A New Method of Preparing Aurone by Marine Actinomycetes and Its Potential Application in Agricultural Fungicides
Abstract
:1. Introduction
2. Results
2.1. Taxonomic Status of Strains
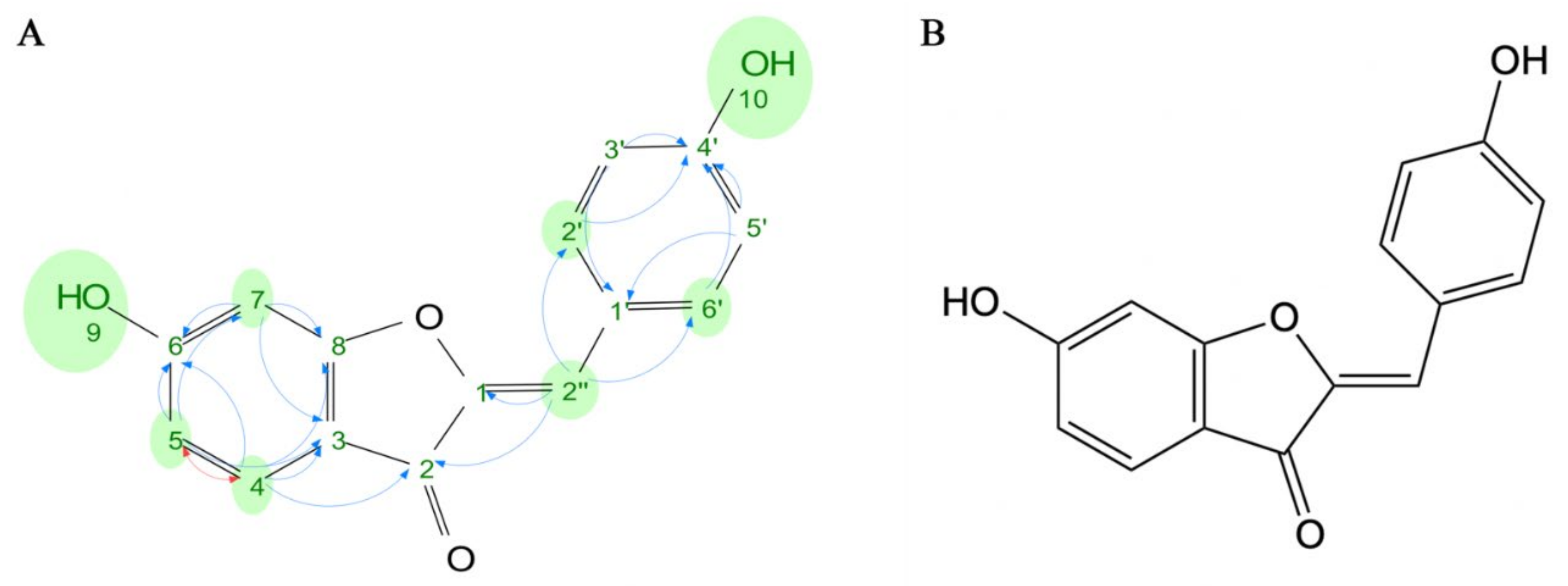
2.2. Structural Elucidation of the Active Compound
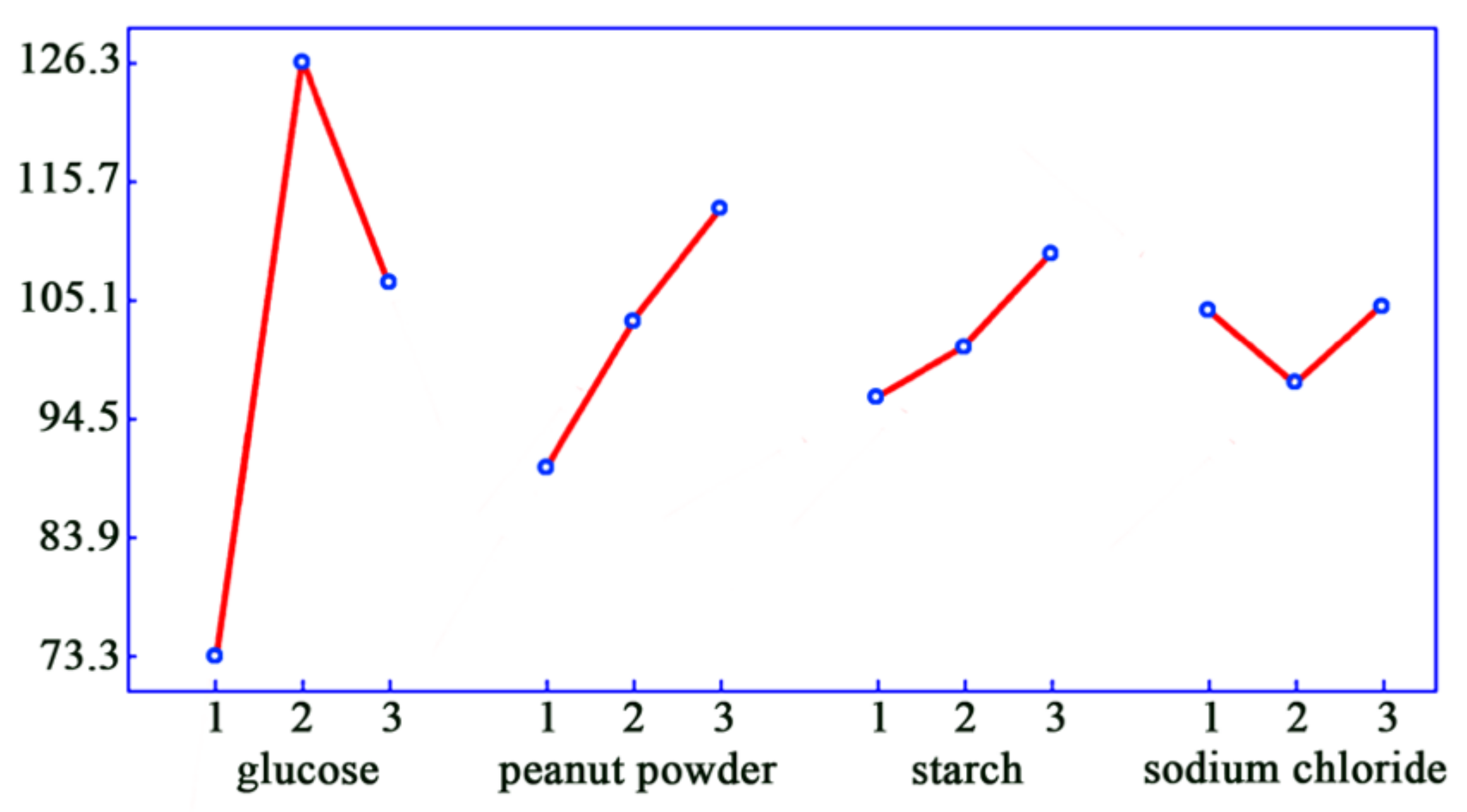
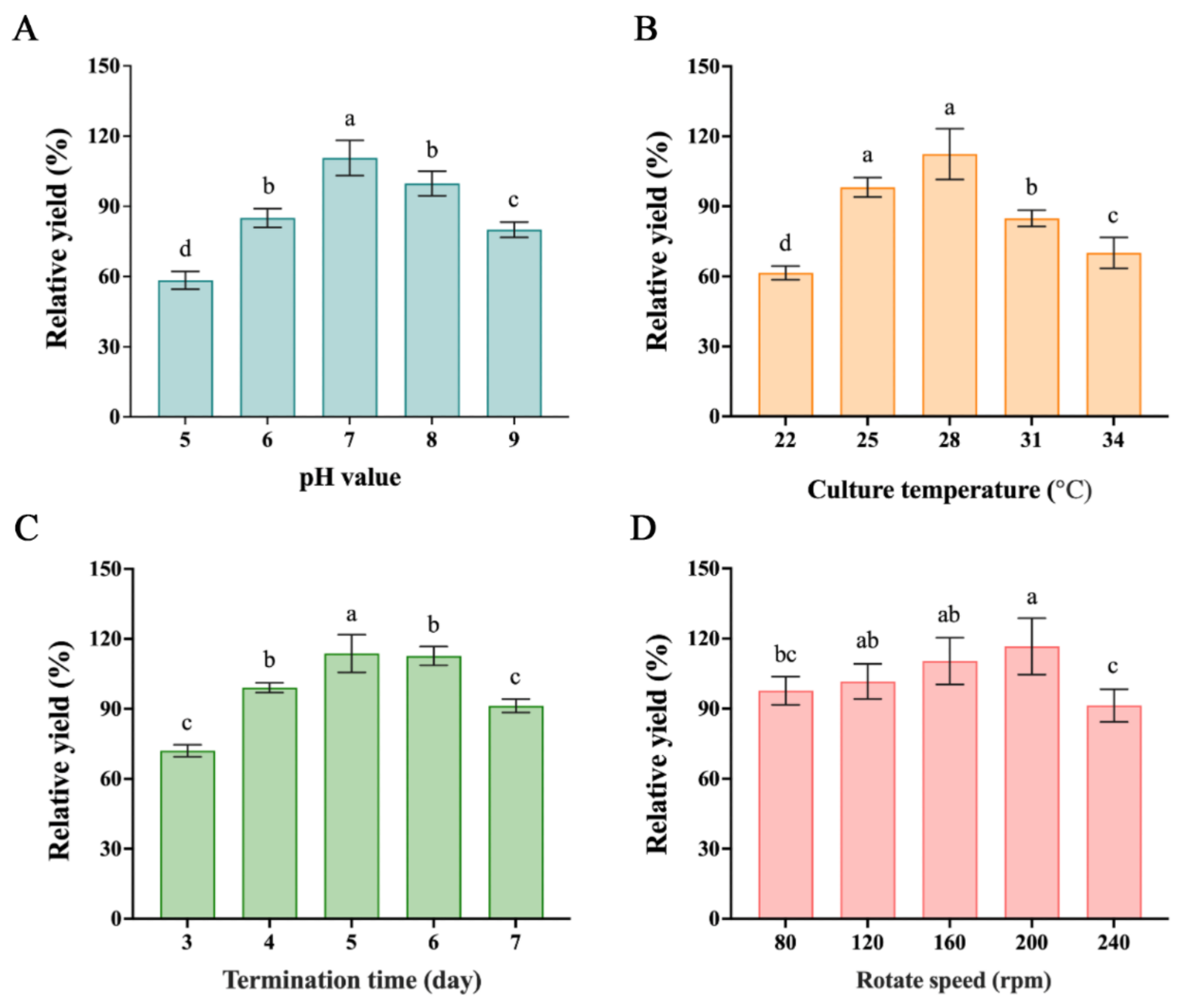
2.3. Optimal Fermentation Medium and Process
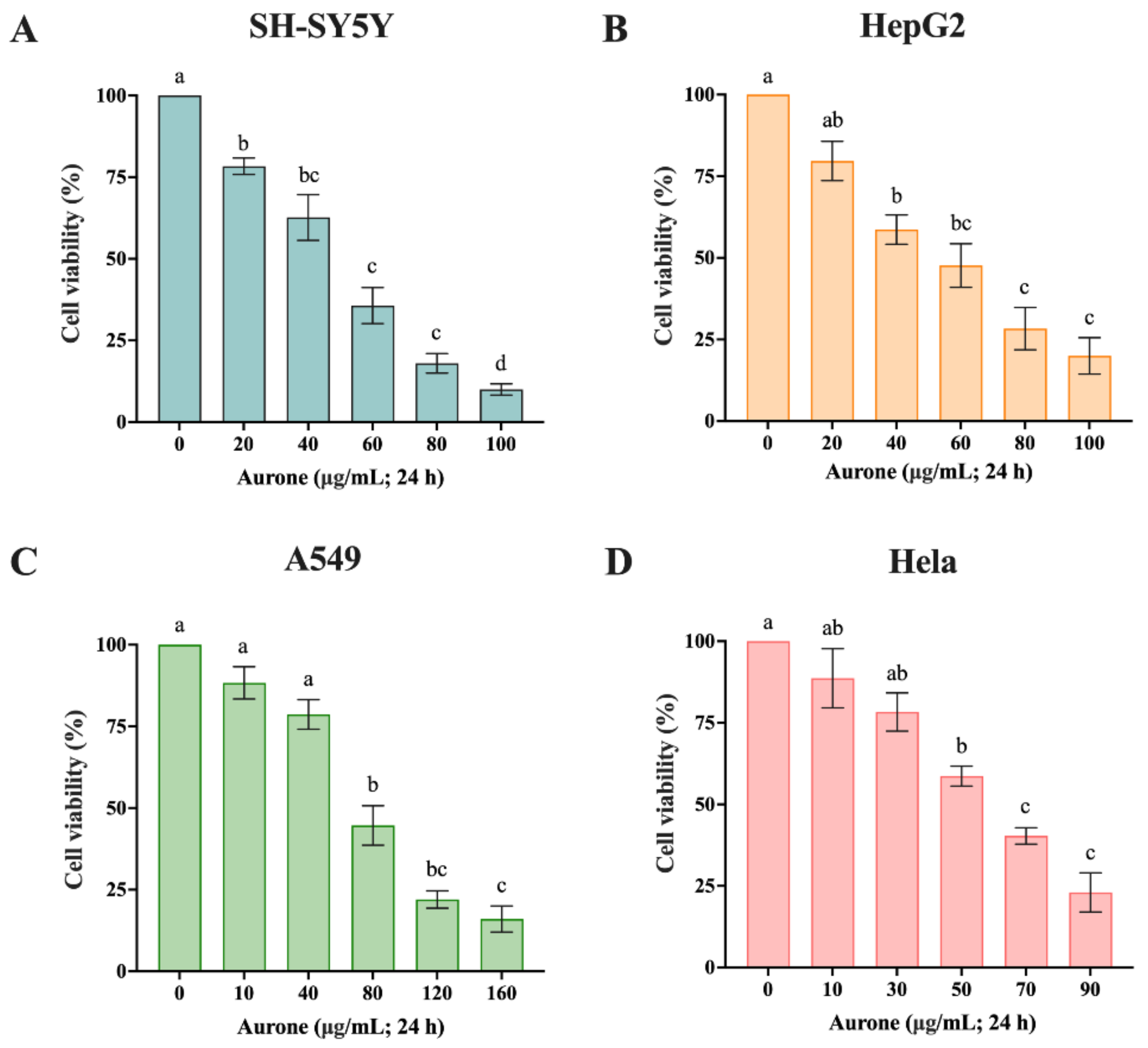
2.4. Cytotoxicity to Four Kinds of Canner Cells
2.5. Fungicidal Activity In Vivo
3. Discussion
4. Material and Methods
4.1. Microorganism Material and Taxonomy
4.2. Optimal Fermentation Experimental Design
4.3. Culture, Extraction and Isolation
4.4. Mass and NMR
4.5. Evaluation of the Cytotoxicity and Fungicidal Activity
4.5.1. Cytotoxicity
4.5.2. Bioassay of Powder Mildew (B. cinerea) in Pot
4.5.3. Bioassay of Cucurbits Powder Mildew (S. fuliginea) in Pot
4.5.4. Bioassay of Rice Blast (P. oryzae) in Pot
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Alsayari, A.; Bin Muhsinah, A.; Hassan, M.Z.; Ahsan, M.J.; Alshehri, J.A.; Begum, N. Aurone: A biologically attractive scaffold as anticancer agent. Eur. J. Med. Chem. 2019, 166, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.; Li, T.; Zhang, B.; Wang, R.; Hao, H.; Zhou, W. Recent advances on synthesis and biological activities of aurones. Bioorganic Med. Chem. 2020, 29, 115895. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.S.; Georgey, H.H.; George, R.F.; Mohamed, E.R. Aurones and furoaurones: Biological activities and synthesis. