Biomaterials Based on Chitosan and Its Derivatives and Their Potential in Tissue Engineering and Other Biomedical Applications—A Review
Abstract
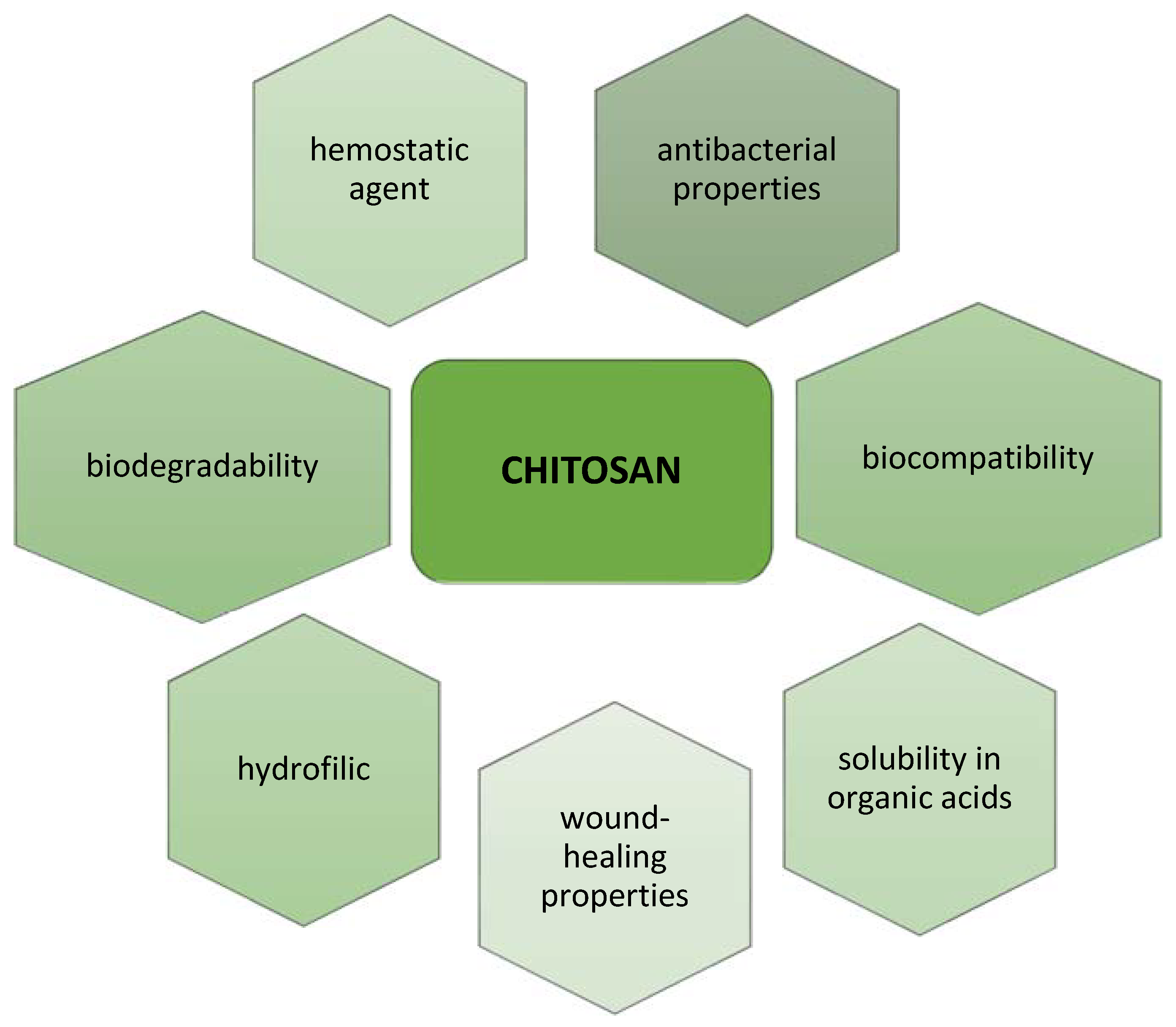
1. Introduction
2. Chitosan and Its Derivatives
3. Chitosan and Its Derivatives in Medicine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; He, X.; Hou, A.; Cheng, C.; Wang, X.; Yue, Y.; Wu, Z.; Wu, H.; Liu, B.; Li, H.; et al. Growth of Cu2O Nanoparticles on Two-Dimensional Zr–Ferrocene–Metal–Organic Framework Nanosheets for Photothermally Enhanced Chemodynamic Antibacterial Therapy. Inorg. Chem. 2022, 61, 9328–9338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, Y.; Peng, F.; Meng, F.; Zha, J.; Ma, L.; Du, Y.; Peng, N.; Ma, L.; Zhang, Q.; et al. Intercalation-Activated Layered MoO3 Nanobelts as Biodegradable Nanozymes for Tumor-Specific Photo-Enhanced Catalytic Therapy. Angew. Chem. Int. Ed. 2022, 61, e202115939. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Hu, T.; Gu, Z.; Williams, G.R.; Strimaite, M.; Zha, J.; Zhou, Z.; Zhang, X.; Tan, C.; Liang, R. Layered Double Hydroxide-Based Nanomaterials for Biomedical Applications. Chem. Soc. Rev. 2022, 51, 6126–6176. [Google Scholar] [CrossRef]
- Tu, D.D.; Chung, Y.G.; Gil, E.S.; Seth, A.; Franck, D.; Cristofaro, V.; Sullivan, M.P.; Di Vizio, D.; Gomez, P.; Adam, R.M.; et al. Bladder Tissue Regeneration Using Acellular Bi-Layer Silk Scaffolds in a Large Animal Model of Augmentation Cystoplasty. Biomaterials 2013, 34, 8681–8689. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, P.; Zhao, J.; Ling, Z.; An, Z.; Fu, Z.; Fu, W.; Zhang, X. Bi-Layer Silk Fibroin Skeleton and Bladder Acellular Matrix Hydrogel Encapsulating Adipose-Derived Stem Cells for Bladder Reconstruction. Biomater. Sci. 2021, 9, 6169–6182. [Google Scholar] [CrossRef]
- Cao, N.; Song, L.; Liu, W.; Fan, S.; Jiang, D.; Mu, J.; Gu, B.; Xu, Y.; Zhang, Y.; Huang, J. Prevascularized Bladder Acellular Matrix Hydrogel/Silk Fibroin Composite Scaffolds Promote the Regeneration of Urethra in a Rabbit Model. Biomed. Mater. 2018, 14, 015002. [Google Scholar] [CrossRef]
- Gasanz, C.; Raventós, C.; Temprana-Salvador, J.; Esteves, M.; Fonseca, C.; de Torres, I.; Morote, J. Use of an Acellular Collagen–Elastin Matrix to Support Bladder Regeneration in a Porcine Model of Peritoneocystoplasty. Cent. Eur. J. Urol. 2018, 71, 353. [Google Scholar] [CrossRef]
- Shi, C.; Chen, W.; Chen, B.; Shan, T.; Jia, W.; Hou, X.; Li, L.; Ye, G.; Dai, J. Bladder Regeneration in a Canine Model Using a Bladder Acellular Matrix Loaded with a Collagen-Binding BFGF. Biomater. Sci. 2017, 5, 2427–2436. [Google Scholar] [CrossRef]
- Jalali, S.; Fereidoni, M.; Shahri, N.M.; Lari, R. Effect of Swim Bladder Matrix Treated with Hyaluronic Acid on Wound Healing: An Animal Model Evaluation. J. Wound Care 2019, 28, 206–213. [Google Scholar] [CrossRef]
- Su, Z.; Ma, H.; Wu, Z.; Zeng, H.; Li, Z.; Wang, Y.; Liu, G.; Xu, B.; Lin, Y.; Zhang, P.; et al. Enhancement of Skin Wound Healing with Decellularized Scaffolds Loaded with Hyaluronic Acid and Epidermal Growth Factor. Mater. Sci. Eng. C 2014, 44, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Mathapati, S.; Bishi, D.K.; Venugopal, J.R.; Cherian, K.M.; Guhathakurta, S.; Ramakrishna, S.; Verma, R.S. Nanofibers Coated on Acellular Tissue-Engineered Bovine Pericardium Supports Differentiation of Mesenchymal Stem Cells into Endothelial Cells for Tissue Engineering. Nanomedicine 2014, 9, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Dolcimascolo, A.; Calabrese, G.; Conoci, S.; Parenti, R. Innovative Biomaterials for Tissue Engineering. In Biomaterial-Supported Tissue Reconstruction or Regeneration; Barbeck, M., Jung, O., Smeets, R., Koržinskas, T., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-83880-377-3. [Google Scholar]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In Vitro Characterization of Chitosan–Gelatin Scaffolds for Tissue Engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef] [PubMed]
- Sencadas, V.; Correia, D.M.; Ribeiro, C.; Moreira, S.; Botelho, G.; Gómez Ribelles, J.L.; Lanceros-Mendez, S. Physical-Chemical Properties of Cross-Linked Chitosan Electrospun Fiber Mats. Polym. Test. 2012, 31, 1062–1069. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef]
- Baranwal, A.; Kumar, A.; Priyadharshini, A.; Oggu, G.S.; Bhatnagar, I.; Srivastava, A.; Chandra, P. Chitosan: An Undisputed Bio-Fabrication Material for Tissue Engineering and Bio-Sensing Applications. Int. J. Biol. Macromol. 2018, 110, 110–123. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.-J.; Chen, S.-Q. Effect of Chitosan Molecular Weight and Deacetylation Degree on Hemostasis. J. Biomed. Mater. Res. 2008, 84B, 131–137. [Google Scholar] [CrossRef]
- Jimtaisong, A.; Saewan, N. Utilization of Carboxymethyl Chitosan in Cosmetics. Int. J. Cosmet. Sci. 2014, 36, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Carboxymethylated Chitins and Chitosans. Carbohydr. Polym. 1988, 8, 1–21. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdara, N.; Ashutosh Tiwari, N. Carboxymethyl Chitosan and Its Applications. Adv. Mater. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Sun, T.; Yao, Q.; Zhou, D.; Mao, F. Antioxidant Activity of N-Carboxymethyl Chitosan Oligosaccharides. Bioorg. Med. Chem. Lett. 2008, 18, 5774–5776. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl Chitosan: Properties and Biomedical Applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.G.; Liu, N.; Liu, C.S.; Liu, C.G.; Meng, X.H.; Yu, L.J.; Kenendy, J.F. Physicochemical Characterization and Antibacterial Property of Chitosan Acetates. Carbohydr. Polym. 2007, 67, 227–232. [Google Scholar] [CrossRef]
