In Vitro and In Vivo Antibiofilm Activity of Red Onion Scales: An Agro-Food Waste
Abstract
:1. Introduction
2. Results
2.1. Screening of the Antibacterial Activity
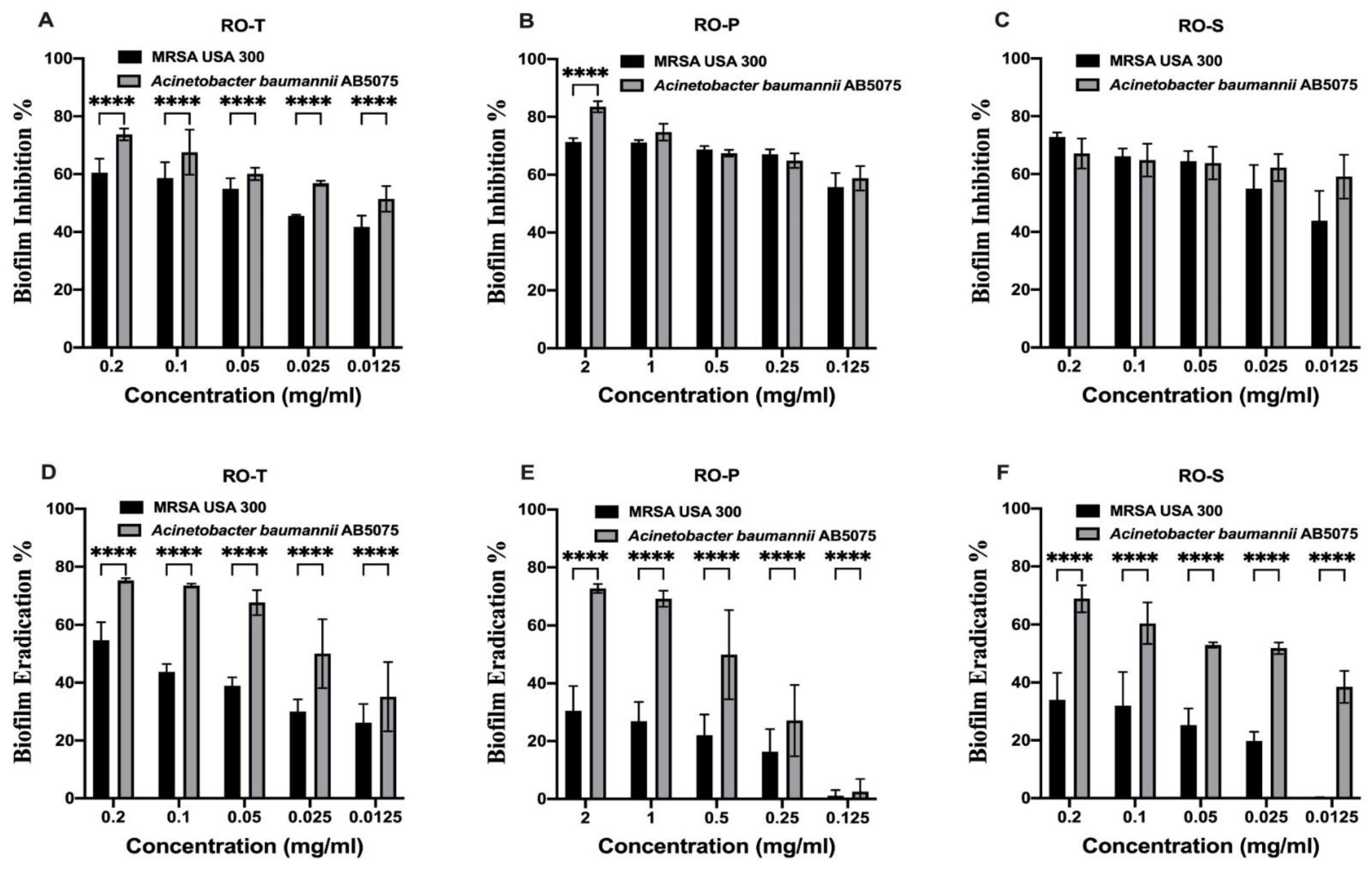
2.2. Effect of the Tested Extracts on the Biofilm Activity
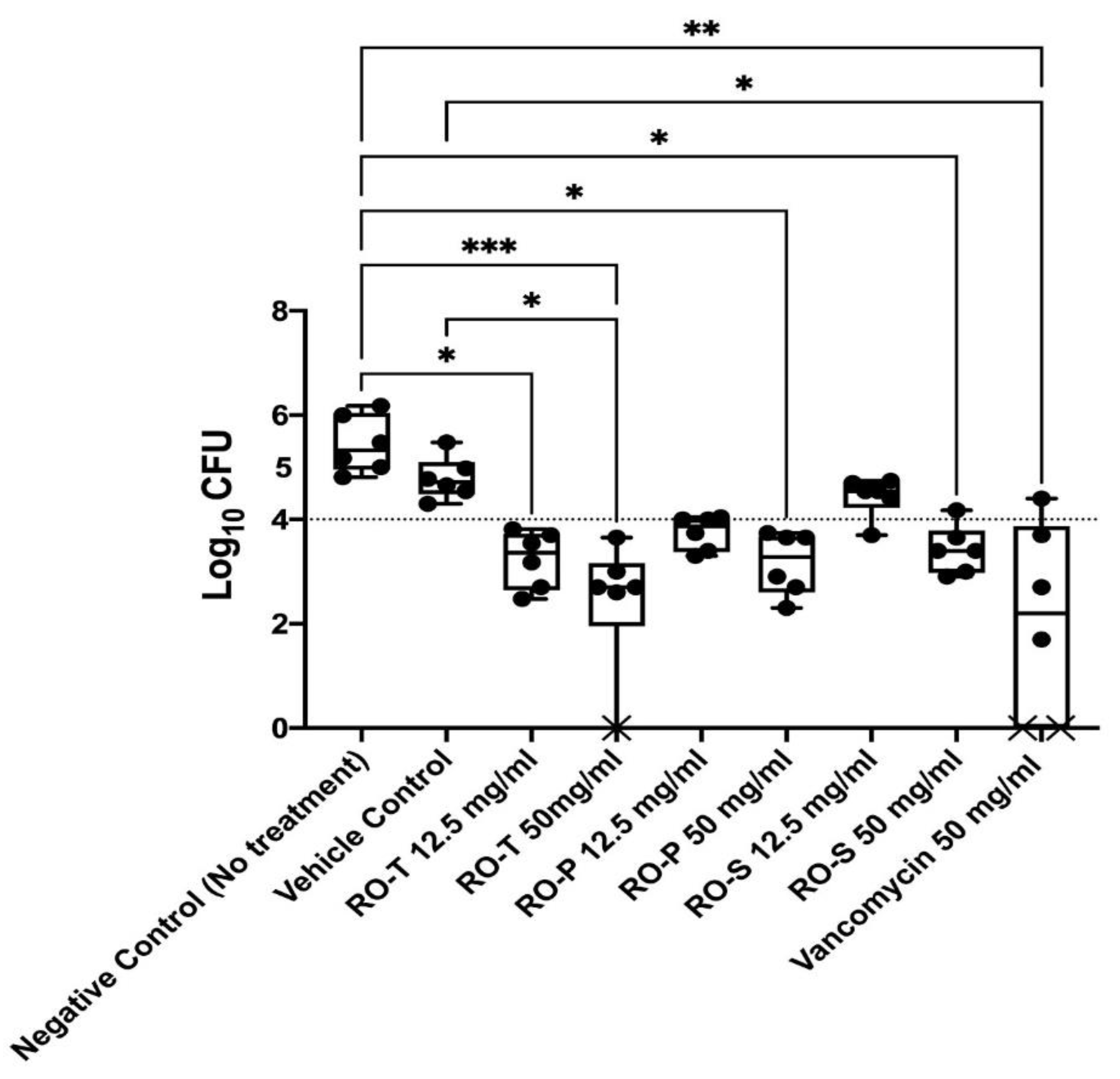
2.3. In Vivo MRSA Vaginal Colonization Model
2.4. Histopathological Analysis
2.5. HPLC Standardization
3. Discussion
4. Materials and Methods
4.1. Plant Material and Chemicals
4.2. Extraction and Fractionation Procedure
4.3. Screening of the Antibacterial Activity
4.4. Effect of the Tested Extracts on the Biofilm Activity
4.5. Biofilm Inhibition Assay
4.6. Biofilm Eradication Assay
4.7. In Vivo MRSA Vaginal Colonization Model
4.8. HPLC Standardization Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Škerget, M.; Majhenič, L.; Bezjak, M.; Knez, Ž. Antioxidant, radical scavenging and antimicrobial activities of red onion (Allium cepa L.) skin and edible part extracts. Chem. Biochem. Eng. Q. 2009, 23, 435–444. [Google Scholar]
- Nile, A.; Nile, S.H.; Cespedes-Acuña, C.L.; Oh, J.-W. Spiraeoside extracted from red onion skin ameliorates apoptosis and exerts potent antitumor, antioxidant and enzyme inhibitory effects. Food Chem. Toxicol. 2021, 154, 112327. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Marinozzi, M.; Baiocchi, C.; Beccari, T.; Calarco, P.; Ceccarini, M.R.; Chielli, M.; Orabona, C.; Orecchini, E.; Ortenzi, R. Bioadhesive polymeric films based on red onion skins extract for wound treatment: An innovative and eco-friendly formulation. Molecules 2020, 25, 318. [Google Scholar] [CrossRef] [Green Version]
- Fredotović, Ž.; Puizina, J.; Nazlić, M.; Maravić, A.; Ljubenkov, I.; Soldo, B.; Vuko, E.; Bajić, D. Phytochemical characterization and screening of antioxidant, antimicrobial and antiproliferative properties of Allium× cornutum clementi and two varieties of Allium cepa L. peel extracts. Plants 2021, 10, 832. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S. Antimicrobial assessment of polyphenolic extracts from onion (Allium cepa L.) skin of fifteen cultivars by sonication-assisted extraction method. Heliyon 2020, 6, e05478. [Google Scholar] [CrossRef] [PubMed]
