Seasonal Variability of Volatilome from Dictyota dichotoma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Variations of Headspace Volatilome of Fresh D. dichotoma
2.2. Variations of Headspace Volatilome of Air-Dried D. dichotoma
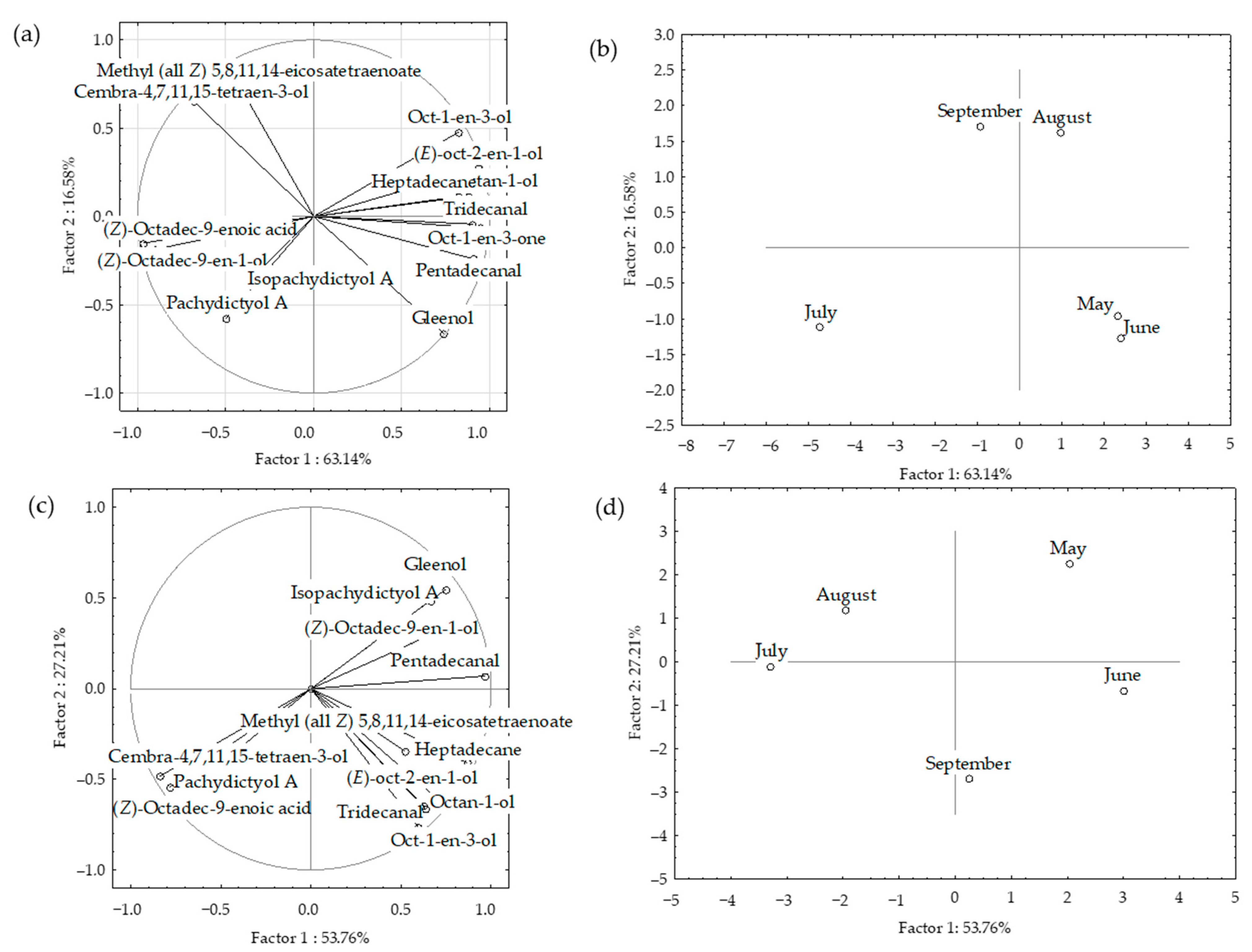
2.3. Statistical Analysis of the Headspace VOCs
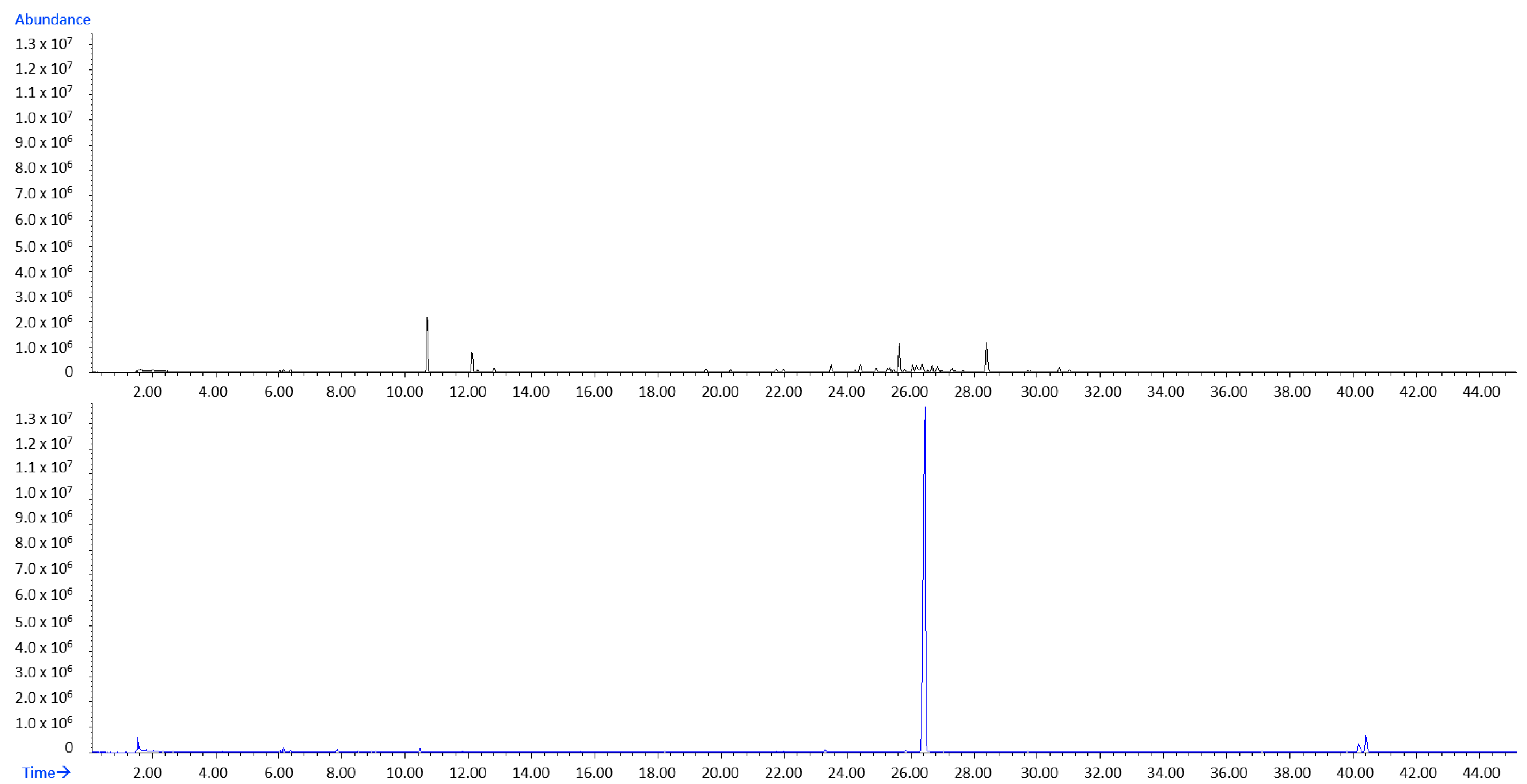
2.4. Variations of Volatilome of Fresh D. dichotoma Obtained by Hydrodistillation
2.5. Variations of Volatilome of Air-Dried D. dichotoma Obtained by Hydrodistillation
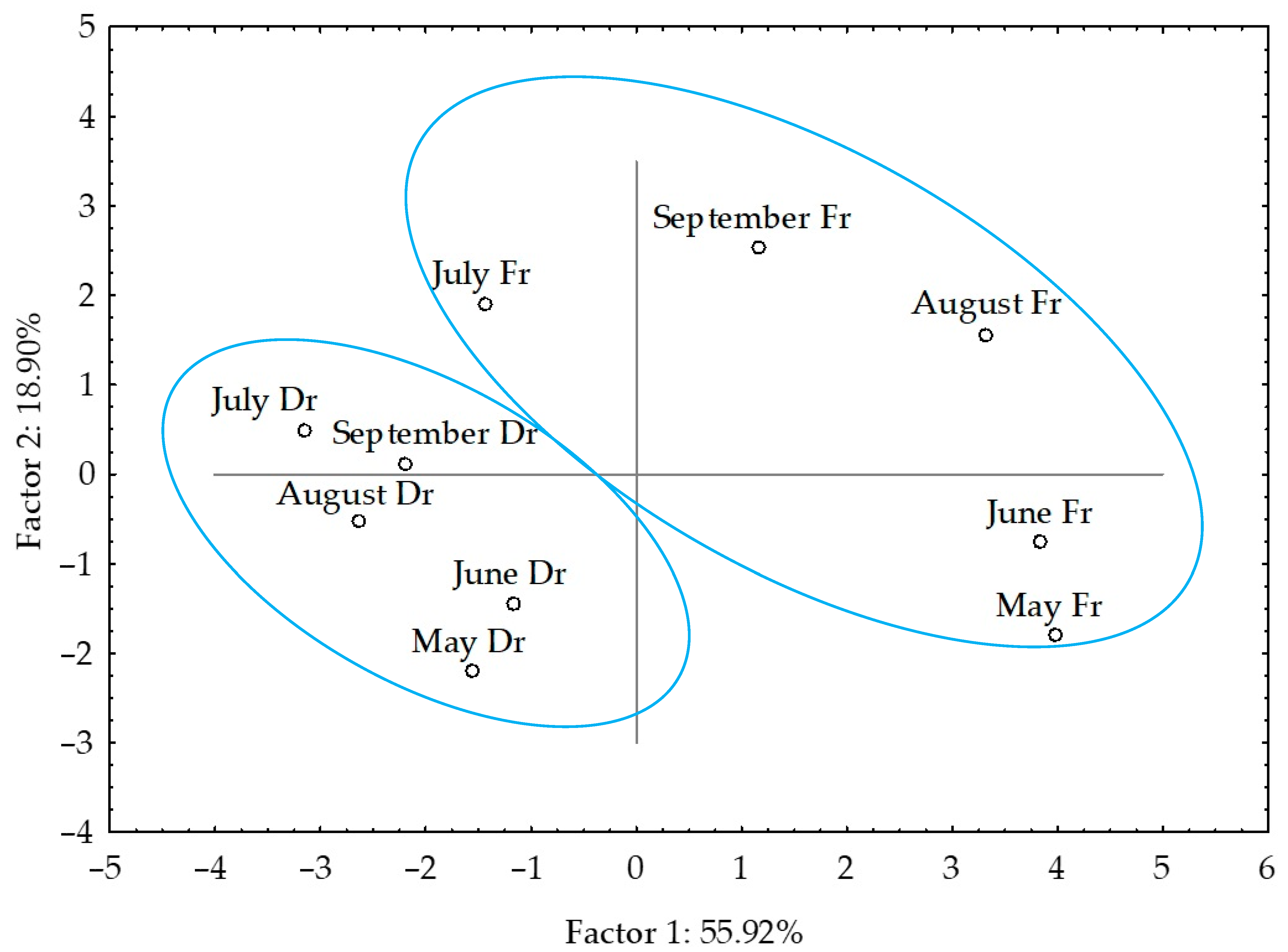
2.6. Statistical Analysis of the VOCs Obtained by Hydrodistillation
3. Materials and Methods
3.1. Sample Collection
3.2. Headspace Solid-Phase Microextraction (HS-SPME)
3.3. Hydrodistillation (HD)
3.4. Gas Chromatography Mass Spectrometry Analysis (GC-MS)
3.5. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siamopoulou, P.; Bimplakis, A.; Iliopoulou, D.; Vagias, C.; Cos, P.; Vanden Berghe, D.; Roussis, V. Diterpenes from the Brown Algae Dictyota dichotoma and Dictyota linearis. Phytochemistry 2004, 65, 2025–2030. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, S.A.; Kalinovsky, A.I.; Fedorov, S.N.; Shubina, L.K.; Stonik, V.A. Diterpenes from the Far-Eastern Brown Alga Dictyota dichotoma. Phytochemistry 2006, 67, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Amico, V.; Oriente, G.; Piattelli, M.; Tringali, C.; Fattorusso, E.; Magno, S.; Mayol, L. Diterpenes Based on the Dolabellane Skeleton from Dictyota dichotoma. Tetrahedron 1980, 36, 1409–1414. [Google Scholar] [CrossRef]
- Durán, R.; Zubía, E.; Ortega, M.J.; Salvá, J. New Diterpenoids from the Alga Dictyota dichotoma. Tetrahedron 1997, 53, 8675–8688. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Zhao, Z.; Xia, X.; Li, B.; Zhang, J.; Yan, X. Diterpenes from the Marine Algae of the Genus Dictyota. Mar. Drugs 2018, 16, 159. [Google Scholar] [CrossRef] [Green Version]
- Abou-El-Wafa, G.; Shaaban, M.; Shaaban, K.; El-Naggar, M.; Maier, A.; Fiebig, H.; Laatsch, H. Pachydictyols B and C: New Diterpenes from Dictyota dichotoma Hudson. Mar. Drugs 2013, 11, 3109–3123. [Google Scholar] [CrossRef]
- De Rosa, S.; De Stefano, S.; Zavodnik, N. Hydroazulenoid Diterpenes from the Brown Alga Dictyota dichotoma Var. Implexa. Phytochemistry 1986, 25, 2179–2181. [Google Scholar] [CrossRef]
- Palermo, J.; Bernardo, J.; Seldes, A. In Dictyol-d-2-Beta-Acetate and Other Diterpenoids from the Brown Alga Dictyota dichotoma. An. Asoc. Quim. Argent. 1994, 82, 355–358. [Google Scholar]
- Rabanal, M.; Ponce, N.M.A.; Navarro, D.A.; Gómez, R.M.; Stortz, C.A. The System of Fucoidans from the Brown Seaweed Dictyota dichotoma: Chemical Analysis and Antiviral Activity. Carbohydr. Polym. 2014, 101, 804–811. [Google Scholar] [CrossRef]
- Bakar, K.; Mohamad, H.; Latip, J.; Tan, H.S.; Herng, G.M. Fatty Acids Compositions of Sargassum Granuliferum and Dictyota dichotoma and Their Anti-Fouling Activities. J. Sustain. Sci. Manag. 2017, 12, 8–16. [Google Scholar]
- Deyab, M.; Ward, F.; Deyab, M.; Elkatony, T.; Student, P.D. Qualitative and Quantitative Analysis of Phytochemical Studies on Brown Seaweed, Dictyota dichotoma. Int. J. Eng. Dev. Res. 2016, 4, 674–678. [Google Scholar]
- Generalić Mekinić, I.; Šimat, V.; Botić, V.; Crnjac, A.; Smoljo, M.; Soldo, B.; Ljubenkov, I.; Čagalj, M.; Skroza, D. Bioactive Phenolic Metabolites from Adriatic Brown Algae Dictyota dichotoma and Padina pavonica (Dictyotaceae). Foods 2021, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Kamenarska, Z.; Gasic, M.J.; Zlatovic, M.; Rasovic, A.; Sladic, D.; Kljajic, Z.; Stefanov, K.; Seizova, K.; Najdenski, H.; Kujumgiev, A.; et al. Chemical Composition of the Brown Alga Padina pavonia (L.) Gaill. from the Adriatic Sea. Bot. Mar. 2002, 45, 339–345. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z.; Roje, M.; Kuś, P.M.; Jokić, S.; Čož-Rakovac, R. Phytochemical Study of the Headspace Volatile Organic Compounds of Fresh Algae and Seagrass from the Adriatic Sea (Single Point Collection). PLoS ONE 2018, 13, e0196462. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, J.; Chen, L.; Shi, L.; Liu, H. Characteristic Volatile Composition of Seven Seaweeds from the Yellow Sea of China. Mar. Drugs 2021, 19, 192. [Google Scholar] [CrossRef]
