Oxyethylated Fluoresceine—(thia)calix[4]arene Conjugates: Synthesis and Visible-Light Photoredox Catalysis in Water–Organic Media
Abstract
:1. Introduction
2. Results and Discussion
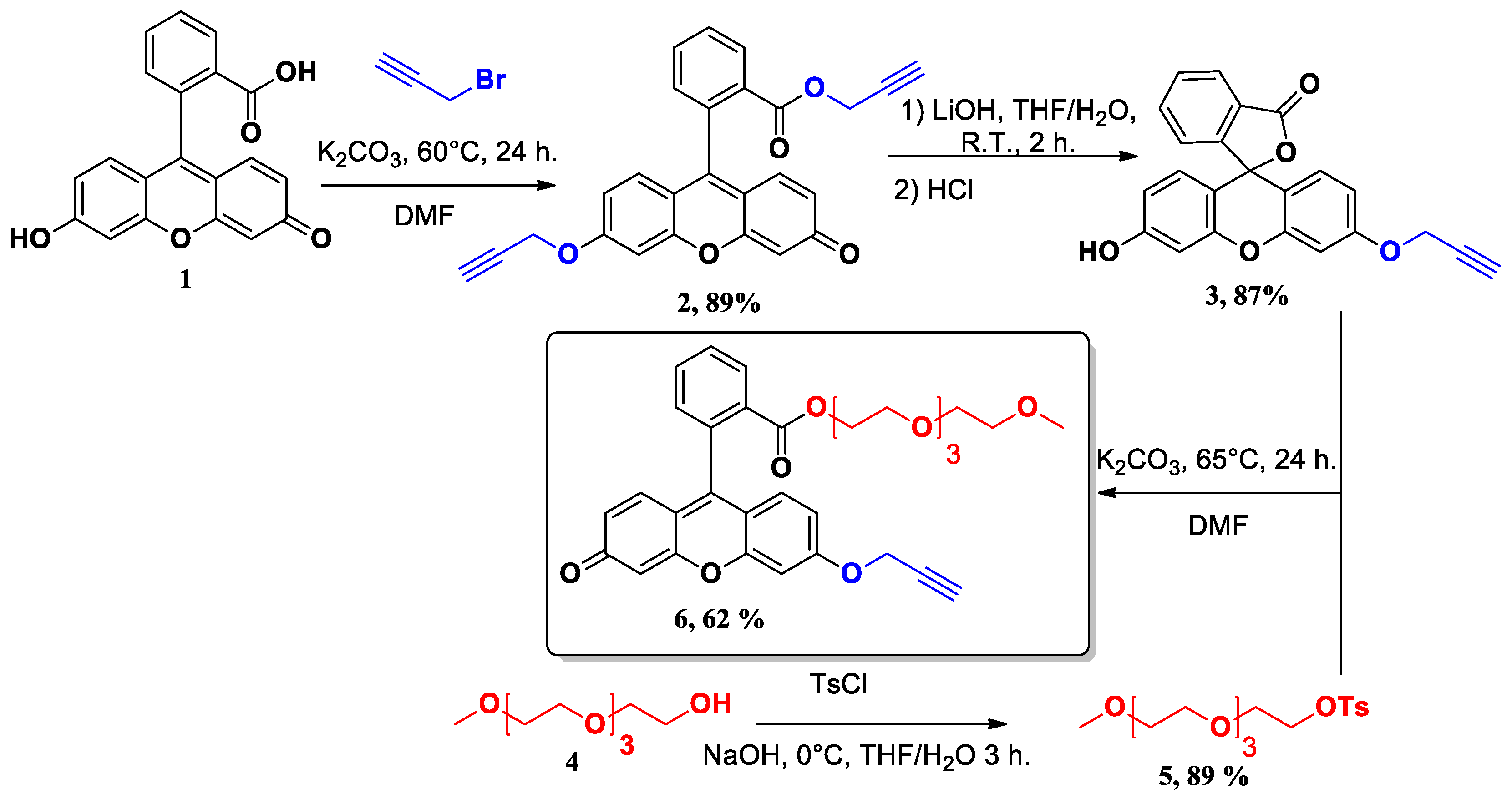
2.1. Synthesis
2.2. Photophysical Properties
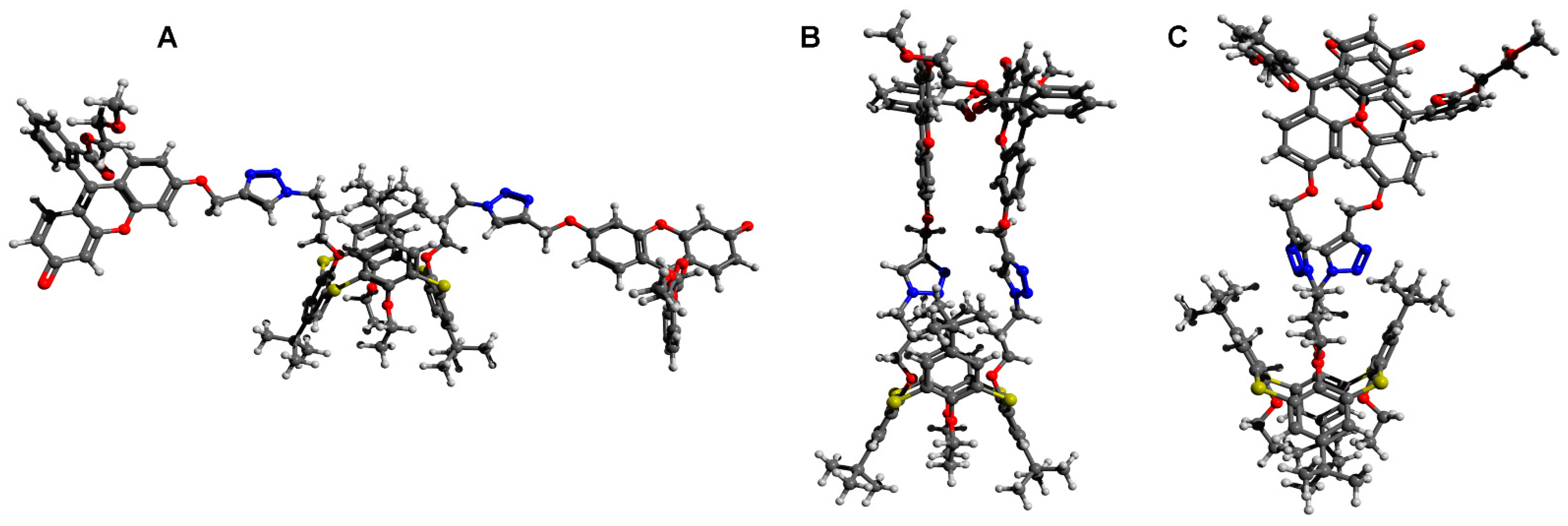
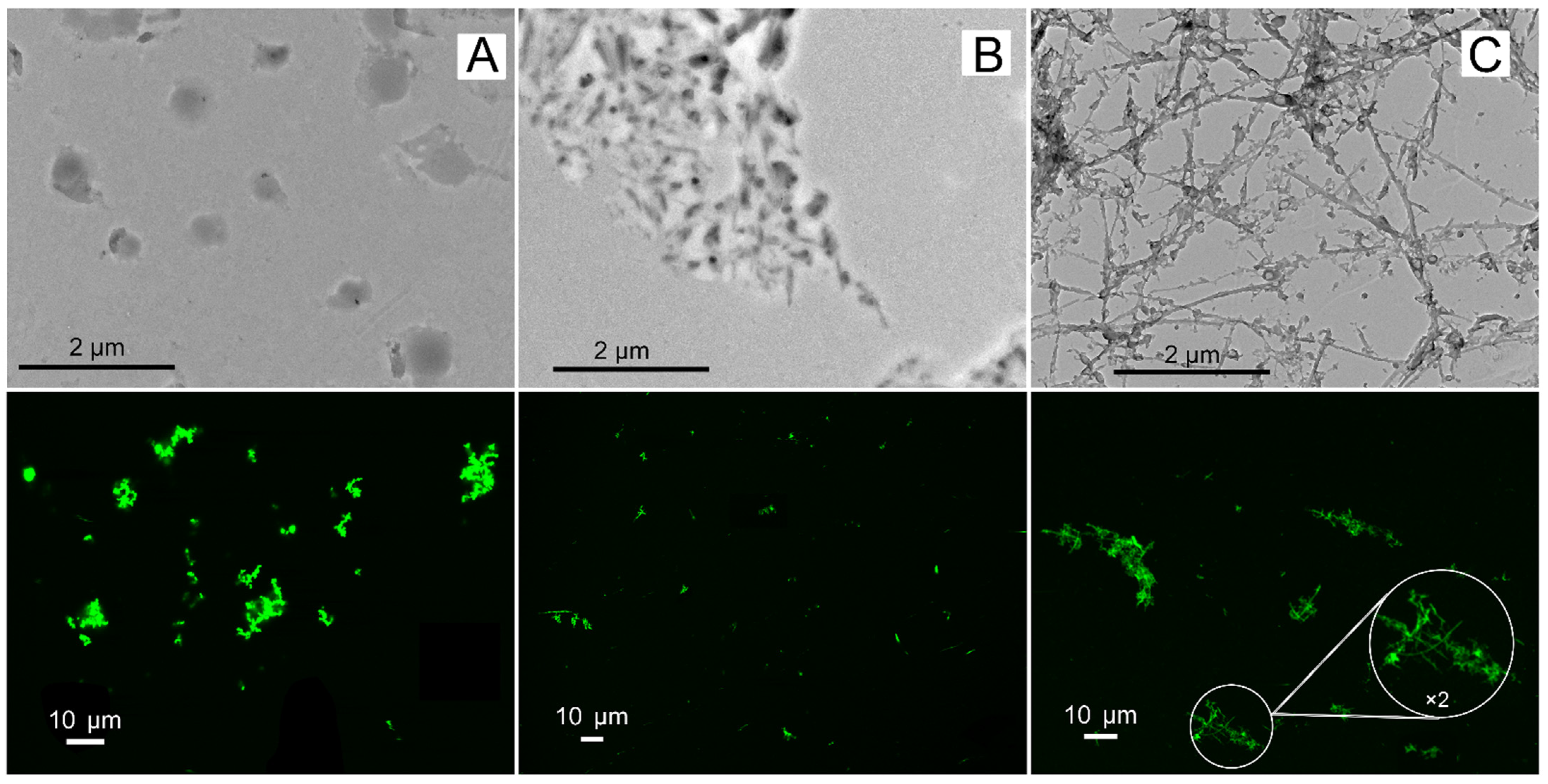
2.3. Aggregation Abilities
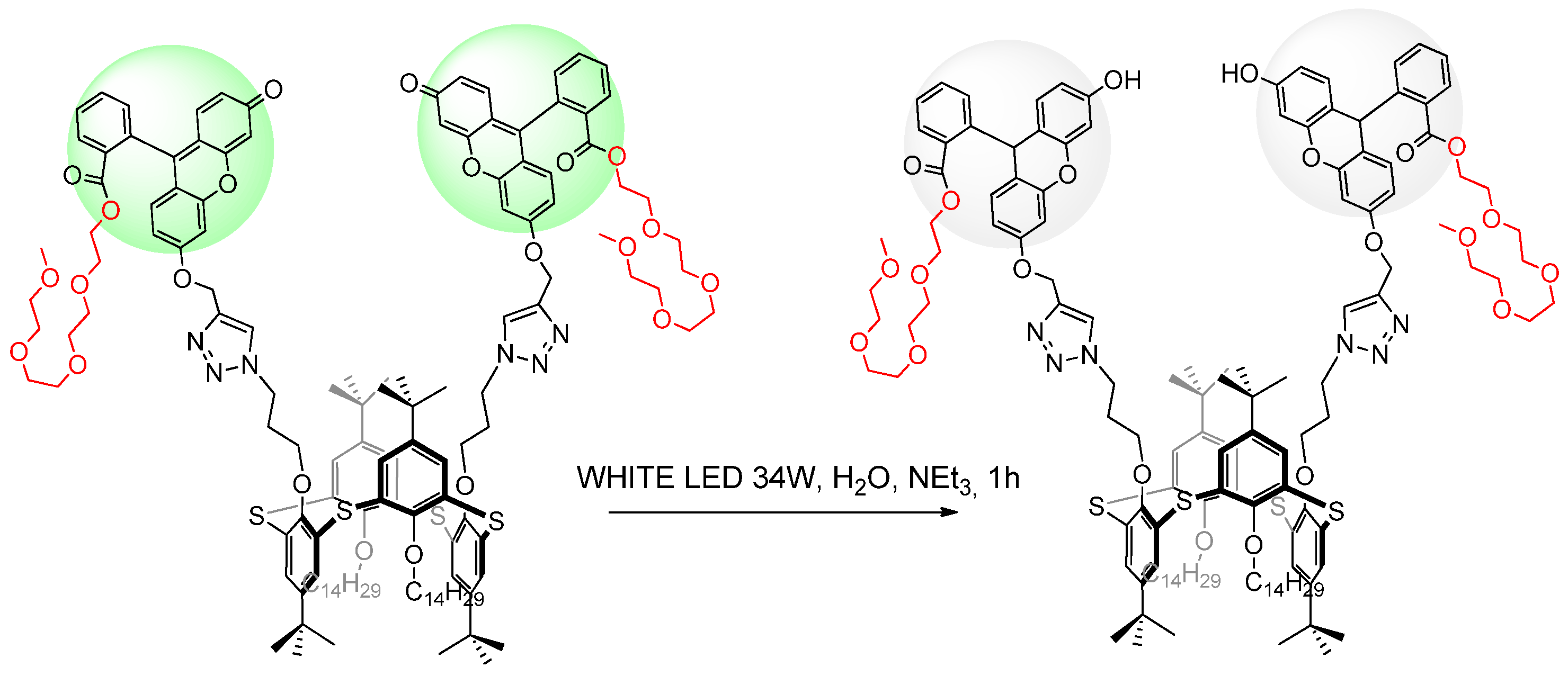
2.4. Photocatalytic Activities
3. Materials and Methods
3.1. Characterisation Methods
3.2. Synthesis
3.3. Photocatalytic Oxidation of Phenylboronic Acid
3.4. Quantum-Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, G.; Li, J.; Zhang, H.; Wang, Y.; Zhang, M. A highly-efficient exciplex-based multifunctional fluorescent film probe to aniline and N-methylphenethylamine vapors. Dyes Pigm. 2022, 210, 110944. [Google Scholar] [CrossRef]
- Correia, B.B.; Brown, T.R.; Reibenspies, J.H.; Lee, H.S.; Hancock, R.D. Exciplex formation as an approach to selective Copper (II) fluorescent sensors. Inorg. Chim. Acta 2020, 506, 119544. [Google Scholar] [CrossRef]
- Daly, B.; Ling, J.; De Silva, A.P. Current developments in fluorescent PET (photoinduced electron transfer) sensors and switches. Chem. Soc. Rev. 2015, 44, 4203–4211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasanna de Silva, A. Crossing the divide: Experiences of taking fluorescent PET (photoinduced electron transfer) sensing/switching systems from solution to solid. Dyes Pigm. 2022, 204, 110453. [Google Scholar] [CrossRef]
- Tian, X.; Murfin, L.C.; Wu, L.; Lewis, S.E.; James, T.D. Fluorescent small organic probes for biosensing. Chem. Sci. 2021, 12, 3406–3426. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.-H.; Todd, M.H.; Rutledge, P.J. Recent Advances in Macrocyclic Fluorescent Probes for Ion Sensing. Molecules 2017, 22, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhmer, V. Calixarenes, Macrocycles with (Almost) Unlimited Possibilities. Angew. Chem. Int. Ed. 1995, 34, 713–745. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, C.; Wang, R. Macrocycle-Surfaced Polymer Nanocapsules: An Emerging Paradigm for Biomedical Applications. Bioconjugate Chem. 2022, 33, 2254–2261. [Google Scholar] [CrossRef]
