Nanostructures as Photothermal Agents in Tumor Treatment
Abstract
:1. Introduction
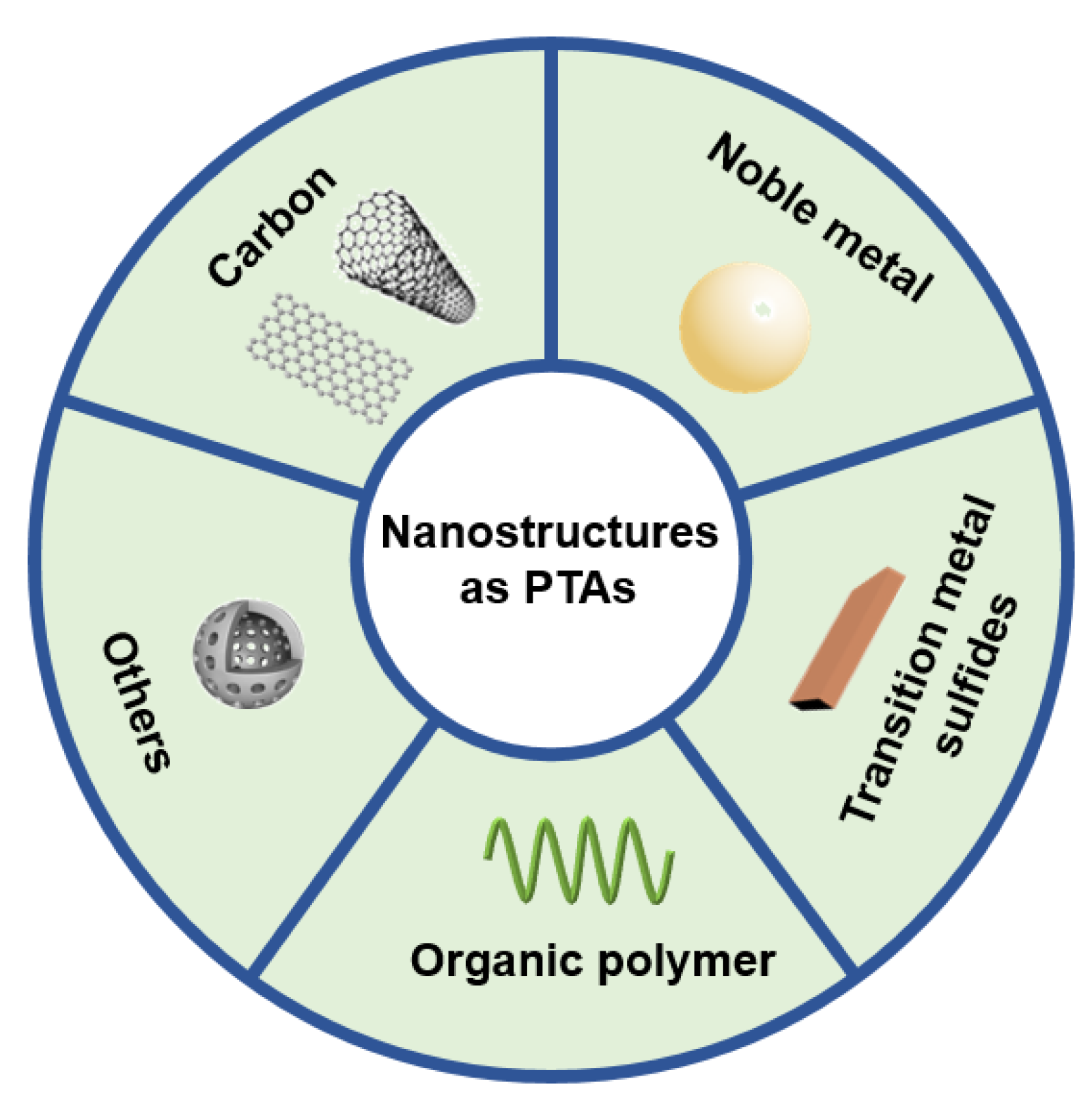
2. Nanostructures for Photothermal Therapy of Tumor
2.1. Carbon Nanostructures
2.1.1. Graphene
2.1.2. Carbon Nanotubes
2.1.3. Carbon-Based Quantum Dots

2.2. Noble Metal Nanostructures
2.2.1. Gold
2.2.2. Silver
2.2.3. Other Noble Metals
2.3. Transition Metal Sulfides
2.4. Organic Polymer
2.5. Other Nanostructures
3. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTT | photothermal therapy |
| PTAs | photothermal agents |
| PCE | photothermal conversion efficiency |
| LSPR | localized surface plasmon resonance |
| GT | gene therapy |
| GO | graphene oxide |
| rGO | reduced graphene oxide |
| CNTs | carbon nanotubes |
| SWCNTs | single-walled carbon nanotubes |
| DWCNTs | double-walled carbon nanotubes |
| MWCNTs | multi-walled carbon nanotubes |
| GQDs | graphene quantum dots |
| CQDs | carbon quantum dots |
| AgNCs | silver nanoclusters |
| POMs | polyoxometalates |
| MoO2 | molybdenum dioxide |
| NIR | near-infrared |
| NIR II | the second near-infrared |
| CT | computed tomography |
| NPs | nanoparticles |
| 0D | zero dimension |
| 2D | two dimensions |
| BSA | bovine serum albumin |
| PANI | polyaniline |
| PA | phytic acid |
| DOX | doxorubicin |
| HCR | hybridization chain reaction |
| PEG | polyethylene glycol |
| PDT | photodynamic therapy |
| ROS | reactive oxygen species |
References
- Vultaggio-Poma, V.; Sarti, A.C.; Di Virgilio, F. Extracellular ATP: A feasible target for cancer therapy. Cells 2020, 9, 2496. [Google Scholar] [CrossRef] [PubMed]
- Chabowski, M.; Jankowska-Polańska, B.; Lomper, K.; Janczak, D. The effect of coping strategy on quality of life in patients with NSCLC. Cancer Manag. Res. 2018, 10, 4085–4093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Liu, L.; Morin, E.E.; Liu, M.; Schwendeman, A. Survey of clinical translation of cancer nanomedicines-lessons learned fromsuccesses and failures. Acc. Chem. Res. 2019, 52, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Parhi, P.; Mohanty, C.; Sahoo, S.K. Nanotechnology-based combinational drug delivery: An emerging approach for cancer therapy. Drug Discov. Today 2012, 17, 1044–1052. [Google Scholar] [CrossRef]
- Li, S.; Jin, Y.; Su, Y.; Li, W.; Xing, Y.; Wang, F.; Hong, Z. Anti-HER2 affibody-conjugated photosensitizer for tumor targeting photodynamic therapy. Mol. Pharm. 2020, 17, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Yan, J.; Cai, S.; He, Q.; Wen, N.; Wang, Y.; Hu, Y.; Peng, D.; Liu, Y.; Liu, Z. Aptamer-pyropheophorbide a conjugates withtumor spheroid targeting and penetration abilities for photodynamic therapy. Mol. Pharm. 2020, 17, 2882–2890. [Google Scholar] [CrossRef]
- Zhou, A.; Wei, Y.; Wu, B.; Chen, Q.; Xing, D. Pyropheophorbide A and c(RGDyK) comodified chitosan-wrapped upconversion nanoparticle for targeted near-Infrared photodynamic therapy. Mol. Pharm. 2012, 9, 1580–1589. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.A.; Del Rincon, S.; Papneja, N.; Miller, W. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lv, J.; Zhuang, Q.; Yang, Z.; Cao, Z.; Xu, L.; Pei, P.; Wang, C.; Wu, H.; Dong, Z. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat. Nanotechnol. 2020, 15, 1043–1052. [Google Scholar] [CrossRef]