- Moore, R.E. Volatile Compounds from Marine Algae. Acc. Chem. Res. 1977, 10, 40–47. [Google Scholar] [CrossRef]
- Müller, D.G.; Gassmann, G.; Boland, W.; Marner, F.; Jaenicke, L. Dictyota dichotoma (Phaeophyceae): Identification of the Sperm Attractant. Science 1981, 212, 1040–1041. [Google Scholar] [CrossRef]
- Kim, S.-K. Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons Ltd.: Chichester, UK, 2012. [Google Scholar]
- Han, J.; Chan, H.W.S.; Calvin, M. Biosynthesis of Alkanes in Nostoc muscorum. J. Am. Chem. Soc. 1969, 91, 5156–5159. [Google Scholar] [CrossRef]
- Youngblood, W.W.; Blumer, M.; Guillard, R.L.; Fiore, F. Saturated and Unsaturated Hydrocarbons in Marine Benthic Algae. Mar. Biol. 1971, 8, 190–201. [Google Scholar] [CrossRef]
- Youngblood, W.W.; Blumer, M. Alkanes and Alkenes in Marine Benthic Algae. Mar. Biol. 1973, 21, 163–172. [Google Scholar] [CrossRef]
- Clark, R.C., Jr.; Blumer, M. Distribution of N-Paraffins in Marine Organisms and Sediment. Limnol. Oceanogr. 1967, 12, 79–87. [Google Scholar] [CrossRef]
- Caamal-Fuentes, E.; Moo-Puc, R.; Freile-Pelegrín, Y.; Robledo, D. Cytotoxic and Antiproliferative Constituents from Dictyota ciliolata, Padina sanctae-crucis and Turbinaria tricostata. Pharm. Biol. 2014, 52, 1244–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayyad, S.-E.N.; Makki, M.S.; Al-kayal, N.S.; Basaif, S.A.; El-Foty, K.O.; Asiri, A.M.; Alarif, W.M.; Badria, F.A. Cytotoxic and Protective DNA Damage of Three New Diterpenoids from the Brown Alga Dictoyota dichotoma. Eur. J. Med. Chem. 2011, 46, 175–182. [Google Scholar] [CrossRef]
- Siless, G.E.; García, M.; Pérez, M.; Blustein, G.; Palermo, J.A. Large-Scale Purification of Pachydictyol A from the Brown Alga Dictyota dichotoma Obtained from Algal Wash and Evaluation of Its Antifouling Activity against the Freshwater Mollusk Limnoperna Fortunei. J. Appl. Phycol. 2018, 30, 629–636. [Google Scholar] [CrossRef]
- Simas, D.L.R.; Kaiser, C.R.; Gestinari, L.M.; Duarte, H.M.; de Paula, J.C.; Soares, A.R. Diterpenes from the Brown Seaweed Dictyota caribaea (Dictyotaceae, Phaeophyceae): The Ecological and Taxonomic Significance. Biochem. Syst. Ecol. 2014, 52, 33–37. [Google Scholar] [CrossRef]
- Pereira, R.; Lourenço, A.; Terra, L.; Abreu, P.; Laneuville Teixeira, V.; Castro, H. Marine Diterpenes: Molecular Modeling of Thrombin Inhibitors with Potential Biotechnological Application as an Antithrombotic. Mar. Drugs 2017, 15, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, A.D.; König, G.M.; Sticher, O. New and Highly Oxidised Hydroazulenoid Diterpenes from the Tropical Marine Brown Alga Dictyota volubilis. Tetrahedron 1993, 49, 571–580. [Google Scholar] [CrossRef]
- Lai, K.-H.; You, W.-J.; Lin, C.-C.; El-Shazly, M.; Liao, Z.-J.; Su, J.-H. Anti-Inflammatory Cembranoids from the Soft Coral Lobophytum crassum. Mar. Drugs 2017, 15, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, I.; Miguel, M.; Mnif, W. A Brief Review on New Naturally Occurring Cembranoid Diterpene Derivatives from the Soft Corals of the Genera Sarcophyton, Sinularia, and Lobophytum Since 2016. Molecules 2019, 24, 781. [Google Scholar] [CrossRef] [Green Version]
- Bozan, B.; Ozek, T.; Kurkcuoglu, M.; Kirimer, N.; Can Baser, K. The Analysis of Essential Oil and Headspace Volatiles of the Flowers of Pelargonium endlicherianum Used as an Anthelmintic in Folk Medicine. Planta Med. 1999, 65, 781–782. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Tabanelli, G.; Özogul, F.; Šimat, V. Maximizing the Antioxidant Capacity of Padina pavonica by Choosing the Right Drying and Extraction Methods. Processes 2021, 9, 587. [Google Scholar] [CrossRef]