- Santoro, O.; Redshaw, C. Metallocalix[n]arenes in catalysis: A 13-year update. Coord. Chem. Rev. 2021, 448, 214173. [Google Scholar] [CrossRef]
- Podyachev, S.N.; Zairov, R.R.; Mustafina, A.R. 1,3-Diketone Calix[4]arene Derivatives—A New Type of Versatile Ligands for Metal Complexes and Nanoparticles. Molecules 2021, 26, 1214. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, X.; Guo, D. Biomedical Applications of Calixarenes: State of the Art and Perspectives. Angew. Chem. Int. Ed. 2020, 60, 2768–2794. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, S.E.; Burilov, V.A.; Antipin, I.S. Thiacalix[4]arene’s Lower Rim Derivatives: Synthesis and Supramolecular Properties. Macroheterocycles 2017, 10, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Quang, D.T. Calixarene-derived fluorescent probes. Chem. Rev. 2007, 107, 3780–3799. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, A.; Singh, H.; Suating, P.; Kim, H.S.; Sunwoo, K.; Shim, I.; Gibb, B.C.; Kim, J.S. Revisiting Fluorescent Calixarenes: From Molecular Sensors to Smart Materials. Chem. Rev. 2019, 119, 9657–9721. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Song, Z.; Yu, L.; Ye, S.; He, H. Fluorescent probes based on macrocyclic hosts: Construction, mechanism and analytical applications. Trends Analyt. Chem. 2020, 133, 116086. [Google Scholar] [CrossRef]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kwon, M.S. Emerging organic photoredox catalysts for organic transformations. Eur. J. Org. Chem. 2020, 2020, 6028–6043. [Google Scholar] [CrossRef]
- Yadav, P.; Anagha Varma, A.; Punnya, A.J.; Gopinath, P. Photoredox-mediated Multicomponent Reactions. Asian J. Org. Chem. 2022, 11, e202200390. [Google Scholar] [CrossRef]
- Grygorenko, O.O.; Volochnyuk, D.M.; Vashchenko, B.V. Emerging Building Blocks for Medicinal Chemistry: Recent Synthetic Advances. Eur. J. Org. Chem. 2021, 2021, 6478. [Google Scholar] [CrossRef]
- Sachdeva, G.; Vaya, D.; Srivastava, C.M.; Kumar, A.; Rawat, V.; Singh, M.; Verma, M.; Rawat, P.; Rao, G.K. Calix[n]arenes and its derivatives as organocatalysts. Coord. Chem. Rev. 2022, 472, 214791. [Google Scholar] [CrossRef]
