---|---|---|
| 25 °C | Drop size, nm | 38.9 ± 3.1 a | 39.4 ± 4.0 a | 60.2 ± 8.3 cd | 65.9 ± 13.5 d | 81.8 ± 9.1 e | 105.7 ± 9.4 f |
| PDL | 0.227 ± 0.013 a | 0.235 ± 0.046 a | 0.339 ± 0.101 abcd | 0.459 ± 0.067 def | 0.559 ± 0.034 f | 0.479 ± 0.032 ef | |
| Turbidity, % | 34.3 ± 0.4 a | 36.8 ± 2.3 ab | 39.18 ± 2.5 abc | 46.3 ± 1.3 def | 50.9 ± 3.7 f | 58.8 ± 1.8 g | |
| pH | 6.3 ± 0.0 c | 6.3 ± 0.1 c | 6.3 ± 0.1 c | 6.2 ± 0.1 bc | 6.1 ± 0.0 b | 5.3 ± 0.4 a | |
| 5 °C | Drop size, nm | 38.9 ± 3.1 a | 42.1 ± 7.6 a | 43.4 ± 4.3 ab | 48.1 ± 10.1 abc | 62.3 ± 3.1 d | 56.2 ± 13.0 bcd |
| PDL 5 °C | 0.227 ± 0.013 a | 0.285 ± 0.092 ab | 0.335 ± 0.072 abc | 0.345 ± 0.102 abcd | 0.412 ± 0.118 cde | 0.389 ± 0.129 bcde | |
| Turbidity, % | 34.3 ± 0.4 a | 39.3 ± 0.8 bc | 42.3 ± 5.1 cd | 42.6 ± 2.4 cd | 44.4 ± 2.5d e | 47.7 ± 7.1 ef | |
| pH | 6.3 ± 0.0 c | 6.3 ± 0.0 c | 6.4 ± 0.1 c | 6.4 ± 0.1 c | 6.3 ± 0.1 c | 6.2 ± 0.1 bc |
| LC50 (mg kg−1) | Confidence Limits at 95% (mg kg−1) | LC95 (mg kg−1) | Confidence Limits at 95% (mg kg−1) | χ2 | df | Sig. | |||
|---|---|---|---|---|---|---|---|---|---|
| lower limit | upper limit | lower limit | upper limit | ||||||
| Essential oil | |||||||||
| C. odorata | 7712.1 | 6122.9 | 9214.9 | 30,043.6 | 23,652.1 | 42,938.7 | 9.573 | 18 | 0.945 |
| Neem | 7667.8 | 6355.5 | 8976.9 | 23,371.3 | 18,208.7 | 35,250.4 | 0.773 | 18 | 1.000 |
| Nanoemulsion | |||||||||
| C. odorata | 9952.4 | 7237.9 | 14,968.1 | - | - | - | 2.701 | 18 | 1.000 |
| Surfactant Mixture | TW80 (%) | SP (%) | HLB |
|---|---|---|---|
| SM 1 | 6 | 94 | 8.98 |
| SM 2 | 22 | 78 | 10.01 |
| SM 3 | 34 | 66 | 10.78 |
| SM 4 | 54 | 46 | 12.06 |
| SM 5 | 67 | 33 | 12.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemus de la Cruz, A.S.; Barrera-Cortés, J.; Lina-García, L.P.; Ramos-Valdivia, A.C.; Santillán, R. Nanoemulsified Formulation of Cedrela odorata Essential Oil and Its Larvicidal Effect against Spodoptera frugiperda (J.E. Smith). Molecules 2022, 27, 2975. https://doi.org/10.3390/molecules27092975
Lemus de la Cruz AS, Barrera-Cortés J, Lina-García LP, Ramos-Valdivia AC, Santillán R. Nanoemulsified Formulation of Cedrela odorata Essential Oil and Its Larvicidal Effect against Spodoptera frugiperda (J.E. Smith). Molecules. 2022; 27(9):2975. https://doi.org/10.3390/molecules27092975
Chicago/Turabian StyleLemus de la Cruz, Ana Sofía, Josefina Barrera-Cortés, Laura Patricia Lina-García, Ana C. Ramos-Valdivia, and Rosa Santillán. 2022. "Nanoemulsified Formulation of Cedrela odorata Essential Oil and Its Larvicidal Effect against Spodoptera frugiperda (J.E. Smith)" Molecules 27, no. 9: 2975. https://doi.org/10.3390/molecules27092975