- Martin, J.D.; Cabral, H.; Stylianopoulos, T.; Jain, R.K. Improving cancer immunotherapy using nanomedicines: Progress, opportunities and challenges. Nat. Rev. Clin. Oncol. 2020, 17, 251–266. [Google Scholar] [CrossRef]
- Holley, R.J.; Wood, S.R.; Bigger, B.W. Delivering hematopoietic stem cell gene therapy treatments for neurological lysosomal diseases. ACS Chem. Neurosci. 2019, 10, 18–20. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Shen, M.; Shi, X. Poly(amidoamine) dendrimer-gold nanohybrids in cancer genetherapy: A concise overview. ACS Appl. Bio. Mater. 2020, 3, 5590–5605. [Google Scholar] [CrossRef]
- He, M.; He, M.; Nie, C.; Yi, J.; Zhang, J.; Chen, T.; Chu, X. mRNA-activated multifunctional DNAzyme nanotweezer for intracellular mRNA sensing and gene therapy. ACS Appl. Mater. Interfaces 2021, 13, 8015–8025. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, J.; Park, J.; Lee, Y.M.; Saravanakumar, G.; Park, K.M.; Choi, W.; Kim, K.; Lee, E.; Kim, C. Tumor vasodilation by N-Heterocyclic carbene-based nitric oxide delivery triggered by high-intensity focused ultrasound and enhanced drug homing to tumor sites for anti-cancer therapy. Biomaterials 2019, 217, 119297. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, H.; Hu, X.; Wang, Y.; Yuan, Y. The efficacy of a new high-intensity focused ultrasound therapy for metastatic pancreatic cancer. Int. J. Hyper. 2021, 38, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Mortezavi, A.; Krauter, J.; Gu, A.; Sonderer, J.; Bruhin, J.; Reeve, K.A.; Held, L.; Donati, O.F.; Rupp, N.J.; Moch, H. Extensive histological sampling following focal therapy of clinically significant prostate cancer with high intensity focused ultrasound. J. Urol. 2019, 202, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, M.; Varvarà, P.; Tranchina, L.; Puleio, R.; Cicero, L.; Cassata, G.; Giammona, G. In vivo efficacy of verteporfin loaded gold nanorods for combined photothermal/photodynamic colon cancer therapy. Int. J. Pharm. 2022, 625, 122134. [Google Scholar] [CrossRef]
- Yang, X.; Wen, L.; Xu, G.; Lin, H.; Wang, S.; Liu, J. Multifunctional organic nanomaterials with ultra-high photothermal conversion efficiency for photothermal therapy and inhibition of cancer metastasis. Bioorg. Chem. 2023, 130, 106220. [Google Scholar] [CrossRef]
- Du, W.; Chen, W.; Wang, J.; Cheng, L.; Wang, J.; Zhang, H.; Song, L.; Hu, Y.; Ma, X. Oxygen-deficient titanium dioxide-loaded black phosphorus nanosheets for synergistic photothermal and sonodynamic cancer therapy. Biomater. Adv. 2022, 136, 212794. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, L.; Wu, J.; Liu, M.; Wang, G.; Liu, B.; Zhang, L. Transcriptional cyclin-dependent kinases: Potential drug targets in cancer therapy. Eur. J. Med. Chem. 2022, 229, 114056. [Google Scholar] [CrossRef]
