Algal-Derived Synthesis of Silver Nanoparticles Using the Unicellular ulvophyte sp. MBIC10591: Optimisation, Characterisation, and Biological Activities
Abstract
1. Introduction
2. Results and Discussions
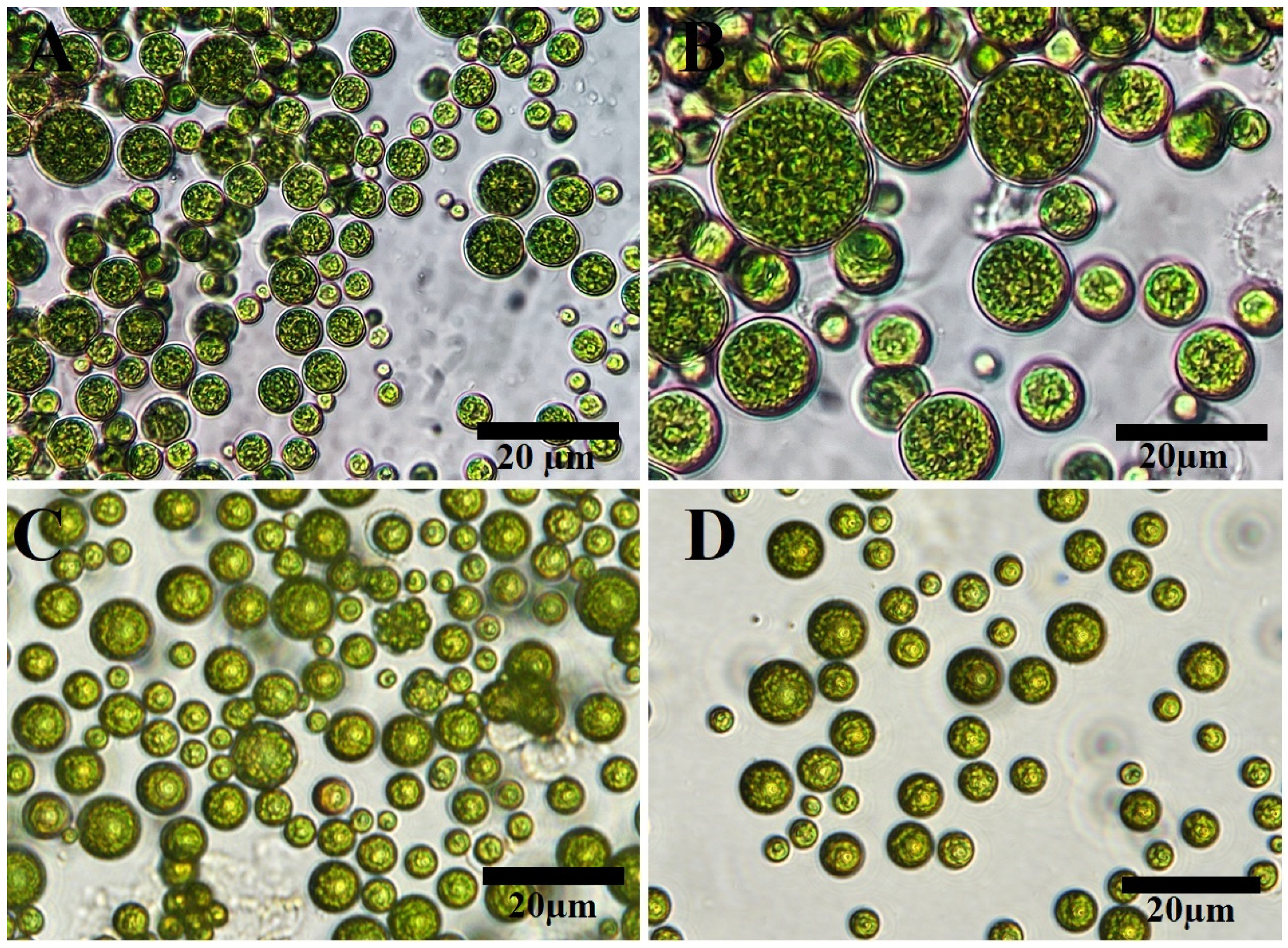
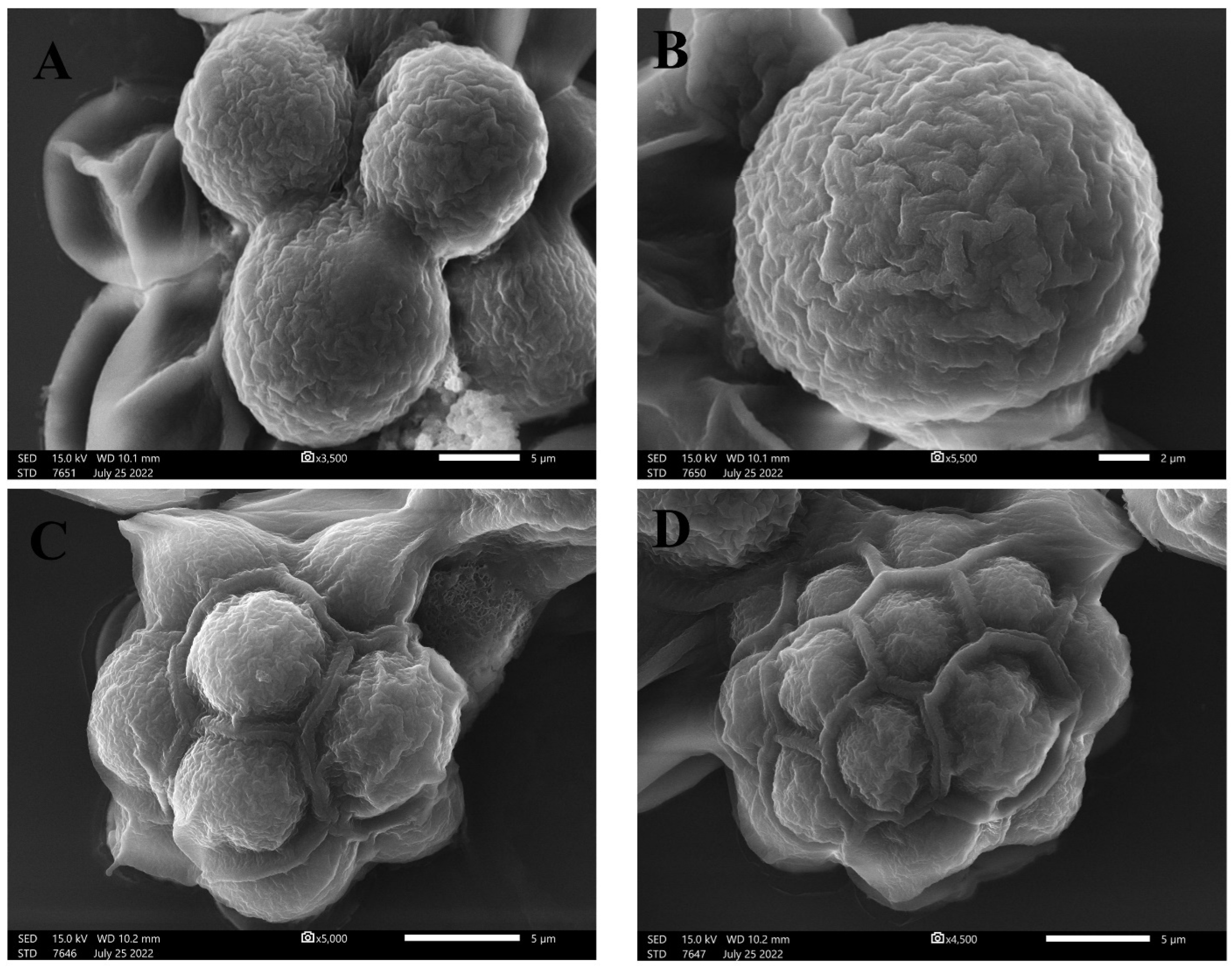
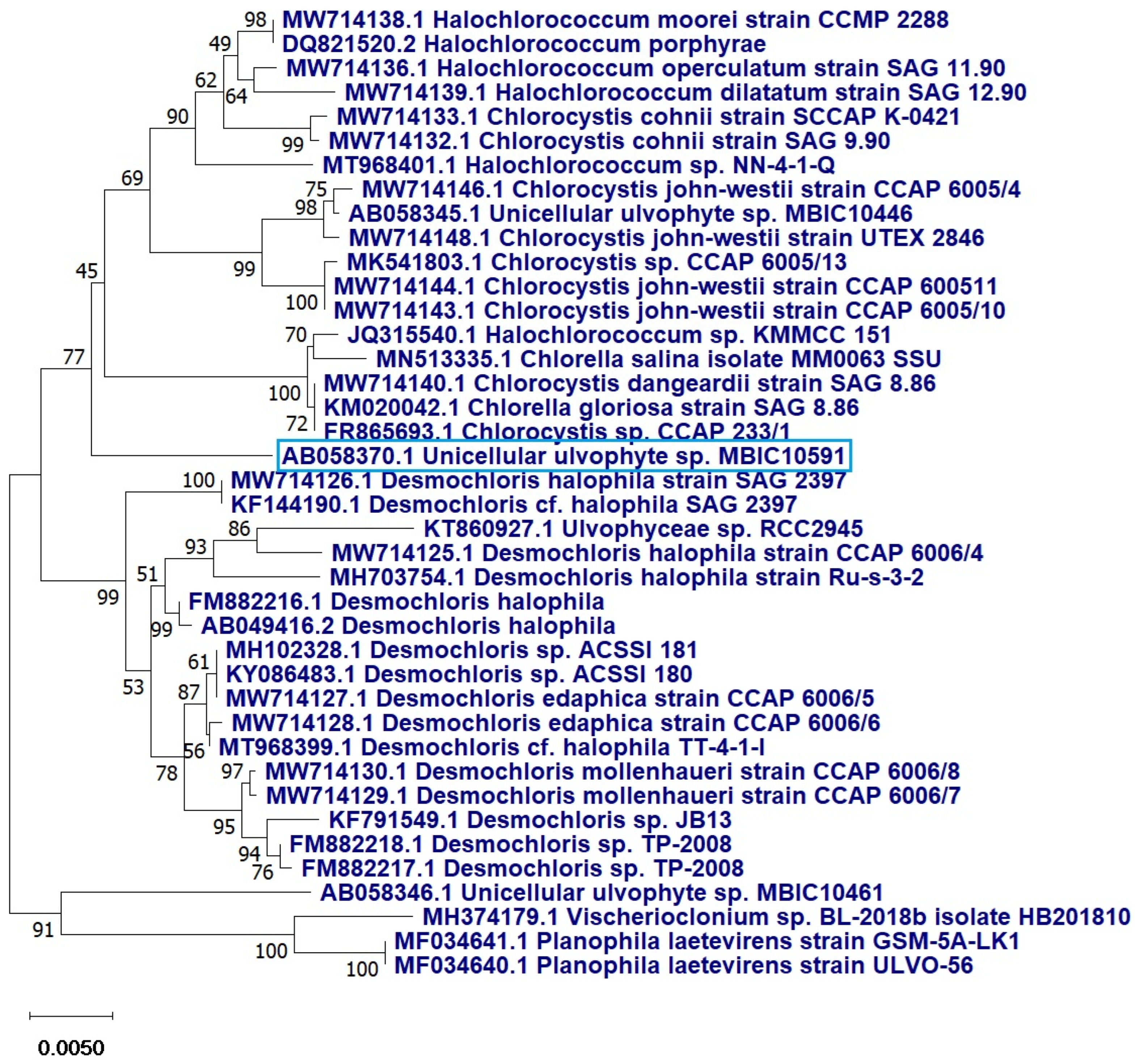
2.1. Algal Identification
2.1.1. Morphological Appearance
2.1.2. Molecular Identification
2.1.3. GC-MS Analysis
2.2. Uv@Ag-NPs Synthesis
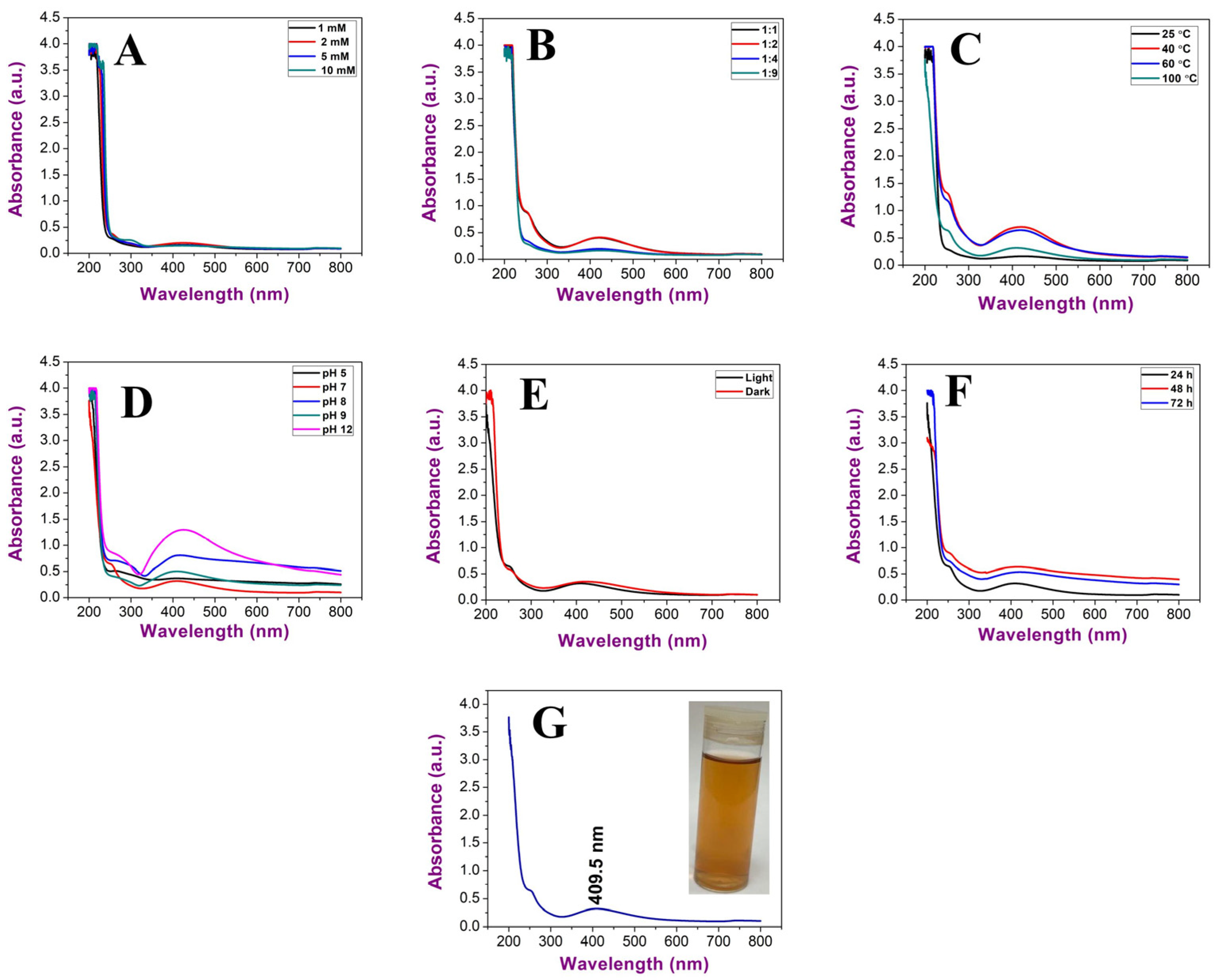
2.2.1. Optimisation Parameters of Uv@Ag-NPs Synthesis
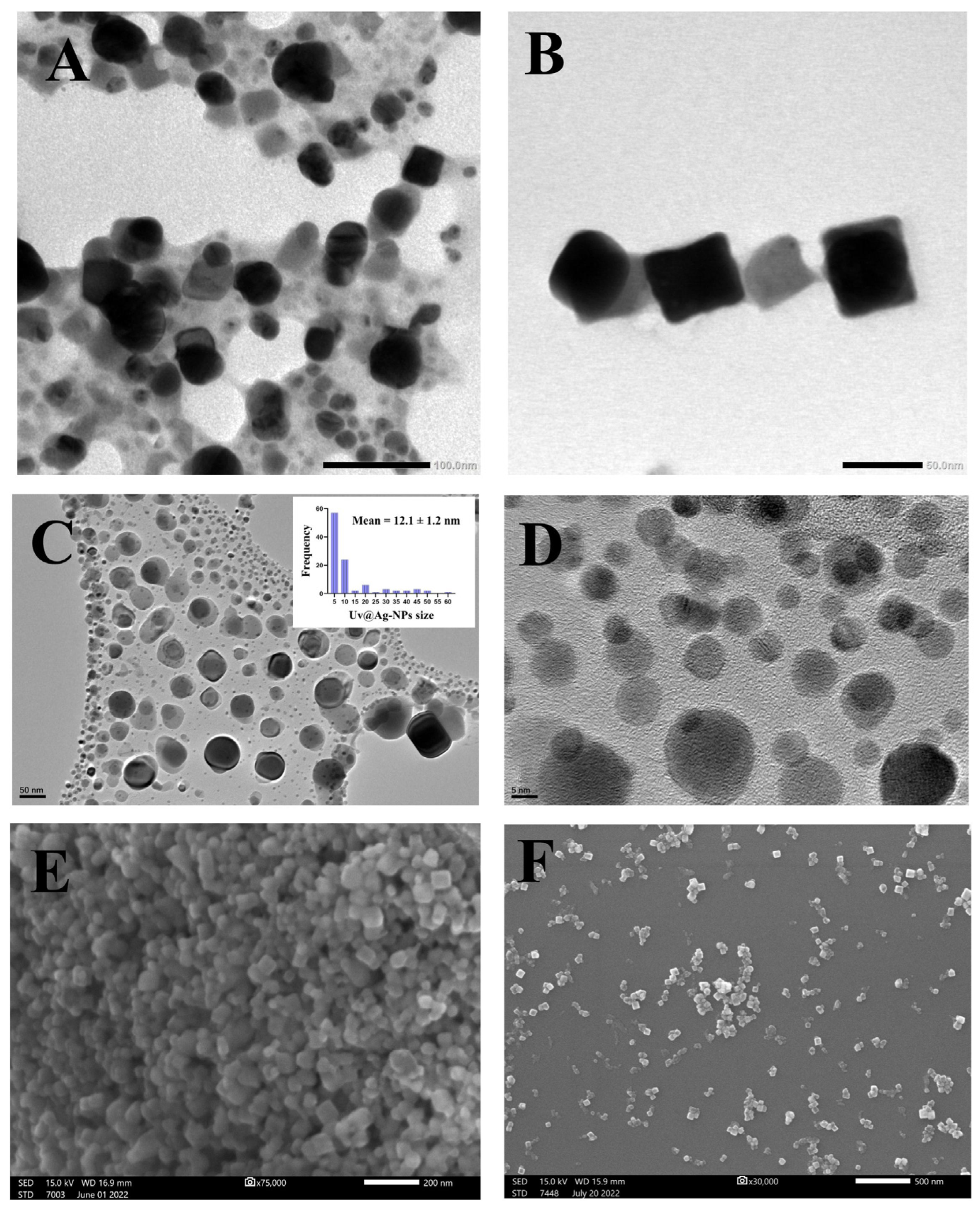
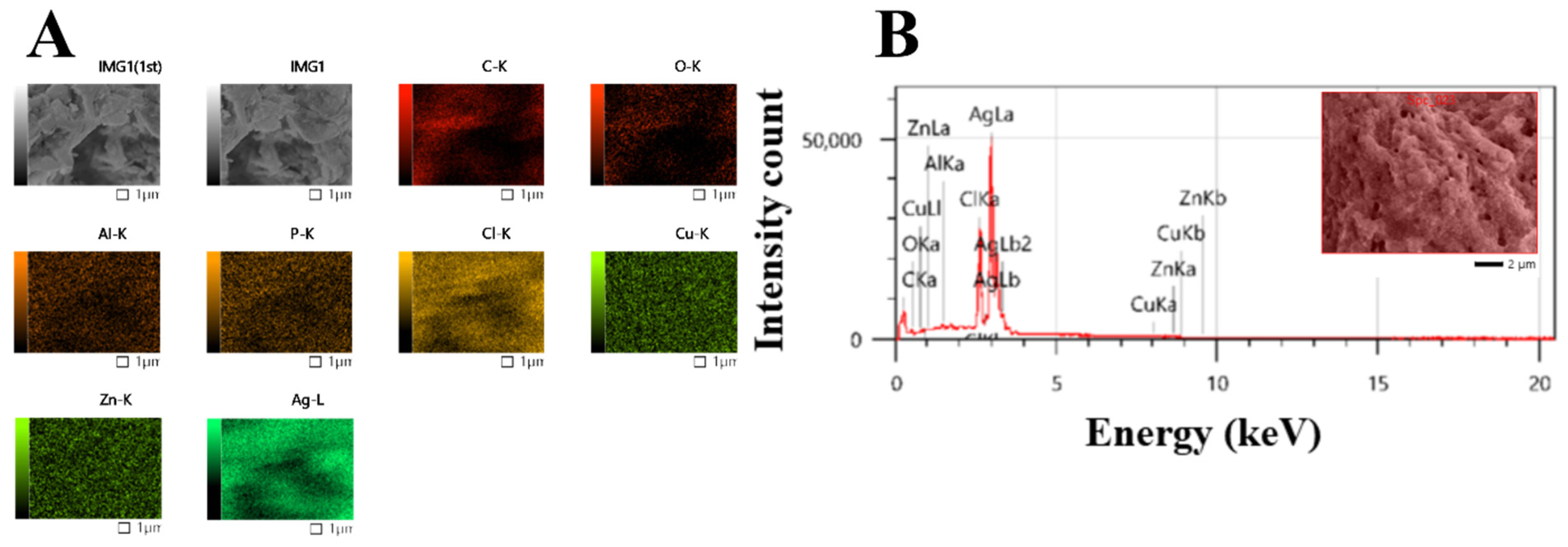
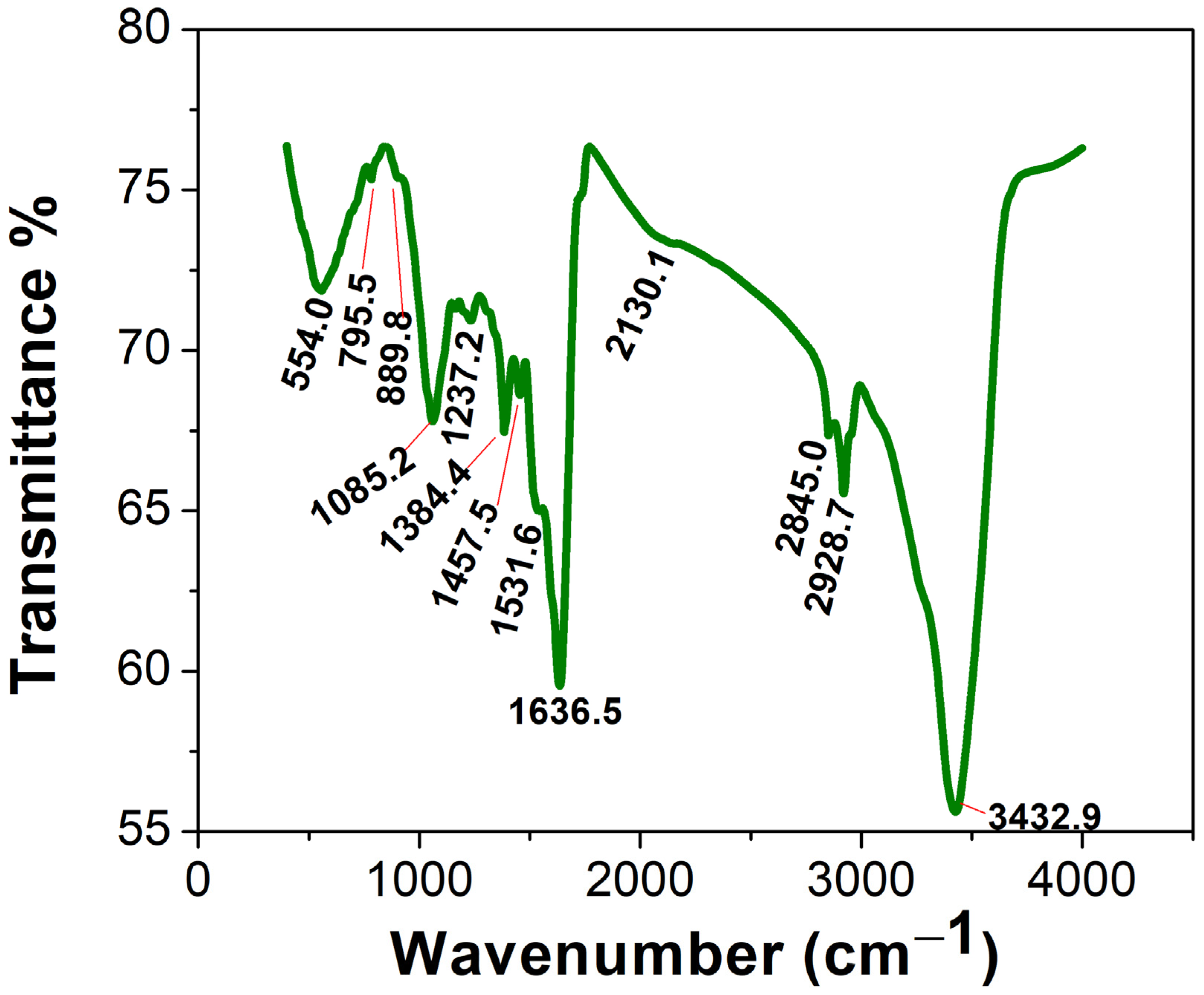
2.2.2. Uv@Ag-NPs Characterisations
TEM, SEM, EDx, and Mapping Analysis
FTIR
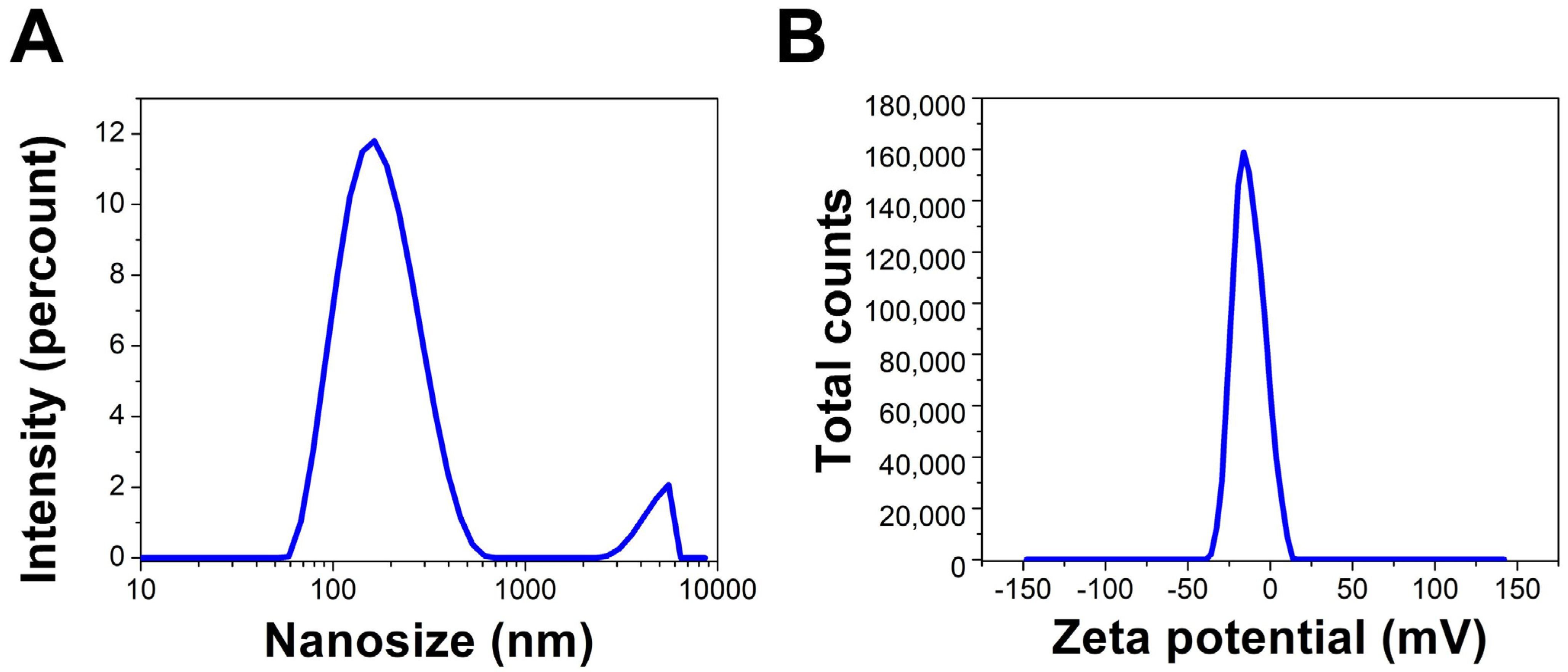
DLS and Zeta Potential
2.3. Antiproliferative Effect of Uv@Ag-NPs
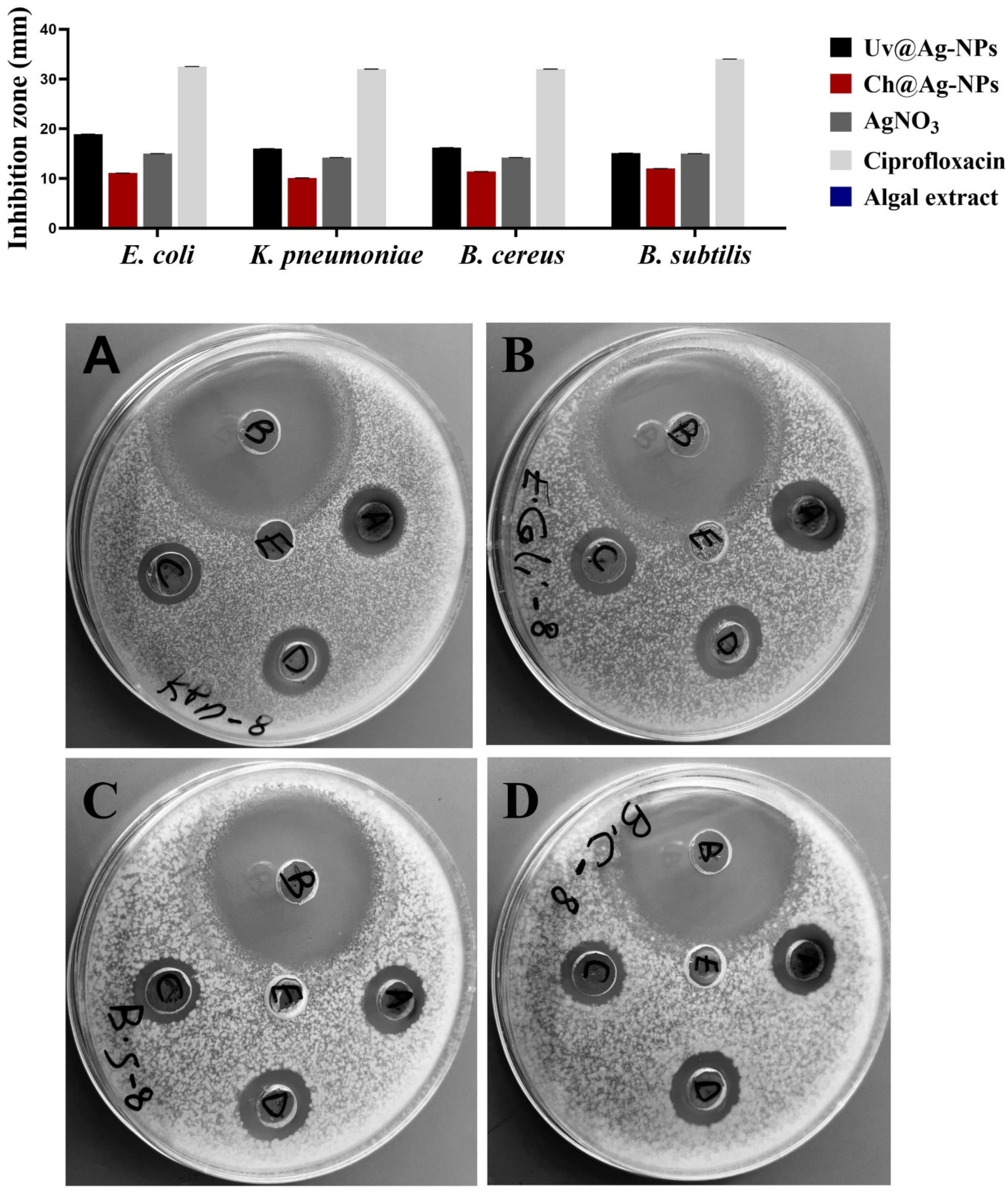
2.4. Biocidal Influence of Uv@Ag-NPs
3. Materials and Methods
3.1. Materials
3.2. Unicellular ulvophyte sp. MBIC105
3.2.1. Isolation and Morphological Estimation
3.2.2. 18s rRNA Identification
3.2.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3.2.4. Algal Aqueous Extract Preparation
3.3. Uv@Ag-NPs Synthesis
3.3.1. Optimisation Parameters for the Biofabrication of Uv@Ag-NPs
Precursor Concentrations and Ratios
Temperature and pH
Illumination and Incubation Duration
3.4. Characterisation of Uv@Ag-NPs
3.4.1. UV Spectroscopy
3.4.2. Morphological and Elemental Composition Analysis of Uv@Ag-NPs
3.4.3. Fourier Transform Infrared Spectroscopy (FTIR) and Zeta Sizer
3.5. Anticancer Activity
3.6. Antibacterial Activity
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-P.; Chen, Y.-F.; Tsai, P.-J.; Huang, I.-H.; Ko, W.-C.; Jan, J.-S. Advances in the Application of Nanomaterials as Treatments for Bacterial Infectious Diseases. Pharmaceutics 2021, 13, 1913. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Albasher, G.; Bin-Meferij, M.M. Oxidative Stress and Apoptotic Responses Elicited by Nostoc-Synthesized Silver Nanoparticles against Different Cancer Cell Lines. Cancers 2020, 12, 2099. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Al-Mureish, A.; Wu, N. Nanotechnology in the treatment of diabetic complications: A comprehensive narrative review. J. Diabetes Res. 2021, 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; Banerjee, P. Green nanotechnology–a new hope for medical biology. Environ. Toxicol. Pharmacol. 2013, 36, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, S.; Lackner, E.; Sosada-Ludwikowska, F.; Singh, V.; Krainer, J.; Wimmer-Teubenbacher, R.; Grammatikopoulos, P.; Köck, A.; Sowwan, M. Atomic-scale structure and chemical sensing application of ultrasmall size-selected Pt nanoparticles supported on SnO 2. Mater. Adv. 2020, 1, 3200–3207. [Google Scholar] [CrossRef]
