A Nationwide Study of Residual Fate of Fluxapyroxad and Its Metabolites in Peanut Crops Across China: Assessment of Human Exposure Potential
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Validation for Tracing Fluxapyroxad and Metabolites in Peanut Matrices
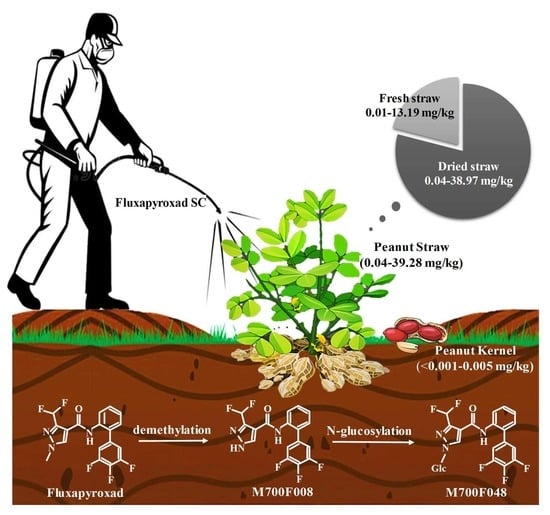
2.2. Occurrence and Degradation of Fluxapyroxad during Peanut Cultivation
2.3. Terminal Magnitude and MRL Comparison of Fluxapyroxad in Peanut
2.4. Dietary Risks of Total Fluxapyroxad under Large–Scale Applications
3. Materials and Methods
3.1. Chemicals, Reagents and Standard Solutions
3.2. Plant Care and Pesticide Application
3.3. Sample Collection and Preparation
3.4. Instrumentations
3.5. Mathematical Calculations
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- FAO. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/zh/#compare (accessed on 2 December 2022).
- Wei, D.; Wu, X.; Ji, M.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y. Carboxin and its major metabolites residues in peanuts: Levels, dietary intake and chronic intake risk assessment. Food Chem. 2019, 275, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, J.; Du, F. Potential use of peanut by-products in food processing: A review. J. Food Sci. Technol. 2012, 49, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.M.; Smith, D.; Rodriquez-Kabana, R. Compendium of Peanut Disease. Am. Phytopathol. Soc. 1984, 2, 73. [Google Scholar]
- Bot, P.; Akgül, D.S.; Ozgonen, H.; Erkilic, A. The Effects of Seed Treatments with Fungicides on Stem Rot Caused by Sclerotium rolfsii Sacc., in Peanut. Pak. J. Bot. 2011, 43, 2991–2996. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef] [Green Version]
- Bi, L.; Xu, J.; Wang, Q.; Li, T.; Wang, J.; Zhou, M.; Duan, Y. Baseline sensitivity and resistance risk assessment of Stemphylium solani to fluxapyroxad. Crop Prot. 2022, 156, 105944. [Google Scholar] [CrossRef]
- USEPA. Pesticide Fact Sheet of Fluxapyroxad. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-138009_02-May-12.pdf (accessed on 2 December 2022).
- EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance fluxapyroxad (BAS 700 F). EFSA J. 2012, 10, 2522. [Google Scholar] [CrossRef]
- Joint FAO/WHO Meeting on Pesticide Residues. FAO Reports for Agricultural Pesticides Residue and Analytical Aspects. Available online: https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report12/JMPR_2012_Report.pdf (accessed on 2 December 2022).
- Joint FAO/WHO Meeting on Pesticide Residues. FAO Specifications and Evaluations for Agricultural Pesticides Residue and Analytical Aspects. Available online: https://www.fao.org/3/cb2755en/cb2755en.pdf (accessed on 2 December 2022).