- Memariani, H.; Memariani, M.; Ghasemian, A. An overview on anti-biofilm properties of quercetin against bacterial pathogens. World J. Microbiol. Biotechnol. 2019, 35, 143. [Google Scholar] [CrossRef]
- Tumuhamye, J.; Steinsland, H.; Bwanga, F.; Tumwine, J.K.; Ndeezi, G.; Mukunya, D.; Namugga, O.; Kasede, A.N.; Sommerfelt, H.; Nankabirwa, V. Vaginal colonization with antimicrobial-resistant bacteria among women in labor in central Uganda: Prevalence and associated factors. Antimicrob. Resist. Infect. Control 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Coolborn, A.F.; Charity, E.-B.C.; Ogbaji, O.S. Inhibition of methicillin resistant Staphylococcus aureus (MRSA) with underutilized medicinal plants extracts. J Int Med Res. 2021, 28, 4953–4964. [Google Scholar]
- Wang, B.; Wei, P.-W.; Wan, S.; Yao, Y.; Song, C.-R.; Song, P.-P.; Xu, G.-B.; Hu, Z.-Q.; Zeng, Z.; Wang, C. Ginkgo biloba exocarp extracts inhibit S. aureus and MRSA by disrupting biofilms and affecting gene expression. J. Ethnopharmacol. 2021, 271, 113895. [Google Scholar] [CrossRef]
- Chiew, S.P.; Thong, O.M.; Yin, K.B. Phytochemical composition, antimicrobial and cytotoxic activities of red onion peel extracts prepared using different methods. Int. J. Integr. Biol. 2014, 15, 49. [Google Scholar]
- Mu, Y.; Zeng, H.; Chen, W. Quercetin inhibits biofilm formation by decreasing the production of EPS and altering the composition of EPS in Staphylococcus epidermidis. Front. Microbiol. 2021, 12, 631058. [Google Scholar] [CrossRef] [PubMed]
- da Costa Júnior, S.D.; de Oliveira Santos, J.V.; de Almeida Campos, L.A.; Pereira, M.A.; Magalhães, N.S.S.; Cavalcanti, I.M.F. Antibacterial and antibiofilm activities of quercetin against clinical isolates of Staphyloccocus aureus and Staphylococcus saprophyticus with resistance profile. Int. J. Environ. Agric. Biotechnol. 2018, 3, 266213. [Google Scholar]
