Decrypting the Potential of Nanotechnology-Based Approaches as Cutting-Edge for Management of Hyperpigmentation Disorder
Abstract
:1. Introduction
2. Fitzpatrick Skin Phototype (FSPT) Classification System
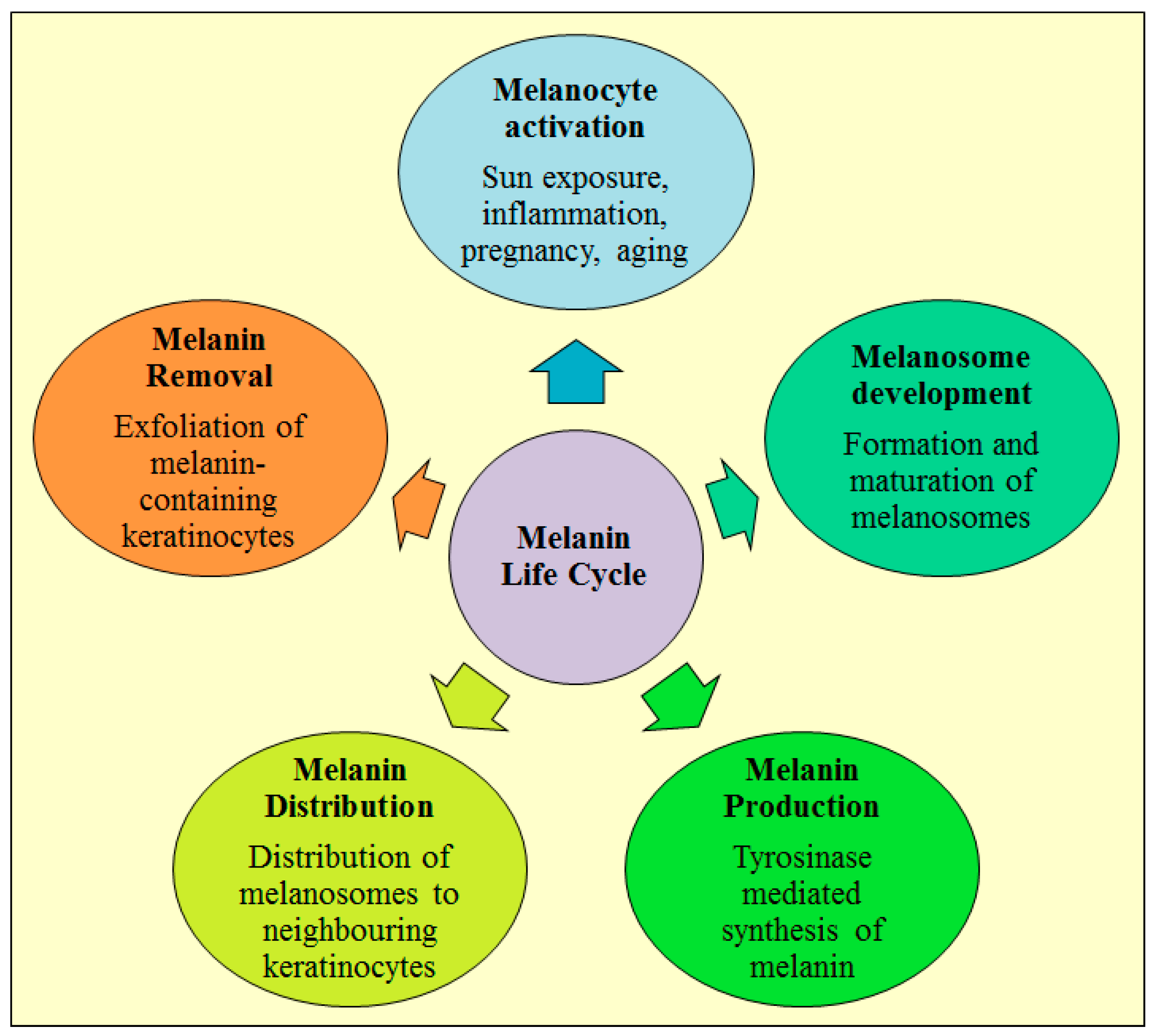
3. Life Cycle of Melanin
4. Pharmacotherapy Approaches for Management of Hyperpigmentation
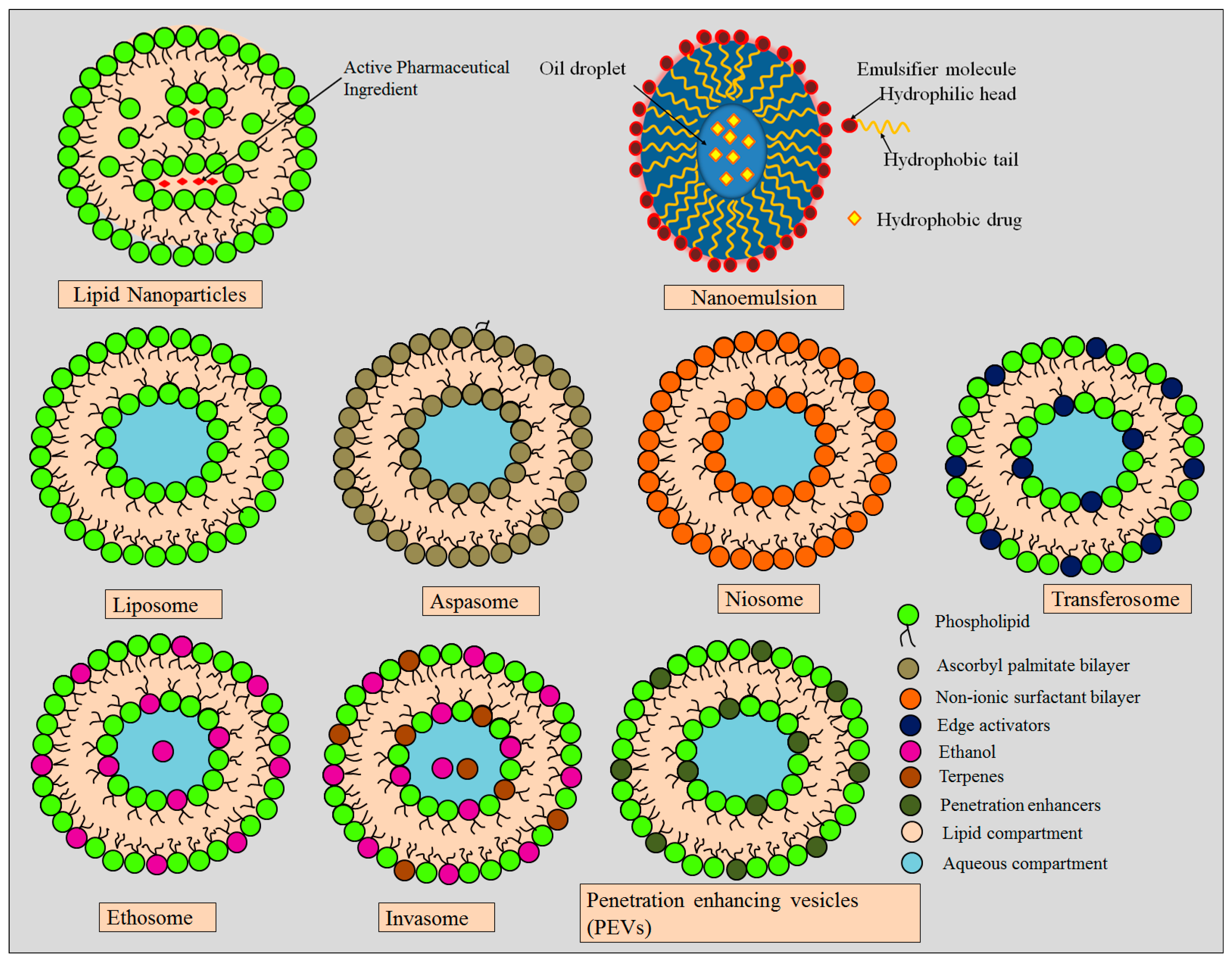
5. Role of Lipid-Based Nanocarriers in Hyperpigmentation
5.1. Lipid Nanoparticles
5.2. Microemulsion and Nanoemulsion
5.3. Liposomes
5.4. Ethosomes
5.5. Niosomes
5.6. Transferosomes
5.7. Aspasomes
5.8. Invasomes
5.9. Penetration-Enhancing Vesicles
6. Role of Inorganic Nanocarriers in Hyperpigmentation
6.1. Gold Nanoparticles
6.2. Fullerenes
7. Role of Polymer-Based Nanocarriers in Hyperpigmentation
7.1. Polymeric Nanoparticles
7.2. Polymerosomes
7.3. Polymeric Micelles or Poloxamers
8. Application of Nanocarriers for Management of Hyperpigmentation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AuNPs | Gold nanoparticles |
| FSPT | Fitzpatrick Skin Phototype |
| LNPs | Lipid nanoparticles |
| LUVs | Large unilamellar vesicles |
| MASI | Melasma area and severity index |
| MEs | Microemulsions |
| MVVs | Multivesicular vesicles |
| NEs | Nanoemulsions |
| NLCs | Nanostructured lipid carriers |
| PEVs | Penetration enhancing vesicles |
| PMCP | Partially myristoylated chitosan pyrrolidone carboxylate |
| SLNs | Solid lipid nanoparticles |
| SUVs | Small unilamellar vesicles |
References
- Miot, L.D.B.; Miot, H.A.; da Silva, M.G.; Marques, M.E.A. Physiopathology of Melasma. An. Bras. Dermatol. 2009, 84, 623–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, P.M.; Miot, H.A. Female Pattern Hair Loss: A Clinical and Pathophysiological Review. An. Bras. Dermatol. 2015, 90, 529–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimes, P.E.; Ijaz, S.; Nashawati, R.; Kwak, D. New Oral and Topical Approaches for the Treatment of Melasma. Int. J. Women’s Dermatol. 2019, 5, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Puri, P.; Jain, R.K.; Singh, A.; Desai, A. Melasma in Men: A Clinical, Aetiological and Histological Study. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Bagherani, N.; Gianfaldoni, S.; Smoller, B. An Overview on Melasma. Pigment. Disord. 2015, 2, 218. [Google Scholar]
- Katsambas, A.; Antoniou, C.H. Melasma. Classification and Treatment. J. Eur. Acad. Dermatol. Venereol. 1995, 4, 217–223. [Google Scholar] [CrossRef]
- Damevska, K. New Aspects of Melasma/Novi Aspekti Melazme. Serb. J. Dermatol. Venereol. 2014, 6, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.-Y. An Updated Review of Melasma Pathogenesis. Dermatol. Sin. 2014, 32, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Hatem, S.; El Hoffy, N.M.; Elezaby, R.S.; Nasr, M.; Kamel, A.O.; Elkheshen, S.A. Background and Different Treatment Modalities for Melasma: Conventional and Nanotechnology-Based Approaches. J. Drug Deliv. Sci. Technol. 2020, 60, 101984. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Halder, R.M.; Nootheti, P.K. Ethnic Skin Disorders Overview. J. Am. Acad. Dermatol. 2003, 48, S143–S148. [Google Scholar] [CrossRef]
- Hexsel, D.; Lacerda, D.A.; Cavalcante, A.S.; Filho, C.A.S.M.; Kalil, C.L.P.V.; Ayres, E.L.; Azulay-Abulafia, L.; Weber, M.B.; Serra, M.S.; Lopes, N.F.P. Epidemiology of Melasma in B Razilian Patients: A Multicenter Study. Int. J. Dermatol. 2014, 53, 440–444. [Google Scholar] [CrossRef]
- Handel, A.C.; Miot, L.D.B.; Miot, H.A. Melasma: A Clinical and Epidemiological Review. An. Bras. Dermatol. 2014, 89, 771–782. [Google Scholar] [CrossRef]
- Videira, I.F.d.S.; Moura, D.F.L.; Magina, S. Mechanisms Regulating Melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, D. Topical Treatment of Melasma. Indian J. Dermatol. 2009, 54, 303. [Google Scholar] [CrossRef]
- Boissy, R.E. Melanosome Transfer to and Translocation in the Keratinocyte. Exp. Dermatol. 2003, 12, 5–12. [Google Scholar] [CrossRef]
- Victor, F.C.; Gelber, J.; Rao, B. Melasma: A Review. J. Cutan. Med. Surg. Inc. Med. Surg. Dermatol. 2004, 8, 97–102. [Google Scholar] [CrossRef]
- Milette, F. Review of The Pigmentary System: Physiology and Pathophysiology by James J. Nordlund, Raymond E. Boissy, Vincent J. Hearing; et al. Dermatol. Pract. Concept. 2014, 4, 89. [Google Scholar] [CrossRef] [Green Version]
- Espósito, A.C.C.; Cassiano, D.P.; da Silva, C.N.; Lima, P.B.; Dias, J.A.F.; Hassun, K.; Bagatin, E.; Miot, L.D.B.; Miot, H.A. Update on Melasma—Part I: Pathogenesis. Dermatol. Ther. (Heidelb). 2022, 12, 1967–1988. [Google Scholar] [CrossRef]
- Lee, A. Recent Progress in Melasma Pathogenesis. Pigment Cell Melanoma Res. 2015, 28, 648–660. [Google Scholar] [CrossRef]
- Mohiuddin, A.K. Skin Lightening & Management of Hyperpigmentation. Int. J. Res. Biol. Pharm. 2019, 2, 1–37. [Google Scholar]
- Nii, D.; Espósito, A.C.; Peres, G.; Schmitt, J.V.; Miot, H. Tinted Sunscreens Lead to a Smaller Amount of the Product Applied on the Face. Int. J. Dermatol. 2020, 59, e438–e439. [Google Scholar] [CrossRef] [PubMed]
- Virador, V.; Matsunaga, N.; Matsunaga, J.; Valencia, J.; Oldham, R.J.; Kameyama, K.; Peck, G.L.; Ferrans, V.J.; Vieira, W.D.; Abdel-Malek, Z.A. Production of Melanocyte-specific Antibodies to Human Melanosomal Proteins: Expression Patterns in Normal Human Skin and in Cutaneous Pigmented Lesions. Pigment Cell Res. 2001, 14, 289–297. [Google Scholar] [CrossRef] [PubMed]