- Zuo, M.; Velmurugan, K.; Wang, K.; Tian, X.; Hu, X.Y. Insight into functionalized-macrocycles-guided supramolecular photocatalysis. Beilstein J. Org. Chem. 2021, 17, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Velmurugan, K.; Tian, X.; Zuo, M.; Wang, K.; Hu, X.Y. Tetraphenylethylene-embedded pillar[5]arene-based orthogonal self-assembly for efficient photocatalysis in water. Beilstein J. Org. Chem. 2022, 18, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, C.L.; Manfredi, N.; Montini, T.; Baldini, L.; Abbotto, A.; Fornasiero, P. Calix[4]arene-based molecular photosensitizers for sustainable hydrogen production and other solar applications. Curr. Opin. Green Sustain. Chem. 2021, 32, 100534. [Google Scholar] [CrossRef]
- Burilov, V.A.; Nugmanov, R.I.; Ibragimova, R.R.; Solovieva, S.E.; Antipin, I.S. «Click chemistry» in the synthesis of new amphiphilic 1,3-alternate thiacalixarenes. Mendeleev Commun. 2015, 25, 177–179. [Google Scholar] [CrossRef]
- Burilov, V.; Valiyakhmetova, A.; Mironova, D.; Sultanova, E.; Evtugyn, V.; Osin, Y.; Katsyuba, S.; Burganov, T.; Solovieva, S.; Antipin, I. Novel amphiphilic conjugates of p-tert-butylthiacalix[4]arene with 10,12-pentacosadiynoic acid in 1,3-alternate stereoisomeric form. Synthesis and chromatic properties in the presence of metal ions. New J. Chem. 2018, 42, 2942–2951. [Google Scholar] [CrossRef]
- Burilov, V.A.; Fatikhova, G.A.; Dokuchaeva, M.N.; Nugmanov, R.I.; Mironova, D.A.; Dorovatovskii, P.V.; Khrustalev, V.N.; Solovieva, S.E.; Antipin, I.S. Synthesis of new p-tert-butylcalix[4]arene-based polyammonium triazolyl amphiphiles and their binding with nucleoside phosphates. Beilstein J. Org. Chem. 2018, 14, 1980–1993. [Google Scholar] [CrossRef] [Green Version]
- Burilov, V.A.; Artemenko, A.A.; Garipova, R.I.; Amirova, R.R.; Fatykhova, A.M.; Borisova, J.A.; Mironova, D.A.; Sultanova, E.D.; Evtugyn, V.G.; Solovieva, S.E.; et al. New Calix[4]arene—Fluoresceine Conjugate by Click Approach—Synthesis and Preparation of Photocatalytically Active Solid Lipid Nanoparticles. Molecules 2022, 27, 2436. [Google Scholar] [CrossRef]
- Chen, L.; Hu, T.-S.; Zhu, J.; Wu, H.; Yao, Z.-J. Application of a Regioselective Mannich Reaction on Naringenin and its Use in Fluorescent Labeling. Synlett 2006, 8, 1225–1229. [Google Scholar] [CrossRef]
