Comparison of the Nucleation Kinetics Obtained from the Cumulative Distributions of the Metastable Zone Width and Induction Time Data
Abstract
:1. Introduction
2. Theoretical Derivations
3. Results and Discussions
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Nomenclature
| Notation | |
| pre-exponential nucleation factor | |
| cooling rate | |
| initial concentration of solute molecules | |
| equilibrium concentration of solute molecules | |
| nucleation rate | |
| Boltzmann constant | |
| molar mass | |
| Avogadro number | |
| gas constant | |
| supersaturation ratio, | |
| temperature | |
| initial saturated temperature for the initial saturated solute concentration | |
| temperature at the MSZW limit at | |
| time | |
| induction time | |
| time at the MSZW limit | |
| solution volume | |
| volume of the solute molecule | |
| Greek letters | |
| interfacial energy (J/m2) | |
| crystal density (kg/m3) | |
| MSZW (K). | |
References
- Mullin, J.W. Crystallization, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 1993. [Google Scholar]
- Kashchiev, D. Nucleation: Basic Theory with Applications; Butterworth-Heinemann: Oxford, UK, 2000. [Google Scholar]
- Kashchiev, D.; van Rosmalen, G.M. Review: Nucleation in solutions revisited. Cryst. Res. Technol. 2003, 38, 555–574. [Google Scholar] [CrossRef]