- Sagar, N.A.; Pareek, S.; Gonzalez-Aguilar, G.A. Quantification of flavonoids, total phenols and antioxidant properties of onion skin: A comparative study of fifteen Indian cultivars. J. Food Sci. Technol. 2020, 57, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Downes, K.; Chope, G.A.; Terry, L.A. Effect of curing at different temperatures on biochemical composition of onion (Allium cepa L.) skin from three freshly cured and cold stored UK-grown onion cultivars. Postharvest Biol. Technol. 2009, 54, 80–86. [Google Scholar] [CrossRef]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.; López-Andréu, F.J.; Downes, K.; Terry, L.; Esteban, R. Study of bioactive compound content in different onion sections. Plant Foods Hum. Nutr. 2011, 66, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Sousa, L.G.; Castro, J.; Cerca, N. The Utilization of Essential Oils to Treat Biofilm-Associated Vaginal Infections. In Microbial Biofilms; CRC Press: Boca Raton, FL, USA, 2020; pp. 395–420. [Google Scholar]
- Ly, T.N.; Hazama, C.; Shimoyamada, M.; Ando, H.; Kato, K.; Yamauchi, R. Antioxidative compounds from the outer scales of onion. J. Agric. Food Chem. 2005, 53, 8183–8189. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.; Singh, R.; Prakash, D.; Singh, D.; Sarma, B.; Upadhyay, G.; Singh, H. Polyphenolics from various extracts/fractions of red onion (Allium cepa) peel with potent antioxidant and antimutagenic activities. Food Chem. Toxicol. 2009, 47, 1161–1167. [Google Scholar] [CrossRef]
- Abouzed, T.K.; del Mar Contreras, M.; Sadek, K.M.; Shukry, M.; Abdelhady, D.H.; Gouda, W.M.; Abdo, W.; Nasr, N.E.; Mekky, R.H.; Segura-Carretero, A. Red onion scales ameliorated streptozotocin-induced diabetes and diabetic nephropathy in Wistar rats in relation to their metabolite fingerprint. Diabetes Res. Clin. Pract. 2018, 140, 253–264. [Google Scholar] [CrossRef]
- Roy, P.K.; Song, M.G.; Park, S.Y. The inhibitory effect of quercetin on biofilm formation of Listeria monocytogenes mixed culture and repression of virulence. Antioxidants 2022, 11, 1733. [Google Scholar] [CrossRef]