- El-Kayal, M.; Nasr, M.; Elkheshen, S.; Mortada, N. Colloidal (-)-Epigallocatechin-3-Gallate Vesicular Systems for Prevention and Treatment of Skin Cancer: A Comprehensive Experimental Study with Preclinical Investigation. Eur. J. Pharm. Sci. 2019, 137, 104972. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Nasr, M.; Tawfik, A.A.; Fadel, M.; Sammour, O. Novel Bergamot Oil Nanospanlastics Combined with PUVB Therapy as a Clinically Translatable Approach for Vitiligo Treatment. Drug Deliv. Transl. Res. 2019, 9, 1106–1116. [Google Scholar] [CrossRef]
- Bseiso, E.A.; Nasr, M.; Sammour, O.; Abd El Gawad, N.A. Recent Advances in Topical Formulation Carriers of Antifungal Agents. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 457. [Google Scholar] [CrossRef]
- Hanumanaik, M.; Patel, S.K.; Sree, K.R. Solid Lipid Nanoparticles: A Review. Int. J. Pharm. Sci. Res. 2013, 4, 928–940. [Google Scholar]
- Amer, S.S.; Nasr, M.; Mamdouh, W.; Sammour, O. Insights on the Use of Nanocarriers for Acne Alleviation. Curr. Drug Deliv. 2019, 16, 18–25. [Google Scholar] [CrossRef]
- Hajare, A.A.; Mali, S.S.; Ahir, A.A.; Thorat, J.D.; Salunkhe, S.S.; Nadaf, S.J.; Bhatia, N.M.; Chitranagari, K. Lipid Nanoparticles: A Modern Formulation Approach in Topical Drug Delivery Systems. J. Adv. Drug Deliv. 2014, 1, 30–37. [Google Scholar]
- Abu-Azzam, O.; Nasr, M. In Vitro Anti-Inflammatory Potential of Phloretin Microemulsion as a New Formulation for Prospective Treatment of Vaginitis. Pharm. Dev. Technol. 2020, 25, 930–935. [Google Scholar] [CrossRef]
- Al-Karaki, R.; Awadallah, A.; Tawfeek, H.M.; Nasr, M. Preparation, Characterization and Cytotoxic Activity of New Oleuropein Microemulsion against HCT-116 Colon Cancer Cells. Pharm. Chem. J. 2020, 53, 1118–1121. [Google Scholar] [CrossRef]
- Ramez, S.A.; Soliman, M.M.; Fadel, M.; Nour El-Deen, F.; Nasr, M.; Youness, E.R.; Aboel-Fadl, D.M. Novel Methotrexate Soft Nanocarrier/Fractional Erbium YAG Laser Combination for Clinical Treatment of Plaque Psoriasis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 996–1002. [Google Scholar] [CrossRef]
- Nasr, M.; Abdel-Hamid, S.; Moftah, N.H.; Fadel, M.; Alyoussef, A.A. Jojoba Oil Soft Colloidal Nanocarrier of a Synthetic Retinoid: Preparation, Characterization and Clinical Efficacy in Psoriatic Patients. Curr. Drug Deliv. 2017, 14, 426–432. [Google Scholar] [CrossRef]
- Badawi, A.A.; Nour, S.A.; Sakran, W.S.; El-Mancy, S.M.S. Preparation and Evaluation of Microemulsion Systems Containing Salicylic Acid. AAPS Pharmscitech 2009, 10, 1081–1084. [Google Scholar] [CrossRef]
- Mahdi, Z.H.; Maraie, N.K. Overview on Nanoemulsion as a Recently Developed Approach in Drug Nanoformulation. Res. J. Pharm. Technol. 2019, 12, 5554–5560. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-T.; Kim, M.-H.; Park, J.-H.; Lee, J.-Y.; Cho, H.-J.; Yoon, I.-S.; Kim, D.-D. Microemulsion-Based Hydrogels for Enhancing Epidermal/Dermal Deposition of Topically Administered 20 (S)-Protopanaxadiol: In Vitro and in Vivo Evaluation Studies. J. Ginseng. Res. 2018, 42, 512–523. [Google Scholar] [CrossRef]
- Lovelyn, C. Current State of Nanoemulsions in Drug Delivery. J. Biomater. Nanobiotechnol. 2011, 2, 626–639. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, Properties and Applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.; Nasr, M.; Sammour, O. Nanoemulsion as a Feasible and Biocompatible Carrier for Ocular Delivery of Travoprost: Improved Pharmacokinetic/Pharmacodynamic Properties. Int. J. Pharm. 2020, 583, 119402. [Google Scholar] [CrossRef]
- Parthasarathi, S.; Muthukumar, S.P.; Anandharamakrishnan, C. The Influence of Droplet Size on the Stability, in Vivo Digestion, and Oral Bioavailability of Vitamin E Emulsions. Food Funct. 2016, 7, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Aboofazeli, R. Nanometric-Scaled Emulsions (Nanoemulsions). Iran. J. Pharm. Res. IJPR 2010, 9, 325. [Google Scholar] [PubMed]
- Hatem, S.; Nasr, M.; Moftah, N.H.; Ragai, M.H.; Geneidi, A.S.; Elkheshen, S.A. Melatonin Vitamin C-Based Nanovesicles for Treatment of Androgenic Alopecia: Design, Characterization and Clinical Appraisal. Eur. J. Pharm. Sci. 2018, 122, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Tokudome, Y.; Nakamura, K.; Itaya, Y.; Hashimoto, F. Enhancement of Skin Penetration of Hydrophilic and Lipophilic Compounds by PH-Sensitive Liposomes. J. Pharm. Pharm. Sci. 2015, 18, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Upputuri, R.T.P.; Mandal, A.K.A. Sustained Release of Green Tea Polyphenols from Liposomal Nanoparticles; Release Kinetics and Mathematical Modelling. Iran. J. Biotechnol. 2017, 15, 277. [Google Scholar] [CrossRef] [Green Version]
- Chountoulesi, M.; Naziris, N.; Pippa, N.; Demetzos, C. The Significance of Drug-to-Lipid Ratio to the Development of Optimized Liposomal Formulation. J. Liposome Res. 2018, 28, 249–258. [Google Scholar] [CrossRef]