- Ahmad Khadem, S.; Rey, A.D. Nucleation and growth of cholesteric collagen tactoids: A time-series statistical analysis based on integration of direct numerical simulation (DNS) and long short-term memory recurrent neural network (LSTM-RNN). J. Colloid Interface Sci. 2021, 582, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Azzari, P.; Bagnani, B.; Mezzenga, R. Liquid–liquid crystalline phase separation in biological filamentous colloids: Nucleation, growth and order–order transitions of cholesteric tactoids. Soft Matter 2021, 17, 6627–6636. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ter Horst, J.H. Crystal nucleation rates from probability distributions of induction times. Cryst. Growth Des. 2011, 11, 256–261. [Google Scholar] [CrossRef]
- Kadam, S.S.; Kramer, H.J.M.; Ter Horst, J.H. Combination of a single primary nucleation event and secondary nucleation in crystallization processes. Cryst. Growth Des. 2011, 11, 1271–1277. [Google Scholar] [CrossRef]
- Kadam, S.S.; Kulkarni, S.A.; Coloma Ribera, R.; Stankiewicz, A.I.; Ter Horst, J.H.; Kramer, H.J.M. A new review on the metastable zone width during cooling crystallization. Chem. Eng. Sci. 2012, 72, 10–19. [Google Scholar] [CrossRef]
- Kubota, N. Effect of sample volume on metastable zone width and induction time. J. Cryst. Growth 2012, 345, 27–33. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Kadam, S.S.; Meekes, H.; Stankiewicz, A.I.; Ter Horst, J.H. Crystal nucleation kinetics from induction times and metastable zone widths. Cryst. Growth Des. 2013, 13, 2435–2440. [Google Scholar] [CrossRef]
- Brandel, C.; Ter Horst, J.H. Measuring induction times and crystal nucleation rates. Faraday Discuss 2015, 179, 199–214. [Google Scholar] [CrossRef] [Green Version]
