Planar Elongated B12 Structure in M3B12 Clusters (M = Cu-Au)
Abstract
:1. Introduction
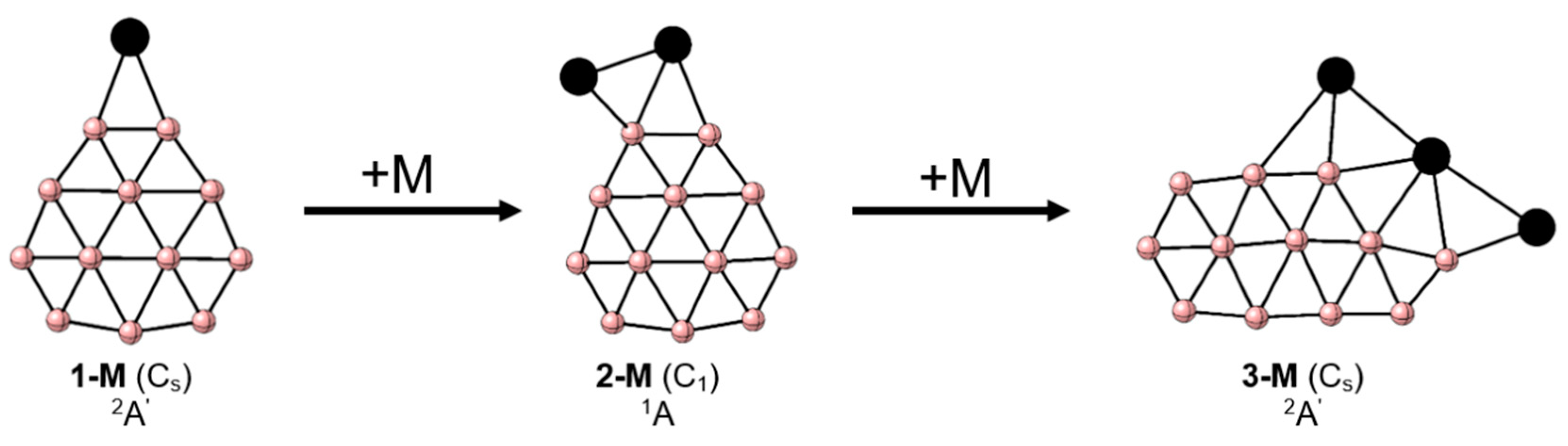
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Akopov, G.; Yeung, M.T.; Kaner, R.B. Rediscovering the Crystal Chemistry of Borides. Adv. Mater. 2017, 29, 1604506. [Google Scholar] [CrossRef] [PubMed]
- Scheifers, J.P.; Zhang, Y.; Fokwa, B.P.T. Boron: Enabling Exciting Metal-Rich Structures and Magnetic Properties. Acc. Chem. Res. 2017, 50, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Goddard, W.A. Improved Ductility of B12 Icosahedra-Based Superhard Materials through Icosahedral Slip. J. Phys. Chem. C 2017, 121, 11831–11838. [Google Scholar] [CrossRef] [Green Version]
- Robinson, P.J.; Liu, G.; Ciborowski, S.; Martinez-Martinez, C.; Chamorro, J.R.; Zhang, X.; McQueen, T.M.; Bowen, K.H.; Alexandrova, A.N. Mystery of Three Borides: Differential Metal-Boron Bonding Governing Superhard Structures. Chem. Mater. 2017, 29, 9892–9896. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Yu, G.; Li, C.Z.; Zhang, S.B.; Zhou, Z.; Chen, Z. Hydrogenation: A Simple Approach to Realize Semiconductor-Half-Metal-Metal Transition in Boron Nitride Nanoribbons. J. Am. Chem. Soc. 2010, 132, 1699–1705. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, L.; Li, J.; Li, H. Metal-Semiconductor Transition of Single-Wall Armchair Boron Nanotubes Induced by Atomic Depression. J. Phys. Chem. C 2017, 121, 26096–26101. [Google Scholar] [CrossRef]
- Bud’ko, S.L.; Lapertot, G.; Petrovic, C.; Cunningham, C.E.; Anderson, N.; Canfield, P.C. Boron Isotope Effect in Superconducting MgB2. Phys. Rev. Lett. 2001, 86, 1877–1880. [Google Scholar] [CrossRef] [Green Version]
- Kazakov, S.M.; Puzniak, R.; Rogacki, K.; Mironov, A.V.; Zhigadlo, N.D.; Jun, J.; Soltmann, C.; Batlogg, B.; Karpinski, J. Carbon Substitution in MgB2 Single Crystals: Structural and Superconducting Properties. Phys. Rev. B Condens. Matter. Mater. Phys. 2005, 71, 024533. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, H. Development of High Boron Content Liposomes and Their Promising Antitumor Effect for Neutron Capture Therapy. Yakugaku Zasshi 2013, 133, 1297–1306. [Google Scholar] [CrossRef] [Green Version]
- Leśnikowski, Z.J. Challenges and Opportunities for the Application of Boron Clusters in Drug Design. J. Med. Chem. 2016, 59, 7738–7758. [Google Scholar] [CrossRef]
- Ohishi, Y.; Kimura, K.; Yamaguchi, M.; Uchida, N.; Kanayama, T. Formation of Hydrogenated Boron Clusters in an External Quadrupole Static Attraction Ion Trap. J. Chem. Phys. 2008, 128, 124304. [Google Scholar] [CrossRef] [PubMed]
- Szwacki, N.G.; Weber, V.; Tymczak, C.J. Aromatic Borozene. Nanoscale Res. Lett. 2009, 4, 1085–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, S.; Shukla, A. Probing Aromaticity of Borozene through Optical and Dielectric Response: A Theoretical Study. Nanoscale Res. Lett. 2010, 5, 714–719. [Google Scholar] [CrossRef] [Green Version]