- Yadav, D.; Sandeep, K.; Pandey, D.; Dutta, R.K. Liposomes for Drug Delivery. J. Biotechnol. Biomater. 2017, 7. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent Advances with Liposomes as Pharmaceutical Carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Banihashemi, M.; Zabolinejad, N.; Jaafari, M.R.; Salehi, M.; Jabari, A. Comparison of Therapeutic Effects of Liposomal Tranexamic Acid and Conventional Hydroquinone on Melasma. J. Cosmet. Dermatol. 2015, 14, 174–177. [Google Scholar] [CrossRef]
- Taghavi, F.; Banihashemi, M.; Zabolinejad, N.; Salehi, M.; Jaafari, M.R.; Marhamati, H.; Golnouri, F.; Dorri, M. Comparison of Therapeutic Effects of Conventional and Liposomal Form of 4% Topical Hydroquinone in Patients with Melasma. J. Cosmet. Dermatol. 2019, 18, 870–873. [Google Scholar] [CrossRef]
- Shigeta, Y.; Imanaka, H.; Ando, H.; Ryu, A.; Oku, N.; Baba, N.; Makino, T. Skin Whitening Effect of Linoleic Acid Is Enhanced by Liposomal Formulations. Biol. Pharm. Bull. 2004, 27, 591–594. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-M.; Kuo, H.-C.; Lin, C.-T.; Kao, E.-S. Inhibitory Effect of Liposome-Encapsulated Anthocyanin on Melanogenesis in Human Melanocytes. Pharm. Biol. 2013, 51, 941–947. [Google Scholar] [CrossRef]
- Ghafarzadeh, M.; Eatemadi, A. Clinical Efficacy of Liposome-Encapsulated Aloe Vera on Melasma Treatment during Pregnancy. J. Cosmet. Laser Ther. 2017, 19, 181–187. [Google Scholar] [CrossRef]
- Huh, S.Y.; SHIN, J.; NA, J.; HUH, C.; YOUN, S.; PARK, K. Efficacy and Safety of Liposome-encapsulated 4-n-butylresorcinol 0.1% Cream for the Treatment of Melasma: A Randomized Controlled Split-face Trial. J. Dermatol. 2010, 37, 311–315. [Google Scholar] [CrossRef]
- Fan, H.; Li, Y.; Huang, Y.; Liu, G.; Xia, Q. Preparation and Evaluation of Phenylethyl Resorcinol Liposome. Integr. Ferroelectr. 2014, 151, 89–98. [Google Scholar] [CrossRef]
- Therdphapiyanak, N.; Jaturanpinyo, M.; Waranuch, N.; Kongkaneramit, L.; Sarisuta, N. Development and Assessment of Tyrosinase Inhibitory Activity of Liposomes of Asparagus Racemosus Extracts. Asian J. Pharm. Sci. 2013, 8, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Panichakul, T.; Rodboon, T.; Suwannalert, P.; Tripetch, C.; Rungruang, R.; Boohuad, N.; Youdee, P. Additive Effect of a Combination of Artocarpus Lakoocha and Glycyrrhiza Glabra Extracts on Tyrosinase Inhibition in Melanoma B16 Cells. Pharmaceuticals 2020, 13, 310. [Google Scholar] [CrossRef]
- Wen, A.-H.; Choi, M.-K.; Kim, D.-D. Formulation of Liposome for Topical Delivery of Arbutin. Arch. Pharm. Res. 2006, 29, 1187–1192. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Ethosomes: New Prospects in Transdermal Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2003, 20. [Google Scholar] [CrossRef]
- Limsuwan, T.; Boonme, P.; Khongkow, P.; Amnuaikit, T. Ethosomes of Phenylethyl Resorcinol as Vesicular Delivery System for Skin Lightening Applications. Biomed Res. Int. 2017, 2017, 8310979. [Google Scholar] [CrossRef] [Green Version]
- Hunter, C.A.; Dolan, T.F.; Coombs, G.H.; Baillie, A.J. Vesicular Systems (Niosomes and Liposomes) for Delivery of Sodium Stibogluconate in Experimental Murine Visceral Leishmaniasis. J. Pharm. Pharmacol. 1988, 40, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Atrux-Tallau, N.; Denis, A.; Padois, K.; Bertholle, V.; Huynh, T.T.N.; Haftek, M.; Falson, F.; Pirot, F. Skin Absorption Modulation: Innovative Non-Hazardous Technologies for Topical Formulations. Open Dermatol. J. 2010, 4, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Mbah, C.C.; Attama, A.A. Vesicular Carriers as Innovative Nanodrug Delivery Formulations. In Organic Materials as Smart Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 519–559. [Google Scholar]
- Divanbeygikermani, M.; Pardakhty, A.; Amanatfard, A. Kojic Acid and Hydroquinone Non-Ionic Surfactant Vesicles for Topical Application. Int. Pharm. Acta 2018, 1, 110. [Google Scholar]
- Buruschat, J.; Amnuaikit, T. Preparation of Phenylethyl Resorcinol Niosomes for Cosmetic Formulation: Effects of BrijTM72 and Cholesterol. Lat. Am. J. Pharm. 2016, 35, 1640–1644. [Google Scholar]
- Fadel, M.; Samy, N.; Nasr, M.; Alyoussef, A.A. Topical Colloidal Indocyanine Green-Mediated Photodynamic Therapy for Treatment of Basal Cell Carcinoma. Pharm. Dev. Technol. 2017, 22, 545–550. [Google Scholar] [CrossRef]
- Kameyama, K.; Sakai, C.; Kondoh, S.; Yonemoto, K.; Nishiyama, S.; Tagawa, M.; Murata, T.; Ohnuma, T.; Quigley, J.; Dorsky, A. Inhibitory Effect of Magnesium L-Ascorbyl-2-Phosphate (VC-PMG) on Melanogenesis in Vitro and in Vivo. J. Am. Acad. Dermatol. 1996, 34, 29–33. [Google Scholar] [CrossRef]
- Celia, C.; Cilurzo, F.; Trapasso, E.; Cosco, D.; Fresta, M.; Paolino, D. Ethosomes® and Transfersomes® Containing Linoleic Acid: Physicochemical and Technological Features of Topical Drug Delivery Carriers for the Potential Treatment of Melasma Disorders. Biomed. Microdevices 2012, 14, 119–130. [Google Scholar] [CrossRef]
- Amnuaikit, T.; Limsuwan, T.; Khongkow, P.; Boonme, P. Vesicular Carriers Containing Phenylethyl Resorcinol for Topical Delivery System; Liposomes, Transfersomes and Invasomes. Asian J. Pharm. Sci. 2018, 13, 472–484. [Google Scholar] [CrossRef]
- Limsuwan, T.; Boonme, P.; Amnuaikit, T. Enhanced Stability of Phenylethyl Resorcinol in Elastic Vesicular Formulations. Trop. J. Pharm. Res. 2018, 17, 1895–1902. [Google Scholar] [CrossRef]