- Ouchi, M.; Inoue, Y.; Wada, K.; Iketani, S.; Hakushi, T.; Weber, E. Molecular design of crown ethers. 4. Syntheses and selective cation binding of 16-crown-5 and 19-crown-6 lariats. J. Org. Chem. 1987, 52, 2420–2427. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2006; p. 673. [Google Scholar] [CrossRef]
- Nagaraja, D.; Melavanki, R.M.; Patil, N.R.; Geethanjali, H.S.; Kusanur, R.A. Solvent effect on the relative quantum yield and fluorescence quenching of a newly synthesized coumarin derivative. Luminescence 2015, 30, 495–502. [Google Scholar] [CrossRef]
- Shen, J.; Snook, R.D. Thermal lens measurement of absolute quantum yields using quenched fluorescent samples as references. Chem. Phys. Lett. 1989, 155, 583–586. [Google Scholar] [CrossRef]
- Magde, D.; Wong, R.; Seybold, P.G. Fluorescence quantum yields and their relation to lifetimes of rhodamine 6G and fluorescein in nine solvents: Improved absolute standards for quantum yields. J. Photochem. Photobiol. A 2002, 75, 327–334. [Google Scholar] [CrossRef]
- Das, S.; Chattopadhyay, A.P.; De, S. Controlling J aggregation in fluorescein by bile salt hydrogels. J. Photochem. Photobiol. A 2008, 197, 402–414. [Google Scholar] [CrossRef]
- Debnath, C.; Saha, M.; Hussain, S.A.; Bhattacharjee, D. Micellar effect of surfactant on the aggregation pattern of a fluorescent dye in ultra-thin film. J. Photochem. Photobiol. A 2018, 364, 696–704. [Google Scholar] [CrossRef]
- Silori, Y.; De, A.K. Tuning effect of local environment to control mechanism of fluorescence depolarization: Rotational diffusion and resonance energy transfer within homo-aggregates of xanthenes. J. Photochem. Photobiol. A 2019, 377, 198–206. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Q.; Wang, H.; Fu, Z.; Zhang, F. Photophysical behavior of lipophilic xanthene dyes without the involvement of photoinduced electron transfer mechanism. J. Photochem. Photobiol. A 2008, 200, 307–313. [Google Scholar] [CrossRef]
- Song, A.; Zhang, J.; Zhang, M.; Shen, T.; Tang, J.A. Spectral properties and structure of fluorescein and its alkyl derivatives in micelles. Colloids Surf. A Physicochem. Eng. Asp. 2000, 167, 253–262. [Google Scholar] [CrossRef]
- Kasha, M. Energy transfer mechanisms and the molecular exciton model for molecular aggregates. Radiat. Res. 1963, 20, 55–70. [Google Scholar] [CrossRef]