- Matilla-Cuenca, L.; Gil, C.; Cuesta, S.; Rapún-Araiz, B.; Žiemytė, M.; Mira, A.; Lasa, I.; Valle, J. Antibiofilm activity of flavonoids on staphylococcal biofilms through targeting BAP amyloids. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Park, S.-H.; Song, M.G.; Park, S.Y. Antimicrobial Efficacy of Quercetin against Vibrio parahaemolyticus biofilm on food surfaces and downregulation of virulence genes. Polymers 2022, 14, 3847. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, P.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Flavonol glucosides in Allium species: A comparative study by means of HPLC–DAD–ESI-MS–MS. Food Chem. 2008, 107, 1668–1673. [Google Scholar] [CrossRef]
- Cahlíková, L.; Ali, B.H.; Havlíková, L.; Ločárek, M.; Siatka, T.; Opletal, L.; Blunden, G. Anthocyanins of Hibiscus sabdiffera calyces from Sudan. Nat. Prod. Commun. 2015, 10, 1934578X1501000120. [Google Scholar] [CrossRef]
- Sedeek, M.S.; Afifi, S.M.; Mansour, M.K.; Hassan, M.; Mehaya, F.M.; Naguib, I.A.; Abourehab, M.A.S.; Farag, M.A. Unveiling Antimicrobial and Antioxidant Compositional Differences between Dukkah and Za’atar via SPME-GCMS and HPLC-DAD. Molecules 2022, 27, 6471. [Google Scholar] [CrossRef]
- Ibrahim, N.M.; Fahim, S.H.; Hassan, M.; Farag, A.E.; Georgey, H.H. Design and synthesis of ciprofloxacin-sulfonamide hybrids to manipulate ciprofloxacin pharmacological qualities: Potency and side effects. Eur. J. Med. Chem. 2022, 228, 114021. [Google Scholar] [CrossRef]
- Ismail, M.M.; Hassan, M.; Moawad, S.S.; Okba, M.M.; Ashour, R.M.; Fayek, N.M.; Saber, F.R. Exploring the Antivirulence Activity of Pulverulentone A, a Phloroglucinol-Derivative from Callistemon citrinus Leaf Extract, against Multi-Drug Resistant Pseudomonas aeruginosa. Antibiotics 2021, 10, 907. [Google Scholar] [CrossRef]
- El-Shiekh, R.A.; Hassan, M.; Hashem, R.A.; Abdel-Sattar, E. Bioguided Isolation of Antibiofilm and Antibacterial Pregnane Glycosides from Caralluma quadrangula: Disarming Multidrug-Resistant Pathogens. Antibiotics 2021, 10, 811. [Google Scholar] [CrossRef]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K.; Development, C.M.; Standardization Working Group of the Subcommittee on Antimicrobial Susceptibility Tests. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.A.; El-Shiekh, R.A.; Hashem, R.A.; Hassan, M. In vivo Antibacterial Activity of Star Anise (Illicium verum Hook.) Extract Using Murine MRSA Skin Infection Model in Relation to Its Metabolite Profile. Infect. Drug Resist. 2021, 14, 33–48. [Google Scholar] [CrossRef]