- Ghasemii, K.; Darroudi, M.; Rahimmanesh, I.; Ghomi, M.; Hassanpour, M.; Sharifi, E.; Yousefiasl, S.; Ahmadi, S.; Zarrabi, A.; Borzacchiello, A.; et al. Advances in aptamer-based drug delivery vehicles for cancer therapy. Biomater. Adv. 2022, 140, 213077. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Feng, X.; Mai, B.; Li, X.; Wang, F.; Liu, J.; Liu, X.; Zhang, K.; Wang, X. Bacterial-based cancer therapy: An emerging toolbox for targeted drug/gene delivery. Biomaterials 2021, 277, 121124. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, S.G.; Amini, M.; Jalilzadeh, N.; Baradaran, B.; Mohammadzadeh, R.; Mokhtarzadeh, A.; Oroojalian, F. Recent advances in nanoparticle-based photothermal therapy for breast cancer. J. Control. Release 2022, 349, 269–303. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Zhao, X.; Yang, F.; Xu, Y.; Yan, F.; Xia, D.; Liu, Y. The effect of near-infrared light-assisted photothermal therapy combined with polymer materials on promoting bone regeneration: A systematic review. Mater. Des. 2022, 217, 110621. [Google Scholar] [CrossRef]
- Yang, K.; Zhao, S.; Li, B.; Wang, B.; Lan, M.; Song, X. Low temperature photothermal therapy: Advances and perspectives. Coord. Chem. Rev. 2022, 454, 214330. [Google Scholar] [CrossRef]
- Yu, X.; Yang, K.; Chen, X.; Li, W. Black hollow silicon oxide nanoparticles as highly efficient photothermal agents in the second near-infrared window for in vivo cancer therapy. Biomaterials 2017, 143, 120–129. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Wang, X.; Hao, Z.; Han, S.; Wang, T.; Zhang, H. Photothermal-based nanomaterials and photothermal-sensing: An overview. Biosens. Bioelectron. 2023, 220, 114883. [Google Scholar] [CrossRef]
- Fu, L.; Jin, W.; Zhang, J.; Zhu, L.; Lu, J.; Zhen, Y.; Zhang, L.; Ouyang, L.; Liu, B.; Yu, H. Repurposing non-oncology small-molecule drugs to improve cancer therapy: Current situation and future directions. Acta Pharm. Sin. B 2022, 12, 532–557. [Google Scholar] [CrossRef]
- Fu, M.; Shen, Y.; Zhou, H.; Liu, X.; Chen, W.; Ma, X. Gallium-based liquid metal micro/nanoparticles for photothermal cancer therapy. J. Mater. Sci. Technol. 2023, 142, 22–33. [Google Scholar] [CrossRef]
- Khot, M.I.; Andrew, H.; Svavarsdottir, H.S.; Armstrong, G.; Quyn, A.J.; Jayne, D.G. A review on the scope of photothermal therapy-based nanomedicines in preclinical models of colorectal cancer. Clin. Color. Cancer 2019, 18, e200–e209. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Saeb, M.R.; Thomas, S. Functionalized theranostic nanocarriers with bio-inspired polydopamine for tumor imaging and chemo-photothermal therapy. J. Control. Release 2019, 309, 203–219. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and bioactive engineered nanomaterials for targeted tumor photothermal therapy: A review. Mater. Sci. Eng. C 2019, 104, 109891. [Google Scholar] [CrossRef]
- Lv, F.; Fan, X.; Liu, D.; Song, F. Photothermal agents based on small organic fluorophores with intramolecular motion. Acta Biomater. 2022, 149, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shi, T.; Zhang, J.; Sun, Y.; Ma, T.; Li, W.; Li, Y.; Qiu, H.; Yin, S. An NIR-II-absorbing photothermal agent containing multiple rotors with enhanced photothermal conversion capacity for multimodal-imaging-guided photothermal therapy. Dyes Pigm. 2022, 210, 110932. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, H.J.; Liu, J.X.; Ma, Y.; Yao, J.T.; Dai, X.H.; Pan, J.M. Double affinity integrated MIPs nanoparticles for specific separation of glycoproteins: A combination of synergistic multiple bindings and imprinting effect. Chem. Eng. J. 2019, 358, 143–152. [Google Scholar] [CrossRef]