- Forte, G.; La Magna, A.; Deretzis, I.; Pucci, R. Ab Initio Prediction of Boron Compounds Arising from Borozene: Structural and Electronic Properties. Nanoscale Res. Lett. 2010, 5, 158–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, H.; Li, S.D. Hydrogenation of B120/−: A Planar-to-Icosahedral Structural Transition in B12Hn0/− (n = 1–6) Boron Hydride Clusters. J. Clust. Sci. 2011, 22, 525–535. [Google Scholar] [CrossRef]
- Ohishi, Y.; Kimura, K.; Yamaguchi, M.; Uchida, N.; Kanayama, T. Hydrogen Detachment from B12Hn+ Clusters by Kinetic Energy. Trans. Mater. Res. Soc. Jpn. 2010, 35, 533–536. [Google Scholar] [CrossRef] [Green Version]
- Ohishi, Y.; Kimura, K.; Yamaguchi, M.; Uchida, N.; Kanayama, T. Synthesis and Formation Mechanism of Hydrogenated Boron Clusters B12Hn with Controlled Hydrogen Content. J. Chem. Phys. 2010, 133, 074305. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Szwacki, N.; Tymczak, C.J. B12H n and B12F N: Planar vs. Icosahedral Structures. Nanoscale Res. Lett. 2012, 7, 236. [Google Scholar] [CrossRef] [Green Version]
- Zhai, H.J.; Wang, L.S.; Alexandrova, A.N.; Boldyrev, A.I.; Zakrzewski, V.G. Photoelectron Spectroscopy and Ab Initio Study of B3− and B4− Anions and Their Neutrals. J. Phys. Chem. A 2003, 107, 9319–9328. [Google Scholar] [CrossRef]
- Alexandrova, A.N.; Boldyrev, A.I.; Zhai, H.J.; Wang, L.S.; Steiner, E.; Fowler, P.W. Structure and Bonding in B6− and B6: Planarity and Antiaromaticity. J. Phys. Chem. A 2003, 107, 1359–1369. [Google Scholar] [CrossRef]
- Alexandrova, A.N.; Boldyrev, A.I.; Zhai, H.J.; Wang, L.S. Electronic Structure, Isomerism, and Chemical Bonding in B7− and B7. J. Phys. Chem. A 2004, 108, 3509–3517. [Google Scholar] [CrossRef]
- Pan, L.L.; Li, J.; Wang, L.S. Low-Lying Isomers of the B9− Boron Cluster: The Planar Molecular Wheel versus Three-Dimensional Structures. J. Chem. Phys. 2008, 129, 024302. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Guajardo, G.; Sergeeva, A.P.; Boldyrev, A.I.; Heine, T.; Ugalde, J.M.; Merino, G. Unravelling Phenomenon of Internal Rotation in B13+ through Chemical Bonding Analysis. Chem. Comm. 2011, 47, 6242–6244. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, A.P.; Zubarev, D.Y.; Zhai, H.J.; Boldyrev, A.I.; Wang, L.S. A Photoelectron Spectroscopic and Theoretical Study of B16 and B162−: An All-Boron Naphthalene. J. Am. Chem. Soc. 2008, 130, 7244–7246. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, A.P.; Averkiev, B.B.; Zhai, H.J.; Boldyrev, A.I.; Wang, L.S. All-Boron Analogues of Aromatic Hydrocarbons: B17− and B18−. J. Chem. Phys. 2011, 134, 224304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Sergeeva, A.P.; Zhai, H.J.; Averkiev, B.B.; Wang, L.S.; Boldyrev, A.I. A Concentric Planar Doubly π-Aromatic B19 Cluster. Nat. Chem. 2010, 2, 202–206. [Google Scholar] [CrossRef]
- Kiran, B.; Bulusu, S.; Zhai, H.J.; Yoo, S.; Zeng, X.C.; Wang, L.S. Planar-to-Tubular Structural Transition in Boron Clusters: B20 as the Embryo of Single-Walled Boron Nanotubes. Proc. Natl. Acad. Sci. USA 2005, 102, 961–964. [Google Scholar] [CrossRef] [Green Version]