- Jelley, E.E. Spectral absorption and fluorescence of dyes in the molecular state. Nature 1936, 138, 1009–1010. [Google Scholar] [CrossRef]
- Gerasimova, M.A.; Tomilin, F.N.; Malyar, E.Y.; Varganov, S.A.; Fedorov, D.G.; Ovchinnikov, S.G.; Slyusareva, E.A. Fluorescence and photoinduced proton transfer in the protolytic forms of fluorescein: Experimental and computational study. Dyes Pigm. 2020, 173, 107851. [Google Scholar] [CrossRef]
- More, K.N.; Lim, T.H.; Kang, J.; Yun, H.; Yee, S.T.; Chang, D.J. Asymmetric and reduced xanthene fluorophores: Synthesis, photochemical properties, and application to activatable fluorescent probes for detection of nitroreductase. Molecules 2019, 24, 3206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-aggregates: From serendipitous discovery to supramolecular engineering of functional dye materials. Angew. Chem. Int. Ed. Engl. 2011, 50, 3376–3410. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.M. Hydrogen bond effects on radiationless electronic transitions in xanthene dyes. Chem. Phys. Lett. 1975, 35, 105–111. [Google Scholar] [CrossRef]
- Hill, J.P.; Shrestha, L.K.; Ishihara, S.; Ji, Q.; Ariga, K. Self-assembly: From amphiphiles to chromophores and beyond. Molecules 2014, 19, 8589–8609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.Q.; Chen, J.R.; Liu, X.P.; Lu, L.Q.; Davis, R.L.; Jørgensen, K.A.; Xiao, W.J. Highly efficient aerobic oxidative hydroxylation of arylboronic acids: Photoredox catalysis using visible light. Angew. Chem. 2012, 124, 808–812. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Luo, Y.; Zhang, D.W.; Wang, H.; Sun, X.W.; Li, Z.T. Iridium complex-linked porous organic polymers for recyclable, broad-scope photocatalysis of organic transformations. Green Chem. 2020, 22, 136–143. [Google Scholar] [CrossRef]
- Luo, D.P.; Huang, Y.F.; Hong, X.Y.; Chen, D.; Li, G.X.; Huang, X.B.; Gao, W.X.; Liu, M.C.; Wu, H.Y. Phthalocyanine Zinc-catalyzed Hydroxylation of Aryl Boronic Acids under Visible Light. Adv. Synth. Catal. 2019, 361, 961–964. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Chen, X.L.; Liu, Y.; Shi, T.; Peng, Y.; Qu, L.; Yu, B. Recyclable Cu@ C3N4-catalyzed hydroxylation of aryl boronic acids in water under visible light: Synthesis of phenols under ambient conditions and room temperature. ACS Sustain. Chem. Eng. 2020, 8, 2682–2687. [Google Scholar] [CrossRef]