- Maggioni, G.M.; Mazzotti, M. Modelling the stochastic behavior of primary nucleation. Faraday Discuss 2015, 179, 359–382. [Google Scholar] [CrossRef]
- Rossi, D.; Gavriilidis, A.; Kuhn, S.; Candel, M.A.; Jones, A.G.; Price, C.; Mazzei, L. Adipic acid primary nucleation kinetics from probability distributions in droplet-based systems under stagnant and flow conditions. Cryst. Growth Des. 2015, 15, 1784–1791. [Google Scholar] [CrossRef] [Green Version]
- Kubota, N. Analysis of the effect of volume on induction time and metastable zone width using a stochastic model. J. Cryst. Growth 2015, 418, 15–24. [Google Scholar] [CrossRef]
- Kubota, N. Monte Carlo simulation of induction time and metastable zone width; stochastic or deterministic? J. Cryst. Growth 2018, 485, 1–7. [Google Scholar] [CrossRef]
- Shiau, L.D. Comparison of the interfacial energy and pre-exponential factor calculated from the induction time and metastable zone width data based on classical nucleation theory. J. Cryst. Growth 2016, 450, 50–55. [Google Scholar] [CrossRef]
- Si, Z.; Li, A.; Yan, Y.; Zhang, X.; Yang, H. Interaction of metastable zone width and induction time based on nucleation potential. Ind. Eng. Chem. Res. 2020, 59, 22597–22604. [Google Scholar] [CrossRef]
- Sangwal, K. New approach to analyze metastable zone width determined by the polythermal method: Physical interpretation of various parameters. Cryst. Growth Des. 2009, 9, 942–950. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Zhang, K.; Wu, S.; Liu, S.; Li, K.; Yu, B.; Gong, J. Nucleation behavior of eszopiclone-butyl acetate solutions from metastable zone widths. Chem. Eng. Sci. 2016, 155, 248–257. [Google Scholar] [CrossRef]