| No. | Compound | RI | May | June | July | August | September | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fr | Dr | Fr | Dr | Fr | Dr | Fr | Dr | Fr | Dr | |||
| Area (%) | ||||||||||||
| 1 | Pentanal a | <900 | - | - | - | 0.20 | - | 0.12 | - | 0.06 | - | 0.07 |
| 2 | Pent-1-en-ol | <900 | - | 0.07 | - | 0.21 | - | - | - | - | - | - |
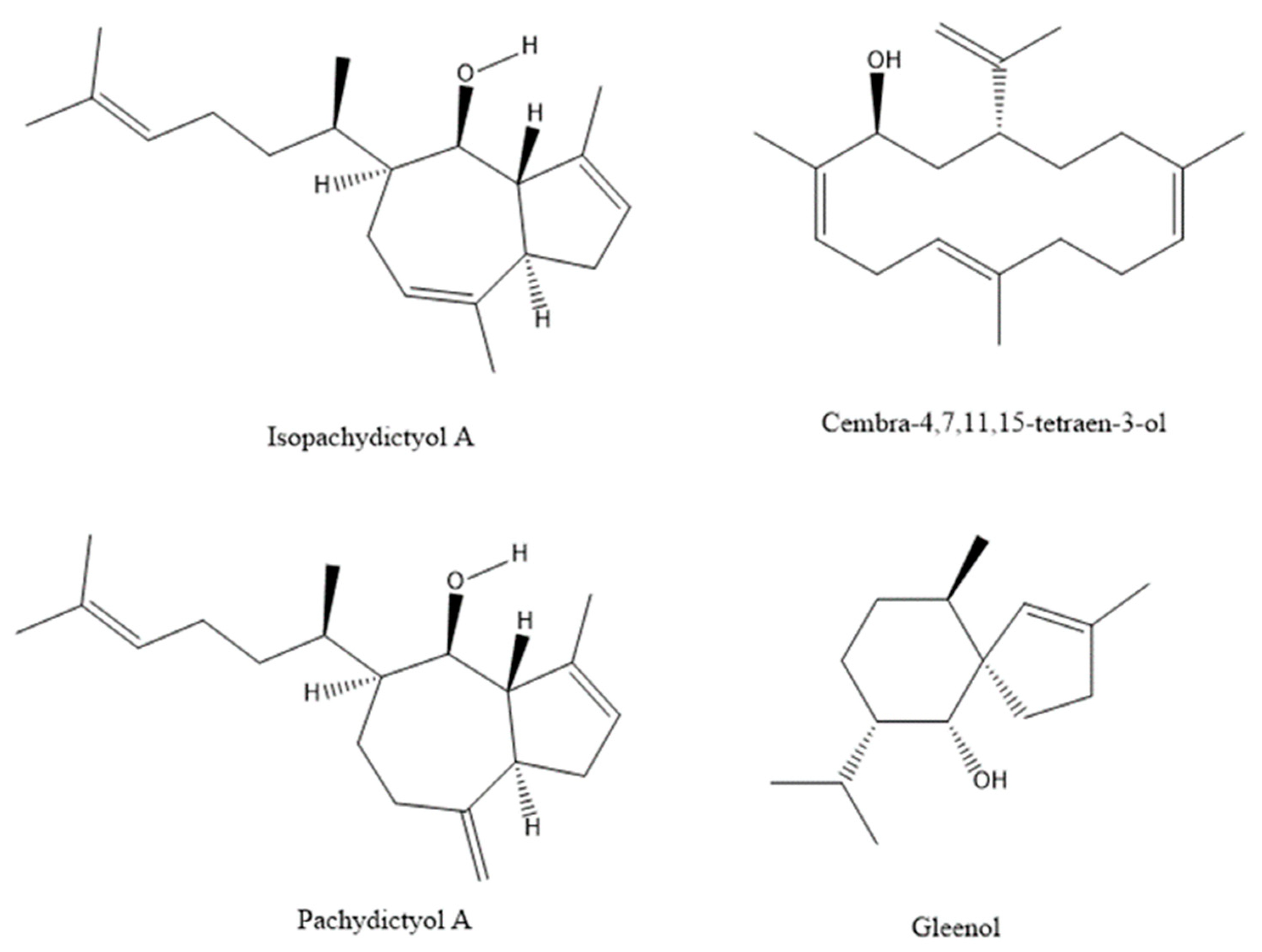
| 3 | Pentan-1-ol a | <900 | - | - | 0.38 | 0.12 | 0.18 | - | - | - | 0.89 | |
| 4 | Hexanal a | <900 | - | 0.05 | - | 0.16 | - | 0.17 | - | 0.13 | 0.52 | 0.06 |
| 5 | Hexan-1-ol a | <900 | - | - | - | - | 0.32 | - | 0.44 | - | 0.39 | - |
| 6 | Heptanal a | 907 | - | 0.08 | - | 0.15 | - | - | - | 0.15 | - | - |
| 7 | Benzaldehyde a | 970 | 0.25 | 0.13 | 4.88 | 1.22 | 3.28 | 0.61 | 2.49 | 0.27 | 3.21 | 0.15 |
| 8 | Oct-1-en-3-one | 979 | 0.22 | 0.16 | 6.36 | 0.28 | 3.78 | 0.22 | 4.19 | 0.11 | 3.89 | 0.16 |
| 9 | Oct-1-en-3-ol | 985 | 0.41 | 0.51 | 16.40 | 0.89 | 12.03 | 1.26 | 15.87 | 0.62 | 19.85 | 0.07 |
| 10 | Octan-3-one a | 991 | 0.55 | - | 0.90 | - | 0.55 | - | 1.44 | - | 1.20 | - |
| 11 | 6-Methylhept-5-en-2-one a | 992 | - | 0.24 | - | 0.60 | - | 0.26 | - | 0.15 | - | - |
| 12 | 2-Pentylfuran a | 996 | - | 0.08 | - | 0.13 | 0.30 | 0.14 | - | 0.08 | - | - |
| 13 | (E,E)-Hepta-2,4-dienal | 1016 | - | - | - | 0.09 | - | - | - | - | - | - |
| 14 | Benzyl alcohol a | 1042 | - | 0.68 | - | 0.74 | 0.52 | 2.24 | - | 0.33 | - | 0.33 |
| 15 | (E)-β-Ocimene | 1044 | - | - | - | - | 0.81 | - | - | - | - | - |
| 16 | Phenylacetaldehyde a | 1052 | - | 0.22 | 0.99 | - | 0.97 | 0.70 | 1.20 | - | 0.56 | - |
| 17 | (E)-Oct-3-en-1-ol | 1056 | - | - | 0.52 | - | - | - | - | - | - | - |
| 18 | γ-Caprolactone a | 1062 | - | - | - | 0.30 | - | - | - | - | - | - |
| 19 | (E)-Oct-2-en-1-al | 1064 | - | - | 2.10 | 0.14 | 1.20 | 0.31 | 1.26 | 0.22 | 4.38 | - |
| 20 | γ-Terpinene a | 1066 | - | - | - | - | 0.31 | - | - | - | - | - |
| 21 | (Z)-Oct-2-en-1-ol | 1073 | - | 0.16 | 6.96 | 0.24 | 6.53 | 0.63 | 7.49 | 0.14 | 10.74 | 0.05 |
| 22 | Octan-1-ol a | 1076 | 0.46 | 0.20 | 4.62 | 0.23 | 3.29 | 0.26 | 4.27 | - | 7.42 | - |
| 23 | Nonanal a | 1108 | - | 0.06 | 0.71 | 0.16 | 0.72 | 0.35 | - | 0.44 | 1.46 | 0.16 |
| 24 | 2,6-Dimethylcyclohexan-1-ol | 1114 | - | 0.45 | 1.24 | 0.43 | 0.61 | 0.29 | 0.63 | 0.07 | 0.35 | - |
| 25 | 4-Ketoisophorone a | 1151 | - | 0.20 | - | - | - | - | - | - | - | - |
| 26 | 6-[(1Z)-butenyl]-1,4-cycloheptadiene] (Dictyopterene D’) | 1158 | 3.01 | - | 0.46 | - | 4.42 | - | 1.57 | - | 0.62 | - |
| 27 | (E)-Non-2-enal | 1165 | - | - | 1.32 | - | 1.27 | 0.30 | 0.76 | 0.19 | 1.97 | 0.09 |
| 28 | [6-Butyl-1,4-cycloheptadiene] (Dictyopterene C’) | 1175 | 0.46 | - | - | - | 0.54 | 1.03 | - | - | - | - |
| 29 | β-Cyclocitral | 1226 | - | 0.10 | 0.72 | 0.16 | 0.38 | 0.22 | 0.47 | - | - | - |
| 30 | Tridecane a | 1300 | - | 0.10 | 0.21 | - | 0.20 | - | 0.14 | - | 0.09 | |
| 31 | (E,E)-Deca-2,4-dienal | 1320 | - | - | 0.89 | - | - | - | - | - | - | - |
| 32 | α-Cubebene | 1355 | 1.98 | - | 0.15 | - | 0.41 | - | 0.30 | - | - | - |
| 33 | α-Copaene a | 1376 | 0.23 | - | - | - | - | - | - | - | - | - |
| 34 | β-Bourbonene | 1388 | 0.63 | 0.12 | 0.57 | 0.28 | 1.45 | 0.44 | 0.95 | 0.14 | 0.38 | 0.06 |
| 35 | β-Cubebene | 1393 | 1.21 | - | 0.88 | - | 0.44 | - | 0.22 | - | 0.16 | - |
| 36 | Tetradecane a | 1400 | - | 0.10 | - | 0.09 | - | - | - | 0.11 | - | - |
| 37 | β-Gurjunene | 1432 | 5.73 | - | - | - | 0.50 | - | - | - | - | - |
| 38 | α-Humulene a | 1455 | 5.85 | - | - | - | - | - | - | - | - | - |
| 39 | epi- Bicyclosesquiphellandrene | 1468 | 2.85 | - | 0.42 | - | 0.47 | - | 0.45 | - | 0.16 | - |
| 40 | γ-Curcumene | 1476 | 1.80 | - | - | - | - | - | - | - | - | - |