- Khakbaz, F.; Mirzaei, M.; Mahani, M. Lecithin sensitized thermo-sensitive niosome using NIR-carbon dots for breast cancer combined chemo-photothermal therapy. J. Photochem. Photobiol. A 2023, 434, 114236. [Google Scholar] [CrossRef]
- Naser Mohammed, S.; Mishaal Mohammed, A.; Al-Rawi, K.F. Novel combination of multi-walled carbon nanotubes and gold nanocomposite for photothermal therapy in human breast cancer model. Steroids 2022, 186, 109091. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Xu, S.; Yue, J.; Guo, Q.; Huang, D.; Yang, B.; Shi, W.; Liang, C.; Xu, W. Intracellular pH-propelled assembly of smart carbon nanodots and selective photothermal therapy for cancer cells. Colloids Surf. B 2020, 188, 110724. [Google Scholar] [CrossRef]
- Zhang, A.; Hai, L.; Wang, T.; Cheng, H.; Li, M.; He, X.; Wang, K. NIR-triggered drug delivery system based on phospholipid coated ordered mesoporous carbon for synergistic chemo-photothermal therapy of cancer cells. Chin. Chem. Lett. 2020, 31, 3158–3162. [Google Scholar] [CrossRef]
- Jia, Q.; Zheng, X.; Ge, J.; Liu, W.; Ren, H.; Chen, S.; Wen, Y.; Zhang, H.; Wu, J.; Wang, P. Synthesis of carbon dots from Hypocrella bambusae for bimodel fluorescence/photoacoustic imaging-guided synergistic photodynamic/photothermal therapy of cancer. J. Colloid Interface Sci. 2018, 526, 302–311. [Google Scholar] [CrossRef]
- Nag, A.; Simorangkir, R.B.V.B.; Gawade, D.R.; Nuthalapati, S.; Buckley, J.L.; O’Flynn, B.; Altinsoy, M.E.; Mukhopadhyay, S.C. Graphene-based wearable temperature sensors: A review. Mater. Des. 2022, 221, 110971. [Google Scholar] [CrossRef]
- Hashemzadeh, H.; Raissi, H. Understanding loading, diffusion and releasing of Doxorubicin and Paclitaxel dual delivery in graphene and graphene oxide carriers as highly efficient drug delivery systems. Appl. Surf. Sci. 2020, 500, 144220. [Google Scholar] [CrossRef]
- Arvas, M.B.; Gürsu, H.; Gencten, M.; Sahin, Y. Supercapacitor applications of novel phosphorus doped graphene-based electrodes. J. Energy Storage 2022, 55, 105766. [Google Scholar] [CrossRef]
- Jin, J.; Li, J.; Tai, Q.; Chen, Y.; Mishra, D.D.; Deng, W.; Xin, J.; Guo, S.; Xiao, B.; Wang, X. Efficient and stable flexible perovskite solar cells based on graphene-AgNWs substrate and carbon electrode without hole transport materials. J. Power Sources 2021, 482, 228953. [Google Scholar] [CrossRef]
- Zaharie-Butucel, D.; Potara, M.; Suarasan, S.; Licarete, E.; Astilean, S. Efficient combined near-infrared-triggered therapy: Phototherapy over chemotherapy in chitosan-reduced graphene oxide-IR820 dye-doxorubicin nanoplatforms. J. Colloid Interface Sci. 2019, 552, 218–229. [Google Scholar] [CrossRef]
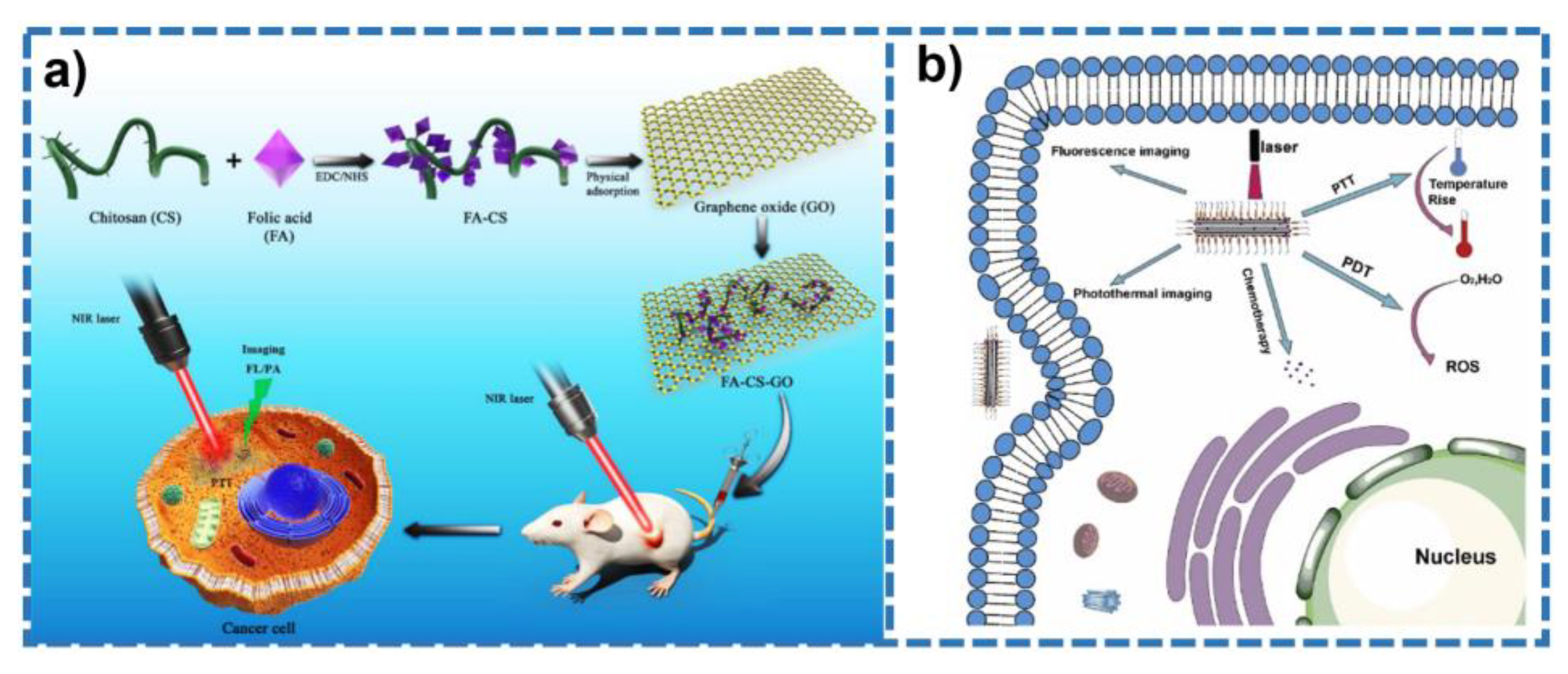
- Jun, S.W.; Manivasagan, P.; Kwon, J.; Nguyen, V.T.; Mondal, S.; Ly, C.D.; Lee, J.; Kang, Y.H.; Kim, C.S.; Oh, J. Folic acid-conjugated chitosan-functionalized graphene oxide for highly efficient photoacoustic imaging-guided tumor-targeted photothermal therapy. Int. J. Biol. Macromol. 2020, 155, 961–971. [Google Scholar] [CrossRef]