- Shiau, L.D.; Wu, D.R. Effect of L-valine impurity oh the nucleation parameters of aqueous L-glutamic acid solutions from metastable zone width data. J. Cryst. Growth 2020, 546, 125790. [Google Scholar] [CrossRef]
- Goh, L.; Chen, K.; Bhamidi, V.; He, G.; Kee, N.C.S.; Kenis, P.J.A.; Zukoski, C.F.; Braatz, R.D. A stochastic model for nucleation kinetics determination in droplet-based microfluidic systems. Cryst. Growth Des. 2010, 10, 2515–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheaffer, R.L.; McClave, J.T. Probability and Statistics for Engineers; Wadsworth, Inc.: Belmont, CA, USA, 1995. [Google Scholar]
- Yang, H.; Rasmuson, A.C. Nucleation of butyl paraben in different solvents. Cryst. Growth Des. 2013, 13, 4226–4238. [Google Scholar] [CrossRef]
- Kakkar, S.; Renuka Devi, K.; Svard, M.; Rasmuson, A. Crystal nucleation of salicylamide and a comparison with salicylic acid. CrystEngComm 2020, 22, 3329–3339. [Google Scholar] [CrossRef]
- Mealey, D.; Croker, D.M.; Rasmuson, A.C. Crystal nucleation of salicylic acid in organic solvents. CrystEngComm 2015, 17, 3961–3973. [Google Scholar] [CrossRef]
- Yang, H. Relation between metastable zone width and induction time of butyl paraben in ethanol. CrystEngComm 2015, 17, 577–586. [Google Scholar] [CrossRef]