- Yu, X.; Cohen, S.M. Photocatalytic metal–organic frameworks for the aerobic oxidation of arylboronic acids. Chem. Commun. 2015, 51, 9880–9883. [Google Scholar] [CrossRef]
- Upadhyay, R.; Singh, D.; Maurya, S.K. Highly efficient heterogeneous V2O5@ TiO2 catalyzed the rapid transformation of boronic acids to phenols. Eur. J. Org. Chem. 2021, 2021, 3925–3931. [Google Scholar] [CrossRef]
- Mahanta, A.; Saikia, T.C.; Bharali, S.J. Titanium dioxide as an efficient heterogeneous catalyst for quick C–B bond cleavage of aryl/hetero arylboronic acid on water at room temperature. Sustain. Chem. Pharm. 2020, 18, 100301. [Google Scholar] [CrossRef]
- Paul, R.; Chandra Shit, S.; Mandal, H.; Rabeah, J.; Kashyap, S.S.; Nailwal, Y.; Shinde, D.B.; Lai, Z.; Mondal, J. Benzothiazole-Linked Metal-Free Covalent Organic Framework Nanostructures for Visible-Light-Driven Photocatalytic Conversion of Phenylboronic Acids to Phenols. ACS Appl. Nano Mater. 2021, 4, 11732–11742. [Google Scholar] [CrossRef]
- Dong, X.; Hao, H.; Zhang, F.; Lang, X. Blue light photocatalysis of carbazole-based conjugated microporous polymers: Aerobic hydroxylation of phenylboronic acids to phenols. Appl. Catal. B 2022, 309, 121210. [Google Scholar] [CrossRef]
- Pitre, S.P.; McTiernan, C.D.; Ismaili, H.; Scaiano, J.C. Mechanistic insights and kinetic analysis for the oxidative hydroxylation of arylboronic acids by visible light photoredox catalysis: A metal-free alternative. J. Am. Chem. Soc. 2013, 135, 13286–13289. [Google Scholar] [CrossRef]
- Xie, H.Y.; Han, L.S.; Huang, S.; Lei, X.; Cheng, Y.; Zhao, W.; Sun, H.; Wen, X.; Xu, Q.L. N-Substituted 3(10 H)-Acridones as Visible-Light, Water-Soluble Photocatalysts: Aerobic Oxidative Hydroxylation of Arylboronic Acids. J. Org. Chem. 2017, 82, 5236–5241. [Google Scholar] [CrossRef]
- Sideri, I.K.; Voutyritsa, E.; Kokotos, C.G. Green Photoorganocatalytic Synthesis of Phenols from Arylboronic Acids. Synlett. 2018, 29, 1324–1328. [Google Scholar] [CrossRef] [Green Version]