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 121–127. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Panchagnula, G.K.; Gottumukkala, A.L.; Subbaraju, G.V. Synthesis, structural revision, and biological activities of 4′-chloroaurone, a metabolite of marine brown alga Spatoglossum variabile. Tetrahedron 2007, 63, 6909–6914. [Google Scholar] [CrossRef]
- Voser, T.M.; Campbell, M.D.; Carroll, A.R. How different are marine microbial natural products compared to their terrestrial counterparts? Nat. Prod. Rep. 2022, 39, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, S.; Pang, X.; Cai, J.; Zhang, X.; Liu, Y.; Zhu, Y.; Zhou, X. Natural products from mangrove sediments-derived microbes: Structural diversity, bioactivities, biosynthesis, and total synthesis. Eur. J. Med. Chem. 2022, 230, 114117. [Google Scholar] [CrossRef]
- El-Bondkly, E.A.M.; El-Bondkly, A.A.M.; El-Bondkly, A.A.M. Marine endophytic fungal metabolites: A whole new world of pharmaceutical therapy exploration. Heliyon 2021, 7, e06362. [Google Scholar] [CrossRef]
- Domratcheva, A.G.; Zhgun, A.A.; Novak, N.V.; Dzhavakhiya, V.V. The Influence of Chemical Mutagenesis on the Properties of the Cyclosporine a High-Producer Strain Tolypocladium inflatum VKM F-3630D. Appl. Biochem. Microbiol. 2018, 54, 53–57. [Google Scholar] [CrossRef]
- Zwergel, C.; Gaascht, F.; Valente, S.; Diederich, M.; Bagrel, D.; Kirsch, G. Aurones: Interesting Natural and Synthetic Compounds with Emerging Biological Potential. Nat. Prod. Commun. 2012, 7, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2011, 28, 1626–1695. [Google Scholar] [CrossRef]