| 41 | α-Amorphene | 1478 | 3.40 | - | 0.44 | - | 0.28 | - | 0.28 | - | 0.03 | - |
| 42 | γ-Muurolene | 1481 | 1.39 | 0.03 | 0.57 | 0.10 | 1.32 | 0.23 | 0.63 | 0.12 | 0.16 | 0.08 |
| 43 | Germacrene D | 1485 | 6.11 | 0.09 | 1.82 | 0.19 | 6.35 | 0.67 | 2.68 | 0.45 | 0.67 | 0.10 |
| 44 | (E)-β-Ionone | 1490 | 2.45 | 0.46 | 1.87 | 0.47 | 0.71 | 0.67 | 0.93 | 0.40 | 0.54 | 0.17 |
| 45 | Pentadec-1-ene | 1495 | 3.98 | 0.10 | 2.03 | 0.15 | 2.47 | 0.16 | 1.53 | 0.17 | 0.92 | 0.09 |
| 46 | Germacrene C | 1498 | 3.30 | - | 0.51 | - | 1.06 | - | 1.04 | - | 0.48 | - |
| 47 | Pentadecane a | 1500 | 7.57 | 85.08 | 9.26 | 83.56 | 23.41 | 74.06 | 16.78 | 75.42 | 4.92 | 92.83 |
| 48 | δ-Cadinene a | 1511 | 6.43 | - | - | - | - | - | - | - | - | - |
| 49 | Tridecanal a | 1514 | 3.12 | 0.07 | 6.73 | 0.22 | 4.57 | 0.19 | 11.08 | 0.96 | 23.24 | 0.77 |
| 50 | γ-Cadinene | 1518 | 1.35 | 0.17 | 1.67 | 0.19 | 1.45 | 0.32 | 2.19 | 0.36 | 1.08 | 0.18 |
| 51 | (E)-Calamenene | 1528 | 3.27 | 0.10 | 0.79 | 0.14 | 1.55 | 0.34 | 0.85 | 0.16 | 0.18 | 0.07 |
| 52 | γ-Selinene | 1530 | 1.03 | - | 1.44 | - | 1.94 | - | 1.23 | - | 0.27 | - |
| 53 | Dihydroactinidiolide | 1533 | - | 0.16 | - | 0.15 | - | 0.20 | - | 0.21 | - | 0.10 |
| 54 | (E)-Cadina-1,4-diene | 1537 | 1.09 | - | 0.32 | - | 0.42 | - | 0.40 | - | 0.16 | - |
| 55 | α-Calacorene | 1548 | 0.58 | - | 0.36 | - | 0.29 | - | 0.28 | - | 0.09 | - |
| 56 | Dactylol | 1557 | 8.10 | - | 0.16 | - | 0.04 | - | 0.18 | - | 0.04 | - |
| 57 | Germacrene-4-ol | 1578 | 0.84 | - | 1.29 | - | 0.09 | - | 0.08 | - | 0.06 | - |
| 58 | Gleenol | 1589 | 0.71 | 0.34 | 1.14 | 0.17 | 0.79 | 0.21 | 0.54 | 0.14 | 0.40 | - |
| 59 | Epicubenol | 1616 | 11.79 | - | 0.56 | - | 0.18 | - | 0.43 | - | 0.87 | - |
| 60 | τ-Cadinol | 1630 | 0.33 | - | 0.09 | - | 0.05 | - | 0.08 | - | 0.06 | - |
| 61 | Cubenol | 1647 | 0.45 | - | 0.45 | - | 0.28 | - | 0.34 | - | 0.16 | - |
| 62 | Heptadecane a | 1700 | 0.18 | 0.16 | 2.51 | 0.15 | 1.62 | 0.29 | 3.53 | 0.28 | 3.42 | 0.19 |
| 63 | Pentadecanal a | 1718 | 0.15 | - | 1.14 | - | 0.88 | - | 1.87 | - | 1.90 | - |
| 64 | Isopachydictyol A | 2127 | 0.92 | - | 3.55 | - | 1.19 | - | 1.79 | - | 0.34 | - |
| No. | Compound | RI | May | June | July | August | September | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fr | Dr | Fr | Dr | Fr | Dr | Fr | Dr | Fr | Dr | |||
| Area (%) | ||||||||||||
| 1 | Dimethyl sulfide | <900 | - | - | - | 0.29 | - | 2.09 | - | 1.03 | - | 3.14 |
| 2 | Pentanal a | <900 | - | - | - | 0.29 | - | 0.31 | - | 0.13 | - | 0.08 |
| 3 | Pent-1-en-3-ol | <900 | - | 0.07 | - | 0.27 | - | 0.10 | - | 0.05 | - | 0.12 |
| 4 | (Z)-Pent-2-en-1-ol | <900 | - | - | 0.71 | - | 0.29 | - | 0.20 | - | 0.43 | - |
| 5 | Hexanal a | <900 | - | 0.05 | 0.53 | 0.20 | 0.41 | 0.32 | 0.19 | 0.27 | 0.31 | 0.09 |
| 6 | 3-Methylbutanoic acid | <900 | - | 0.10 | - | 1.73 | - | 0.30 | - | 0.31 | - | 0.37 |
| 7 | 2-Methylbutanoic acid | <900 | - | - | - | 0.41 | - | - | - | - | - | - |
| 8 | Hexan-1-ol a | <900 | - | - | - | - | 0.37 | - | 0.52 | - | 0.47 | - |
| 9 | Heptanal a | 907 | - | - | - | 0.16 | - | 0.30 | - | 0.36 | - | - |
| 10 | Benzaldehyde a | 970 | 0.20 | 0.06 | 2.04 | 1.11 | 1.31 | 0.67 | 0.99 | 0.23 | 4.00 | 0.13 |
| 11 | 1,3,5-Trimethylbenzene a (Mesitylene) | 976 | - | - | 0.23 | - | 0.16 | - | 0.16 | - | 0.24 | - |
| 12 | Oct-1-en-3-one | 980 | - | 0.21 | - | 0.53 | - | 0.37 | - | 0.13 | - | 0.06 |
| 13 | 3,5,5-Trimethylhex-2-ene | 980 | 0.43 | - | 9.33 | - | 5.06 | - | 6.35 | - | 5.99 | - |
| 14 | Oct-1-en-3-ol | 984 | 0.92 | 0.65 | 27.25 | 1.36 | 17.59 | 1.95 | 23.19 | 0.93 | 24.37 | 0.27 |
| 15 | Octan-3-one | 991 | 0.59 | - | 2.00 | - | 0.98 | - | 2.89 | - | 0.97 | - |
| 16 | 6-Methylhept-5-en-2-one a | 992 | - | 0.29 | - | 0.93 | - | 0.50 | - | 0.22 | - | - |
| 17 | 2-Pentylfuran a | 996 | - | 0.06 | 0.63 | 0.23 | 0.76 | 0.34 | 0.33 | 0.18 | 0.26 | - |