- Li, Q.; Hong, L.; Li, H.; Liu, C. Graphene oxide-fullerene C60 (GO-C60) hybrid for photodynamic and photothermal therapy triggered by near-infrared light. Biosens. Bioelectron. 2017, 89 (Pt 1), 477–482. [Google Scholar] [CrossRef]
- Su, Y.; Wang, N.; Liu, B.; Du, Y.; Li, R.; Meng, Y.; Feng, Y.; Shan, Z.; Meng, S. A phototheranostic nanoparticle for cancer therapy fabricated by BODIPY and graphene to realize photo-chemo synergistic therapy and fluorescence/photothermal imaging. Dyes Pigm. 2020, 177, 108262. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, Y. Theoretical study on paramagnetic amino carbon nanotube as fluorouracil drug delivery system. J. Drug Deliv. Sci. Technol. 2022, 75, 103670. [Google Scholar] [CrossRef]
- Lavagna, L.; Nisticò, R.; Musso, S.; Pavese, M. Functionalization as a way to enhance dispersion of carbon nanotubes in matrices: A review. Mater. Today Chem. 2021, 20, 100477. [Google Scholar] [CrossRef]
- Suo, X.; Eldridge, B.N.; Zhang, H.; Mao, C.; Min, Y.; Sun, Y.; Singh, R.; Ming, X. P-glycoprotein-targeted photothermal therapy of drug-resistant cancer cells using antibody-conjugated carbon nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 33464–33473. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, T.; Cao, Y.; Sun, J.; Zhou, Q.; Chen, H.; Guo, S.; Wang, Y.; Zhen, Y.; Liang, X.J.; et al. Temperature-sensitive lipid-coated carbon nanotubes for synergistic photothermal therapy and gene therapy. ACS Nano 2021, 15, 6517–6529. [Google Scholar] [CrossRef]
- Lu, G.H.; Shang, W.T.; Deng, H.; Han, Z.Y.; Hu, M.; Liang, X.Y.; Fang, C.H.; Zhu, X.H.; Fan, Y.F.; Tian, J. Targeting carbon nanotubes based on IGF-1R for photothermal therapy of orthotopic pancreatic cancer guided by optical imaging. Biomaterials 2019, 195, 13–22. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, S.; Wang, G.; Cui, J.; Lu, Y.; Rong, X.; Gao, C. A review on mechanism, applications and influencing factors of carbon quantum dots based photocatalysis. Ceram. Int. 2022, 48, 35986–35999. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Qian, Y.; Hu, L.; Fang, J.; Tong, W.; Nie, R.; Chen, Q.; Wang, H. Magnetic-induced graphene quantum dots for imaging-guided photothermal therapy in the second near-infrared window. Biomaterials 2020, 232, 119700. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Liang, L.; Yue, J.; Huang, D.; Xu, W.; Shi, W.; Liang, C.; Xu, S. Mitochondria-targeting supra-carbon dots: Enhanced photothermal therapy selective to cancer cells and their hyperthermia molecular actions. Carbon 2020, 156, 558–567. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, L.; Cao, M.; Huang, L.; Yang, K.; Wu, S.; Lan, M.; Niu, G.; Zhang, W. Near-infrared light-triggered lysosome-targetable carbon dots for photothermal therapy of cancer. ACS Appl. Mater. Interfaces 2021, 13, 53610–53617. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Qin, H.; Shen, W.; Li, P.; Fang, F.; Li, X.; Pan, D.; Shen, L. Carbon dot/WS2 heterojunctions for NIR-II enhanced photothermal therapy of osteosarcoma and bone regeneration. Chem. Eng. J. 2020, 383, 123102. [Google Scholar] [CrossRef]