- Kahya, N. Water Soluble Chitosan Derivatives and Their Biological Activities: A Review. Polym. Sci. 2019, 5, 1–16. [Google Scholar] [CrossRef]
- Kato, Y. N-Succinyl-Chitosan as a Drug Carrier: Water-Insoluble and Water-Soluble Conjugates. Biomaterials 2004, 25, 907–915. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, N.; Xu, Q.; Zhang, L.; Zhang, C.; Liu, H.; Yu, Z.; Zhou, S.; Feng, G.; Huang, F. Decellularized Nerve Extracellular Matrix/Chitosan Crosslinked by Genipin to Prepare a Moldable Nerve Repair Material. Cell Tissue Bank 2021, 22, 419–430. [Google Scholar] [CrossRef]
- Xu, J.; Fang, H.; Zheng, S.; Li, L.; Jiao, Z.; Wang, H.; Nie, Y.; Liu, T.; Song, K. A Biological Functional Hybrid Scaffold Based on Decellularized Extracellular Matrix/Gelatin/Chitosan with High Biocompatibility and Antibacterial Activity for Skin Tissue Engineering. Int. J. Biol. Macromol. 2021, 187, 840–849. [Google Scholar] [CrossRef]
- Zhao, W.; Cao, S.; Cai, H.; Wu, Y.; Pan, Q.; Lin, H.; Fang, J.; He, Y.; Deng, H.; Liu, Z. Chitosan/Silk Fibroin Biomimic Scaffolds Reinforced by Cellulose Acetate Nanofibers for Smooth Muscle Tissue Engineering. Carbohydr. Polym. 2022, 298, 120056. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.E.G.; Lardy, B.; Bossard, F.; Soltero, F.A. Chitosan based biomaterials for cartilage tissue engineering: Chondrocyte adhesion and proliferation. Food Hydrocolloids for Health 2021, 1, 100018. [Google Scholar] [CrossRef]
- Karabıyık Acar, Ö.; Bedir, S.; Kayitmazer, A.B.; Kose, G.T. Chondro-Inductive Hyaluronic Acid/Chitosan Coacervate-Based Scaffolds for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2021, 188, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Asghari, F.; Rabiei Faradonbeh, D.; Malekshahi, Z.V.; Nekounam, H.; Ghaemi, B.; Yousefpoor, Y.; Ghanbari, H.; Faridi-Majidi, R. Hybrid PCL/Chitosan-PEO Nanofibrous Scaffolds Incorporated with A. Euchroma Extract for Skin Tissue Engineering Application. Carbohydr. Polym. 2022, 278, 118926. [Google Scholar] [CrossRef] [PubMed]
- Yavari Maroufi, L.; Ghorbani, M. Injectable Chitosan-Quince Seed Gum Hydrogels Encapsulated with Curcumin Loaded-Halloysite Nanotubes Designed for Tissue Engineering Application. Int. J. Biol. Macromol. 2021, 177, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Sadeghianmaryan, A.; Naghieh, S.; Alizadeh Sardroud, H.; Yazdanpanah, Z.; Afzal Soltani, Y.; Sernaglia, J.; Chen, X. Extrusion-Based Printing of Chitosan Scaffolds and Their in Vitro Characterization for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2020, 164, 3179–3192. [Google Scholar] [CrossRef]
- Ma, P.; Wu, W.; Wei, Y.; Ren, L.; Lin, S.; Wu, J. Biomimetic Gelatin/Chitosan/Polyvinyl Alcohol/Nano-Hydroxyapatite Scaffolds for Bone Tissue Engineering. Mater. Des. 2021, 207, 109865. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Noroozi Pesyan, N.; Fathi, M.; Omidi, Y. The Design of Polycaprolactone-Polyurethane/Chitosan Composite for Bone Tissue Engineering. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127895. [Google Scholar] [CrossRef]
- Sanchez, A.G.; Prokhorov, E.; Luna-Barcenas, G.; Hernández-Vargas, J.; Román-Doval, R.; Mendoza, S.; Rojas-Chávez, H. Chitosan-Hydroxyapatite-MWCNTs Nanocomposite Patch for Bone Tissue Engineering Applications. Mater. Today Commun. 2021, 28, 102615. [Google Scholar] [CrossRef]
- Shirzaei Sani, I.; Rezaei, M.; Baradar Khoshfetrat, A.; Razzaghi, D. Preparation and Characterization of Polycaprolactone/Chitosan-g-Polycaprolactone/Hydroxyapatite Electrospun Nanocomposite Scaffolds for Bone Tissue Engineering. Int. J. Biol. Macromol. 2021, 182, 1638–1649. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Miszuk, J.M.; Zhu, M.; Lansakara, T.I.; Tivanski, A.V.; Banas, J.A.; Sun, H. Vanillin-Bioglass Cross-Linked 3D Porous Chitosan Scaffolds with Strong Osteopromotive and Antibacterial Abilities for Bone Tissue Engineering. Carbohydr. Polym. 2021, 271, 118440. [Google Scholar] [CrossRef] [PubMed]
- Scalera, F.; Monteduro, A.G.; Maruccio, G.; Blasi, L.; Gervaso, F.; Mazzotta, E.; Malitesta, C.; Piccirillo, C. Sustainable Chitosan-Based Electrical Responsive Scaffolds for Tissue Engineering Applications. Sustain. Mater. Technol. 2021, 28, e00260. [Google Scholar] [CrossRef]
- Mirmusavi, M.H.; Ahmadian, M.; Karbasi, S. Polycaprolactone-Chitosan/Multi-Walled Carbon Nanotube: A Highly Strengthened Electrospun Nanocomposite Scaffold for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2022, 209, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, J.; Jadbabaee, S.; Far, F.M.; Lukolayeh, M.E.; Kırboğa, K.K.; Rezaei, F.S.; Barati, A. Decellularized Alstroemeria Flower Stem Modified with Chitosan for Tissue Engineering Purposes: A Cellulose/Chitosan Scaffold. Int. J. Biol. Macromol. 2022, 204, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.U.; Iqbal, D.N.; Iqbal, M.; Ezzine, S.; Arshad, A.; Zeeshan, R.; Chaudhry, A.A.; Alshawwa, S.Z.; Nazir, A.; Khan, A.F. HPMC Crosslinked Chitosan/Hydroxyapatite Scaffolds Containing Lemongrass Oil for Potential Bone Tissue Engineering Applications. Arab. J. Chem. 2022, 15, 103850. [Google Scholar] [CrossRef]
- Wasupalli, G.K.; Verma, D. Thermosensitive Injectable Hydrogel Based on Chitosan-Polygalacturonic Acid Polyelectrolyte Complexes for Bone Tissue Engineering. Carbohydr. Polym. 2022, 294, 119769. [Google Scholar] [CrossRef]