| 18 | Octanal a | 1007 | - | - | - | - | 0.25 | - | - | - | - | - |
| 19 | (E,E)-Hepta-2,4-dienal | 1017 | - | 0.03 | 0.33 | 0.11 | 0.21 | 0.16 | - | 0.11 | 0.77 | - |
| 20 | p-Cymene a | 1032 | - | - | - | - | 0.21 | - | - | - | - | - |
| 21 | (E)-3-Ethyl-2-methylhexa-1,3-diene | 1040 | - | - | 0.91 | - | 0.44 | - | 0.65 | - | 0.66 | - |
| 22 | Benzyl alcohol a | 1041 | - | 0.56 | - | 0.77 | 0.40 | 2.43 | - | - | - | - |
| 23 | (E)-β-Ocimene | 1044 | - | - | - | - | 1.98 | - | - | - | - | - |
| 24 | Phenylacetaldehyde a | 1052 | - | 0.05 | 1.00 | 0.35 | 0.96 | 1.01 | 1.11 | 0.45 | 0.78 | 0.38 |
| 25 | (E)-Oct-3-en-1-ol | 1056 | - | - | 0.40 | - | - | - | - | - | - | - |
| 26 | γ-Caprolactone a (5-Ethyloxolan-2-one) | 1061 | - | 0.22 | - | - | - | - | - | - | - | - |
| 27 | (E)-Oct-2-enal | 1065 | 0.28 | 0.06 | 5.54 | 0.24 | 4.06 | 0.43 | 3.13 | 0.32 | 2.51 | - |
| 28 | (E)-Oct-2-en-1-ol | 1073 | 0.19 | 0.19 | 6.93 | 0.25 | 6.53 | 1.02 | 8.35 | 0.24 | 15.97 | 0.06 |
| 29 | Octan-1-ol a | 1076 | 0.47 | 0.24 | 3.92 | 0.27 | 3.00 | 0.33 | 4.21 | 0.17 | 9.98 | 0.04 |
| 30 | (E,Z)-Octa-3,5-dien-2-one | 1098 | - | - | 1.80 | - | - | - | - | - | - | - |
| 31 | Nonanal a | 1108 | 0.20 | 0.07 | 1.51 | 0.11 | 1.40 | 0.57 | 0.51 | 0.81 | 1.39 | 0.18 |
| 32 | 2,6-Dimethylcyclohexan-1-ol | 1114 | - | 0.66 | 1.37 | 0.70 | 0.50 | 0.46 | 0.50 | 0.16 | 0.57 | 0.04 |
| 33 | Oct-1-en-3-yl acetate | 1116 | - | - | 0.60 | - | 0.25 | - | 0.60 | - | 0.37 | - |
| 34 | 4-Ketoisophorone a | 1150 | - | 0.16 | - | 0.08 | - | - | - | - | - | - |
| 35 | 6-[(1Z)-Butenyl]-1,4-cycloheptadiene] (Dictyopterene D’) | 1158 | 8.22 | 0.07 | 1.53 | 0.09 | 7.21 | 1.74 | 2.77 | 0.11 | 0.63 | 0.10 |
| 36 | (Z)-Non-2-enal | 1165 | - | - | 1.80 | - | 1.70 | 0.32 | 1.05 | 0.24 | 1.63 | 0.10 |
| 37 | [6-Butyl-1,4-cycloheptadiene] (Dictyopterene C’) | 1175 | 1.69 | - | - | - | 0.98 | 2.32 | 0.76 | - | - | - |
| 38 | (Z)-Dec-4-enal | 1197 | - | 0.06 | - | 0.18 | - | 0.20 | - | 0.23 | - | 0.07 |
| 39 | β-Cyclocitral | 1226 | - | 0.11 | 0.55 | 0.20 | 0.28 | 0.29 | 0.34 | 0.13 | 0.51 | 0.04 |
| 40 | Tridecane a | 1300 | - | 0.21 | - | 0.45 | - | 0.25 | - | 0.23 | - | 0.09 |
| 41 | (E,E)-Deca-2,4-dienal | 1321 | - | - | 0.42 | - | - | - | - | - | - | - |
| 42 | α-Cubebene | 1355 | 1.56 | 0.07 | - | 0.08 | - | 0.12 | - | 0.05 | - | 0.00 |
| 43 | β-Bourbonene | 1389 | 1.65 | 0.18 | 0.41 | 0.50 | 1.50 | 0.81 | 0.97 | 0.41 | 0.04 | |
| 44 | β-Cubebene | 1394 | 1.82 | 0.20 | - | - | 0.24 | - | - | - | - | - |
| 45 | Tetradec-1-ene | 1395 | - | 0.10 | - | 0.26 | - | 0.30 | - | 0.22 | - | 0.05 |
| 46 | Tetradecane a | 1400 | - | 0.15 | - | 0.12 | - | 0.16 | - | 0.12 | - | 0.07 |
| 47 | β-Gurjunene | 1432 | 3.92 | - | - | - | - | - | - | - | - | - |
| 48 | β-Cubebene | 1434 | - | - | - | - | 0.28 | - | - | - | - | - |
| 49 | α-Humulene a | 1455 | 3.86 | - | - | - | - | - | - | - | - | - |
| 50 | β-Farnesene | 1462 | 0.18 | - | - | - | - | - | - | - | - | - |
| 51 | epi-Bicyclosesquiphellandrene | 1468 | 2.28 | - | - | - | - | - | - | - | - | - |
| 52 | γ-Curcumene | 1476 | 1.79 | - | - | - | - | - | - | - | - | - |
| 53 | α-Amorphene | 1478 | 2.51 | - | - | - | - | - | - | - | - | - |
| 54 | γ-Muurolene | 1481 | 1.07 | - | - | - | 0.57 | 0.21 | 0.37 | 0.15 | - | - |
| 55 | Germacrene D | 1485 | 14.39 | - | 1.33 | 0.27 | 5.48 | 0.82 | 2.95 | 0.67 | 0.56 | 0.11 |
| 57 | (E)-β-Ionone | 1490 | 1.84 | 0.43 | 0.69 | 0.44 | 0.38 | 0.49 | 0.56 | 0.36 | 0.41 | 0.20 |
| 58 | Pentadec-1-ene | 1495 | 3.69 | 0.14 | 1.10 | 0.16 | 1.45 | 0.16 | 1.36 | 0.15 | 0.38 | 0.06 |
| 59 | Germacrene C | 1498 | 4.59 | - | - | - | 0.64 | - | 0.80 | - | 0.27 | - |
| 60 | Pentadecane a | 1500 | 5.20 | 84.97 | 9.15 | 79.15 | 9.13 | 62.41 | 11.51 | 72.00 | 2.14 | 88.23 |
| 61 | δ-Cadinene a | 1511 | 3.45 | - | - | - | - | - | - | - | - | - |
| 62 | Tridecanal a | 1514 | 2.81 | - | 3.76 | - | 3.60 | - | 7.01 | 1.04 | 13.89 | 0.74 |