- Kumar, A.V.P.; Dubey, S.K.; Tiwari, S.; Puri, A.; Hejmady, S.; Gorain, B.; Kesharwani, P. Recent advances in nanoparticles mediated photothermal therapy induced tumor regression. Int. J. Pharm. 2021, 606, 120848. [Google Scholar] [CrossRef]
- Chen, J.; Ma, Y.; Du, W.; Dai, T.; Wang, Y.; Jiang, W.; Wan, Y.; Wang, Y.; Liang, G.; Wang, G. Furin-instructed intracellular gold nanoparticle aggregation for tumor photothermal therapy. Adv. Funct. Mater. 2020, 30, 2001566. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.; Sun, W.; Lin, X.; Wang, R.; Li, C.; Zong, L.; Fu, Z.; Liu, H.; Xu, S. Robust emission in near-infrared II of lanthanide nanoprobes conjugated with Au (LNPs-Au) for temperature sensing and controlled photothermal therapy. Chem. Eng. J. 2023, 452, 139504. [Google Scholar] [CrossRef]
- Song, Y.; Qu, Z.; Li, J.; Shi, L.; Zhao, W.; Wang, H.; Sun, T.; Jia, T.; Sun, Y. Fabrication of the biomimetic DOX/Au@Pt nanoparticles hybrid nanostructures for the combinational chemo/photothermal cancer therapy. J. Alloys Compd. 2021, 881, 160592. [Google Scholar] [CrossRef]
- Yim, W.; Borum, R.M.; Zhou, J.; Mantri, Y.; Wu, Z.; Zhou, J.; Jin, Z.; Creyer, M.; Jokerst, J.V. Ultrasmall gold nanorod-polydopamine hybrids for enhanced photoacoustic imaging and photothermal therapy in second near-infrared window. Nanotheranostics 2022, 6, 79–90. [Google Scholar] [CrossRef] [PubMed]
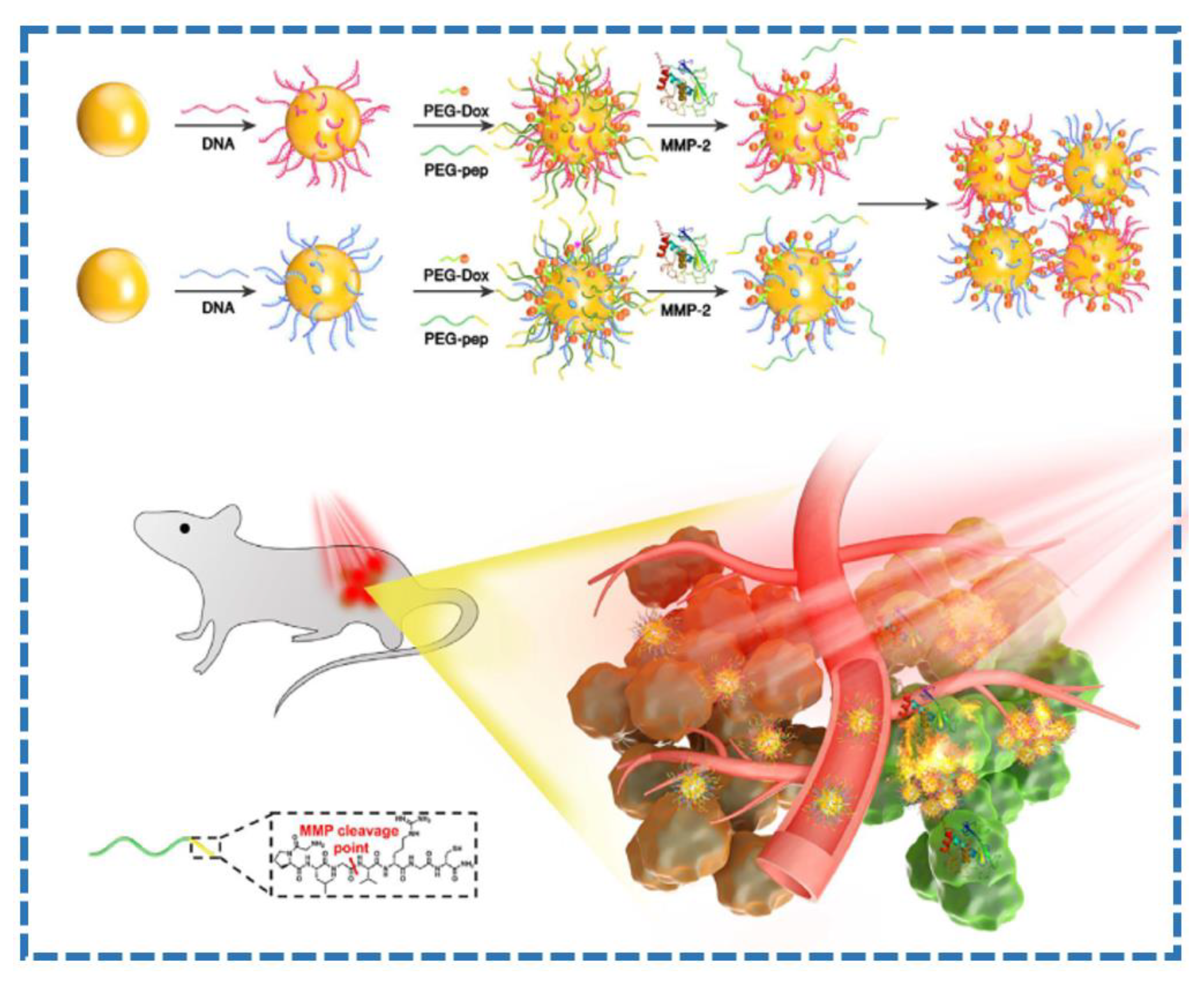
- Yang, K.; Liu, Y.; Wang, Y.; Ren, Q.; Guo, H.; Matson, J.B.; Chen, X.; Nie, Z. Enzyme-induced in vivo assembly of gold nanoparticles for imaging-guided synergistic chemo-photothermal therapy of tumor. Biomaterials 2019, 223, 119460. [Google Scholar] [CrossRef] [PubMed]
- Boca, S.C.; Potara, M.; Gabudean, A.-M.; Juhem, A.; Baldeck, P.L.; Astilean, S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett. 2011, 311, 131–140. [Google Scholar] [CrossRef]
- Mondal, S.; Montaño-Priede, J.L.; Nguyen, V.T.; Park, S.; Choi, J.; Doan, V.H.M.; Vo, T.M.T.; Vo, T.H.; Large, N.; Kim, C.-S.; et al. Computational analysis of drug free silver triangular nanoprism theranostic probe plasmonic behavior for in-situ tumor imaging and photothermal therapy. J. Adv. Res. 2022, 41, 23–38. [Google Scholar] [CrossRef]
- He, F.; Luo, Z.; Wen, Z.; Huangfu, H.; Feng, Y.; Qi, X.; Duan, Y. A bent fiber optic sensor based on gold nanoparticle-decorated silver film for integration of cytosensor and plasmonic photothermal therapy: A simulation study. Optik 2022, 271, 170227. [Google Scholar] [CrossRef]
- Rivas Aiello, M.B.; Azcarate, J.C.; Zelaya, E.; David Gara, P.; Bosio, G.N.; Gensch, T.; Martire, D.O. Photothermal therapy with silver nanoplates in HeLa cells studied by in situ fluorescence microscopy. Biomater. Sci. 2021, 9, 2608–2619. [Google Scholar] [CrossRef]
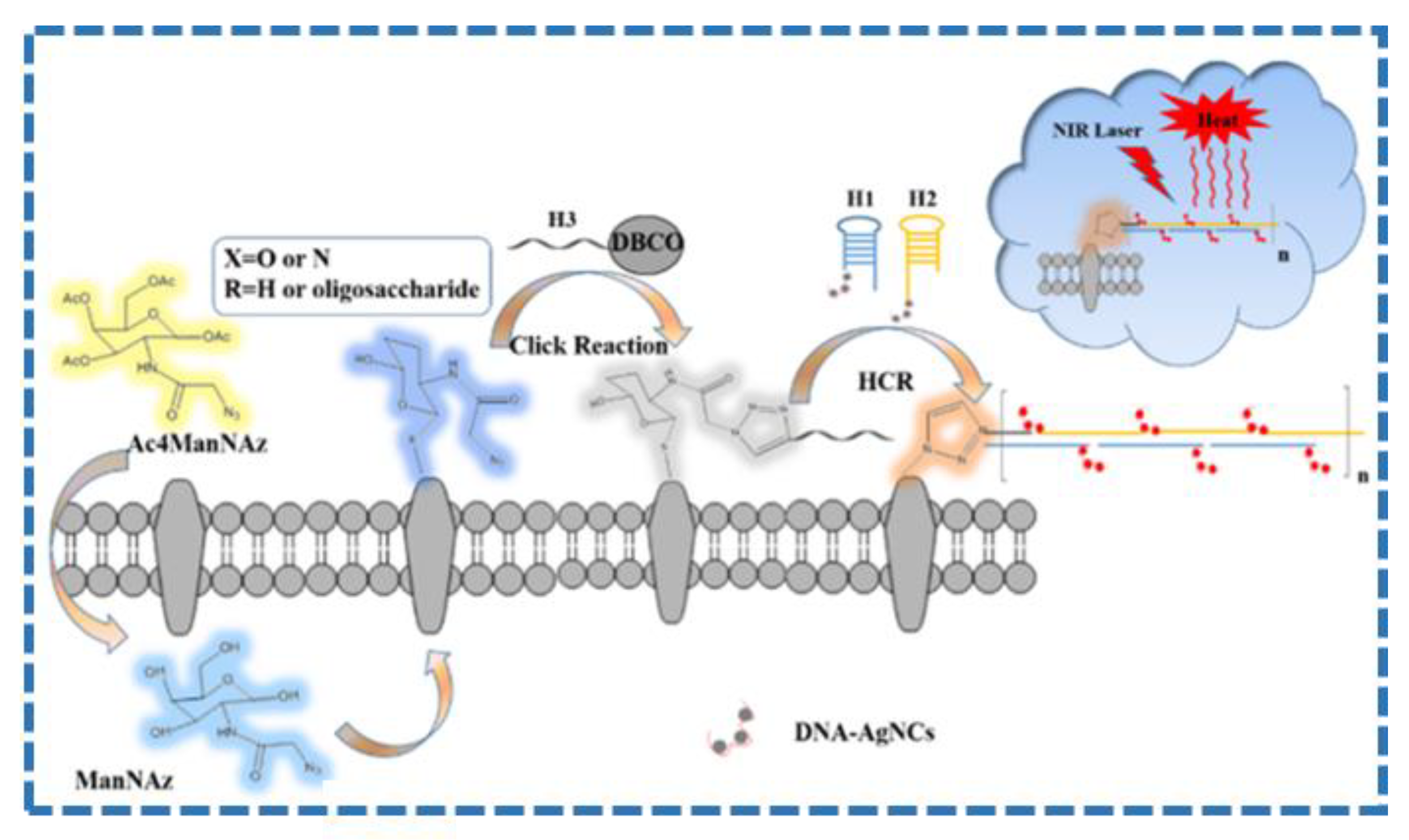