- Piazza, Z.A.; Li, W.L.; Romanescu, C.; Sergeeva, A.P.; Wang, L.S.; Boldyrev, A.I. A Photoelectron Spectroscopy and Ab Initio Study of B21−: Negatively Charged Boron Clusters Continue to Be Planar at 21. J. Chem. Phys. 2012, 136, 104310. [Google Scholar] [CrossRef] [Green Version]
- Sergeeva, A.P.; Piazza, Z.A.; Romanescu, C.; Li, W.L.; Boldyrev, A.I.; Wang, L.S. B22− and B23−: All-Boron Analogues of Anthracene and Phenanthrene. J. Am. Chem. Soc. 2012, 134, 18065–18073. [Google Scholar] [CrossRef]
- Popov, I.A.; Piazza, Z.A.; Li, W.L.; Wang, L.S.; Boldyrev, A.I. A Combined Photoelectron Spectroscopy and Ab Initio Study of the Quasi-Planar B24− Cluster. J. Chem. Phys. 2013, 139, 144307. [Google Scholar] [CrossRef]
- Piazza, Z.A.; Popov, I.A.; Li, W.L.; Pal, R.; Cheng Zeng, X.; Boldyrev, A.I.; Wang, L.S. A Photoelectron Spectroscopy and Ab Initio Study of the Structures and Chemical Bonding of the B25− Cluster. J. Chem. Phys. 2014, 141, 034303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.M.; Jian, T.; Cheng, L.J.; Li, W.L.; Chen, Q.; Li, R.; Zhai, H.J.; Li, S.D.; Boldyrev, A.I.; Li, J.; et al. B26−: The Smallest Planar Boron Cluster with a Hexagonal Vacancy and a Complicated Potential Landscape. Chem. Phys. Lett. 2017, 683, 336–341. [Google Scholar] [CrossRef]
- Li, W.L.; Pal, R.; Piazza, Z.A.; Zeng, X.C.; Wang, L.S. B27−: Appearance of the Smallest Planar Boron Cluster Containing a Hexagonal Vacancy. J. Chem. Phys. 2015, 142, 204305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.R.; Jian, T.; Li, W.L.; Miao, C.Q.; Wang, Y.J.; Chen, Q.; Luo, X.M.; Wang, K.; Zhai, H.J.; Li, S.D.; et al. Competition between Quasi-Planar and Cage-like Structures in the B29− Cluster: Photoelectron Spectroscopy and: Ab Initio Calculations. Phys. Chem. Chem. Phys. 2016, 18, 29147–29155. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhao, Y.F.; Li, W.L.; Jian, T.; Chen, Q.; You, X.R.; Ou, T.; Zhao, X.Y.; Zhai, H.J.; Li, S.D.; et al. Observation and Characterization of the Smallest Borospherene, B28− and B28. J. Chem. Phys. 2016, 144, 064307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.L.; Zhao, Y.F.; Hu, H.S.; Li, J.; Wang, L.S. [B30]−: A Quasiplanar Chiral Boron Cluster. Angew. Chem. Int. Ed. 2014, 53, 5540–5545. [Google Scholar] [CrossRef]
- Chen, Q.; Li, W.L.; Zhao, X.Y.; Li, H.R.; Feng, L.Y.; Zhai, H.J.; Li, S.D.; Wang, L.S. B33− and B34−: Aromatic Planar Boron Clusters with a Hexagonal Vacancy. Eur. J. Inorg. Chem. 2017, 2017, 4546–4551. [Google Scholar] [CrossRef] [Green Version]
- Li, W.L.; Chen, Q.; Tian, W.J.; Bai, H.; Zhao, Y.F.; Hu, H.S.; Li, J.; Zhai, H.J.; Li, S.D.; Wang, L.S. The B35 Cluster with a Double-Hexagonal Vacancy: A New and More Flexible Structural Motif for Borophene. J. Am. Chem. Soc. 2014, 136, 12257–12260. [Google Scholar] [CrossRef]
- Piazza, Z.A.; Hu, H.S.; Li, W.L.; Zhao, Y.F.; Li, J.; Wang, L.S. Planar Hexagonal B36 as a Potential Basis for Extended Single-Atom Layer Boron Sheets. Nat. Commun. 2014, 5, 3113. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Tian, W.J.; Feng, L.Y.; Lu, H.G.; Mu, Y.W.; Zhai, H.J.; Li, S.D.; Wang, L.S. Planar B38− and B37− Clusters with a Double-Hexagonal Vacancy: Molecular Motifs for Borophenes. Nanoscale 2017, 9, 4550–4557. [Google Scholar] [CrossRef]