- Tao, F.F.; Nguyen, L.; Zhang, S. Introduction: Synthesis and catalysis on metal nanoparticles. In Metal Nanoparticles for Catalysis: Advances and Applications; Royal Society of Chemistry: Philadelphia, PA, USA, 2014. [Google Scholar]
- Ahmed, S.F.; Mofijur, M.; Rafa, N.; Chowdhury, A.T.; Chowdhury, S.; Nahrin, M.; Islam, A.S.; Ong, H.C. Green approaches in synthesising nanomaterials for environmental nanobioremediation: Technological advancements, applications, benefits and challenges. Environ. Res. 2022, 204, 111967. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Redhwan, A.; Bin-Meferij, M.M. Cyanobacteria—A Promising Platform in Green Nanotechnology: A Review on Nanoparticles Fabrication and Their Prospective Applications. Int. J. Nanomed. 2020, 15, 6033–6066. [Google Scholar] [CrossRef]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A review of current research into the biogenic synthesis of metal and metal oxide nanoparticles via marine algae and seagrasses. J. Nanosci. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Almohawes, Z.N.; Alahdal, H.; Momenah, M.A.; Bin-Meferij, M.M. Green Synthesis of Hexagonal Silver Nanoparticles Using a Novel Microalgae Coelastrella aeroterrestrica Strain BA_Chlo4 and Resulting Anticancer, Antibacterial, and Antioxidant Activities. Pharmaceutics 2022, 14, 2002. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.A.; Hassan, S.S.U.; Bungau, S.; Si, Y.; Xu, H.; Rahman, M.H.; Behl, T.; Gitea, D.; Pavel, F.-M.; Corb Aron, R.A. Chemically diverse and biologically active secondary metabolites from marine Phylum chlorophyta. Mar. Drugs 2020, 18, 493. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Tiwari, R.; Singh, V.K.; Singh, P.; Khadim, S.R.; Singh, U.; Srivastava, V.; Hasan, S.; Asthana, R. Green synthesis of gold nanoparticles from Dunaliella salina, its characterization and in vitro anticancer activity on breast cancer cell line. J. Drug Deliv. Sci. Technol. 2019, 51, 164–176. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, T.; Paliwal, C.; Maurya, R.; Ghosh, A.; Mishra, S. Green synthesis, characterization and antioxidant potential of silver nanoparticles biosynthesized from de-oiled biomass of thermotolerant oleaginous microalgae Acutodesmus dimorphus. RSC Adv. 2016, 6, 72269–72274. [Google Scholar] [CrossRef]