- Wu, J.; Li, N.; Yao, Y.; Tang, D.; Yang, D.; Ong’Achwa Machuki, J.; Li, J.; Yu, Y.; Gao, F. DNA-stabilized silver nanoclusters for label-free Ffuorescence imaging of cell surface glycans and fluorescence guided photothermal therapy. Anal. Chem. 2018, 90, 14368–14375. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Zhao, X.; Zhou, Z.; Wang, Y.; Cheng, Y.; Huang, Q.; Zhang, Q. Hyaluronic acid-encapsulated platinum nanoparticles for targeted photothermal therapy of breast cancer. J. Biomed. Nanotechnol. 2017, 13, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Fan, T.; Yan, Y.; Zhang, S.; Zhou, Y.; Deng, H.; Cai, X.; Xiao, J.; Song, D.; Zhang, Q.; et al. One stone with two birds: Phytic acid-capped platinum nanoparticles for targeted combination therapy of bone tumors. Biomaterials 2019, 194, 130–138. [Google Scholar] [CrossRef]
- Ming, J.; Zhang, J.; Shi, Y.; Yang, W.; Li, J.; Sun, D.; Xiang, S.; Chen, X.; Chen, L.; Zheng, N. A trustworthy CpG nanoplatform for highly safe and efficient cancer photothermal combined immunotherapy. Nanoscale 2020, 12, 3916–3930. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, R.; Rajalakshmi, P.S.; Thomas, A.; Rengan, A.K. Doxorubicin loaded polyvinylpyrrolidone-copper sulfide nanoparticles enabling mucoadhesiveness and chemo-photothermal synergism for effective killing of breast cancer cells. Materialia 2021, 19, 101195. [Google Scholar]
- Rajasekar, S.; Martin, E.M.; Kuppusamy, S.; Vetrivel, C. Chitosan coated molybdenum sulphide nanosheet incorporated with tantalum oxide nanomaterials for improving cancer photothermal therapy. Arab. J. Chem. 2020, 13, 4741–4750. [Google Scholar] [CrossRef]
- Manivasagan, P.; Joe, A.; Han, H.-W.; Thambi, T.; Selvaraj, M.; Chidambaram, K.; Kim, J.; Jang, E.-S. Recent advances in multifunctional nanomaterials for photothermal-enhanced Fenton-based chemodynamic tumor therapy. Mater. Today Bio. 2022, 13, 100197. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Choi, H.W.; Mo, S.J.; Chung, B.G. Dual-stimuli responsive mesoporous copper (II) sulfide nanocomposite for chemo-photothermal synergistic therapy. Microporous Mesoporous Mater. 2020, 302, 110228. [Google Scholar] [CrossRef]
- Cai, H.; Dai, X.; Guo, X.; Zhang, L.; Cao, K.; Yan, F.; Ji, B.; Liu, Y. Ataxia telangiectasia mutated inhibitor-loaded copper sulfide nanoparticles for low-temperature photothermal therapy of hepatocellular carcinoma. Acta Biomater. 2021, 127, 276–286. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Williams, G.R.; Yang, Y.; Niu, S.; Qian, Q.; Zhu, L.M. Dual-responsive molybdenum disulfide/copper sulfide-based delivery systems for enhanced chemo-photothermal therapy. J. Colloid Interface Sci. 2019, 539, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.T.; Zhang, W.; Wang, W.B.; Wu, Y.J.; Zhou, L.; Cao, F. One-pot bottom-up fabrication of biocompatible PEGylated WS(2) nanoparticles for CT-guided photothermal therapy of tumors in vivo. Biochem. Biophys. Res. Commun. 2019, 511, 587–591. [Google Scholar] [CrossRef]
- Bian, W.; Wang, Y.; Pan, Z.; Chen, N.; Li, X.; Wong, W.-L.; Liu, X.; He, Y.; Zhang, K.; Lu, Y.J. Review of functionalized nanomaterials for photothermal therapy of cancers. ACS Appl. Nano Mater. 2021, 4, 11353–11385. [Google Scholar] [CrossRef]