- Lee, M.-H.; Lee, K.-K.; Park, M.-H.; Hyun, S.-S.; Kahn, S.-Y.; Joo, K.-S.; Kang, H.-C.; Kwon, W.-T. In Vivo Anti-Melanogenesis Activity and in Vitro Skin Permeability of Niacinamide-Loaded Flexible Liposomes (BounsphereTM). J. Drug Deliv. Sci. Technol. 2016, 31, 147–152. [Google Scholar] [CrossRef]
- Bian, S.; Choi, M.-K.; Lin, H.; Zheng, J.; Chung, S.-J.; Shim, C.-K.; Kim, D.-D. Deformable Liposomes for Topical Skin Delivery of Arbutin. J. Pharm. Investig. 2006, 36, 299–304. [Google Scholar]
- Amer, S.S.; Nasr, M.; Abdel-Aziz, R.T.A.; Moftah, N.H.; El Shaer, A.; Polycarpou, E.; Mamdouh, W.; Sammour, O. Cosm-Nutraceutical Nanovesicles for Acne Treatment: Physicochemical Characterization and Exploratory Clinical Experimentation. Int. J. Pharm. 2020, 577, 119092. [Google Scholar] [CrossRef]
- Aboul-Einien, M.H.; Kandil, S.M.; Abdou, E.M.; Diab, H.M.; Zaki, M.S.E. Ascorbic Acid Derivative-Loaded Modified Aspasomes: Formulation, in Vitro, Ex Vivo and Clinical Evaluation for Melasma Treatment. J. Liposome Res. 2020, 30, 54–67. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Shamma, R.N.; Farouk, F.; Nasralla, S.M. Dapsone-Loaded Invasomes as a Potential Treatment of Acne: Preparation, Characterization, and in Vivo Skin Deposition Assay. Aaps Pharmscitech 2018, 19, 2174–2184. [Google Scholar] [CrossRef]
- Paolino, D.; Lucania, G.; Mardente, D.; Alhaique, F.; Fresta, M. Ethosomes for Skin Delivery of Ammonium Glycyrrhizinate: In Vitro Percutaneous Permeation through Human Skin and in Vivo Anti-Inflammatory Activity on Human Volunteers. J. Control. Release 2005, 106, 99–110. [Google Scholar] [CrossRef]
- Dragicevic-Curic, N.; Scheglmann, D.; Albrecht, V.; Fahr, A. Temoporfin-Loaded Invasomes: Development, Characterization and in Vitro Skin Penetration Studies. J. Control. Release 2008, 127, 59–69. [Google Scholar] [CrossRef]
- Shah, S.M.; Ashtikar, M.; Jain, A.S.; Makhija, D.T.; Nikam, Y.; Gude, R.P.; Steiniger, F.; Jagtap, A.A.; Nagarsenker, M.S.; Fahr, A. LeciPlex, Invasomes, and Liposomes: A Skin Penetration Study. Int. J. Pharm. 2015, 490, 391–403. [Google Scholar] [CrossRef]
- Manconi, M.; Caddeo, C.; Sinico, C.; Valenti, D.; Mostallino, M.C.; Lampis, S.; Monduzzi, M.; Fadda, A.M. Penetration Enhancer-Containing Vesicles: Composition Dependence of Structural Features and Skin Penetration Ability. Eur. J. Pharm. Biopharm. 2012, 82, 352–359. [Google Scholar] [CrossRef]
- Aldalaen, S.; Nasr, M.; El-Gogary, R.I. Angiogenesis and Collagen Promoting Nutraceutical-Loaded Nanovesicles for Wound Healing. J. Drug Deliv. Sci. Technol. 2020, 56, 101548. [Google Scholar] [CrossRef]
- Kapoor, A.; Mishra, S.K.; Verma, D.K.; Pandey, P. Chemical Penetration Enhancers for Transdermal Drug Delivery System. J. Drug Deliv. Ther. 2018, 8, 62–66. [Google Scholar] [CrossRef]
- Bsieso, E.A.; Nasr, M.; Moftah, N.H.; Sammour, O.A.; Abd El Gawad, N.A. Could Nanovesicles Containing a Penetration Enhancer Clinically Improve the Therapeutic Outcome in Skin Fungal Diseases? Nanomedicine 2015, 10, 2017–2031. [Google Scholar] [CrossRef] [PubMed]
- Schlich, M.; Fornasier, M.; Nieddu, M.; Sinico, C.; Murgia, S.; Rescigno, A. 3-Hydroxycoumarin Loaded Vesicles for Recombinant Human Tyrosinase Inhibition in Topical Applications. Colloids Surf. B Biointerfaces 2018, 171, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Edison, T.N.J.I.; Karuppusamy, I.; Kathirvel, B. Inorganic Nanoparticles: A Potential Cancer Therapy for Human Welfare. Int. J. Pharm. 2018, 539, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Swierczewska, M.; Lee, S.; Chen, X. Inorganic Nanoparticles for Multimodal Molecular Imaging. Mol. Imaging 2011, 10, 2011–7290. [Google Scholar] [CrossRef]
- Khodakiya, A.S.; Chavada, J.R.; Jivani, N.P.; Patel, B.N.; Khodakiya, M.S.; Ramoliya, A.P. Microemulsions as Enhanced Drug Delivery Carrier: An Overview. Am. J. Pharmtech. Res. 2012, 206–226. [Google Scholar]
- Jiménez-Pérez, Z.E.; Singh, P.; Kim, Y.-J.; Mathiyalagan, R.; Kim, D.-H.; Lee, M.H.; Yang, D.C. Applications of Panax Ginseng Leaves-Mediated Gold Nanoparticles in Cosmetics Relation to Antioxidant, Moisture Retention, and Whitening Effect on B16BL6 Cells. J. Ginseng Res. 2018, 42, 327–333. [Google Scholar] [CrossRef]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of Nanotechnology in Targeted Drug Delivery and Imaging: A Concise Review. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 193–212. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; El-Refaie, W.M.; El-Massik, M.A.; Abdallah, O.Y. Lecithin-Based Nanostructured Gels for Skin Delivery: An Update on State of Art and Recent Applications. J. Control. Release 2014, 180, 10–24. [Google Scholar] [CrossRef]
- Xiao, L.; Matsubayashi, K.; Miwa, N. Inhibitory Effect of the Water-Soluble Polymer-Wrapped Derivative of Fullerene on UVA-Induced Melanogenesis via Downregulation of Tyrosinase Expression in Human Melanocytes and Skin Tissues. Arch. Dermatol. Res. 2007, 299, 245–257. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Rangari, A.T.; Ravikumar, P. Polymeric Nanoparticles Based Topical Drug Delivery: An Overview. Asian J. Biomed. Pharm. Sci. 2015, 5, 5. [Google Scholar] [CrossRef]
- Samadzadeh, S.; Mousazadeh, H.; Ghareghomi, S.; Dadashpour, M.; Babazadeh, M.; Zarghami, N. In Vitro Anticancer Efficacy of Metformin-Loaded PLGA Nanofibers towards the Post-Surgical Therapy of Lung Cancer. J. Drug Deliv. Sci. Technol. 2021, 61, 102318. [Google Scholar] [CrossRef]