- Mawed, S.A.; Centoducati, G.; Farag, M.R.; Alagawany, M.; Abou-Zeid, S.M.; Elhady, W.M.; El-Saadony, M.T.; Di Cerbo, A.; Al-Zahaby, S.A. Dunaliella salina Microalga Restores the Metabolic Equilibrium and Ameliorates the Hepatic Inflammatory Response Induced by Zinc Oxide Nanoparticles (ZnO-NPs) in Male Zebrafish. Biology 2022, 11, 1447. [Google Scholar] [CrossRef]
- Caliskan, G.; Mutaf, T.; Agba, H.C.; Elibol, M. Green Synthesis and Characterization of Titanium Nanoparticles Using Microalga, Phaeodactylum tricornutum. Geomicrobiol. J. 2022, 39, 83–96. [Google Scholar] [CrossRef]
- Princy, K.; Gopinath, A. Optimization of physicochemical parameters in the biofabrication of gold nanoparticles using marine macroalgae Padina tetrastromatica and its catalytic efficacy in the degradation of organic dyes. J. Nanostructure Chem. 2018, 8, 333–342. [Google Scholar] [CrossRef]
- Alves, M.F.; Murray, P.G. Biological Synthesis of Monodisperse Uniform-Size Silver Nanoparticles (AgNPs) by Fungal Cell-Free Extracts at Elevated Temperature and pH. J. Fungi 2022, 8, 439. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Abdelmeguid, N.E.; Al-Zaban, M.I.; Baz, L.; Bin-Meferij, M.M. Lichens—A Potential Source for Nanoparticles Fabrication: A Review on Nanoparticles Biosynthesis and Their Prospective Applications. J. Fungi 2021, 7, 291. [Google Scholar] [CrossRef]
- Khan, S.; Singh, S.; Gaikwad, S.; Nawani, N.; Junnarkar, M.; Pawar, S.V. Optimization of process parameters for the synthesis of silver nanoparticles from Piper betle leaf aqueous extract, and evaluation of their antiphytofungal activity. Environ. Sci. Pollut. Res. 2020, 27, 27221–27233. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wang, J.; Wei, J. Effect of temperature on the size of biosynthesized silver nanoparticle: Deep insight into microscopic kinetics analysis. Arab. J. Chem. 2020, 13, 1011–1019. [Google Scholar] [CrossRef]
- Aziz, N.; Faraz, M.; Pandey, R.; Shakir, M.; Fatma, T.; Varma, A.; Barman, I.; Prasad, R. Facile algae-derived route to biogenic silver nanoparticles: Synthesis, antibacterial, and photocatalytic properties. Langmuir 2015, 31, 11605–11612. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.A.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver nanoparticles: Therapeutical uses, toxicity, and safety issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Acevedo, N.; Zapata-Giraldo, J.; Botero, L.E.; Quintero, J.; Zárate-Triviño, D.; Saldarriaga, J.; Pérez, V.Z. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater. Res. 2019, 23, 27. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Al-Zaban, M.I. Novel Biogenic Silver Nanoparticle-Induced Reactive Oxygen Species Inhibit the Biofilm Formation and Virulence Activities of Methicillin-Resistant Staphylococcus aureus (MRSA) Strain. Front. Bioeng. Biotechnol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Sommer, V.; Mikhailyuk, T.; Glaser, K.; Karsten, U. Uncovering unique green algae and cyanobacteria isolated from biocrusts in highly saline potash tailing pile habitats, using an integrative approach. Microorganisms 2020, 8, 1667. [Google Scholar] [CrossRef]
- Huda, J.A.-T.; Mohammed, Y.H.; Imad, H.H. Phytochemical analysis of Urtica dioica leaves by fourier-transform infrared spectroscopy and gas chromatography-mass spectrometry. J. Pharmacogn. Phytother. 2015, 7, 238–252. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Qin, J.; Zheng, X.-Y.; Bai, Y.; Yang, K.; Xie, L.-P. Diallyl trisulfide induces Bcl-2 and caspase-3-dependent apoptosis via downregulation of Akt phosphorylation in human T24 bladder cancer cells. Phytomedicine 2010, 17, 363–368. [Google Scholar] [CrossRef]
- Junwei, L.; Juntao, C.; Changyu, N.; Peng, W. Molecules and functions of rosewood: Pterocarpus cambodianus. Arab. J. Chem. 2018, 11, 763–770. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Odjadjare, E.C.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chlorella sorokiniana and Chlorella minutissima exhibit antioxidant potentials, inhibit cholinesterases and modulate disaggregation of β-amyloid fibrils. Electron. J. Biotechnol. 2019, 40, 1–9. [Google Scholar] [CrossRef]
- Ahmad, N.; Ang, B.C.; Amalina, M.A.; Bong, C.W. Influence of precursor concentration and temperature on the formation of nanosilver in chemical reduction method. Sains Malays. 2018, 47, 157–168. [Google Scholar]
- Traiwatcharanon, P.; Timsorn, K.; Wongchoosuk, C. Flexible room-temperature resistive humidity sensor based on silver nanoparticles. Mater. Res. Express 2017, 4, 085038. [Google Scholar] [CrossRef]
- Hamal, D.B.; Klabunde, K.J. Synthesis, characterization, and visible light activity of new nanoparticle photocatalysts based on silver, carbon, and sulfur-doped TiO2. J. Colloid Interface Sci. 2007, 311, 514–522. [Google Scholar] [CrossRef]
- Ball, R.; Weitz, D.; Witten, T.; Leyvraz, F. Universal kinetics in reaction-limited aggregation. Phys. Rev. Lett. 1987, 58, 274. [Google Scholar] [CrossRef]
- Husain, S.; Sardar, M.; Fatma, T. Screening of cyanobacterial extracts for synthesis of silver nanoparticles. World J. Microbiol. Biotechnol. 2015, 31, 1279–1283. [Google Scholar] [CrossRef]
- Kusumaningrum, H.P.; Zainuri, M.; Marhaendrajaya, I.; Subagio, A. Nanosilver microalgae biosynthesis: Cell appearance based on SEM and EDX methods. J. Phys. Conf. Ser. 2018, 1025, 012084. [Google Scholar] [CrossRef]