- Weng, W.Z.; Liang, H.; Zhang, B. Visible-light-mediated aerobic oxidation of organoboron compounds using in situ generated hydrogen peroxide. Org. Lett. 2018, 20, 4979–4983. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Ghorai, S.; Cortes-Clerget, M. The hydrophobic effect applied to organic synthesis: Recent synthetic chemistry “in water”. Chem. Eur. J. 2018, 24, 6672–6695. [Google Scholar] [CrossRef]
- Luo, W.; Yang, J.D.; Cheng, J.P. Toward Rational Understandings of α-C–H Functionalization: Energetic Studies of Representative Tertiary Amines. Iscience 2020, 23, 100851. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, T.; Liu, H.; Gao, X.; Wang, C.; Wang, G. Desulfurization through Photocatalytic Oxidation: A Critical Review. ChemSusChem 2021, 14, 492. [Google Scholar] [CrossRef]
- Oster, G.; Oster, G.K.; Karg, G. Extremely long-lived intermediates in photochemical reactions of dyes in non-viscous media. J. Phys. Chem. 1962, 66, 2514–2517. [Google Scholar] [CrossRef]
- Marchesi, E.; Rota, C.; Fann, Y.C.; Chignell, C.F.; Mason, R.P. Photoreduction of the fluorescent dye 29-79-dichlorofluorescein: A spin trapping and direct electron spin resonance study with implications for oxidative stress measurements. Free Radic. Biol. Med. 1999, 26, 148–161. [Google Scholar] [CrossRef]
- Hyman, L.M.; Franz, K.J. Probing oxidative stress: Small molecule fluorescent sensors of metal ions, reactive oxygen species, and thiols. Coord. Chem. Rev. 2012, 256, 2333–2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burilov, V.A.; Mironova, D.A.; Ibragimova, R.R.; Solovieva, S.E.; König, B.; Antipin, I.S. Thiacalix[4] arene-functionalized vesicles as phosphorescent indicators for pyridoxine detection in aqueous solution. RSC Adv. 2015, 5, 101177–101185. [Google Scholar] [CrossRef]
- Burilov, V.A.; Mironova, D.A.; Grygoriev, I.A.; Valiyakhmetova, A.M.; Solovieva, S.E.; Antipin, I.S. Synthesis of Water-Soluble Polyammonium Thiacalix[4] arene Derivative and Its Interaction with Calf Thymus DNA. Russ. J. Gen. Chem. 2020, 90, 99–104. [Google Scholar] [CrossRef]
- Laikov, D.N. Fast evaluation of density functional exchange-correlation terms using the expansion of the electron density in auxiliary basis sets. Chem. Phys. Lett. 1997, 281, 151–156. [Google Scholar] [CrossRef]
- Laikov, D.N. A new class of atomic basis functions for accurate electronic structure calculations of molecules. Chem. Phys. Lett. 2005, 416, 116–120. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef] [Green Version]