- Jaque, D.; Martinez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martin Rodriguez, E.; Garcia Sole, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yang, Y.; Liang, M.; Ma, Y.; Sun, N.; Gao, X.; Li, J. Pt@polydopamine nanoparticles as nanozymes for enhanced photodynamic and photothermal therapy. Chem. Commun. 2021, 57, 255–258. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, X.; Chen, T.; Sun, S.; Shi, Z.; Liu, J.; Ji, X.; Zeng, X.; Guan, J.; Mei, L.; et al. Renal-clearable ultrasmall polypyrrole nanoparticles with size-regulated property for second near-infrared light-mediated photothermal therapy. Adv. Funct. Mater. 2021, 31, 2008362. [Google Scholar] [CrossRef]
- Tian, Q.; Li, Y.; Jiang, S.; An, L.; Lin, J.; Wu, H.; Huang, P.; Yang, S. Tumor pH-responsive albumin/polyaniline assemblies for amplified photoacoustic imaging and augmented photothermal therapy. Small 2019, 15, e1902926. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Xu, Q.; Wang, Y.; Ji, J. Polydopamine nanoparticles with different sizes for NIR-promoted gene delivery and synergistic photothermal therapy. Colloids Surf. B 2021, 208, 112125. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, X.; Li, W.; Zhang, Q.; Bai, H.; Li, J.; Xi, G. Highly stable molybdenum dioxide nanoparticles with strong plasmon resonance are promising in photothermal cancer therapy. Biomaterials 2018, 163, 43–54. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Q.; Li, J.; Peng, S.; Wang, X.; Cai, R. Near-infrared photoactivated nanomedicines for photothermal synergistic cancer therapy. Nano Today 2021, 37, 101073. [Google Scholar] [CrossRef]
- Fernandes, N.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Overview of the application of inorganic nanomaterials in cancer photothermal therapy. Biomater. Sci. 2020, 8, 2990–3020. [Google Scholar] [CrossRef]
- Yao, Z.Y.; Li, Z.H.; Xie, X.L.; Zou, Y.; Ren, X.M. Reversible crystal-amorphous transformation assisted by loss and adsorption of coordination water molecules and ionic conduction in two isomorphous decavanadate-type polyoxometalates. J. Solid State Chem. 2022, 314, 123309. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Li, S.; Zhang, L.; Sun, M. A review of application and prospect for polyoxometalate-based composites in electrochemical sensor. Inorg. Chem. Commun. 2022, 135, 109084. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, J.; Huang, H.; Cao, C.; Yin, J.; Xu, W.; Wang, W.; Song, X.; Zhang, Y.; Dong, X. Fe-doped polyoxometalate as acid-aggregated nanoplatform for NIR-II photothermal-enhanced chemodynamic therapy. Adv. Healthcare Mater. 2020, 9, e2000005. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhou, F.; Wang, C.; Hu, L.; Guo, P. Nanostructures as Photothermal Agents in Tumor Treatment. Molecules 2023, 28, 277. https://doi.org/10.3390/molecules28010277
Chen Y, Zhou F, Wang C, Hu L, Guo P. Nanostructures as Photothermal Agents in Tumor Treatment. Molecules. 2023; 28(1):277. https://doi.org/10.3390/molecules28010277
Chicago/Turabian StyleChen, Yuqian, Futing Zhou, Chenshuai Wang, Linlin Hu, and Pengfei Guo. 2023. "Nanostructures as Photothermal Agents in Tumor Treatment" Molecules 28, no. 1: 277. https://doi.org/10.3390/molecules28010277
APA StyleChen, Y., Zhou, F., Wang, C., Hu, L., & Guo, P. (2023). Nanostructures as Photothermal Agents in Tumor Treatment. Molecules, 28(1), 277. https://doi.org/10.3390/molecules28010277