- Oger, E.; Crawford, N.R.M.; Kelting, R.; Weis, P.; Kappes, M.M.; Ahlrichs, R. Boron Cluster Cations: Transition from Planar to Cylindrical Structures. Angew. Chem. Int. Ed. 2007, 46, 8503–8506. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.B.; Tam, N.M.; Nguyen, M.T. The Boron Conundrum: The Case of Cationic Clusters Bn+ with n = 2–20. Theor Chem Acc 2012, 131, 1241. [Google Scholar] [CrossRef]
- Dong, X.; Jalife, S.; Vásquez-Espinal, A.; Ravell, E.; Pan, S.; Cabellos, J.L.; Liang, W.Y.; Cui, Z.H.; Merino, G. Li2B12 and Li3B12: Prediction of the Smallest Tubular and Cage-like Boron Structures. Angew. Chem. Int. Ed. 2018, 57, 4627–4631. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Juárez, G.; Ravell, E.; Arcudia, J.; Zarate, X.; Cui, Z.; Merino, G.; Barroso, J. Structural Effects of Alkali-Metals on the B12 Skeleton. Phys. Chem. Chem. Phys. 2020, 22, 17344–17350. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Osorio, E.; Ferraro, F.; Puga, G.; Donald, K.J.; Harrison, J.G.; Merino, G.; Tiznado, W. Isomerization Energy Decomposition Analysis for Highly Ionic Systems: Case Study of Starlike E5Li7+ Clusters. Chem. Eur. J. 2013, 19, 2305–2310. [Google Scholar] [CrossRef]
- Barroso, J.; Pan, S.; Merino, G. Structural Transformations in Boron Clusters Induced by Metal Doping. Chem. Soc. Rev. 2022, 51, 1098–1123. [Google Scholar] [CrossRef]
- Solar-Encinas, J.; Leyva-Parra, L.; Yáñez, O.; Inostroza, D.; Barrios-Llacuachaqui, J.R.; Vásquez-Espinal, A.; Orellana, W.; Tiznado, W. Bowl-Shaped CuB12− Cluster. A Viable Global Minimum with Twofold Aromaticity. ChemPhysChem 2022, 23, e202200366. [Google Scholar] [CrossRef]
- Bai, H.; Zhai, H.-J.; Li, S.-D.; Wang, L.-S. Photoelectron Spectroscopy of Aromatic Compound Clusters of the B12 All-Boron Benzene: B12Au− and B12(BO)−. Phys. Chem. Chem. Phys. 2013, 15, 9646–9653. [Google Scholar] [CrossRef]
- Liu, L.; Moreno, D.; Osorio, E.; Castro, A.C.; Pan, S.; Chattaraj, P.K.; Heine, T.; Merino, G. Structure and Bonding of IrB12−: Converting a Rigid Boron B12 Platelet to a Wankel Motor. RSC Adv. 2016, 6, 27177–27182. [Google Scholar] [CrossRef]
- Shao, X.; Qu, X.; Liu, S.; Yang, L.; Yang, J.; Liu, X.; Zhong, X.; Sun, S.; Vaitheeswaran, G.; Lv, J. Structure Evolution of Chromium-Doped Boron Clusters: Toward the Formation of Endohedral Boron Cages. RSC Adv. 2019, 9, 2870–2876. [Google Scholar] [CrossRef]
- Li, S.X.; Zhang, Z.P.; Long, Z.W.; Chen, D.L. Structures, Electronic, and Spectral Properties of Doped Boron Clusters MB120/− (M = Li, Na, and K). ACS Omega 2020, 5, 20525–20534. [Google Scholar] [CrossRef] [PubMed]
- Popov, I.A.; Li, W.L.; Piazza, Z.A.; Boldyrev, A.I.; Wang, L.S. Complexes between Planar Boron Clusters and Transition Metals: A Photoelectron Spectroscopy and Ab Initio Study of CoB12− and RhB12−. J. Phys. Chem. A 2014, 118, 8098–8105. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Luo, Z.; Bi, J.; Jin, S.; Zhang, Z.; Lu, C. Structural Evolution and Relative Stability of Vanadium-Doped Boron Clusters. J. Phys. Condens. Matter 2022, 34, 445302. [Google Scholar] [CrossRef]