- Chen, X.; Dong, F.; Xu, J.; Liu, X.; Wu, X.; Zheng, Y. Effective Monitoring of Fluxapyroxad and Its Three Biologically Active Metabolites in Vegetables, Fruits, and Cereals by Optimized QuEChERS Treatment Based on UPLC-MS/MS. J. Agric. Food Chem. 2016, 64, 8935–8943. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, X.; Zhu, Y.; Dong, F.; Xu, J.; Li, M.; Zheng, Y. A statistical approach to determine fluxapyroxad and its three metabolites in soils, sediment and sludge based on a combination of chemometric tools and a modified quick, easy, cheap, effective, rugged and safe method. J. Chromatogr. A 2014, 1358, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Chen, X.; Liu, X.; Xu, J.; Li, Y.; Shan, W.; Zheng, Y. Simultaneous determination of five pyrazole fungicides in cereals, vegetables and fruits using liquid chromatography/tandem mass spectrometry. J. Chromatogr. A. 2012, 1262, 98–106. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Jia, C.; Zhao, E.; Chen, L.; Yu, P.; Jing, J.; Zheng, Y. Concentrations and dissipation of difenoconazole and fluxapyroxad residues in apples and soil, determined by ultrahigh-performance liquid chromatography electrospray ionization tandem mass spectrometry. Environ. Sci. Pollut. Res. Int. 2016, 23, 5618–5626. [Google Scholar] [CrossRef]
- Noh, H.H.; Lee, J.Y.; Park, H.K.; Lee, J.W.; Jo, S.H.; Lim, J.B.; Shin, H.G.; Kwon, H.; Kyung, K.S. Dissipation, persistence, and risk assessment of fluxapyroxad and penthiopyrad residues in perilla leaf (Perilla frutescens var. japonica Hara). PLoS ONE 2019, 14, e0212209. [Google Scholar] [CrossRef] [PubMed]
- SANTE. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11312_2021.pdf (accessed on 2 December 2022).
- Renew, J.E.; Huang, C.H. Simultaneous determination of fluoroquinolone, sulfonamide, and trimethoprim antibiotics in wastewater using tandem solid phase extraction and liquid chromatography-electrospray mass spectrometry. J. Chromatogr. A. 2004, 1042, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dong, F.; Liu, X.; Xu, J.; Meng, W.; Liu, N.; Chen, Z.; Tao, Y.; Zheng, Y.-Q. Simultaneous determination of fipronil and its major metabolites in corn and soil by ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Methods. 2014, 6, 1788. [Google Scholar] [CrossRef]
- Peanut Caltivation. Available online: https://baike.baidu.com/item/%E8%90%BD%E8%8A%B1%E7%94%9F/805835 (accessed on 2 December 2022).
- Wu, J. Incorporating Temperature Effects on Pesticide Degradation into a Management Model. J. Environ. Qual. 1999, 28, 92–100. [Google Scholar] [CrossRef]
- Fang, H.; Yu, Y.L.; Wang, X.; Shan, M.; Wu, X.M.; Yu, J.Q. Dissipation of Chlorpyrifos in Pakchoi-Vegetated Soil in A Greenhouse. J. Environ. Sci. 2006, 18, 760–764. Available online: https://pubmed.ncbi.nlm.nih.gov/17078557 (accessed on 2 December 2022).
- Caracciolo, A.B.; Bottoni, P.; Grenni, P. Microcosm studies to evaluate microbial potential to degrade pollutants in soil and water ecosystems. Microchem. J. 2013, 107, 126–130. [Google Scholar] [CrossRef]
- CAC. Joint FAO/WHO Food Standards Programme Codex Committee on Pesticide Residues. Available online: https://thenhf.com/joint-fao-who-food-standards-programme-codex-committee-on-pesticide-residues/ (accessed on 2 December 2022).
- eCFR. Code of Federal Regulations. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-E/part-180/subpart-C/section-180.564 (accessed on 2 December 2022).
- JFCRF. Maximum Residue Limits for Pesticides in Food (Affirmative List System) Database. Available online: http://db.ffcr.or.jp/front (accessed on 2 December 2022).
- Jin, S. Survey Report on the Nutrition and Health of Chinese Residents: Data Set on the Status of Nutrition and Health in 2002; People’s Hygiene Press: Beijing, China, 2008; p. 533. [Google Scholar]
- Lu, C.; Schenck, F.J.; Pearson, M.A.; Wong, J.W. Assessing children’s dietary pesticide exposure: Direct measurement of pesticide residues in 24-hr duplicate food samples. Environ. Health Perspect. 2010, 118, 1625–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, E.; Turrero, N.; Kolia, M.; Konate, Y.; de Alencastro, L. Levels of 48 pesticides in vegetables and water and evaluation of health risk resulting from dietary intake in gardening areas in Burkina Faso. In Proceedings of the 11th Eurpean Pesticide Residue Workshop (EPRW), Limassol, Cyprus, 24–27 May 2016; Ecole Polytechnique Federale de Lausanne: Lausanne, Switzerland, 2016. [Google Scholar]
- OECD. Organization for Economic Co-operation and Development Guidelines for the Testing of Chemicals. Test No. 509: Crop Field Trial. Available online: http://www.oecd.org/termsandconditions/ (accessed on 2 December 2022).