- Sacco, P.; Borgogna, M.; Travan, A.; Marsich, E.; Paoletti, S.; Asaro, F.; Grassi, M.; Donati, I. Polysaccharide-Based Networks from Homogeneous Chitosan-Tripolyphosphate Hydrogels: Synthesis and Characterization. Biomacromolecules 2014, 15, 3396–3405. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, K.; Du, L.; Cheng, Z.; Zhang, T.; Ding, J.; Li, W.; Xu, B.; Zhu, M. Construction of Chitosan Scaffolds with Controllable Microchannel for Tissue Engineering and Regenerative Medicine. Mater. Sci. Eng. C 2021, 126, 112178. [Google Scholar] [CrossRef]
- Baysan, G.; Colpankan Gunes, O.; Akokay, P.; Husemoglu, R.B.; Ertugruloglu, P.; Ziylan Albayrak, A.; Cecen, B.; Havitcioglu, H. Loofah-Chitosan and Poly (−3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV) Based Hydrogel Scaffolds for Meniscus Tissue Engineering Applications. Int. J. Biol. Macromol. 2022, 221, 1171–1183. [Google Scholar] [CrossRef]
- Ali, A.; Hasan, A.; Negi, Y.S. Effect of Carbon Based Fillers on Xylan/Chitosan/Nano-HAp Composite Matrix for Bone Tissue Engineering Application. Int. J. Biol. Macromol. 2022, 197, 1–11. [Google Scholar] [CrossRef]
- Narmatha, C.P.; Khaleel, B.S.; Sugantha, K.V. Multifunctional Organic and Inorganic Hybrid Bionanocomposite of Chitosan/Poly(Vinyl Alcohol)/Nanobioactive Glass/Nanocellulose for Bone Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2022, 135, 105427. [Google Scholar] [CrossRef]
- Phatchayawat, P.P.; Khamkeaw, A.; Yodmuang, S.; Phisalaphong, M. 3D Bacterial Cellulose-Chitosan-Alginate-Gelatin Hydrogel Scaffold for Cartilage Tissue Engineering. Biochem. Eng. J. 2022, 184, 108476. [Google Scholar] [CrossRef]
- Christy, P.N.; Basha, S.K.; Kumari, V.S. Nano Zinc Oxide and Nano Bioactive Glass Reinforced Chitosan/Poly(Vinyl Alcohol) Scaffolds for Bone Tissue Engineering Application. Mater. Today Commun. 2022, 31, 103429. [Google Scholar] [CrossRef]
- Leite, M.L.; Anselmi, C.; Soares, I.P.M.; Manso, A.P.; Hebling, J.; Carvalho, R.M.; de Souza Costa, C.A. Calcium Silicate-Coated Porous Chitosan Scaffold as a Cell-Free Tissue Engineering System for Direct Pulp Capping. Dent. Mater. 2022, 38, 1763–1776. [Google Scholar] [CrossRef]
- Shamekhi, M.A.; Mirzadeh, H.; Mahdavi, H.; Rabiee, A.; Mohebbi-Kalhori, D.; Baghaban Eslaminejad, M. Graphene Oxide Containing Chitosan Scaffolds for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2019, 127, 396–405. [Google Scholar] [CrossRef]
- Kashi, M.; Baghbani, F.; Moztarzadeh, F.; Mobasheri, H.; Kowsari, E. Green Synthesis of Degradable Conductive Thermosensitive Oligopyrrole/Chitosan Hydrogel Intended for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2018, 107, 1567–1575. [Google Scholar] [CrossRef]
- Vishwanath, V.; Pramanik, K.; Biswas, A. Optimization and Evaluation of Silk Fibroin-Chitosan Freeze-Dried Porous Scaffolds for Cartilage Tissue Engineering Application. J. Biomater. Sci. Polym. Ed. 2016, 27, 657–674. [Google Scholar] [CrossRef]
- Kar, S.; Kaur, T.; Thirugnanam, A. Microwave-Assisted Synthesis of Porous Chitosan–Modified Montmorillonite–Hydroxyapatite Composite Scaffolds. Int. J. Biol. Macromol. 2016, 82, 628–636. [Google Scholar] [CrossRef]
- Tithito, T.; Suntornsaratoon, P.; Charoenphandhu, N.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M.; Pon-On, W. Fabrication of Biocomposite Scaffolds Made with Modified Hydroxyapatite Inclusion of Chitosan-Grafted-Poly(Methyl Methacrylate) for Bone Tissue Engineering. Biomed. Mater. 2019, 14, 025013. [Google Scholar] [CrossRef]
- Fiqrianti, I.; Widiyanti, P.; Manaf, M.; Savira, C.; Cahyani, N.; Bella, F. Poly-L-Lactic Acid (PLLA)-Chitosan-Collagen Electrospun Tube for Vascular Graft Application. JFB 2018, 9, 32. [Google Scholar] [CrossRef]
- Pezeshki-Modaress, M.; Zandi, M.; Rajabi, S. Tailoring the Gelatin/Chitosan Electrospun Scaffold for Application in Skin Tissue Engineering: An in Vitro Study. Prog. Biomater. 2018, 7, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Hassanajili, S.; Azarpira, N.; Bagher Karimi, M.; Geramizadeh, B. Development of Thermal-Crosslinkable Chitosan/Maleic Terminated Polyethylene Glycol Hydrogels for Full Thickness Wound Healing: In Vitro and in Vivo Evaluation. Eur. Polym. J. 2019, 118, 113–127. [Google Scholar] [CrossRef]
- Madni, A.; Khan, R.; Ikram, M.; Naz, S.S.; Khan, T.; Wahid, F. Fabrication and Characterization of Chitosan–Vitamin C–Lactic Acid Composite Membrane for Potential Skin Tissue Engineering. Int. J. Polym. Sci. 2019, 2019, 4362395. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, T.; Wang, Y.; Liu, J.; Zhang, J.; Yao, R.; Wu, F. Modulating Cationicity of Chitosan Hydrogel to Prevent Hypertrophic Scar Formation during Wound Healing. Int. J. Biol. Macromol. 2020, 154, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mao, J.; Guo, Z.; Hu, Y.; Wang, S. Polyvinyl Alcohol/Carboxymethyl Chitosan Hydrogel Loaded with Silver Nanoparticles Exhibited Antibacterial and Self-Healing Properties. Int. J. Biol. Macromol. 2022, 220, 211–222. [Google Scholar] [CrossRef]
- Chang, G.; Dang, Q.; Liu, C.; Wang, X.; Song, H.; Gao, H.; Sun, H.; Zhang, B.; Cha, D. Carboxymethyl Chitosan and Carboxymethyl Cellulose Based Self-Healing Hydrogel for Accelerating Diabetic Wound Healing. Carbohydr. Polym. 2022, 292, 119687. [Google Scholar] [CrossRef]
- Mishra, A.H.; Mishra, D. Evidences of Biomimetic and Nonantibiotic Characteristics of the Zinc–Carboxymethyl Chitosan–Genipin Organometallic Complex and Its Biocompatibility Aspects. Biomacromolecules 2020, 21, 688–700. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B.; Li, M.; Li, J.; Wan, Y. Enhanced Dual Network Hydrogels Consisting of Thiolated Chitosan and Silk Fibroin for Cartilage Tissue Engineering. Carbohydr. Polym. 2020, 227, 115335. [Google Scholar] [CrossRef]