- Kemper, F.; Beckert, E.; Eberhardt, R.; Tünnermann, A. Light filter tailoring–the impact of light emitting diode irradiation on the morphology and optical properties of silver nanoparticles within polyethylenimine thin films. RSC Adv. 2017, 7, 41603–41609. [Google Scholar] [CrossRef]
- Kannan, R.; Stirk, W.; Van Staden, J. Synthesis of silver nanoparticles using the seaweed Codium capitatum P.C. Silva (Chlorophyceae). South Afr. J. Bot. 2013, 86, 1–4. [Google Scholar] [CrossRef]
- Borah, D.; Das, N.; Das, N.; Bhattacharjee, A.; Sarmah, P.; Ghosh, K.; Chandel, M.; Rout, J.; Pandey, P.; Ghosh, N.N. Alga-mediated facile green synthesis of silver nanoparticles: Photophysical, catalytic and antibacterial activity. Appl. Organomet. Chem. 2020, 34, e5597. [Google Scholar] [CrossRef]
- Al-Zahrani, S.; Astudillo-Calderón, S.; Pintos, B.; Pérez-Urria, E.; Manzanera, J.A.; Martín, L.; Gomez-Garay, A. Role of synthetic plant extracts on the production of silver-derived nanoparticles. Plants 2021, 10, 1671. [Google Scholar] [CrossRef] [PubMed]
- Ajeboriogbon, A.; Adewuyi, B.; Talabi, H. Synthesis of silver nanoparticles from selected plants extract. Acta Tech. Corviniensis-Bull. Eng. 2019, 12, 55–58. [Google Scholar]
- Rani, K.; Lekha, C. Green synthesis and characterization of silver nanoparticles using leaf and stem aqueous extract of Pauzolzia Bennettiana and their antioxidant activity. J. Pharmacogn. Phytochem. 2018, 7, 129–132. [Google Scholar]
- Yadav, A.; Theivasanthi, T.; Paul, P.; Upadhyay, K. Extracellular biosynthesis of silver nanoparticles from plant growth promoting rhizobacteria Pseudomonas sp. arXiv 2015, arXiv:1511.03130. [Google Scholar]
- Oliveira, G.Z.S.; Lopes, C.A.P.; Sousa, M.H.; Silva, L.P. Synthesis of silver nanoparticles using aqueous extracts of Pterodon emarginatus leaves collected in the summer and winter seasons. Int. Nano Lett. 2019, 9, 109–117. [Google Scholar] [CrossRef]
- Huq, M. Green Synthesis of Silver Nanoparticles Using Pseudoduganella eburnea MAHUQ-39 and Their Antimicrobial Mechanisms Investigation against Drug Resistant Human Pathogens. Int. J. Mol. Sci. 2020, 21, 1510. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Hussein, M.H.; El-Sawah, A.A. Phycobiliprotein-mediated synthesis of biogenic silver nanoparticles, characterization, in vitro and in vivo assessment of anticancer activities. Sci. Rep. 2018, 8, 8925. [Google Scholar] [CrossRef]