- Mogheri, F.; Jokar, E.; Afshin, R.; Akbari, A.A.; Dadashpour, M.; Firouzi-amandi, A.; Serati-Nouri, H.; Zarghami, N. Co-Delivery of Metformin and Silibinin in Dual-Drug Loaded Nanoparticles Synergistically Improves Chemotherapy in Human Non-Small Cell Lung Cancer A549 Cells. J. Drug Deliv. Sci. Technol. 2021, 66, 102752. [Google Scholar] [CrossRef]
- Amirsaadat, S.; Jafari-Gharabaghlou, D.; Alijani, S.; Mousazadeh, H.; Dadashpour, M.; Zarghami, N. Metformin and Silibinin Co-Loaded PLGA-PEG Nanoparticles for Effective Combination Therapy against Human Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102107. [Google Scholar] [CrossRef]
- Adlravan, E.; Nejati, K.; Karimi, M.A.; Mousazadeh, H.; Abbasi, A.; Dadashpour, M. Potential Activity of Free and PLGA/PEG Nanoencapsulated Nasturtium Officinale Extract in Inducing Cytotoxicity and Apoptosis in Human Lung Carcinoma A549 Cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102256. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Tong, R.; Cheng, J. Anticancer Polymeric Nanomedicines. J. Macromol. Sci. Part C Polym. Rev. 2007, 47, 345–381. [Google Scholar] [CrossRef]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric Nanoparticles, Nanospheres and Nanocapsules, for Cutaneous Applications. Drug Target Insights 2007, 2, 117739280700200000. [Google Scholar] [CrossRef]
- Singh, R.; Lillard Jr, J.W. Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Abd-Allah, H.; Abdel-Aziz, R.T.A.; Nasr, M. Chitosan Nanoparticles Making Their Way to Clinical Practice: A Feasibility Study on Their Topical Use for Acne Treatment. Int. J. Biol. Macromol. 2020, 156, 262–270. [Google Scholar] [CrossRef]
- Ayumi, N.S.; Sahudin, S.; Hussain, Z.; Hussain, M.; Samah, N.H.A. Polymeric Nanoparticles for Topical Delivery of Alpha and Beta Arbutin: Preparation and Characterization. Drug Deliv. Transl. Res. 2019, 9, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.-S.; Park, H.-J.; Lee, J. Stabilization of Glabridin by Chitosan Nano-Complex. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 457–462. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Jou, C.-H.; Hung, C.-C.; Yang, M.-C. Cellular Fusion and Whitening Effect of a Chitosan Derivative Coated Liposome. Colloids Surf. B Biointerfaces 2012, 90, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Urbán-Morlán, Z.; Mendoza-Elvira, S.E.; Hernández-Cerón, R.S.; Alcalá-Alcalá, S.; Ramírez-Mendoza, H.; Ciprián-Carrasco, A.; Piñón-Segundo, E.; Quintanar-Guerrero, D. Preparation of Ethyl Cellulose Nanoparticles by Solvent-Displacement Using the Conventional Method and a Recirculation System. J. Mex. Chem. Soc. 2015, 59, 173–180. [Google Scholar] [CrossRef]
- Duarah, S.; Durai, R.D.; Narayanan, V.B. Nanoparticle-in-Gel System for Delivery of Vitamin C for Topical Application. Drug Deliv. Transl. Res. 2017, 7, 750–760. [Google Scholar] [CrossRef]
- Cho, H.K.; Cheong, I.W.; Lee, J.M.; Kim, J.H. Polymeric Nanoparticles, Micelles and Polymersomes from Amphiphilic Block Copolymer. Korean J. Chem. Eng. 2010, 27, 731–740. [Google Scholar] [CrossRef]
- Lee, J.S.; Feijen, J. Polymersomes for Drug Delivery: Design, Formation and Characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P. Polymersomes in Nanomedicine-A Review. Curr. Nanosci. 2017, 13, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Mabrouk, E.; Cuvelier, D.; Pontani, L.-L.; Xu, B.; Lévy, D.; Keller, P.; Brochard-Wyart, F.; Nassoy, P.; Li, M.-H. Formation and Material Properties of Giant Liquid Crystal Polymersomes. Soft Matter 2009, 5, 1870–1878. [Google Scholar] [CrossRef]
- Riess, G. Micellization of Block Copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Ling, P.; Zhang, T. Polymeric Micelles, a Promising Drug Delivery System to Enhance Bioavailability of Poorly Water-Soluble Drugs. J. Drug Deliv. 2013, 2013, 340315. [Google Scholar] [CrossRef]
- Seino, H.; Arai, Y.; Nagao, N.; Ozawa, N.; Hamada, K. Efficient Percutaneous Delivery of the Antimelanogenic Agent Glabridin Using Cationic Amphiphilic Chitosan Micelles. PLoS ONE 2016, 11, e0164061. [Google Scholar] [CrossRef]
- Khezri, K.; Saeedi, M.; Morteza-Semnani, K.; Akbari, J.; Rostamkalaei, S.S. An Emerging Technology in Lipid Research for Targeting Hydrophilic Drugs to the Skin in the Treatment of Hyperpigmentation Disorders: Kojic Acid-Solid Lipid Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2020, 48, 841–853. [Google Scholar] [CrossRef]
- Sanna, V.; Gavini, E.; Cossu, M.; Rassu, G.; Giunchedi, P. Solid Lipid Nanoparticles (SLN) as Carriers for the Topical Delivery of Econazole Nitrate: In-Vitro Characterization, Ex-Vivo and in-Vivo Studies. J. Pharm. Pharmacol. 2007, 59, 1057–1064. [Google Scholar] [CrossRef]
- Bhalekar, M.R.; Pokharkar, V.; Madgulkar, A.; Patil, N.; Patil, N. Preparation and Evaluation of Miconazole Nitrate-Loaded Solid Lipid Nanoparticles for Topical Delivery. Aaps Pharmscitech 2009, 10, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Marto, J.; Sangalli, C.; Capra, P.; Perugini, P.; Ascenso, A.; Gonçalves, L.; Ribeiro, H. Development and Characterization of New and Scalable Topical Formulations Containing N-Acetyl-d-Glucosamine-Loaded Solid Lipid Nanoparticles. Drug Dev. Ind. Pharm. 2017, 43, 1792–1800. [Google Scholar] [CrossRef]