- Janarthanan, G.; Tran, H.N.; Cha, E.; Lee, C.; Das, D.; Noh, I. 3D Printable and Injectable Lactoferrin-Loaded Carboxymethyl Cellulose-Glycol Chitosan Hydrogels for Tissue Engineering Applications. Mater. Sci. Eng. C 2020, 113, 111008. [Google Scholar] [CrossRef]
- Li, T.; Song, X.; Weng, C.; Wang, X.; Gu, L.; Gong, X.; Wei, Q.; Duan, X.; Yang, L.; Chen, C. Silk Fibroin/Carboxymethyl Chitosan Hydrogel with Tunable Biomechanical Properties Has Application Potential as Cartilage Scaffold. Int. J. Biol. Macromol. 2019, 137, 382–391. [Google Scholar] [CrossRef]
- Rui, Q.; Gao, J.; Yin, Z.-Z.; Li, J.; Cai, W.; Wu, D.; Kong, Y. A Biodegradable PH and Glutathione Dual-Triggered Drug Delivery System Based on Mesoporous Silica, Carboxymethyl Chitosan and Oxidized Pullulan. Int. J. Biol. Macromol. 2022, 224, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Chen, S.; Wang, R.; Zhang, K.; Lin, X.; Mai, S. Antibacterial Activity and Bonding Performance of Carboxymethyl Chitosan–Containing Dental Adhesive System. Int. J. Adhes. Adhes. 2022, 119, 103269. [Google Scholar] [CrossRef]
- Yin, H.; Song, P.; Chen, X.; Huang, Q.; Huang, H. A Self-Healing Hydrogel Based on Oxidized Microcrystalline Cellulose and Carboxymethyl Chitosan as Wound Dressing Material. Int. J. Biol. Macromol. 2022, 221, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Han, J.; Mo, J.; Dong, P.; Zhuo, Y.; Feng, Y. Biocompatiable Silk Fibroin/Carboxymethyl Chitosan/Strontium Substituted Hydroxyapatite/Cellulose Nanocrystal Composite Scaffolds for Bone Tissue Engineering. Int. J. Biol. Macromol. 2019, 136, 1247–1257. [Google Scholar] [CrossRef]
- Kashyap, P.K.; Chauhan, S.; Negi, Y.S.; Goel, N.K.; Rattan, S. Biocompatible Carboxymethyl Chitosan-Modified Glass Ionomer Cement with Enhanced Mechanical and Anti-Bacterial Properties. Int. J. Biol. Macromol. 2022, 223, 1506–1520. [Google Scholar] [CrossRef]
- Xu, C.; Guan, S.; Wang, S.; Gong, W.; Liu, T.; Ma, X.; Sun, C. Biodegradable and Electroconductive Poly(3,4-Ethylenedioxythiophene)/Carboxymethyl Chitosan Hydrogels for Neural Tissue Engineering. Mater. Sci. Eng. C 2018, 84, 32–43. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, H.; Zheng, W.; Zhou, Q. Carboxymethyl Chitosan-Based Hydrogels Containing Fibroblast Growth Factors for Triggering Diabetic Wound Healing. Carbohydr. Polym. 2022, 287, 119336. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Tao, H.; Jin, L.; Wan, Z.; Dai, F.; Xiang, W.; Deng, H. Carboxymethyl Chitosan/Sodium Alginate-Based Micron-Fibers Fabricated by Emulsion Electrospinning for Periosteal Tissue Engineering. Mater. Des. 2020, 194, 108849. [Google Scholar] [CrossRef]
- Liu, T.; Feng, Z.; Li, Z.; Lin, Z.; Chen, L.; Li, B.; Chen, Z.; Wu, Z.; Zeng, J.; Zhang, J.; et al. Carboxymethyl Chitosan/Sodium Alginate Hydrogels with Polydopamine Coatings as Promising Dressings for Eliminating Biofilm and Multidrug-Resistant Bacteria Induced Wound Healing. Int. J. Biol. Macromol. 2022, in press. [Google Scholar] [CrossRef]
- Osorio Echavarría, J.; Gómez Vanegas, N.A.; Orozco, C.P.O. Chitosan/Carboxymethyl Cellulose Wound Dressings Supplemented with Biologically Synthesized Silver Nanoparticles from the Ligninolytic Fungus Anamorphous Bjerkandera Sp. R1. Heliyon 2022, 8, e10258. [Google Scholar] [CrossRef]
- Maji, S.; Agarwal, T.; Das, J.; Maiti, T.K. Development of Gelatin/Carboxymethyl Chitosan/Nano-Hydroxyapatite Composite 3D Macroporous Scaffold for Bone Tissue Engineering Applications. Carbohydr. Polym. 2018, 189, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-T.; Lu, T.-W.; Chen, C.-H.; Lu, K.-Y.; Mi, F.-L. Development of Nanocomposite Scaffolds Based on Biomineralization of N,O-Carboxymethyl Chitosan/Fucoidan Conjugates for Bone Tissue Engineering. Int. J. Biol. Macromol. 2018, 120, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Lu, D.-Q.; Chen, L.; Yang, R.; Liu, D.; Zhang, B. Diselenide-Crosslinked Carboxymethyl Chitosan Nanoparticles for Doxorubicin Delivery: Preparation and in Vivo Evaluation. Carbohydr. Polym. 2022, 292, 119699. [Google Scholar] [CrossRef] [PubMed]
- Medeiros Borsagli, F.G.L.; de Souza, A.J.M.; Paiva, A.E. Ecofriendly Multifunctional Thiolated Carboxymethyl Chitosan-Based 3D Scaffolds with Luminescent Properties for Skin Repair and Theragnostic of Tissue Regeneration. Int. J. Biol. Macromol. 2020, 165, 3051–3064. [Google Scholar] [CrossRef] [PubMed]
- Murugan, E.; Akshata, C.R.; Ilangovan, R.; Mohan, M. Evaluation of Quaternization Effect on Chitosan-HAP Composite for Bone Tissue Engineering Application. Colloids Surf. B Biointerfaces 2022, 218, 112767. [Google Scholar] [CrossRef]
- Zhou, M.; Liao, J.; Li, G.; Yu, Z.; Xie, D.; Zhou, H.; Wang, F.; Ren, Y.; Xu, R.; Dai, Y.; et al. Expandable Carboxymethyl Chitosan/Cellulose Nanofiber Composite Sponge for Traumatic Hemostasis. Carbohydr. Polym. 2022, 294, 119805. [Google Scholar] [CrossRef]
- Yu, N.; Li, Y.; Wang, Y.; Xu, H.; Ye, F.; Fu, Q. Healing Effect of Carboxymethyl Chitosan-Plantamajoside Hydrogel on Burn Wound Skin. Burns 2022, 48, 902–914. [Google Scholar] [CrossRef]
- Ghahremanzadeh, F.; Alihosseini, F.; Semnani, D. Investigation and Comparison of New Galactosylation Methods on PCL/Chitosan Scaffolds for Enhanced Liver Tissue Engineering. Int. J. Biol. Macromol. 2021, 174, 278–288. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Abdelhameed, M.F.; Zaghloul, S.; Montaser, A.S. In Vivo Assessment of the Durable, Green and in Situ Bio-Functional Cotton Fabrics Based Carboxymethyl Chitosan Nanohybrid for Wound Healing Application. Int. J. Biol. Macromol. 2022, 209, 485–497. [Google Scholar] [CrossRef]