- Rahman, M.M.; Abd El-Aty, A.M.; Choi, J.-H.; Kim, S.-W.; Shin, S.C.; Shim, J.-H. Consequences of the matrix effect on recovery of dinotefuran and its metabolites in green tea during tandem mass spectrometry analysis. Food Chem. 2015, 168, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Rashid, M.; Liu, J.; Hu, M.; Zhong, G. Identification of multi-insecticide residues using GC-NPD and the degradation kinetics of chlorpyrifos in sweet corn and soils. Food Chem. 2016, 212, 420–426. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Principles and Methods for the Risk Assessment of Chemicals in Food. Available online: https://www.who.int/foodsafety/chem/dietaryexposure (accessed on 2 December 2022).



| Compound | Matrix | Regression Equation | R2 | Matrix Effect (%) | LOQ (μg/kg) | Mean Recoveries ± SD (%, n = 6) | ||
|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | ||||||
| Fluxapyroxad | Acetonitrile | y = 1309x + 27.166 | 0.9991 | – | – | – | – | – |
| Peanut kernel | y = 4400.2x + 34.488 | 0.9999 | −30.3 | 0.001 | 110 ± 6.9 | 99 ± 13.0 | 98 ± 6.3 | |
| Peanut straw | y = 2532.1x + 46.288 | 0.9996 | −48.3 | 0.001 | 105 ± 16 | 86 ± 8.4 | 105 ± 3.1 | |
| M700F008 | Acetonitrile | y = 30,737x + 652.69 | 0.9994 | – | – | – | – | – |
| Peanut kernel | y = 74,053x + 751.33 | 0.9999 | −16.2 | 0.001 | 95 ± 5.4 | 112 ± 8.7 | 96 ± 11.0 | |
| Peanut straw | y = 58,882x + 745.08 | 0.9999 | −47.8 | 0.001 | 102 ± 3.1 | 103 ± 3.3 | 106 ± 2.6 | |
| M700F048 | Acetonitrile | y = 15,270x + 132.41 | 0.9999 | – | – | – | – | – |
| Peanut kernel | y = 33,924x − 374.84 | 0.9967 | +10.6 | 0.001 | 79 ± 7.7 | 99 ± 10.0 | 85 ± 6.2 | |
| Peanut straw | y = 26,018x + 47.223 | 0.9996 | −41.3 | 0.001 | 106 ± 15.0 | 101 ± 7.1 | 105 ± 3.1 | |
| Location | Trial Sites | Peanut Cultivars | Fluxapyroxad | M700F008 | M700F048 | Fluxapyroxad (Sum) | ||||
| Peanut Kernel | Peanut Straw | Peanut Kernel | Peanut Straw | Peanut Kernel | Peanut Straw | Peanut Kernel | Peanut Straw | |||
| Heilongjiang | Trial #1 (126.22 °E, 45.80 °N) | Xiaolihong | ≤0.002 | 0.49–0.60 | 0.002–0.003 | 0.22–0.32 | ≤0.001 | 0.54–1.17 | 0.004–0.005 | 1.43–1.74 |
| Inner Mongolia | Trial #2 (112.78 °E, 40.62 °N) | Luhua No.11 | ≤0.001 | 0.22–0.47 | <0.001 | 0.05–0.06 | <0.001 | 0.18–0.29 | 0.003 | 0.40–0.68 |
| Beijing | Trial #3 (116.37 °E, 39.44 °N) | Ji You 4 | <0.001 | 0.12–0.22 | <0.001 | 0.16–0.29 | <0.001 | 0.81–1.09 | 0.003 | 0.95–1.28 |
| Shandong | Trial #4 (117.12 °E, 36.49 °N) | Luhua No.14 | ≤0.001 | 0.05–0.23 | ≤0.001 | 0.09–0.15 | <0.001 | 0.50–0.92 | 0.003 | 0.50–1.05 |
| Henan | Trial #5 (113.68 °E, 34.96 °N) | Yuhua 22 | ≤0.001 | 6.51–29.67 | <0.001 | 1.69–5.69 | <0.001 | 1.29–3.13 | 0.003 | 10.51–33.04 |
| Sichuan | Trial #6 (103.10 °E, 30.95 °N) | Tianfu 9 | 0.002–0.003 | 0.43–0.87 | 0.001 | 0.17–0.33 | <0.001 | 0.29–0.81 | 0.003–0.005 | 0.82–1.80 |
| Anhui | Trial #7 (116.78 °E, 34.18 °N) | Luhua No.14 | ≤0.001 | 1.40–37.38 | ≤0.001 | 0.42–0.77 | ≤0.001 | 1.53–2.60 | 0.003 | 3.64–39.28 |