- Ghanbarzadeh, S.; Hariri, R.; Kouhsoltani, M.; Shokri, J.; Javadzadeh, Y.; Hamishehkar, H. Enhanced Stability and Dermal Delivery of Hydroquinone Using Solid Lipid Nanoparticles. Colloids Surf. B Biointerfaces 2015, 136, 1004–1010. [Google Scholar] [CrossRef]
- Al-Amin, M.; Cao, J.; Naeem, M.; Banna, H.; Kim, M.-S.; Jung, Y.; Chung, H.Y.; Moon, H.R.; Yoo, J.-W. Increased Therapeutic Efficacy of a Newly Synthesized Tyrosinase Inhibitor by Solid Lipid Nanoparticles in the Topical Treatment of Hyperpigmentation. Drug Des. Dev. Ther. 2016, 10, 3947. [Google Scholar] [CrossRef] [Green Version]
- Shrotriya, S.; Ranpise, N.; Satpute, P.; Vidhate, B. Skin Targeting of Curcumin Solid Lipid Nanoparticles-Engrossed Topical Gel for the Treatment of Pigmentation and Irritant Contact Dermatitis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1471–1482. [Google Scholar] [CrossRef] [Green Version]
- Ayuningtyas, I.N.; Mun’im, A.; Sutriyo, S. The Study of Safety and Skin Whitening Efficacy of Melinjo (Gnetum Gnemon L.) Seed Extract-Loaded Lipid Particle Gel. Pharmacogn. Res. 2018, 10, 432–436. [Google Scholar]
- Kumar, A.; Rao, R. Enhancing Efficacy and Safety of Azelaic Acid via Encapsulation in Cyclodextrin Nanosponges: Development, Characterization and Evaluation. Polym. Bull. 2021, 78, 5275–5302. [Google Scholar] [CrossRef]
- ElHoffy, N.; Elezaby, R.; Nasr, M.; Osama, A.; Elkheshen, S. Optimization of the Colloidal Properties of Chitosan Nanoparticles Encapsulating Alpha-Arbutin. Arch. Pharm. Sci. Ain Shams Univ. 2022, 6, 17–28. [Google Scholar]
- Hatem, S.; Elkheshen, S.A.; Kamel, A.O.; Nasr, M.; Moftah, N.H.; Ragai, M.H.; Elezaby, R.S.; El Hoffy, N.M. Functionalized Chitosan Nanoparticles for Cutaneous Delivery of a Skin Whitening Agent: An Approach to Clinically Augment the Therapeutic Efficacy for Melasma Treatment. Drug Deliv. 2022, 29, 1212–1231. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Duan, N.; Song, S.; Nie, D.; Yu, M.; Wang, J.; Xi, Z.; Li, J.; Sheng, Y.; Xu, C. Transfersomes Improved Delivery of Ascorbic Palmitate into the Viable Epidermis for Enhanced Treatment of Melasma. Int. J. Pharm. 2021, 608, 121059. [Google Scholar] [CrossRef]
- Radmard, A.; Saeedi, M.; Morteza-Semnani, K.; Hashemi, S.M.H.; Nokhodchi, A. An Eco-Friendly and Green Formulation in Lipid Nanotechnology for Delivery of a Hydrophilic Agent to the Skin in the Treatment and Management of Hyperpigmentation Complaints: Arbutin Niosome (Arbusome). Colloids Surf. B Biointerfaces 2021, 201, 111616. [Google Scholar] [CrossRef]
- Fachinetti, N.; Rigon, R.B.; Eloy, J.O.; Sato, M.R.; Dos Santos, K.C.; Chorilli, M. Comparative Study of Glyceryl Behenate or Polyoxyethylene 40 Stearate-Based Lipid Carriers for Trans-Resveratrol Delivery: Development, Characterization and Evaluation of the in Vitro Tyrosinase Inhibition. AAPS PharmSciTech 2018, 19, 1401–1409. [Google Scholar] [CrossRef]
- Wu, P.-S.; Lin, C.-H.; Kuo, Y.-C.; Lin, C.-C. Formulation and Characterization of Hydroquinone Nanostructured Lipid Carriers by Homogenization Emulsification Method. J. Nanomater. 2017, 2017, 3282693. [Google Scholar] [CrossRef] [Green Version]
- Tofani, R.P.; Sumirtapura, Y.C.; Darijanto, S.T. Formulation, Characterisation, and in Vitro Skin Diffusion of Nanostructured Lipid Carriers for Deoxyarbutin Compared to a Nanoemulsion and Conventional Cream. Sci. Pharm. 2016, 84, 634–645. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.-S.; Na, Y.-G.; Choi, J.-H.; Kim, I.; Lee, E.; Kim, S.-Y.; Lee, J.-Y.; Cho, C.-W. The Improvement of Skin Whitening of Phenylethyl Resorcinol by Nanostructured Lipid Carriers. Nanomaterials 2017, 7, 241. [Google Scholar] [CrossRef]
- Aliasgharlou, L.; Ghanbarzadeh, S.; Azimi, H.; Zarrintan, M.H.; Hamishehkar, H. Nanostructured Lipid Carrier for Topical Application of N-Acetyl Glucosamine. Adv. Pharm. Bull. 2016, 6, 581. [Google Scholar] [CrossRef] [Green Version]
- Parveen, R.; Akhtar, N.; Mahmood, T. Topical Microemulsion Containing Punica Granatum Extract: Its Control over Skin Erythema and Melanin in Healthy Asian Subjects. Adv. Dermatol. Allergol. Dermatol. Alergol. 2014, 31, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Pakpayat, N.; Nielloud, F.; Fortuné, R.; Tourne-Peteilh, C.; Villarreal, A.; Grillo, I.; Bataille, B. Formulation of Ascorbic Acid Microemulsions with Alkyl Polyglycosides. Eur. J. Pharm. Biopharm. 2009, 72, 444–452. [Google Scholar] [CrossRef]
- Azhar, S.N.A.S.; Ashari, S.E.; Salim, N. Development of a Kojic Monooleate-Enriched Oil-in-Water Nanoemulsion as a Potential Carrier for Hyperpigmentation Treatment. Int. J. Nanomed. 2018, 13, 6465. [Google Scholar] [CrossRef] [Green Version]
- Al-Edresi, S.; Baie, S. Formulation and Stability of Whitening VCO-in-Water Nano-Cream. Int. J. Pharm. 2009, 373, 174–178. [Google Scholar] [CrossRef]

| Skin Type | Skin Texture | Aptitude to Tan |
|---|---|---|
| I | Blond/red hair, blond/red complexion, blue/green eyes | Never tan, always burn |
| II | Blond skin, blue eyes | Usually burn, difficulty in tan |
| III | White skin with a deeper hue | Average tan, early burn |
| IV | Moderate brown skin | Burns minimally, tans simply |