- Liu, H.; Mao, J.; Yao, K.; Yang, G.; Cui, L.; Cao, Y. A Study on a Chitosan-Gelatin-Hyaluronic Acid Scaffold as Artificial Skin in Vitro and Its Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2004, 15, 25–40. [Google Scholar] [CrossRef]
- Jin, Z.; Hu, G.; Zhao, K. Mannose-Anchored Quaternized Chitosan/Thiolated Carboxymethyl Chitosan Composite NPs as Mucoadhesive Carrier for Drug Delivery. Carbohydr. Polym. 2022, 283, 119174. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-Biocomposite Scaffolds of Chitosan, Carboxymethyl Cellulose and Silver Nanoparticle Modified Cellulose Nanowhiskers for Bone Tissue Engineering Applications. Int. J. Biol. Macromol. 2018, 111, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Xu, L.; Dai, J.; Yi, X.; He, J.; Li, H. N, O-Carboxymethyl Chitosan/Oxidized Cellulose Composite Sponge Containing ε-Poly-l-Lysine as a Potential Wound Dressing for the Prevention and Treatment of Postoperative Adhesion. Int. J. Biol. Macromol. 2022, 209, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gu, Z.; Li, W.; Guo, L.; Wang, L.; Guo, L.; Ma, S.; Han, B.; Chang, J. PH-Sensitive O-Carboxymethyl Chitosan/Sodium Alginate Nanohydrogel for Enhanced Oral Delivery of Insulin. Int. J. Biol. Macromol. 2022, 223, 433–445. [Google Scholar] [CrossRef]
- Sharifi, F.; Atyabi, S.M.; Norouzian, D.; Zandi, M.; Irani, S.; Bakhshi, H. Polycaprolactone/Carboxymethyl Chitosan Nanofibrous Scaffolds for Bone Tissue Engineering Application. Int. J. Biol. Macromol. 2018, 115, 243–248. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, L.; Li, H.; Xia, L.; Hu, H.; Wang, S.; Liu, C.; Chi, J.; Yang, Y.; Song, F.; et al. Preparation, Biocompatibility, and Wound Healing Effects of O-Carboxymethyl Chitosan Nonwoven Fabrics in Partial-Thickness Burn Model. Carbohydr. Polym. 2022, 280, 119032. [Google Scholar] [CrossRef]
- Yang, Y.; Campbell Ritchie, A.; Everitt, N.M. Recombinant Human Collagen/Chitosan-Based Soft Hydrogels as Biomaterials for Soft Tissue Engineering. Mater. Sci. Eng. C 2021, 121, 111846. [Google Scholar] [CrossRef]
- Shakir, M.; Jolly, R.; Khan, M.S.; Iram, N.; Sharma, T.K.; Al-Resayes, S.I. Synthesis and Characterization of a Nano-Hydroxyapatite/Chitosan/Polyethylene Glycol Nanocomposite for Bone Tissue Engineering: Nano-hydroxyapatite/chitosan/polyethylene glycol nanocomposite. Polym. Adv. Technol. 2015, 26, 41–48. [Google Scholar] [CrossRef]
- Chi, J.; Jiang, Z.; Chen, X.; Peng, Y.; Liu, W.; Han, B.; Han, B. Studies on Anti-Hepatocarcinoma Effect, Pharmacokinetics and Tissue Distribution of Carboxymethyl Chitosan Based Norcantharidin Conjugates. Carbohydr. Polym. 2019, 226, 115297. [Google Scholar] [CrossRef]
- Rao, K.M.; Sudhakar, K.; Suneetha, M.; Won, S.Y.; Han, S.S. Fungal-Derived Carboxymethyl Chitosan Blended with Polyvinyl Alcohol as Membranes for Wound Dressings. Int. J. Biol. Macromol. 2021, 190, 792–800. [Google Scholar] [CrossRef]
- Xie, M.; Zeng, Y.; Wu, H.; Wang, S.; Zhao, J. Multifunctional Carboxymethyl Chitosan/Oxidized Dextran/Sodium Alginate Hydrogels as Dressing for Hemostasis and Closure of Infected Wounds. Int. J. Biol. Macromol. 2022, 219, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lan, Y.; Guo, H.; Cheng, L.; Fan, J.; Cai, X.; Zhang, L.; Chen, R.; Zhou, H. Ophthalmic Drug-Loaded N,O-Carboxymethyl Chitosan Hydrogels: Synthesis, in Vitro and in Vivo Evaluation. Acta Pharm. Sin. 2010, 31, 1625–1634. [Google Scholar] [CrossRef] [PubMed]


| Composition | Method | Application | In Vivo/In Vitro | Advantages | Ref. |
|---|---|---|---|---|---|
| Chitosan, genipin | Crosslinking, freeze-drying | Spinal cord tissue engineering | In vivo (rats) | Low cytotoxicity, high histocompatibility, good mechanical properties | [30] |
| Decellularized extracellular matrix/gelatin/and chitosan, EDC/NHS | Crosslinking, freeze-drying | Skin tissue engineering | In vitro (L929 fibroblasts) | The high modulus of elasticity, biodegradability, non-cytotoxic | [31] |
| Cellulose acetate nanofibers/chitosan/fibroin silk cryogel scaffold, genipin | Electrospinning, crosslinking, freeze-drying | Smooth muscle tissue engineering | In vitro (smooth muscle cell) | Good mechanical properties, good proliferation | [32] |
| Chitosan/poly (ethylene oxide) | Electrospinning scaffold | Cartilage tissue engineering | In vitro (chondrocyte cells) | Good cell adhesion and proliferation | [33] |
| Hyaluronic acid/chitosan coacervate-based scaffolds | Centrifuge, incubation | Cartilage tissue engineering | In vitro | Good proliferation and cell viability | [34] |
| PCL/chitosan-PEO with A. euchroma extract | Two-nozzle electrospinning | Skin tissue engineering | In vitro (HDF cells) | Good proliferation and cell viability | [35] |
| Hydrogels of chitosan/oxidized-modified quince seed gum/curcumin-loaded | Encapsulation | Tissue engineering | In vitro (NIH3T3 fibroblast cells) | Improved thermal stability, swelling ratio, and degradation rate of hydrogels, non-cytotoxicity, good proliferation | [36] |
| Chitosan scaffolds, sodium hydroxide-crosslinking agent | 3D print | Cartilage tissue engineering | In vitro (ATDC5 cells) | Higher elastic modulus, good biocompatibility | [37] |