- Nakayama, T. Enzymology of aurone biosynthesis. J. Biosci. Bioeng. 2002, 94, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, S.; Li, Y.; Zheng, M.; Xi, X.; Cao, H.; Cui, X.; Guo, H.; Han, C. Yield enhancement strategies of rare pharmaceutical metabolites from endophytes. Biotechnol. Lett. 2018, 40, 797–807. [Google Scholar] [CrossRef]
- Deepa, T.; Gangwane, A.K.; Sayyed, R.Z.; El Enshasy, H.A.; Zaidel, D.N.A. UV Induced Mutagenesis Elevates the Production of Laccase in Enterobacter cloacae. J. Sci. Ind. Res. 2020, 79, 442–448. [Google Scholar]
- Zhou, W.-W.; Ma, B.; Tang, Y.-J.; Zhong, J.-J.; Zheng, X. Enhancement of validamycin A production by addition of ethanol in fermentation of Streptomyces hygroscopicus 5008. Bioresour. Technol. 2012, 114, 616–621. [Google Scholar] [CrossRef]
- Siddique, S.; Syed, Q.; Adnan, A.; Nadeem, M.; Irfan, M.; Qureshi, F.A. Production of Avermectin B1b From Streptomyces avermitilis 41445 by Batch Submerged Fermentation. Jundishapur J. Microbiol. 2013, 6, e7198. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Zhou, P.; Fan, P.; Zhu, Y.; Tong, Y.; Wang, H.; Yu, L. Four-stage dissolved oxygen strategy based on multi-scale analysis for improving spinosad yield by Saccharopolyspora spinosa ATCC49460. Microb. Biotechnol. 2015, 8, 561–568. [Google Scholar] [CrossRef]
- Xie, D.; Cai, X.; Yang, C.; Xie, L.; Qin, G.; Zhang, M.; Huang, Y.; Gong, G.; Chang, X.; Chen, H. Studies on the control effect of Bacillus subtilis on wheat powdery mildew. Pest Manag. Sci. 2021, 77, 4375–4382. [Google Scholar] [CrossRef]