| Hubei | Trial #8 (114.32 °E, 30.48 °N) | Baisha 1016 | ≤0.001 | 0.61–0.84 | ≤0.001 | 0.34–0.54 | <0.001 | 0.42–0.83 | 0.003 | 1.26–1.85 |
| Hunan | Trial #9 (113.25 °E, 28.27 °N) | Baisha 1016 | <0.001 | 5.53–12.31 | ≤0.001 | 0.91–1.82 | ≤0.001 | 5.21–9.23 | 0.003 | 10.22–20.84 |
| Guangxi | Trial #10 (107.45 °E, 22.83 °N) | Guihua 17 | ≤0.001 | 0.004–0.07 | 0.001–0.002 | 0.02–0.05 | ≤0.001 | 0.03–0.32 | 0.003–0.004 | 0.04–0.35 |
| Trial Sites | Crop Cultivars | Climate Factors | Soil Properties | ||||
|---|---|---|---|---|---|---|---|
| Climate Type | A.T. a (°C) | A.R. b (mm) | Soil Type | pH | O.M. c (%) | ||
| Trial #1 Zhaodong, Heilongjiang (126.22 °E, 45.80 °N) | Xiaolihong | Cold temperate monsoon climate | 20.8 | 524 | Phaeozem | 7.9 | 4.8 |
| Trial #2 Wulanchabu, Inner Mongolia (112.78 °E, 40.62 °N) | Luhua 11 | Mid–temperate continental monsoon climate | 18.4 | 300 | Clay | 8.2 | 3.8 |
| Trial #3 Tongzhou, Beijing (116.81 °E, 39.44 °N) | Jiyou 4 | Warm temperate subhumid continental monsoon climate | 25.2 | 650 | Loam | 8.6 | 1.6 |
| Trial #4 Jinan, Shandong (117.20 °E, 36.49 °N) | Luhua 14 | Warm temperate continental monsoon climate | 26.4 | 628 | Loam | 7.1 | 1.0 |
| Trial #5 Xinxiang, Henan (113.68 °E, 34.96 °N) | Yuhua 22 | Warm temperate continental monsoon climate | 25.6 | 606 | Chao soil | 7.1 | 1.2 |
| Trial #6 Pengzhou, Sichuan (103.10 °E, 30.95 °N) | Tianfu 9 | Subtropical humid climate | 25.1 | 1003 | Loam | 6.5 | 1.5 |
| Trial #7 Suzhou, Anhui (116.78 °E, 34.18 °N) | Luhua 14 | Warm temperate subhumid monsoon climate | 26.6 | 941 | Sandy loam | 7.4 | 1.1 |
| Trial #8 Wuhan, Hubei (114.32 °E, 30.48 °N) | Baisha 1016 | Subtropical monsoon climate | 28.8 | 1050 | Sandy loam | 6.8 | 1.9 |
| Trial #9 Changsha, Hunan (113.25 °E, 28.27 °N) | Baisha 1016 | Subtropical monsoon climate | 29.1 | 1410 | Clay | 5.4 | 2.7 |
| Trial #10 Nanning, Guangxi (107.45 °E, 22.83 °N) | Guihua 17 | Subtropical monsoon climate | 29.3 | 1435 | Laterite | 6.4 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, L.; Ren, X.; Kang, S.; Li, W.; Chen, Z. A Nationwide Study of Residual Fate of Fluxapyroxad and Its Metabolites in Peanut Crops Across China: Assessment of Human Exposure Potential. Molecules 2023, 28, 194. https://doi.org/10.3390/molecules28010194
Wang X, Chen L, Ren X, Kang S, Li W, Chen Z. A Nationwide Study of Residual Fate of Fluxapyroxad and Its Metabolites in Peanut Crops Across China: Assessment of Human Exposure Potential. Molecules. 2023; 28(1):194. https://doi.org/10.3390/molecules28010194
Chicago/Turabian StyleWang, Xi, Li Chen, Xin Ren, Shanshan Kang, Wei Li, and Zenglong Chen. 2023. "A Nationwide Study of Residual Fate of Fluxapyroxad and Its Metabolites in Peanut Crops Across China: Assessment of Human Exposure Potential" Molecules 28, no. 1: 194. https://doi.org/10.3390/molecules28010194
APA StyleWang, X., Chen, L., Ren, X., Kang, S., Li, W., & Chen, Z. (2023). A Nationwide Study of Residual Fate of Fluxapyroxad and Its Metabolites in Peanut Crops Across China: Assessment of Human Exposure Potential. Molecules, 28(1), 194. https://doi.org/10.3390/molecules28010194