- Mukaratirwa-Muchanyereyi, N.; Gusha, C.; Mujuru, M.; Guyo, U.; Nyoni, S. Synthesis of Silver nanoparticles using plant extracts from Erythrina abyssinica aerial parts and assessment of their anti-bacterial and anti-oxidant activities. Results Chem. 2022, 4, 100402. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Mahdavi, M.; Abdollahi, Y. Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method. Int. J. Mol. Sci. 2012, 13, 6639–6650. [Google Scholar] [CrossRef]
- Mahajan, A.; Arya, A.; Chundawat, T.S. Green synthesis of silver nanoparticles using green alga (Chlorella vulgaris) and its application for synthesis of quinolines derivatives. Synth. Commun. 2019, 49, 1926–1937. [Google Scholar] [CrossRef]
- Ardani, H.; Imawan, C.; Handayani, W.; Djuhana, D.; Harmoko, A.; Fauzia, V. Enhancement of the stability of silver nanoparticles synthesized using aqueous extract of Diospyros discolor Willd. leaves using polyvinyl alcohol. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012056. [Google Scholar] [CrossRef]
- Rathod, D.; Golinska, P.; Wypij, M.; Dahm, H.; Rai, M. A new report of Nocardiopsis valliformis strain OT1 from alkaline Lonar crater of India and its use in synthesis of silver nanoparticles with special reference to evaluation of antibacterial activity and cytotoxicity. Med. Microbiol. Immunol. 2016, 205, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Mishra, A.K.; Nayak, D.; Patra, B.; Bratovcic, A.; Avula, S.K.; Mohanta, T.K.; Murugan, K.; Saravanan, M. Exploring Dose-Dependent Cytotoxicity Profile of Gracilaria edulis-Mediated Green Synthesized Silver Nanoparticles against MDA-MB-231 Breast Carcinoma. Oxidative Med. Cell. Longev. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Raman, J.; Reddy, G.R.; Lakshmanan, H.; Selvaraj, V.; Gajendran, B.; Nanjian, R.; Chinnasamy, A.; Sabaratnam, V. Mycosynthesis and characterization of silver nanoparticles from Pleurotus djamor var. roseus and their in vitro cytotoxicity effect on PC3 cells. Process Biochem. 2015, 50, 140–147. [Google Scholar] [CrossRef]
- Ebrahimzadeh, Z.; Salehzadeh, A.; Naeemi, A.; Jalali, A. Silver nanoparticles biosynthesized by Anabaena flos-aquae enhance the apoptosis in breast cancer cell line. Bull. Mater. Sci. 2020, 43, 92. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Kaur, A.; Preet, S.; Kumar, V.; Kumar, R.; Kumar, R. Synergetic effect of vancomycin loaded silver nanoparticles for enhanced antibacterial activity. Colloids Surf. B Biointerfaces 2019, 176, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.-A.-R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Abdelmeguid, N.E.; Ali, M.A.; Bin-Meferij, M.M.; Khalil, M.I. Synthesis of silver nanoparticles using a novel cyanobacteria Desertifilum sp. extract: Their antibacterial and cytotoxicity effects. Int. J. Nanomed. 2020, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Al-Zaban, M.I. Lethal Mechanisms of Nostoc-Synthesized Silver Nanoparticles Against Different Pathogenic Bacteria. Int. J. Nanomed. 2020, 15, 10499. [Google Scholar] [CrossRef]