- le Chen, B.; Sun, W.G.; Kuang, X.Y.; Lu, C.; Xia, X.X.; Shi, H.X.; Maroulis, G. Structural Stability and Evolution of Medium-Sized Tantalum-Doped Boron Clusters: A Half-Sandwich-Structured TaB12− Cluster. Inorg. Chem. 2018, 57, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xia, X.; Lu, C.; Kuang, X.; Hermann, A. Probing the Structural and Electronic Properties of Zirconium Doped Boron Clusters: Zr Distorted B12 Ligand Framework. Phys. Chem. Chem. Phys. 2018, 20, 23740–23746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.; Chen, B.; Kuang, X.; Lu, C.; Sun, W.; Xia, X.; Gutsev, G.L. Structural and Electronic Properties of Medium-Sized Aluminum-Doped Boron Clusters AlBn and Their Anions. J. Phys. Chem. C 2019, 123, 6276–6283. [Google Scholar] [CrossRef]
- Yañez, O.; Inostroza, D.; Usuga-Acevedo, B.; Vásquez-Espinal, A.; Pino-Rios, R.; Tabilo-Sepulveda, M.; Garza, J.; Barroso, J.; Merino, G.; Tiznado, W. Evaluation of Restricted Probabilistic Cellular Automata on the Exploration of the Potential Energy Surface of Be6B11−. Theor. Chem. Acc. 2020, 139, 41. [Google Scholar] [CrossRef]
- Thimmakondu, V.S.; Sinjari, A.; Inostroza, D.; Vairaprakash, P.; Thirumoorthy, K.; Roy, S.; Anoop, A.; Tiznado, W. Why an Integrated Approach between Search Algorithms and Chemical Intuition Is Necessary? Phys. Chem. Chem. Phys. 2022, 24, 11680–11686. [Google Scholar] [CrossRef]
- Oña, O.B.; Alcoba, D.R.; Torre, A.; Lain, L.; Torres-Vega, J.J.; Tiznado, W. Orbital Localization Criterion as a Complementary Tool in the Bonding Analysis by Means of Electron Localization Function: Study of the Sin(BH)5 -N2− (n = 0–5) Clusters. J. Phys. Chem. A 2013, 117, 12953–12958. [Google Scholar] [CrossRef]
- Oña, O.B.; Torres-Vega, J.J.; Torre, A.; Lain, L.; Alcoba, D.R.; Vásquez-Espinal, A.; Tiznado, W. Chemical Bonding Analysis in Boron Clusters by Means of Localized Orbitals According to the Electron Localization Function Topology. Theor. Chem. Acc. 2015, 134, 28. [Google Scholar] [CrossRef]
- Zhai, H.-J.; Kiran, B.; Li, J.; Wang, L.-S. Hydrocarbon Analogues of Boron Clusters—Planarity, Aromaticity and Antiaromaticity. Nat. Mater. 2003, 2, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Yañez, O.; Báez-Grez, R.; Inostroza, D.; Rabanal-León, W.A.; Pino-Rios, R.; Garza, J.; Tiznado, W. AUTOMATON: A Program That Combines a Probabilistic Cellular Automata and a Genetic Algorithm for Global Minimum Search of Clusters and Molecules. J. Chem. Theory Comput. 2019, 15, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Fuentealba, P.; Von Szentpaly, L.; Preuss, H.; Stoll, H. Pseudopotential Calculations for Alkaline-Earth Atoms. J. Phys. B At. Mol.Opt. 1985, 18, 1287–1296. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Riplinger, C.; Pinski, P.; Becker, U.; Valeev, E.F.; Neese, F. Sparse Maps—A Systematic Infrastructure for Reduced-Scaling Electronic Structure Methods. II. Linear Scaling Domain Based Pair Natural Orbital Coupled Cluster Theory. J. Chem. Phys. 2016, 144, 024109. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System, Version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, 73–78. [Google Scholar] [CrossRef]