| 63 | γ-Cadinene | 1518 | 1.20 | - | 0.70 | - | 0.80 | - | 1.41 | 0.30 | 0.77 | - |
| 64 | (E)-Calamenene | 1528 | 2.04 | 0.23 | 0.27 | - | 0.56 | 0.17 | 0.28 | 0.14 | 0.21 | - |
| 65 | γ-Selinene | 1530 | 0.77 | - | 0.15 | - | 0.04 | - | 0.56 | - | 0.46 | - |
| 66 | Dihydroactinidiolide | 1533 | - | 0.10 | - | - | - | - | - | - | - | - |
| 67 | (E)-Cadina-1,4-diene | 1537 | 0.89 | - | 0.20 | - | 0.16 | - | 0.17 | - | 0.37 | - |
| 68 | α-Calacorene | 1552 | 0.19 | - | 0.05 | - | 0.07 | - | - | - | - | - |
| 69 | Dactylol | 1557 | 15.38 | - | 0.11 | - | 0.04 | - | 0.04 | - | 0.05 | - |
| 70 | Germacrene-4-ol | 1578 | 0.34 | - | 0.20 | - | 0.04 | - | 0.06 | - | 0.06 | - |
| 71 | Gleenol | 1589 | 0.58 | 0.26 | 0.65 | - | 0.24 | 0.18 | 0.27 | - | - | - |
| 72 | Hexadecane a | 1600 | - | 0.08 | - | 0.08 | - | 0.21 | - | 0.13 | - | 0.07 |
| 73 | Epicubenol | 1616 | 2.56 | - | 0.30 | - | 0.09 | - | 0.22 | - | 0.22 | - |
| 74 | τ-Cadinol | 1630 | 0.22 | - | 0.10 | - | - | - | - | - | - | - |
| 75 | Cubenol | 1647 | 0.21 | - | 0.18 | - | 0.09 | - | 2.18 | - | 0.10 | - |
| 76 | Heptadecane a | 1700 | 0.14 | 0.14 | 0.91 | 0.13 | 0.86 | 0.21 | 1.89 | 0.37 | 2.35 | 0.22 |
| 77 | Pentadecanal a | 1718 | 0.12 | - | 0.41 | - | 0.43 | - | 0.87 | - | 1.36 | - |
| 78 | Isopachydictyol A | 2127 | 0.34 | - | 0.71 | - | 0.31 | - | 0.57 | - | - | - |
| No. | Compound | RI | May | June | July | August | September | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fr | Dr | Fr | Dr | Fr | Dr | Fr | Dr | Fr | Dr | |||
| Area (%) | ||||||||||||
| 1 | (E)-Hex-2-enal | <900 | 0.14 | 0.03 | 0.12 | 0.05 | - | - | - | 0.51 | - | - |
| 2 | (Z)-Hex-3-en-1-ol | <900 | 0.01 | - | 0.01 | - | 0.01 | 0.08 | 0.10 | 0.05 | ||
| 3 | Ethylbenzene | <900 | 0.10 | - | 0.08 | - | - | - | - | - | - | - |
| 4 | Hexan-1-ol a | <900 | - | - | - | - | 0.01 | - | 0.27 | - | 0.15 | - |
| 5 | 1,4-Xylene | <900 | 0.16 | - | 0.02 | - | 0.01 | - | - | - | - | - |
| 6 | (E,E)-Octa-1,3,5-triene | <900 | 0.17 | - | 0.15 | - | 0.01 | - | - | - | 0.16 | - |
| 7 | (E)-Hept-4-enal | 901 | 0.09 | 0.03 | 0.05 | 0.04 | - | - | - | - | - | |
| 8 | Heptanal a | 904 | 0.05 | 0.06 | 0.08 | 0.10 | - | 0.03 | 0.10 | - | 0.04 | 0.05 |
| 9 | Benzaldehyde a | 968 | 0.05 | 0.06 | 0.06 | 0.09 | - | 0.03 | 0.10 | 0.01 | 0.12 | 0.11 |
| 10 | Heptan-1-ol a | 974 | 0.06 | - | 0.06 | - | - | - | 0.07 | - | - | - |
| 11 | 3,5,5-Trimethylhex-2-ene | 978 | - | 0.09 | - | 0.18 | - | 0.04 | - | 0.01 | - | 0.12 |
| 12 | Oct-1-en-3-one | 978 | 2.30 | - | 2.27 | - | 0.13 | - | 1.34 | - | 1.57 | - |
| 13 | Oct-1-en-3-ol | 983 | 3.64 | 0.17 | 5.26 | 0.58 | 0.40 | 0.20 | 5.41 | 0.09 | 4.55 | 0.49 |
| 14 | Octan-3-one | 990 | 0.15 | - | 0.22 | - | 0.01 | - | 0.21 | - | 0.23 | - |
| 15 | 2-Pentylfuran a | 995 | 0.14 | 0.04 | 0.20 | 0.09 | 0.01 | 0.03 | 0.12 | 0.14 | 0.11 | |
| 16 | Octan-3-ol | 997 | - | - | 0.04 | - | - | - | - | - | - | - |
| 17 | (E,Z)-Hepta-2,4-dienal | 999 | 0.06 | - | 0.11 | - | - | - | - | - | - | - |
| 18 | 2-[(E)-Pent-1-enyl]furan | 1005 | 0.07 | - | 0.07 | - | - | - | - | - | - | - |
| 19 | (E,E)-Hepta-2,4-dienal | 1014 | 0.15 | - | 0.14 | - | 0.02 | - | 0.10 | - | - | - |
| 20 | Limonene a | 1035 | - | - | - | - | - | - | 0.15 | - | 0.07 | - |
| 21 | (3E)-3-Ethyl-2-methylhexa-1,3-diene | 1038 | 0.01 | - | 0.06 | - | - | - | - | - | - | - |
| 22 | Benzyl alcohol a | 1042 | - | - | - | - | - | - | - | - | - | 0.05 |
| 23 | Phenylacetaldehyde a | 1050 | 0.11 | 0.14 | 0.23 | 0.26 | 0.02 | 0.07 | 0.30 | 0.02 | 0.28 | 0.14 |
| 24 | (E)-Oct-2-enal | 1063 | 0.42 | - | 0.77 | 0.09 | 0.08 | 0.04 | 1.09 | 0.02 | 0.91 | 0.15 |
| 25 | (E)-Oct-2-en-1-ol | 1073 | 2.87 | 0.08 | 3.49 | 0.28 | 0.22 | 0.11 | 2.99 | 0.03 | 2.73 | 0.19 |
| 26 | Octan-1-ol a | 1076 | 2.57 | 0.07 | 2.42 | 0.25 | 0.14 | 0.06 | 1.55 | 0.01 | 2.25 | 0.16 |