| V | Skin colour: dark brown | Hardly ever burns, tans very easily |
| VI | Skin colour: black | Always tans darkly and never burns |
| Drug (Technique) | Excipients | Outcome & Significance | Ref. |
|---|---|---|---|
| Solid Lipid Nanoparticles | |||
| Kojic acid (high speed homogenisation and ultra-probe sonication method) | Cholesterol, Glyceryl monostearate, Tween 20, Span 60 | Enhanced the cutaneous delivery of Kojic acid with increased concentration and controlled drug release into deeper skin layers | [115] |
| Econazole nitrate (High-shear homogenization method) | Precirol ATO 5, Tween 80 | Increased permeation of drug within 1 h of its application and possessed better penetration of drug into deeper layers of skin after 3 h | [116] |
| Miconazole nitrate (High-pressure homogenization) | Compritol 888, Propylene glycol, Tween 80, Glyceryl monostearate | Improved skin targeting effect and accumulative absorption of drug in the skin | [117] |
| N-Acetyl-D-Glucosamine (High shear homogenization) | Cetyl Palmitate, Phosphatidylcholine, Hydrogenated Castor Oil | Improvement of skin hydration and elasticity in several skin disorders | [118] |
| Hydroquinone (Hot melt homogenization method) | Poloxamer 407, Glycerol Palmitostearate | Excellent physicochemical stability and improved drug localisation in the skin | [119] |
| Tyrosinase inhibitor-(Z)-5-(2,4-dihydroxy benzylidene)thiazolidine-2,4-dione (MHY498) (o/w emulsion solvent-evaporation method) | Compritol 888 ATO, Phosphatidylcholine, Poloxamer 188 | MHY-SLNs exhibited prolonged release and increased skin permeation and effectively prevented UVB-induced melanogenesis | [120] |
| Curcumin (Pre-emulsion technique followed by ultrasonic probe sonication method) | Precirol ATO5, Tween-80 | Curcumin-SLN exhibited controlled drug release up to 24 h. It showed potential for skin targeting and has potential in skin depigmentation | [121] |
| Melinjo (Gnetum gnemon L.) seed extract (High-shear homogenization and hot-melted technique) | Glyceryl monostearate, Brij CS25 | Melinjo seed extract produced skin whitening effect without causing irritation | [122] |
| Nanosponges | |||
| Azelaic acid (Melt method) | β-Cyclodextrin, diphenyl carbonate | Beta cyclodextrin nanosponges increased the solubility and depigmenting action of Azelaic acid via antioxidant and antityrosinase effects | [123] |
| Polymeric Nanoparticles | |||
| Alpha-arbutin (Ionic gelation method) | Chitosan, tripolyphosphate sodium salt | Loading of α-arbutin into chitosan produced significant higher entrapment efficiency as compared to α-arbutin loading into tripolyphosphate | [124] |
| Alpha-arbutin (Ionic gelation method) | Chitosan, tripolyphosphate sodium salt, hyaluronic acid, collagen | Alpha-arbutin loaded chitosan nanoparticles hydrogels revealed better therapeutic effectiveness in melasma as compared to free drug hydrogels and also exhibited improved drug deposition into deep skin layers | [125] |
| Vitamin C (Modified solvent evaporation technique) | Ethyl cellulose, Pluronic F127 | Polymeric nanoparticles showed sustained release behaviour till 8 h and significantly improved the therapeutic response and decreased adverse effects | [107] |
| Glabridin (Pressure homogenization method) | Partially myristoylated chitosan pyrrolidone carboxylate (PMCP), Polyquaternium-64, Tween 60, Butylene glycol, Cetyl ethylhexanoate, | PMCP was found efficient transdermal drug carrier for enhancing the permeation of Glabridin into epidermis of skin and suppressed the synthesis of melanin in skin | [114] |
| Aspasomes | |||
| Mg ascorbyl phosphate (Film hydration method) | Lecithin, cholesterol | Aspasome based cream exhibited enhanced drug permeation and skin retention and showed clinical effectiveness in melasma equivalent to 15% trichloroacetic acid | [74] |
| Transferosomes | |||
| Ascorbic palmitate (Thin-film hydration method) | Soybean phosphatidylcholine, Sodium deoxycholate | The permeation of ascorbic palmitate from transferosomes based drug delivery was higher which leads to 14.1-fold increase in ascorbic palmitate accumulation in epidermis in comparison to plain drug | [126] |
| Ethosomes | |||
| Phenylethyl Resorcinol (Thin-film hydration method) | Soybean phosphatidylcholine, Cholesterol | Ethosomes showed increased tyrosinase inhibition activity and also decreased melatonin content as compared to other formulations in B16 melanoma cells | [60] |
| Niosomes | |||
| Arbutin (Ultrasonic technique) | Cholesterol, Tween 20, Span 20 | Research study illustrated higher drug deposition in skin layers and revealed no signs of cytotoxicity in in-vitro cytotoxicity test and non-irritancy on Wistar rats | [127] |
| Nanostructured Lipid Carrier | |||
| Trans-Resveratrol (High shear homogenization technique) | Glyceryl behenate, Poloxamer 407, PEG-40 stearate, Castor oil, Caprylic/capric triglycerides | NLCs developed with PEG-40 stearate leads to 1.31 and 1.83-fold higher tyrosinase inhibition as compared to NLCs prepared with glyceryl behenate and plain trans-resveratrol solution | [128] |
| Hydroquinone (Homogenization emulsification method) | Sodium hydrogen sulfite, Bees wax, Caprylic/capric triglyceride, Lecithin, Span 80 | Hydroquinone loaded in NLC showed enhanced permeability, improved light stability and exhibited higher tyrosinase inhibition rate | [129] |