- Truhlar, D.G. Basis-Set Extrapolation. Chem. Phys. Lett. 1998, 294, 45–48. [Google Scholar] [CrossRef]
- Neese, F.; Hansen, A.; Liakos, D.G. Efficient and Accurate Approximations to the Local Coupled Cluster Singles Doubles Method Using a Truncated Pair Natural Orbital Basis. J. Chem. Phys. 2009, 131, 064103. [Google Scholar] [CrossRef] [PubMed]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. Natural Bond Orbital Analysis Program: NBO 6.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2013. [Google Scholar]
- Zubarev, D.Y.; Boldyrev, A.I. Developing Paradigms of Chemical Bonding: Adaptive Natural Density Partitioning. Phys. Chem. Chem. Phys. 2008, 10, 5207–5217. [Google Scholar] [CrossRef] [PubMed]
- Zubarev, D.Y.; Boldyrev, A.I. Revealing Intuitively Assessable Chemical Bonding Patterns in Organic Aromatic Molecules via Adaptive Natural Density Partitioning. J. Org. Chem. 2008, 73, 9251–9258. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic Total Energy Using Regular Approximations. J. Chem. Phys. 1994, 101, 9783–9792. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-Type Basis Sets for the Elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Baerends, E.J.; Ziegler, T.; Atkins, A.J.; Autschbach, J.; Baseggio, O.; Bashford, D.; Bérces, A.; Bickelhaupt, F.M.; Bo, C.; Boerritger, P.M.; et al. ADF2012. 01; SCM, Theoretical Chemistry; Vrije Universiteit: Armsterdam, The Netherland, 2012. [Google Scholar]
- Jusélius, J.; Sundholm, D.; Gauss, J. Calculation of Current Densities Using Gauge-Including Atomic Orbitals. J. Chem. Phys. 2004, 121, 3952–3963. [Google Scholar] [CrossRef] [PubMed]
- Fliegl, H.; Taubert, S.; Lehtonen, O.; Sundholm, D. The Gauge Including Magnetically Induced Current Method. J. Chem. Phys. 2011, 13, 20500–20518. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient Implementation of the Gauge-Independent Atomic Orbital Method for NMR Chemical Shift Calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Ayachit, U.; Geveci, B.; Moreland, K.; Patchett, J.; Ahrens, J. The ParaView visualization application. In High Performance Visualization—Enabling Extreme-Scale Scientific Insight; Bethel, E.W., Childs, H., Hansen, C., Eds.; Taylor & Francis: Abingdon, UK, 2012; pp. 383–400. [Google Scholar]
- Ahrens, J.; Geveci, B.; Law, C. Paraview: An End-User Tool for Large Data Visualization. In The Visualization Handbook; Elsevier Academic Press Cambridge: Cambridge, MA, USA, 2005; Volume 717. [Google Scholar]
- Abramowitz, M. Handbook of Mathematical Functions, with Formulas, Graphs, and Mathematical Tables; Dover Publications, Inc.: New York, NY, USA, 1974. [Google Scholar]
- Keith, T.A. AIMAll, Version 19.10. 12; TK Gristmill Software: Overland Park, KS, USA, 2019.