- Romero, D.; Pérez-García, A.; Rivera, M.E.; Cazorla, F.M.; De Vicente, A. Isolation and evaluation of antagonistic bacteria towards the cucurbit powdery mildew fungus Podosphaera fusca. Appl. Microbiol. Biotechnol. 2004, 64, 263–269. [Google Scholar] [CrossRef]
- Katsantonis, D.; Kadoglidou, K.; Dramalis, C.; Puigdollers, P. Rice blast forecasting models and their practical value: A review. Phytopathol. Mediterr. 2017, 56, 187–216. [Google Scholar] [CrossRef]
- Li, M.; Zhang, P.; Xu, W.; Yuan, J.; Li, Q.; Tao, L.; Li, Z.; Zhang, Y. Avermectin induces toxic effects in insect nontarget cells involves DNA damage and its associated programmed cell death. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 249, 109130. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Xu, W.; Gao, J.; Cao, H.; Yang, M.; Wang, B.; Hao, Y.; Tao, L. Cytotoxic effects of Avermectin on human HepG2 cells in vitro bioassays. Environ. Pollut. 2017, 220, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
| Medium | Visual Status | Arobic Myclium | Inner Myclium | Soluble Pigment |
|---|---|---|---|---|
| NA | abundant | gray | coral red | red |
| Glucose-aginine agar | Rare | cineritious | blue gray | ivory yellow |
| Salt-starch agar | abundant | pale yellow | yellow | none |
| Oats powder agar | Rare | light purple | gray | none |
| PDA | Rare | white clam | gray | black |
| Yeast-malt agar | abundant | aurora red | litchi white | yellow |
| Santas agar | abundant | lady gray | shallow tobacco | none |