| Gelatin/chitosan/polyvinyl alcohol/nano-hydroxyapatite | Freeze-drying | Bone tissue engineering | In vitro (BMSCs cells) | Improved surface bioactivity and biomimetic structure, high osteogenic differentiation ability | [38] |
| Polycaprolactone–polyurethane/chitosan | Freeze-drying, drying in oven | Bone tissue engineering | In vitro (hBMSCs) | Non-cytotoxicity, good mechanical properties, good promotion of the formation of calcium levels, good gene expression | [39] |
| Chitosan–hydroxyapatite–carbon | Drying in oven | Bone tissue engineering | In vitro (human osteoblasts) | Good biocompatibility with human osteoblasts, good mechanical properties | [40] |
| Polycaprolactone/chitosan-g-polycaprolactone/hydroxyapatite | Electrospinning, drying in oven | Bone tissue engineering | In vitro (NIH3T3 fibroblast cells) | High cell viability and proliferation, good mechanical properties | [41] |
| Chitosan–vanillin–BG (CVB) | Freeze-drying | Bone tissue engineering | In vivo (female mice | Good biocompatibility, bioactivity, strong antibacterial ability, good promotion of | [42] |
| osteoblastic differentiation, ectopic bone formation in vivo | |||||
| Chitosan-pyrolyzed cork | Freeze-drying | Electrically active biological tissue engineering | In vitro (SH-SY5Y neuroblastoma cell) | Good biocompatibility, high mechanical strength | [43] |
| Polycaprolactone (PCL)–chitosan/carboxyl carbon | Electrospinning | Cartilage tissue engineering | In vitro (chondrocytes cells) | High porosity, good mechanical properties, good biocompatibility | [44] |
| Decellularized Alstroemeria flower stem/chitosan | Freeze-drying | Tissue engineering | In vitro (MC3T3 cells) | Good cell attachment, proliferation and migration, good mechanical properties | [45] |
| Chitosan/hydroxypropyl methyl cellulose/hydroxyapatite/ lemon grass oil | Freeze gelation method | Bone tissue engineering | In vitro (MC3T3 cells) | Antimicrobial activity (S. aureus), non-toxic | [46] |
| Chitosan/βGP/NaHCO3/ HAp/PECs/gelatin | Gelation in a water batch | Bone tissue engineering | In vitro (MG63 cells) | Good cellular proliferation, osteogenic differentiation | [47] |
| Chitosan–tripolyphosphate | Exploiting dialysis technique, freeze-drying | Tissue engineering | In vitro (NIH3T3 fibroblast cells) | Good biocompatibility, good mechanical properties | [48] |
| Chitosan scaffolds with controllable microchannel | Combining a 3D printing microfiber template-leaching method and a freeze-drying method | Tissue engineering | In vitro (NIH3T3 fibroblast cells), in vivo (rats) | Good cell proliferation and distribution, improved cell, tissue growth and vascular formation | [49] |
| Chitosan/loofah/Poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) | Electrospinning, freeze-drying | Tissue engineering | In vitro (human mesenchymal stem cells) | Good cell proliferation and migration, good mechanical and | [50] |
| viscoelastic properties, differentiation into adipogenic, osteogenic, and chondrogenic tissues | |||||
| Xylan/chitosan/nano-HAp/graphene oxide/reduced graphene oxide | Freeze-drying | Bone tissue engineering | In vitro (MG-63 cell) | Improved mineralization tendency, osteogenic differentiation capability | [51] |
| Hybrid bionanocomposite of chitosan/poly(vinyl alcohol)/nanobioactive glass/nanocellulose | Drying in oven | Bone tissue engineering | In vitro (red blood cells) | Good porosity, better antibacterial effect (E. coli, S. aureus), improved hemocompatibility | [52] |
| Bacterial cellulose/chitosan/alginate/gelatin | Stirring with heat | Cartilage tissue engineering | In vitro (human mesenchymal stem cells) | Good compressive strength, stability, biocompatibility, good cell proliferation | [53] |
| Chitosan/poly(vinyl alcohol)/nano bioactive glass/nano zinc oxide | Drying in oven | Bone tissue engineering | In vitro (red blood cells) | Better tensile strength, good hemocompatibility, antimicrobial activity (Enterococcus faecalis, Salmonella typhi) | [54] |
| Calcium silicate-coated porous chitosan | Freeze-drying | Dental tissue engineering | In vitro (human dental pulp cells) | Good cell proliferation and mineralization | [55] |
| Graphene-oxide-containing chitosan | Freeze-drying | Cartilage tissue engineering | In vitro (chondro-cytes cells) | Improved physical and mechanical properties, good proliferation | [56] |
| Injectable chitosan/beta glycerophosphate/pyrrole oligomers | Stirring | Cartilage tissue engineering | In vitro (fibroblastoid cell CHO-K1) | Good biodegradability, biocompatibility, electro-activity, swelling ratio, and pore size values | [57] |
| Silk fibroin–chitosan | Freeze-drying | Cartilage tissue engineering | In vitro (human mesenchymal stem cell) | Good porosity, good compressive strength, proliferation, cell viability | [58] |
| Chitosan/ modified montmorillonite/hydroxyapatite | Microwave irradiation, gas-foaming method, freeze-drying | Bone tissue engineering | In vitro (MG 63 osteoblast cell) | Non-cytotoxic, good biodegradation, swelling properties, and good mechanical properties | [59] |
| Chitosan-grafted-poly(methyl methacrylate)/hydroxyapatite scaffold | Freeze-drying | Bone tissue engineering | In vitro (UMR-106 osteoblast-like cells) | Good viability, proliferation, and cells attachment, good mechanical properties, good drug delivery | [60] |
| Poly-L-lactic acid/chitosan/collagen | Electrospinning | Vascular tissue engineering | In vitro (lymphocyte T cell) | Good cell viability and hemolysis, good mechanical properties, and bust pressure | [61] |
| Gelatin/chitosan | Electrospinning | Skin tissue engineering | In vitro (human dermal fibroblast cells) | Very good porosity, good mechanical properties, non-cytotoxic, spindle-like shape cells | [62] |