| Deoxyarbutin (High-shear homogenisation and ultrasonication) | Cetyl palmitate, Myristyl myristate, Poloxamer 188, PEG-400, Sodium sulfite | Increased efficacy of deoxyarbutin to inhibit tyrosinase activity during melanogenesis in skin | [130] |
| Phenylethyl Resorcinol (Hot-melted ultrasonic method) | Glyceryl monostearate, Olive oil, Lecithin, Tween 80, Polyvinyl alcohol | Phenylethyl Resorcinol loaded NLCs have particle size, polydispersity index, encapsulation efficiency and loading capacity of 57.9 ± 1.3 nm, 0.24 ± 0.01, 93.1 ± 4.2% and 8.5 ± 0.4%, respectively and exhibited sustained release pattern | [131] |
| N-Acetyl Glucosamine (Hot homogenization technique) | Miglyol, Precirol, Poloxamer, Tween 80 | N-Acetyl Glucosamine loaded NLCs have particle size of 190 nm, loading capacity of 9% and revealed significant decrease in melanin distribution pattern | [132] |
| Liposomes | |||
| Niacinamide (High-pressure homogenization method) | Phosphatidylcholine, Cholesterol, Ceramide, Dipotassium glycyrrhizate | Flexible liposomes synthesized in this research demonstrated higher deformability, safety, skin permeability, and anti-melanogenesis activity in comparison to conventional liposomes | [71] |
| Tranexamic Acid (Fusion method) | Soybean phosphatidylcholine, Cholesterol, Propylene glycol | Immense reduction in MASI scores was observed in patients treated with 5% liposomal tranexamic acid in comparison with patients treated with 4% hydroquinone cream and no serious adverse effects were observed in patients treated with liposomes | [49] |
| Anthocyanin (Vaporization and dehydration-hydration of organic solution) | Lecithin, Cholesterol | Encapsulation of anthocyanin into liposomes enhanced its stability and reduced melanogenesis by inhibition of tyrosinase and suppression of protein expression of tyrosinase and microphthalmia-associated transcription factor | [52] |
| Asparagus racemosus extracts (Chloroform-film, Reverse-phase evaporation, Polyol dilution, Freeze-drying of monophase solution methods) | Lecithin, Phospholipon, Diosgenin, Cholesterol, Propylene glycol | Liposomes had particle size in range of 0.26–13.83 μm and zeta potentials of −1.5 to −39.3 mV. The liposomes prepared by polyol dilution containing lecithin had maximum entrapment efficiency and in-vitro tyrosinase inhibitory activity of 69.08% and 25%, respectively | [55] |
| Phenylethyl Resorcinol (Injection method) | Soybean lecithin, Tween 80 | Liposomes had particle size in range of 160∼170 nm, drug loading of 2.45 ± 0.03% and had excellent stability | [56] |
| Arbutin (Film dispersion method) | Soybean Phosphatidylcholine, Cholesterol | The deposition of arbutin in epidermis/dermis layer of skin from liposome was higher in comparison to plain arbutin | [58] |
| Microemulsion | |||
| Punica granatum extract (Spontaneous emulsification technique phase titration method) | Tween 80, Propylene Glycol, Palm oil | Microemulsion revealed skin compatibility and exhibited reduction in skin melanin content in healthy male volunteers | [133] |
| Ascorbic acid (Hydrophilic lipophilic deviation concept) | Dioctylcyclohexane, Sorbitan monolaurate, Decylglucoside, Mineral oil | Microemulsion showed transcutaneous penetration of ascorbic acid which illustrated ascorbic acid-loaded microemulsion as suitable cosmetic for skin whitening potential | [134] |
| Nanoemulsion | |||
| Kojic monooleate (High and low energy emulsification technique) | Lemon essential oil, Castor oil, Tween 80, Xanthan gum | Cytotoxicity assay of nanoemulsion on mouse embryonic fibroblast cell line revealed safety and suitability of formulation in cosmeceutical application | [135] |
| Virgin coconut oil (Condensation method) | Squalene oil, Emulium Kappa, Propylene glycol | Addition of squalene oil caused reduction in ostwald ripening and increased stability of formulation | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Sharma, N.; Zahoor, I.; Behl, T.; Antil, A.; Gupta, S.; Anwer, M.K.; Mohan, S.; Bungau, S.G. Decrypting the Potential of Nanotechnology-Based Approaches as Cutting-Edge for Management of Hyperpigmentation Disorder. Molecules 2023, 28, 220. https://doi.org/10.3390/molecules28010220
Singh S, Sharma N, Zahoor I, Behl T, Antil A, Gupta S, Anwer MK, Mohan S, Bungau SG. Decrypting the Potential of Nanotechnology-Based Approaches as Cutting-Edge for Management of Hyperpigmentation Disorder. Molecules. 2023; 28(1):220. https://doi.org/10.3390/molecules28010220
Chicago/Turabian StyleSingh, Sukhbir, Neelam Sharma, Ishrat Zahoor, Tapan Behl, Anita Antil, Sumeet Gupta, Md Khalid Anwer, Syam Mohan, and Simona Gabriela Bungau. 2023. "Decrypting the Potential of Nanotechnology-Based Approaches as Cutting-Edge for Management of Hyperpigmentation Disorder" Molecules 28, no. 1: 220. https://doi.org/10.3390/molecules28010220
APA StyleSingh, S., Sharma, N., Zahoor, I., Behl, T., Antil, A., Gupta, S., Anwer, M. K., Mohan, S., & Bungau, S. G. (2023). Decrypting the Potential of Nanotechnology-Based Approaches as Cutting-Edge for Management of Hyperpigmentation Disorder. Molecules, 28(1), 220. https://doi.org/10.3390/molecules28010220