| 27 | (E,E)-Octa-3,5-dien-2-one | 1080 | 0.20 | - | 0.28 | - | - | - | - | - | 0.27 | - |
| 28 | (E,Z)-Octa-3,5-dien-2-one | 1097 | 0.18 | - | 0.24 | 0.21 | - | - | 0.14 | - | 0.08 | 0.05 |
| 29 | Nonanal a | 1107 | 0.18 | 0.07 | 0.22 | 0.15 | 0.02 | 0.04 | 0.15 | 0.02 | 0.17 | 0.11 |
| 30 | (E,E)-Octa-2,4-dienal | 1113 | 0.19 | - | 0.24 | 0.11 | 0.01 | 0.03 | 0.25 | 0.13 | 0.04 | |
| 31 | Bicyclo [3.2.0]hept-6-ene | 1120 | 0.69 | - | 0.76 | - | 0.55 | 0.25 | 1.39 | 0.04 | 2.07 | 0.20 |
| 32 | 2-Hydroxyisophorone (2-Hydroxy-3,5,5-trimethylcyclohex-2-en-1-one) | 1122 | 0.10 | - | 0.09 | - | 0.02 | - | 0.07 | - | 0.08 | - |
| 33 | 4-Ketoisophorone a | 1150 | - | - | - | 0.05 | - | - | - | - | - | - |
| 34 | (E,Z)-Nona-2,6-dienal | 1158 | 0.90 | 0.17 | 0.78 | 0.32 | 0.21 | 0.12 | 0.66 | 0.04 | 0.79 | 0.26 |
| 35 | (E)-Non-2-enal | 1165 | 0.40 | 0.13 | 0.64 | 0.31 | 0.04 | 0.08 | 0.64 | 0.05 | 0.50 | 0.25 |
| 36 | [6-Butyl-1,4-cycloheptadiene] (Dictyopterene C’) | 1174 | 0.08 | - | 0.09 | - | 0.04 | - | 0.12 | - | 0.13 | - |
| 37 | 2,4-Dimethylbenzaldehyde a | 1180 | 0.13 | - | 0.17 | 0.09 | 0.03 | - | 0.24 | - | 0.11 | 0.08 |
| 38 | 3,4-Dimethylphenol | 1197 | - | - | - | 0.06 | - | 0.03 | - | - | - | 0.07 |
| 39 | β-Cyclocitral | 1226 | 0.14 | 0.05 | 0.13 | 0.11 | - | 0.04 | 0.10 | 0.01 | 0.07 | 0.08 |
| 40 | β-Cyclohomocitral | 1263 | 0.05 | - | 0.07 | 0.10 | - | 0.03 | - | 0.02 | - | 0.08 |
| 41 | Indole a | 1296 | 0.15 | 0.12 | 0.26 | 0.21 | 0.01 | 0.04 | 0.20 | 0.04 | 0.10 | 0.11 |
| 42 | (E,E)-Deca-2,4-dienal | 1320 | 0.31 | - | 0.47 | 0.10 | 0.02 | 0.04 | 0.23 | 0.01 | 0.11 | 0.08 |
| 43 | β-Bourbonene | 1388 | 0.12 | 0.13 | 0.10 | 0.29 | 0.01 | 0.03 | 0.08 | 0.05 | - | 0.08 |
| 44 | β-Cubebene | 1393 | 0.22 | 0.07 | 0.37 | - | 0.02 | 0.04 | - | 0.03 | - | - |
| 45 | Tetradec-1-ene | 1395 | - | - | - | 0.21 | - | - | - | - | - | - |
| 46 | Dodecanal a | 1412 | 0.27 | 0.08 | 0.24 | 0.11 | 0.02 | 0.02 | 0.21 | 0.02 | - | 0.09 |
| 47 | α-Ionone | 1432 | 0.11 | 0.08 | 0.15 | 0.14 | - | 0.04 | 0.16 | 0.02 | - | 0.05 |
| 48 | (Z)-Geranylacetone | 1458 | - | - | - | 0.06 | - | - | - | - | - | - |
| 49 | Alloaromadendrene | 1465 | 0.07 | - | 0.11 | 0.07 | 0.02 | 0.03 | 0.24 | 0.02 | - | 0.06 |
| 50 | Dodecan-1-ol a | 1479 | 0.19 | - | - | - | 0.02 | - | - | - | - | - |
| 51 | α-Amorphene | 1481 | 0.06 | 0.09 | 0.16 | 0.11 | 0.01 | 0.03 | 0.23 | - | - | - |
| 52 | Germacrene D | 1485 | 0.98 | 0.59 | 1.10 | 0.81 | 0.08 | 0.18 | 0.54 | 0.37 | 0.11 | 0.41 |
| 53 | β-Ionone | 1489 | 0.87 | 0.56 | 0.93 | 1.10 | 0.07 | 0.31 | 0.92 | 0.16 | 0.40 | 0.69 |
| 54 | epi-Bicyclosesquiphellandrene | 1495 | 0.36 | - | 0.68 | - | 0.03 | - | 0.13 | - | 0.05 | - |
| 55 | Pentadec-1-ene | 1495 | - | 0.22 | - | 0.48 | - | 0.14 | - | 0.15 | - | 0.18 |
| 56 | (E)-β-Guaiene | 1498 | 0.32 | - | 0.32 | - | 0.03 | - | 0.20 | - | 0.07 | - |
| 57 | Tridecan-2-one | 1499 | - | 0.27 | - | 0.47 | - | 0.08 | - | - | - | 0.14 |
| 58 | Pentadecane a | 1500 | 0.27 | 0.87 | 0.28 | 1.08 | 0.15 | 0.05 | 0.16 | 0.09 | - | 0.20 |
| 59 | Tridecanal a | 1514 | 4.89 | 0.62 | 4.66 | 1.07 | 0.93 | 0.09 | 5.12 | 0.49 | 1.62 | 1.45 |
| 60 | γ-Cadinene | 1520 | 0.37 | 0.22 | 0.52 | 0.38 | 0.04 | 0.07 | 0.47 | 0.12 | 0.21 | 0.24 |
| 61 | β-Cadinene | 1528 | 0.11 | 0.07 | 0.08 | 0.11 | 0.04 | 0.10 | 0.13 | 0.04 | - | 0.12 |
| 63 | Dactylol | 1562 | 0.06 | - | 0.11 | 0.07 | - | - | - | - | - | - |
| 64 | Germacrene-4-ol | 1580 | 0.29 | 0.27 | 0.44 | 0.45 | 0.02 | 0.05 | - | - | - | - |
| 65 | Caryophyllene oxide | 1587 | 0.05 | - | 0.09 | - | - | - | - | - | - | - |
| 66 | Gleenol | 1590 | 3.83 | 1.87 | 3.61 | 1.63 | 0.29 | 0.53 | 0.49 | 0.39 | 0.09 | 0.13 |
| 67 | α-Guaiol | 1592 | 0.26 | - | 0.14 | - | 0.02 | 0.03 | - | - | - | - |
| 68 | Hexadecane a | 1600 | 0.22 | 0.17 | 0.27 | 0.20 | 0.04 | - | - | - | - | - |
| 69 | Tetradecanal a | 1616 | 0.34 | 0.24 | 0.39 | 0.24 | 0.06 | - | 0.32 | 0.07 | - | - |