| l-chitosan/maleic terminated polyethylene glycol | Freeze-drying | Skin tissue engineering | In vitro (HFFF2 cells), in vivo (rats) | Porous structure, high swelling ratio, biocompatibility, fully closed wound with improved vascularization | [63] |
| Chitosan–vitamin C–lactic acid | Freeze-drying | Skin tissue engineering | In vitro (NIH3T3 fibroblast cells) | Good cell attachment, proliferation and spreading | [64] |
| Composition | Method | Application | In Vivo/In Vitro | Advantages | Ref. |
|---|---|---|---|---|---|
| Carboxymethyl chitosan/genipin | Stirring | Skin tissue engineering | In vitro (HSFs cells) in vivo (rats) | Good cell attachment and proliferation, good wound healing promotion | [65] |
| Polyvinyl alcohol, carboxymethyl chitosan with silver nanoparticles and borax | Stirring | Skin tissue engineering | In vitro (L929 cells) | Antibacterial properties, good mechanical properties, non-cytotoxic | [66] |
| Carboxymethyl chitosan/carboxymethyl cellulose hydrogel with heparin and glutaraldehyde | Stirring | Skin tissue engineering | In vivo (rats with diabetes) | Accelerated open wound healing | [67] |
| Carboxymethyl chitosan/genipin/Zn scaffolds | Freeze-drying | Dental tissue engineering | In vitro (dental pulp stem cells) | Antibacterial properties, good cell proliferation | [68] |
| Thiolated chitosan and silk fibroin | Incubating at 37 °C | Cartilage tissue engineering | In vitro (chondrocytes cells) | Good mechanical properties, high porosity, good cell proliferation | [69] |
| Lactoferrin-loaded carboxymethyl cellulose glycol chitosan | Stirring, 3D printing | Tissue engineering applications | In vitro (mouse osteoblastic cells) | Good biocompatibility, good physician properties | [70] |
| Silk fibroin/carboxymethyl chitosan hydrogel crosslinking by horseradish peroxidase | Stirring | Cartilage tissue engineering | In vitro (chondrocytes cells) | Good biocompatibility, biodegradability, good mechanical and rheological properties | [71] |
| Carboxymethyl chitosan/oxidized pullulan with methotrexate-loaded mesoporous silica | Stirring | Drug delivery | In vitro (human hepatoma SMMC-7721 and hepatic LO2 cells) | Good biocompatibility, non-cytotoxic, good drug release | [72] |
| Polymerized CMC-modified adhesive | Mixing the powder with the adhesive | Dental tissue engineering | Antibacterial test | Good antibacterial properties (S. mutans) | [73] |
| Oxidized microcrystalline cellulose/ carboxymethyl chitosan | Stirring | Skin tissue engineering | In vitro blood compatibility test | Good mechanical, self-healing characteristic, good coagulation | [74] |
| Silk fibroin/carboxymethyl chitosan/strontium substituted hydroxyapatite/cellulose | Freeze-drying | Bone tissue engineering | In vitro (BMSCs cells) | Non-toxic, good hemocompatibility, good gene expression (osteogenic gene markers), high porosity | [75] |
| Carboxymethyl chitosan-modified glass ionomer cement | Mixing | Dental tissue engineering | In vitro (NIH 3 T3 fibroblast cells) | Good biocompatibility, good attachment, and cell proliferation, better mechanical properties | [76] |
| Poly(3,4-ethylenedioxythiophene)/ carboxymethyl chitosan | Vibration | Neural tissue engineering | Good biodegradation and electroconductivity, good compressive modulus, better cell adhesion, viability and proliferation | [77] | |
| Benzaldehyde-terminated 4-arm PEG/carboxymethyl chitosan/basic fibroblast growth factor | Stirring | Skin tissue engineering | In vitro (blood cells) | Excellent biocompatibility, fast hemostasis capacity, strong wet-tissue adhesion, self-mending, and antibacterial property | [78] |
| Polycaprolactone /carboxymethyl chitosan/sodium alginate micron-fibrous | Emulsion electrospinning | Periosteal tissue engineering | In vitro (osteoblasts cells) | Excellent tensile strength, no significant cytotoxicity, good cell adhesion | [79] |
| Carboxymethyl chitosan/sodium alginate hydrogels with polydopamine coatings | Immersion | Skin tissue engineering | In vitro (human umbilical vein endothelial cells), in vivo (rats with MRSA) | Antibacterial, anti-inflammatory properties, good antibacterial properties (Methicillin-resistant Staphylococcus aureus), fast wound healing | [80] |
| Chitosan/carboxymethyl cellulose with silver nanoparticles | Stirring | Skin tissue engineering | In vitro (human skin fibroblasts) | Good mechanical properties, good antibacterial properties (E.coli), non-cytotoxic | [81] |
| Gelatin/carboxymethyl chitosan/nano-hydroxyapatite | Freeze-drying | Bone tissue engineering | In vitro (human Wharton’s jelly MSC microtissue) | High porosity, slow enzymatic degradation, good mechanical properties, good viability, the proliferation of human Wharton’s jelly MSC microtissue | [82] |
| N,O-carboxymethyl chitosan/fucoidan | Freeze-drying | Bone tissue engineering | In vitro (L929 cells) | Good mineralization, good physical properties, good cell proliferation and mineralization | [83] |
| Diselenide-crosslinked carboxymethyl chitosan nanoparticles with doxorubicin | Stirring, dialysis | Drug delivery | In vitro (tumor cells) | High drug encapsulation efficiency, high drug accumulation, and cytotoxicity in tumor cells | [84] |
| Thiolated carboxymethyl chitosan-based 3D scaffolds | Freeze-drying | Theragnostic of tissue regeneration | In vitro (human dermo fibroblast cells) | High porosity, good mechanical properties, non-cytotoxic | [85] |