- Yang, H.; Rasmuson, A.C. Solubility of butyl paraben in methanol, ethanol, propanol, ethyl acetate, acetone, and acetonitrile. J. Chem. Eng. Data 2010, 55, 5091–5093. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, G.; Yan, X.; Hu, C.; Zhou, X. Solid-liquid equilibrium of dicyandiamide in different solvents. Fluid Phase Equilibria 2014, 363, 228–232. [Google Scholar] [CrossRef]
- Nordstrom, F.L.; Rasmuson, A.C. Solubility and melting properties of salicylic acid. J. Chem. Eng. Data 2006, 51, 1668–1671. [Google Scholar] [CrossRef]

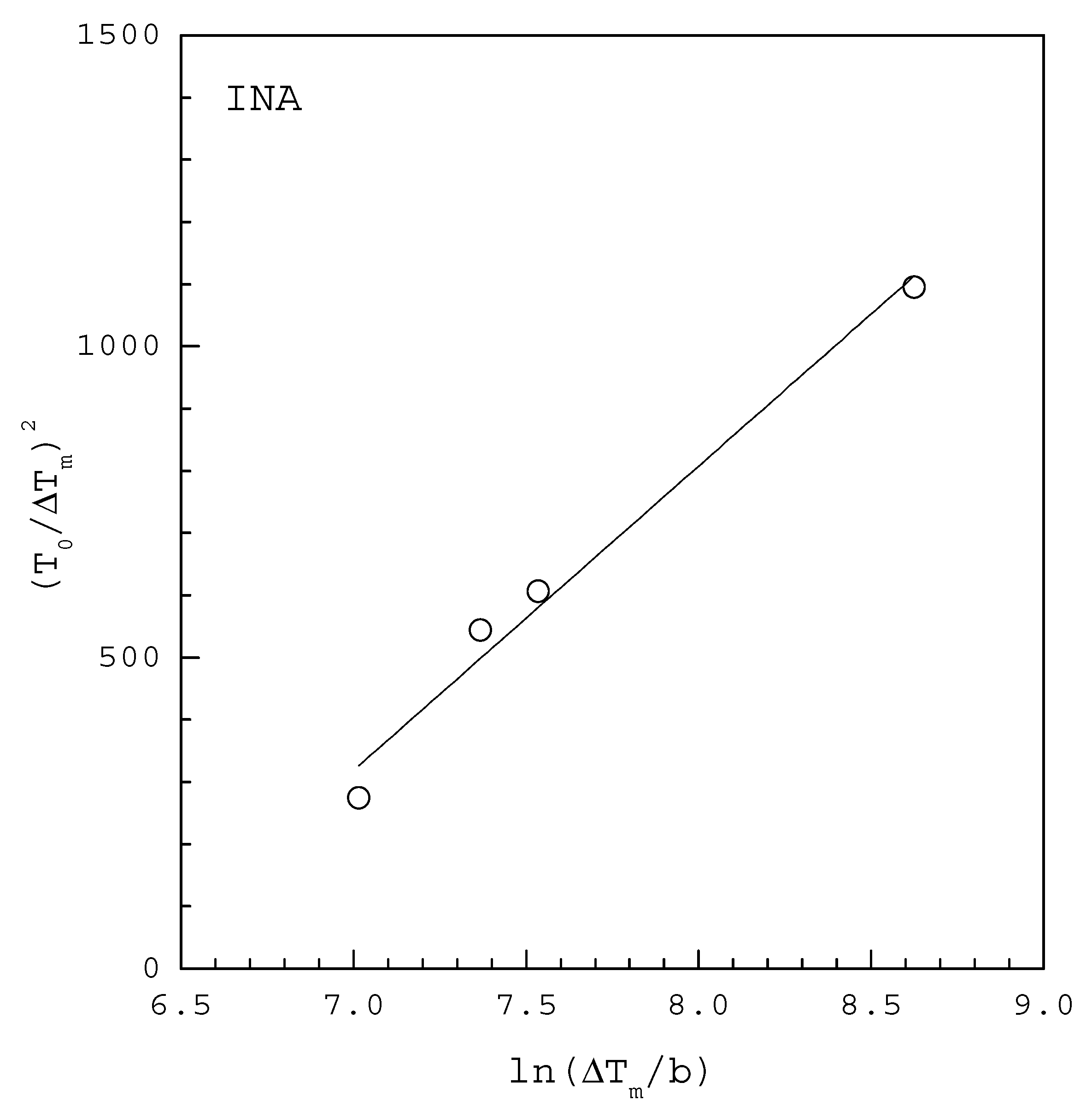
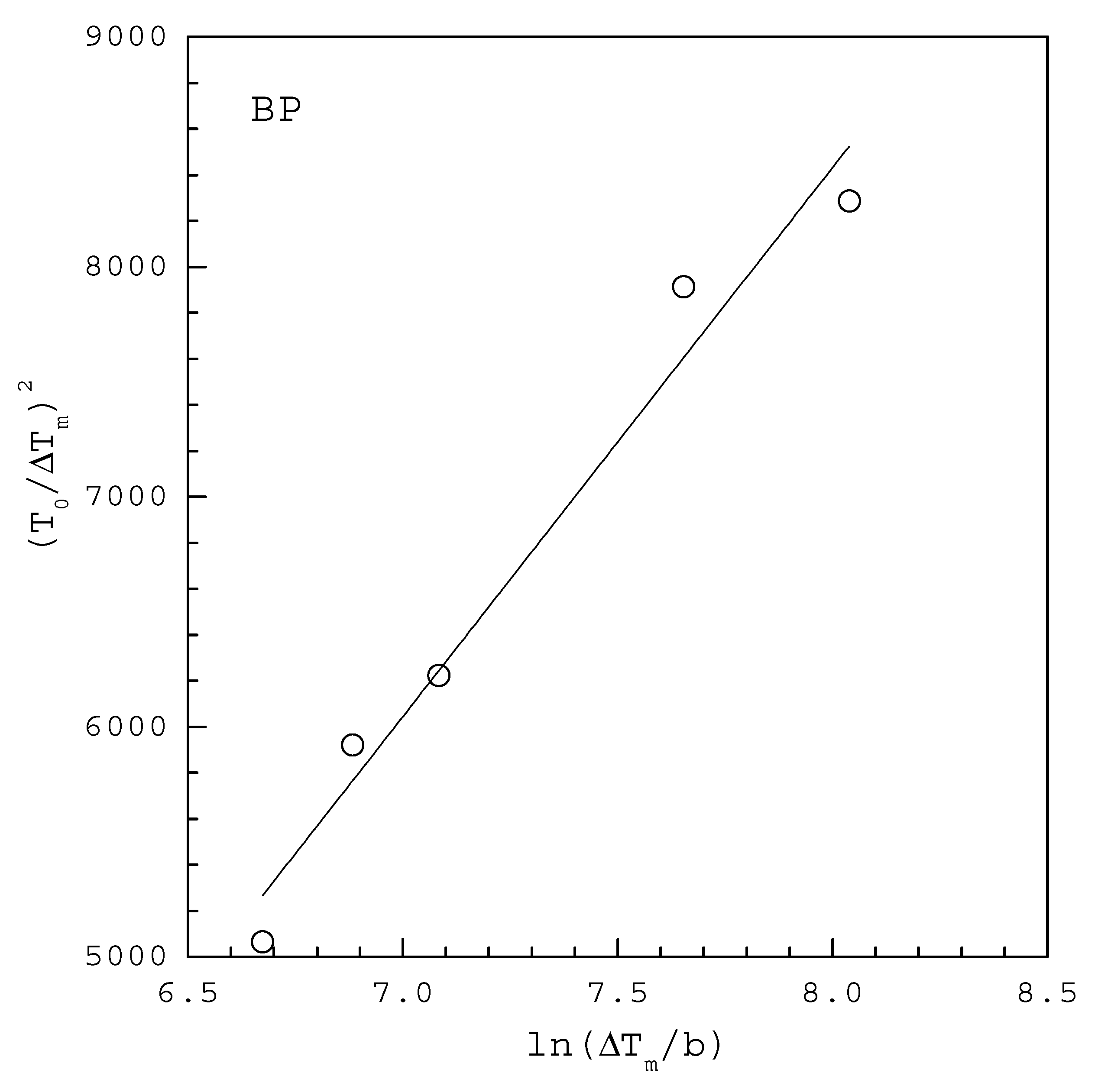
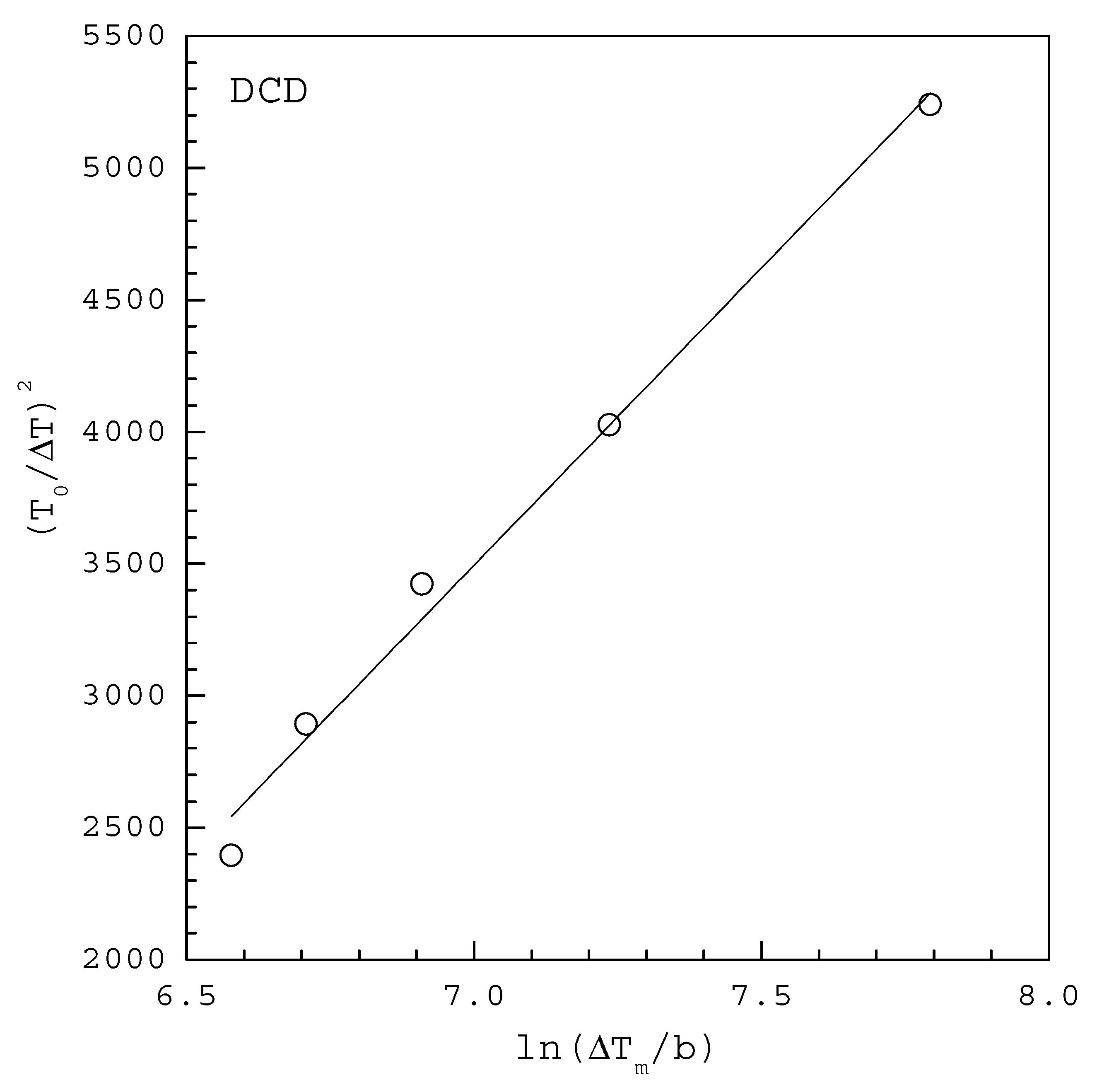

| INA | 2.96 | 3499 | 0.975 | 3.60 | 6600 |
| BP | 0.86 | 4588 | 0.961 | 1.15 | 2752 |
| DCD | 3.24 | 86 | 0.988 | 2.77 | 58 |
| SA in ACN | 1.62 | 63 | 0.935 | 1.71 | 285 |
| SA in EtAc | 2.26 | 140 | 0.866 | 2.03 | 144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiau, L.-D. Comparison of the Nucleation Kinetics Obtained from the Cumulative Distributions of the Metastable Zone Width and Induction Time Data. Molecules 2022, 27, 3007. https://doi.org/10.3390/molecules27093007
Shiau L-D. Comparison of the Nucleation Kinetics Obtained from the Cumulative Distributions of the Metastable Zone Width and Induction Time Data. Molecules. 2022; 27(9):3007. https://doi.org/10.3390/molecules27093007
Chicago/Turabian StyleShiau, Lie-Ding. 2022. "Comparison of the Nucleation Kinetics Obtained from the Cumulative Distributions of the Metastable Zone Width and Induction Time Data" Molecules 27, no. 9: 3007. https://doi.org/10.3390/molecules27093007