| Saccarose-nitrate agar | abundant | cineritious | light megenta | light red |
| Biochemistry Characteristics | TXC6 | The Use of Carbon Source | TXC6 |
|---|---|---|---|
| Glutin hydrolyzation | Positive | L-arabinose | negative |
| Milk peptonization | Positive | D-glucose | positive |
| Starch hydrolyzation | Positive | L-rhamnose | negative |
| Nitrate reduction | Positive | D-fructose | positive |
| Cellulose usage | Negative | D-xylose | negative |
| Black pigment | Yield | sucrose | negative |
| H2S | Yield | D-mannitol | positive |
| Position | 1H-NMR | 13C-NMR |
|---|---|---|
| 1 | - | 145.77 |
| 2 | - | 181.31 |
| 3 | - | 113.21 |
| 4 | 7.60 (d, J = 8.4 Hz) | 125.78 |
| 5 | 6.70 (dd, J = 8.4, 2.0 Hz) | 112.92 |
| 6 | - | 167.56 |
| 7 | 6.78 (d, J = 2.0 Hz) | 98.57 |
| 8 | - | 166.21 |
| 1′ | - | 123.09 |
| 2′, 6′ | 7.79–7.84 (m) | 133.33 |
| 3′, 5′ | 6.88–6.91 (m) | 116.12 |
| 4′ | - | 159.32 |
| 2″ | 6.73 (s) | 111.45 |
| 9-OH | 11.13 (s) | - |
| 10-OH | 10.15 (s) | - |
| Carbon Source | Relative Yield * (%) | Nitrogen Source | Relative Yield (%) |
|---|---|---|---|
| Glucose | 100 | Soybean powder | 100 |
| Fructose | 81.25 ± 0.75 | Peanut powder | 108.30 ± 1.73 |
| Maltose | 83.75 ± 1.02 | Yeast extract powder | 105.65 ± 0.98 |
| Sucrose | 27.50 ± 0.33 | Peptone | 102.65 ± 1.25 |
| Starch | 93.75 ± 2.18 | Potassium nitrate | 68.35 ± 0.67 |
| Factor Level | Glucose (A)/g·L−1 | Peanut Powder (B)/g·L−1 | Starch (C)/g·L−1 | Sodium Chloride (D)/g·L−1 |
|---|---|---|---|---|
| 1 | 2.0 | 1.0 | 0.5 | 0.5 |
| 2 | 4.0 | 2.0 | 1.0 | 1 |