| Quaternized chitosan/hydroxyapatite curcumin-loaded | Stirring | Bone tissue engineering | In vitro (MG-63 cells) | Good mechanical strength, drug release, good biocompatibility and cell proliferation | [86] |
| Carboxymethyl chitosan/cellulose nanofiber | Freeze-drying, drying in the oven | Skin tissue engineering | In vivo (rats) | Good blood absorption, and excellent coagulation ability | [87] |
| Carboxymethyl chitosan–plantamajoside | Stirring | Skin tissue engineering | In vitro (L929 cells), in vivo (rats with burn wounds) | Good porosity, good cell viability, proliferation, significantly improved wound healing, granulation tissue proliferation | [88] |
| Polycaprolactone/galactosylated chitosan | Freeze-drying, electrospinning | Liver tissue engineering | In vitro (HepG2 cells) | Non-cytotoxic, good cell growth, and proliferation | [89] |
| Cotton fabric/carboxymethyl chitosan/silver nitrate | Pad–dry–cure method, drying in oven | Skin tissue engineering | In vivo (rats with wounds) | Good wound healing properties, antibacterial properties (E. coli, S. aureus) | [90] |
| Chitosan–gelatin–hyaluronic acid | Freeze-drying | Skin tissue engineering | In vitro (fibroblast and keratinocytes cells) | Good mechanical properties, flexible scaffold/cells, artificial skin, good cell proliferation in co-cultures | [91] |
| Mannose-anchored quaternized chitosan/thiolated carboxymethyl chitosan | Freeze-drying | Drug delivery | In vitro (293T cells) | Non-cytotoxic, high hydrophilicity, good drug release and stability | [92] |
| Chitosan, carboxymethyl cellulose and silver-nanoparticle-modified cellulose nanowhiskers | Freeze-drying | Bone tissue engineering | In vitro (MG63 cells) | Good mechanical properties, high porosity, excellent antimicrobial activity (E. coli), good biomineralization | [93] |
| N, O-carboxymethyl chitosan/oxidized cellulose containing ε-poly-L-lysine | Freeze-drying | Skin tissue engineering | In vitro (NIH 3T3 cells), in vivo (rabbit) | Good antibacterial properties (E. coli, S. aureus), excellent biological security and compatibility in vitro and in vivo | [94] |
| O-carboxymethyl chitosan/sodium alginate with insulin | Stirring | Drug delivery | In vitro (L929 mouse fibroblast cells), in vivo (rats) | High drug loading capacity and high effectively released drugs as oral drugs, lower glucose level compared with insulin injections | [95] |
| Polycaprolactone/carboxymethyl chitosan | Electrospinning | Bone tissue engineering | In vitro (human osteoblast cells MG63) | Good biocompatibility, good cell proliferation | [96] |
| O-carboxymethyl chitosan nonwoven fabrics | Chitosan needle-punched nonwoven reaction with chloroacetic acid | Skin tissue engineering | In vitro (L929 mouse fibroblast cells), in vivo (rats with a partial-thickness burn) | Good mechanical properties, good cell migration, and proliferation, good healing rate, good angiogenesis | [97] |
| Recombinant human collagen/carboxylated chitosan | Stirring | Soft tissue engineering | In vitro (NIH 3T3 cells), in vivo (rats with open wounds) | Good biocompatibility, non-cytotoxic, acceleration of the cell infiltration and wound closure | [98] |
| Nano-hydroxyapatite/chitosan/polyethylene glycol | Stirring, filtration, drying in the oven | Bone tissue engineering | In vitro (murine fibroblast L929 cells) | Good thermal stability and swelling ratio, non-cytotoxic | [99] |
| Norcantharidin-conjugated carboxymethyl chitosan | Vacuum-dried | Drug delivery | In vitro (BEL-7402 cells), in vivo (mice with H22 cells, tumor cells) | Inhibitory effects on the proliferation and migration of cells, changes in cell structure, reduction in the distribution of norcantharidin in heart and kidney tissues, diminished systemic toxicity | [100] |
| Poly (vinyl alcohol) and fungal mushroom-derived carboxymethyl chitosan | Solution casting technique | Skin tissue engineering | In vitro (skin fibroblasts and keratinocytes) | Good antibacterial properties (E. coli, S. aureus), good biocompatibility, good hemolysis | [101] |
| Carboxymethyl chitosan/oxidized dextran/sodium alginate | Mixing with a double-barreled syringe | Skin tissue engineering | In vitro (L929 cells), in vivo (rat liver injury model and mouse tail amputation model) | Red blood cells could adhere to the surface of hydrogel, good hemostasis, good antibacterial properties (S. aureus) | [102] |
| N,O-carboxymethyl chitosan | Stirring | Drug delivery | In vivo (rabbit) | Good drug delivery, non-cytotoxic to the cornea, good degradability | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szulc, M.; Lewandowska, K. Biomaterials Based on Chitosan and Its Derivatives and Their Potential in Tissue Engineering and Other Biomedical Applications—A Review. Molecules 2023, 28, 247. https://doi.org/10.3390/molecules28010247
Szulc M, Lewandowska K. Biomaterials Based on Chitosan and Its Derivatives and Their Potential in Tissue Engineering and Other Biomedical Applications—A Review. Molecules. 2023; 28(1):247. https://doi.org/10.3390/molecules28010247
Chicago/Turabian StyleSzulc, Marta, and Katarzyna Lewandowska. 2023. "Biomaterials Based on Chitosan and Its Derivatives and Their Potential in Tissue Engineering and Other Biomedical Applications—A Review" Molecules 28, no. 1: 247. https://doi.org/10.3390/molecules28010247
APA StyleSzulc, M., & Lewandowska, K. (2023). Biomaterials Based on Chitosan and Its Derivatives and Their Potential in Tissue Engineering and Other Biomedical Applications—A Review. Molecules, 28(1), 247. https://doi.org/10.3390/molecules28010247