- Halgren, T.A. MMFF VI. MMFF94s option for energy minimization studies. J. Comput. Chem. 1999, 20, 720–729. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]




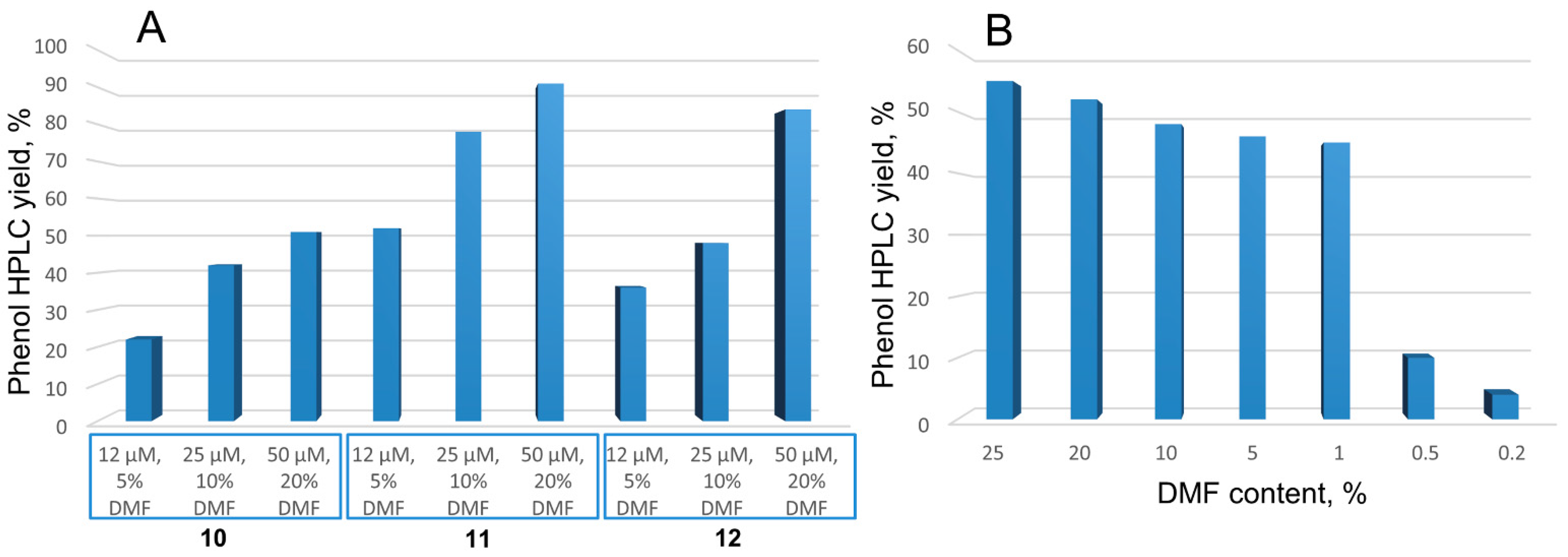
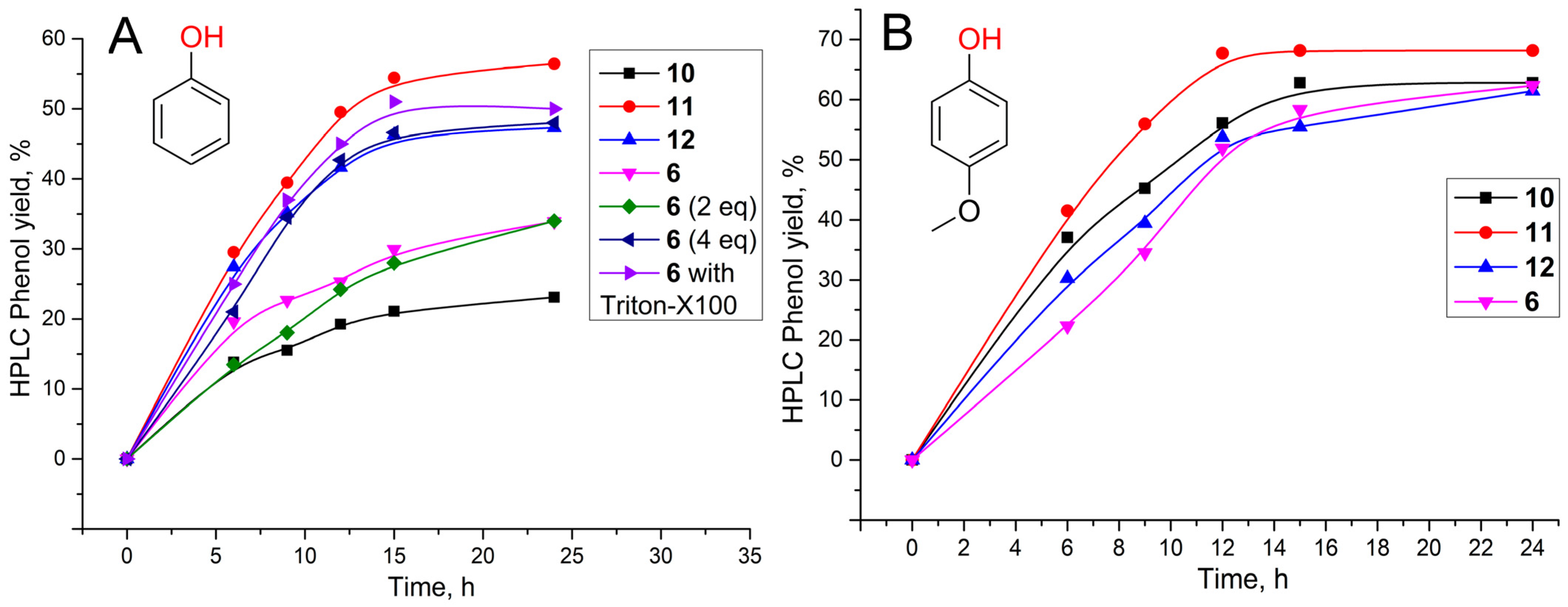
| System | , % (Water with 1% Dimethylformamide (DMF)) | , % (DMF) | , % (Toluene) |
|---|---|---|---|
| 6 | 80.3 | 24.0 | 23.2 |
| 10 | 30.2 | 31.1 | 7.8 |
| 11 | 27.9 | 34.3 | 15.8 |
| 12 | 28.8 | 29.5 | 14.9 |
| System | D Average, nm | d1, nm | d2, nm | PDI |
|---|---|---|---|---|
| 10 | 260 ± 22 | 258 ± 56 (100%) | 0.210 ± 0.091 | |
| 11 | 166 ± 1 | 177 ± 8 (100%) | 0.144 ± 0.021 | |
| 12 | 89 ± 2 | 148 ± 10 (75 ± 4%) | 36 ± 2 (25 ± 4%) | 0.390 ± 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burilov, V.; Fatykhova, A.; Mironova, D.; Sultanova, E.; Nugmanov, R.; Artemenko, A.; Volodina, A.; Daminova, A.; Evtugyn, V.; Solovieva, S.; et al. Oxyethylated Fluoresceine—(thia)calix[4]arene Conjugates: Synthesis and Visible-Light Photoredox Catalysis in Water–Organic Media. Molecules 2023, 28, 261. https://doi.org/10.3390/molecules28010261
Burilov V, Fatykhova A, Mironova D, Sultanova E, Nugmanov R, Artemenko A, Volodina A, Daminova A, Evtugyn V, Solovieva S, et al. Oxyethylated Fluoresceine—(thia)calix[4]arene Conjugates: Synthesis and Visible-Light Photoredox Catalysis in Water–Organic Media. Molecules. 2023; 28(1):261. https://doi.org/10.3390/molecules28010261
Chicago/Turabian StyleBurilov, Vladimir, Aigul Fatykhova, Diana Mironova, Elza Sultanova, Ramil Nugmanov, Alina Artemenko, Anastasia Volodina, Amina Daminova, Vladimir Evtugyn, Svetlana Solovieva, and et al. 2023. "Oxyethylated Fluoresceine—(thia)calix[4]arene Conjugates: Synthesis and Visible-Light Photoredox Catalysis in Water–Organic Media" Molecules 28, no. 1: 261. https://doi.org/10.3390/molecules28010261
APA StyleBurilov, V., Fatykhova, A., Mironova, D., Sultanova, E., Nugmanov, R., Artemenko, A., Volodina, A., Daminova, A., Evtugyn, V., Solovieva, S., & Antipin, I. (2023). Oxyethylated Fluoresceine—(thia)calix[4]arene Conjugates: Synthesis and Visible-Light Photoredox Catalysis in Water–Organic Media. Molecules, 28(1), 261. https://doi.org/10.3390/molecules28010261