| 70 | τ-Cadinol | 1630 | - | 0.06 | - | - | - | - | - | - | - | - |
| 71 | Cubenol | 1647 | 0.31 | 0.19 | 0.41 | 0.26 | 0.07 | 0.10 | 0.50 | 0.13 | 0.16 | 0.21 |
| 72 | α-Cadinol | 1660 | 0.32 | 0.14 | 0.38 | 0.22 | 0.06 | 0.12 | 0.53 | 0.14 | 0.20 | 0.21 |
| 73 | (E)-Heptadec-8-ene | 1683 | - | 0.16 | - | 0.15 | - | - | - | - | - | - |
| 74 | (Z,E)-Farnesol | 1688 | 1.40 | 0.99 | 1.20 | 1.23 | 0.16 | 0.22 | - | 0.29 | - | 0.35 |
| 75 | (E)-Heptadec-8-ene | 1696 | 0.68 | 0.33 | 0.69 | 0.56 | 0.08 | - | 0.23 | 0.09 | - | - |
| 76 | Heptadecane a | 1700 | 3.60 | 0.97 | 3.63 | 1.28 | 1.30 | 0.11 | 4.61 | 0.51 | 1.72 | 1.32 |
| 77 | Pentadecanal a | 1718 | 1.49 | 0.98 | 1.38 | 0.84 | 0.42 | 0.07 | 1.26 | 0.16 | 0.49 | 0.60 |
| 78 | Tetradecanoic acid a | 1770 | 0.19 | 0.84 | 0.42 | 1.75 | 0.04 | 4.94 | - | 12.96 | 0.26 | 3.93 |
| 79 | (Z,E)-Farnesyl acetate | 1799 | 0.78 | 0.55 | 0.73 | 0.78 | 0.27 | 0.35 | 1.29 | 0.85 | 0.25 | 0.84 |
| 80 | 6,10,14-Trimethylpentadecan-2-one a | 1850 | - | 0.29 | - | 0.18 | - | - | - | - | - | - |
| 81 | Nonadec-1-ene | 1897 | - | 0.40 | - | 0.39 | - | - | - | 0.11 | - | 0.16 |
| 82 | Cembrene | 1930 | 0.08 | 0.27 | 0.12 | 0.28 | 0.09 | 0.07 | - | 0.09 | - | 0.18 |
| 83 | Palmitoleic acid a | 1962 | - | - | - | - | - | - | - | 1.23 | - | - |
| 84 | Cembrene A | 1969 | 0.42 | 0.81 | 0.59 | 1.07 | 0.44 | 0.85 | 0.61 | 1.17 | 0.35 | 1.00 |
| 85 | Hexadecanoic acid a | 1975 | - | 1.07 | - | 1.32 | - | 0.26 | - | 0.54 | - | - |
| 86 | Ethyl hexadecanoate a | 1987 | - | 0.49 | - | 0.61 | - | 0.43 | - | 1.12 | - | 0.54 |
| 87 | (Z)-Octadecen-9-al | 1997 | - | 3.93 | - | 4.69 | - | 2.25 | - | 1.66 | - | 3.15 |
| 88 | Octadecanal a | 2025 | - | - | - | 0.32 | - | - | - | - | - | - |
| 89 | Methyl octadecyl ether | 2036 | - | 3.33 | - | 5.09 | - | 4.33 | - | 3.44 | - | 3.68 |
| 90 | Methyl (all Z) eicosa-5,8,11,14, 17-pentaenoate | 2040 | 0.34 | 0.21 | - | - | - | - | 0.22 | - | 0.25 | - |
| 91 | Methyl (all Z) eicosa-5,8,11,14-tetraenoate | 2045 | 8.97 | 8.20 | 6.51 | 7.49 | 9.73 | 6.95 | 11.01 | 7.28 | 10.31 | 8.70 |
| 92 | (Z,Z,Z)-Octadeca-9,12,15-trien-1-ol | 2055 | 1.08 | 0.25 | 1.10 | - | - | - | - | - | - | - |
| 93 | (Z)-Octadec-9-en-1-ol | 2061 | 1.58 | 0.66 | 1.55 | 0.66 | 4.44 | - | 2.43 | - | 1.81 | - |
| 94 | (E)-Phytol | 2116 | 1.05 | - | - | - | - | - | - | - | - | - |
| 95 | Pachydictyol A | 2120 | 1.86 | 2.25 | 5.19 | 2.60 | 5.54 | 5.12 | 2.09 | 2.04 | 2.93 | 3.67 |
| 96 | Isopachydictyol A | 2128 | 19.72 | 25.03 | 15.01 | 18.79 | 18.88 | 16.02 | 15.77 | 17.92 | 16.91 | 19.15 |
| 97 | (Z)-Octadec-9-enoic acid | 2147 | 1.90 | 6.61 | 2.85 | 6.28 | 6.15 | 10.81 | 2.71 | 9.08 | 3.73 | 11.05 |
| 98 | (E)-Geranylgeraniol | 2184 | 0.34 | 0.50 | 0.44 | 0.58 | 1.36 | 0.75 | 0.72 | 0.74 | 0.66 | 0.64 |
| 99 | Cembra-4,7,11,15-tetraen-3-ol | 2230 | 13.08 | 17.38 | 16.89 | 16.85 | 27.10 | 26.90 | 24.07 | 22.43 | 33.56 | 25.64 |
| May | June | July | August | September | |
|---|---|---|---|---|---|
| Sea temperature (°C) | 20.1 | 25.0 | 26.2 | 28.1 | 23.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radman, S.; Čagalj, M.; Šimat, V.; Jerković, I. Seasonal Variability of Volatilome from Dictyota dichotoma. Molecules 2022, 27, 3012. https://doi.org/10.3390/molecules27093012
Radman S, Čagalj M, Šimat V, Jerković I. Seasonal Variability of Volatilome from Dictyota dichotoma. Molecules. 2022; 27(9):3012. https://doi.org/10.3390/molecules27093012
Chicago/Turabian StyleRadman, Sanja, Martina Čagalj, Vida Šimat, and Igor Jerković. 2022. "Seasonal Variability of Volatilome from Dictyota dichotoma" Molecules 27, no. 9: 3012. https://doi.org/10.3390/molecules27093012
APA StyleRadman, S., Čagalj, M., Šimat, V., & Jerković, I. (2022). Seasonal Variability of Volatilome from Dictyota dichotoma. Molecules, 27(9), 3012. https://doi.org/10.3390/molecules27093012