- Sundholm, D.; Berger, R.J.F.; Fliegl, H. Analysis of the Magnetically Induced Current Density of Molecules Consisting of Annelated Aromatic and Antiaromatic Hydrocarbon Rings. Phys. Chem. Chem. Phys. 2016, 18, 15934–15942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inostroza, D.; García, V.; Yañez, O.; Torres-Vega, J.J.; Vásquez-Espinal, A.; Pino-Rios, R.; Báez-Grez, R.; Tiznado, W. On the NICS Limitations to Predict Local and Global Current Pathways in Polycyclic Systems. New J. Chem. 2021, 45, 8345–8351. [Google Scholar] [CrossRef]





| System | Cu3B12 | Ag3B12 | Au3B12 |
|---|---|---|---|
| ΔEiso | 13.0 | 9.8 | −0.2 |
| ΔEdist (M33+) | −82.1 | −75.7 | −73.2 |
| ΔEdist (B123−) | 7.6 | 6.7 | 8.2 |
| ΔΔEint | 87.5 | 78.9 | 64.8 |
| ΔΔEorb | 115.3 | 85.2 | 86.8 |
| ΔΔVelstat | −7.9 | −22.5 | −70.6 |
| ΔΔEPauli | −19.9 | 16.4 | 49.0 |
| ΔΔEdisp | 0.0 | −0.2 | −0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solar-Encinas, J.; Vásquez-Espinal, A.; Leyva-Parra, L.; Yañez, O.; Inostroza, D.; Valenzuela, M.L.; Orellana, W.; Tiznado, W. Planar Elongated B12 Structure in M3B12 Clusters (M = Cu-Au). Molecules 2023, 28, 236. https://doi.org/10.3390/molecules28010236
Solar-Encinas J, Vásquez-Espinal A, Leyva-Parra L, Yañez O, Inostroza D, Valenzuela ML, Orellana W, Tiznado W. Planar Elongated B12 Structure in M3B12 Clusters (M = Cu-Au). Molecules. 2023; 28(1):236. https://doi.org/10.3390/molecules28010236
Chicago/Turabian StyleSolar-Encinas, José, Alejandro Vásquez-Espinal, Luis Leyva-Parra, Osvaldo Yañez, Diego Inostroza, Maria Luisa Valenzuela, Walter Orellana, and William Tiznado. 2023. "Planar Elongated B12 Structure in M3B12 Clusters (M = Cu-Au)" Molecules 28, no. 1: 236. https://doi.org/10.3390/molecules28010236
APA StyleSolar-Encinas, J., Vásquez-Espinal, A., Leyva-Parra, L., Yañez, O., Inostroza, D., Valenzuela, M. L., Orellana, W., & Tiznado, W. (2023). Planar Elongated B12 Structure in M3B12 Clusters (M = Cu-Au). Molecules, 28(1), 236. https://doi.org/10.3390/molecules28010236