| 3 | 6.0 | 3.0 | 2.0 | 2 |
| Experimental Number | Factors and Levels | Yield/mg·L−1 | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 1 | 1 | 1 | 1 | 1 | 57.67 ± 4.51 |
| 2 | 1 | 2 | 2 | 2 | 68.33 ± 3.21 |
| 3 | 1 | 3 | 3 | 3 | 94.00 ± 4.58 |
| 4 | 2 | 1 | 2 | 3 | 115.33 ± 4.93 |
| 5 | 2 | 2 | 3 | 1 | 136.67 ± 4.72 |
| 6 | 2 | 3 | 1 | 2 | 127.00 ± 9.84 |
| 7 | 3 | 1 | 3 | 2 | 97.33 ± 2.08 |
| 8 | 3 | 2 | 1 | 3 | 104.33 ± 7.09 |
| 9 | 3 | 3 | 2 | 1 | 118.33 ± 3.05 |
| R | 53 | 23 | 13 | 7 | |
| R’ | 47.73 | 20.72 | 11.71 | 6.03 | |
| F value | 224.20 | 41.54 | 13.68 | 4.87 | |
| p value | 0 | 0 | 0.0002 | 0.0204 | |
| Treatment Chemicals | Concentration/mg·L−1 | Pathogens and Control Effect/% | |||||
|---|---|---|---|---|---|---|---|
| P. orizae | B. cinerea | S. fuliginea | |||||
| Disease Index | Control Effect | Disease Index | Control Effect | Disease Index | Control Effect | ||
| CK | 0 | 55.80 ± 1.89 a | / | 78.72 ± 2.03 a | / | 79.12 ± 2.10 a | / |
| Purified aurone | 25 | 27.42 ± 4.25 b | 51.01 ± 6.26 e | 42.07 ± 3.01 b | 46.57 ± 3.66 e | 53.09 ± 3.39 b | 32.91 ± 4.09 f |
| 50 | 16.08 ± 0.86 d | 71.18 ± 1.17 c | 22.67 ± 1.90 d | 71.20 ± 2.38 c | 23.47 ± 1.89 d | 70.35 ± 2.25 d | |
| 100 | 1.02 ± 0.38 f | 98.16 ± 0.71 a | 1.20 ± 0.75 f | 98.48 ± 0.96 a | 5.61 ± 1.36 f | 92.91 ± 1.68 b | |
| Crude extra ointment | 25 | 20.57 ± 1.07 c | 63.15 ± 1.50 d | 29.30 ± 2.00 c | 62.79 ± 2.39 d | 34.08 ± 2.95 c | 56.92 ± 3.76 e |
| 50 | 10.03 ± 1.05 e | 82.04 ± 1.76 b | 14.66 ± 1.12 e | 81.37 ± 1.51 b | 16.82 ± 1.97 e | 78.74 ± 2.40 c | |