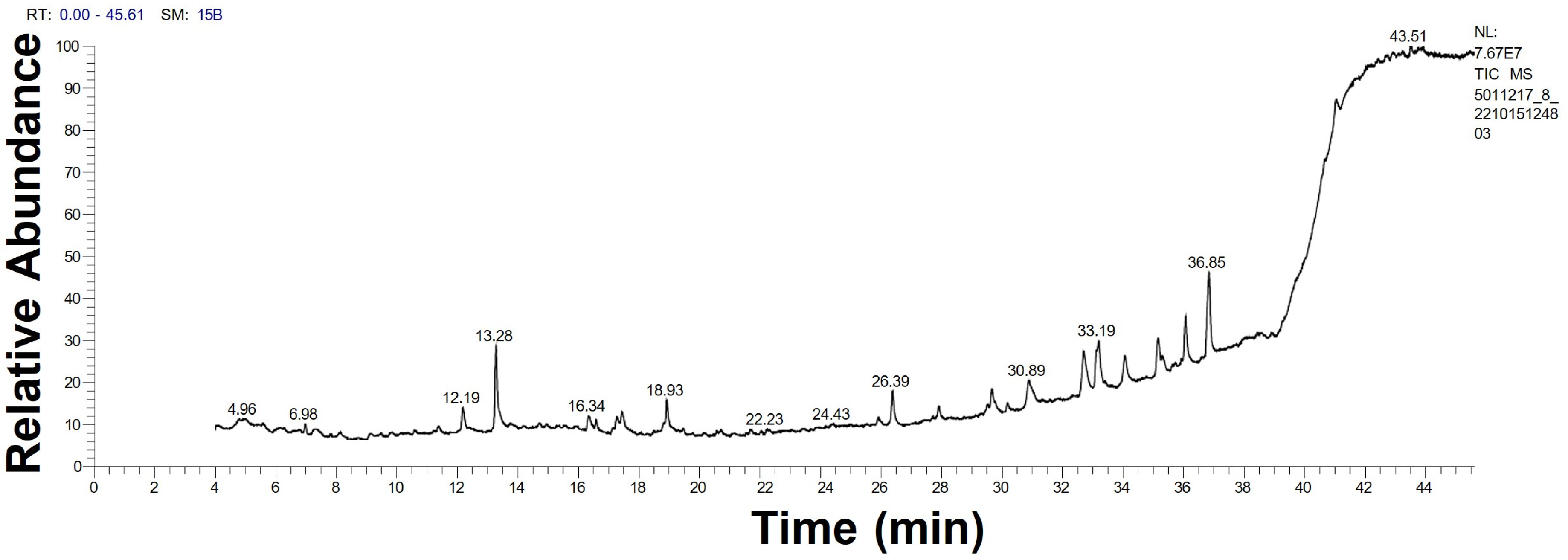


| No. | Biomolecule Name | Retention Time | Area % | Mentioned Factor | Molecular Formula | Molecular Weight |
|---|---|---|---|---|---|---|
| 1 | D-fructose, diethyl mercaptal, pentaacetate | 4.04, 4.09, 16.34 | 0.49, 0.63, 3.25 | 659, 655, 659 | C20H32O10S2 | 496 |
| 2 | Methyl 4,7,10,13-hexadecatetraenoate | 6.15 | 0.89 | 708 | C17H26O2 | 262 |
| 3 | 2-Aminoethanethiolsulphuric acid | 6.99, 11.39, 21.7 | 1.10, 0.96, 0.71 | 699, 727, 760 | C2H7NO3S2 | 157 |
| 4 | 25,26,27-Trinorcholecalcifer-24-al | 7.34 | 2.05 | 766 | C24H36O2 | 356 |
| 5 | Trisulfide, di-2-propenyl | 12.19 | 3.34 | 760 | C6H10S3 | 178 |
| 6 | 1,2-Diacetin | 13.28 | 9.63 | 927 | C7H12O5 | 176 |
| 7 | [5,5-dimethyl-6-(3-methyl-buta-1,3-dienyl)-7-oxa-bicyclo [4.1.0]hept-1-yl]-methanol | 16.59 | 1.22 | 748 | C14H22O2 | 222 |
| 8 | 3a,4,7,7a-Tetrahydrodimethyl-4,7-methanoindene | 17.26 | 1.58 | 775 | C12H16 | 160 |
| 9 | Phenol, 2,6-bis(1,1-dimethylethyl)- | 17.45 | 2.06 | 749 | C14H22O | 206 |
| 10 | Methyl 6,9-octadecadiynoate | 18.83 | 0.94 | 739 | C19H30O2 | 290 |
| 11 | 1H-Cycloprop[e]azulen-7-ol, decahydro-1,1,7-trimethyl-4-methylene-, [1ar-(1aalpha,4aalpha,7beta,7abeta,7balpha)]- | 18.93 | 3.61 | 899 | C15H24O | 220 |
| 12 | 3-Oxo-20-methyl-11-à-hydroxyconanine-1,4-diene | 19.47 | 0.73 | 757 | C22H31NO2 | 341 |
| 13 | 2,5-Octadecadiynoic acid, methyl Ester | 20.72 | 0.76 | 786 | C19H30O2 | 290 |
| 14 | (5e,7e)-9,10-Secocholesta-5,7,10-triene-3,25,26-triol # | 22.23 | 0.64 | 753 | C27H44O3 | 416 |
| 15 | 9-Oximino-2,7-diethoxyfluorene | 25.92, 27.92 | 1.43, 1.75 | 745, 747 | C17H17NO3 | 283 |
| 16 | Methyl 14-methylpentadecanoate | 26.39 | 4.38 | 742 | C17H34O2 | 270 |
| 17 | 1-Heptatriacotanol | 29.52 | 0.85 | 780 | C37H76O | 536 |
| 18 | 9-Octadecenoic acid (z)-, 2-Hydroxy-1-(hydroxymethyl)ethyl Ester | 29.67 | 3.40 | 792 | C21H40O4 | 356 |
| 19 | Cyclopropanebutanoic acid, 2-[[2-[[2-[(2 pentylcyclopropyl)meth yl]cyclopropyl]methyl]cyclopropyl] methyl]-, methyl ester | 30.18 | 1.23 | 813 | C25H42O2 | 374 |
| 20 | 9-(2′,2′-Dimethylpropanoilhydrazono)-3,6-dichloro-2,7-bis-[2-(diethylamino)-ethoxy]fluorene | 30.89 | 5.84 | 761 | C30H42Cl2N4O3 | 576 |