| 100 | 0.00 ± 0.00 f | 100.00 ± 0.00 a | 0.00 ± 0.00 f | 100.00 ± 0.00 a | 0.00 ± 0.00 g | 100.00 ± 0.00 a | |
| Tricyclazole | 20 | 0.38 ± 0.28 f | 99.32 ± 0.49 a | / | / | / | / |
| Carbendazim | 500 | / | / | 0.39 ± 0.24 f | 99.50 ± 0.31 a | / | / |
| Difenoconazole | 100 | / | / | / | / | 0.45 ± 0.44 g | 99.43 ± 0.56 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Li, X.; Wang, W.; Wang, X.; Aihaiti, P.; Lin, T.; Fu, Z.; Xu, R.; Wu, M.; Li, Z.; et al. A New Method of Preparing Aurone by Marine Actinomycetes and Its Potential Application in Agricultural Fungicides. Molecules 2023, 28, 17. https://doi.org/10.3390/molecules28010017
Liu B, Li X, Wang W, Wang X, Aihaiti P, Lin T, Fu Z, Xu R, Wu M, Li Z, et al. A New Method of Preparing Aurone by Marine Actinomycetes and Its Potential Application in Agricultural Fungicides. Molecules. 2023; 28(1):17. https://doi.org/10.3390/molecules28010017
Chicago/Turabian StyleLiu, Bin, Xiaomeng Li, Weiguo Wang, Xin Wang, Pahaiding Aihaiti, Tingtang Lin, Zishuo Fu, Rui Xu, Mengqi Wu, Zhong Li, and et al. 2023. "A New Method of Preparing Aurone by Marine Actinomycetes and Its Potential Application in Agricultural Fungicides" Molecules 28, no. 1: 17. https://doi.org/10.3390/molecules28010017
APA StyleLiu, B., Li, X., Wang, W., Wang, X., Aihaiti, P., Lin, T., Fu, Z., Xu, R., Wu, M., Li, Z., & Zhang, Y. (2023). A New Method of Preparing Aurone by Marine Actinomycetes and Its Potential Application in Agricultural Fungicides. Molecules, 28(1), 17. https://doi.org/10.3390/molecules28010017