| 21 | 1,2-Benzenedicarboxylic acid | 32.70, 34.07, 35.15, 36.85, 36.07 | 7.78, 3.83, 4.94, 6.02, 12.89 | 789, 783, 776, 795, 779 | C24H38O4 | 390 |
| 22 | Widdrol hydroxyether | 33.12, 33.19 | 3.17, 5.39 | 770, 752 | C15H26O2 | 238 |
| 23 | 9,12,15-octadecatrienoic acid, 2,3-bis [(trimethylsilyl)oxy]propyl ester, (z,z,z) | 35.31 | 1.25 | 734 | C27H52O4Si2 | 496 |
| 24 | Tetraneurin-a-diol | 35.73 | 0.71 | 805 | C15H20O5 | 280 |
| Element | Line | Mass% | Atom% |
|---|---|---|---|
| C | K | 6.93 ± 0.02 | 32.29 ± 0.09 |
| O | K | 1.81 ± 0.02 | 6.32 ± 0.08 |
| Al | K | 0.3 ± 0.01 | 0.63 ± 0.02 |
| Cl | K | 12.18 ± 0.03 | 19.22 ± 0.05 |
| Cu | K | 1.13 ± 0.04 | 1.00 ± 0.04 |
| Zn | K | 0.91 ± 0.05 | 0.78 ± 0.04 |
| Ag | L | 76.74 ± 0.11 | 39.78 ± 0.06 |
| Total | 100 | 100 |
| Microorganisms | Drugs (µg/mL) | ||||
|---|---|---|---|---|---|
| Uv@Ag-NPs | Ch@Ag-NPs | AgNO3 | Algal Extract | Ciprofloxacin | |
| IZD (mm) | |||||
| E. coli | 18.9 ± 0.03 | 11.1 ± 0.14 | 15.0 ± 0.13 | 0.0 ± 0.0 | 32.5 ± 0.03 |
| K. pneumoniae | 16.0 ±0.01 | 10.1 ± 0.03 | 14.2 ± 0.45 | 0.0 ± 0.0 | 32.0 ± 0.03 |
| B. cereus | 16.2 ± 0.18 | 11.4 ± 0.05 | 14.2 ± 0.03 | 0.0 ± 0.0 | 32.0 ± 0.06 |
| B. subtilis | 15.1 ± 0.04 | 12.0 ± 0.01 | 15.0 ± 0.19 | 0.0 ± 0.0 | 34.0 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamida, R.S.; Ali, M.A.; Alkhateeb, M.A.; Alfassam, H.E.; Momenah, M.A.; Bin-Meferij, M.M. Algal-Derived Synthesis of Silver Nanoparticles Using the Unicellular ulvophyte sp. MBIC10591: Optimisation, Characterisation, and Biological Activities. Molecules 2023, 28, 279. https://doi.org/10.3390/molecules28010279
Hamida RS, Ali MA, Alkhateeb MA, Alfassam HE, Momenah MA, Bin-Meferij MM. Algal-Derived Synthesis of Silver Nanoparticles Using the Unicellular ulvophyte sp. MBIC10591: Optimisation, Characterisation, and Biological Activities. Molecules. 2023; 28(1):279. https://doi.org/10.3390/molecules28010279
Chicago/Turabian StyleHamida, Reham Samir, Mohamed Abdelaal Ali, Mariam Abdulaziz Alkhateeb, Haifa Essa Alfassam, Maha Abdullah Momenah, and Mashael Mohammed Bin-Meferij. 2023. "Algal-Derived Synthesis of Silver Nanoparticles Using the Unicellular ulvophyte sp. MBIC10591: Optimisation, Characterisation, and Biological Activities" Molecules 28, no. 1: 279. https://doi.org/10.3390/molecules28010279
APA StyleHamida, R. S., Ali, M. A., Alkhateeb, M. A., Alfassam, H. E., Momenah, M. A., & Bin-Meferij, M. M. (2023). Algal-Derived Synthesis of Silver Nanoparticles Using the Unicellular ulvophyte sp. MBIC10591: Optimisation, Characterisation, and Biological Activities. Molecules, 28(1), 279. https://doi.org/10.3390/molecules28010279