- Albash, R.; Abdellatif, M.M.; Hassan, M.; Badawi, N.M. Tailoring Terpesomes and Leciplex for the Effective Ocular Conveyance of Moxifloxacin Hydrochloride (Comparative Assessment): In-vitro, Ex-vivo, and In-vivo Evaluation. Int. J. Nanomed. 2021, 16, 5247–5263. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.A.; Eladawy, S.A.; El-Enin, A.S.M.A.; Hussein, Z.M. Development and investigation of timolol maleate niosomal formulations for the treatment of glaucoma. J. Pharm. Investig. 2020, 50, 59–70. [Google Scholar] [CrossRef]
- Deng, L.; Schilcher, K.; Burcham Lindsey, R.; Kwiecinski Jakub, M.; Johnson Paige, M.; Head Steven, R.; Heinrichs David, E.; Horswill Alexander, R.; Doran Kelly, S. Identification of Key Determinants of Staphylococcus aureus Vaginal Colonization. mBio 2019, 10, e02321-19. [Google Scholar] [CrossRef] [Green Version]
- Au-Patras, K.A.; Au-Doran, K.S. A Murine Model of Group B Streptococcus Vaginal Colonization. JoVE 2016, 117, e54708. [Google Scholar] [CrossRef]

| Minimum Bactericidal Concentration (MBC) mg/mL * | ||||
|---|---|---|---|---|
| MRSA USA300 | Acinetobacter baumannii AB5075 | Escherichia coli ATCC87 | Pseudomonas aeruginosa PAO1 | |
| RO-T | 0.651 ± 0.226 | 4.688 ± 2.706 | 9.375 ± 5.413 | 16.667 ± 7.217 |
| RO-P | 4.167 ± 1.804 | 6.25 ± 0 | 25 ± 0 | # |
| RO-S | 0.325 ± 0.113 | 0.651 ± 0.226 | 7.292 ± 4.774 | 12.5 ± 0 |
| Biofilm Inhibition | Biofilm Eradication | ||||||
|---|---|---|---|---|---|---|---|
| Log IC50 | IC50 (mg/mL) | Log EC50 | EC50 (mg/mL) | ||||
| mean | SE | mean | mean | SE | mean | ||
| RO-T | MRSA USA300 | −1.195 | 0.164 | 0.064 | −1.797 | 0.187 | 0.016 |
| Acinetobacter baumannii AB5075 | −0.818 | 0.173 | 0.152 | −0.761 | 0.224 | 0.174 | |
| RO-P | MRSA USA300 | 0.311 | 0.153 | 2.048 | −1.573 | 0.305 | 0.027 |
| Acinetobacter baumannii AB5075 | 0.476 | 0.189 | 2.990 | −0.818 | 0.303 | 0.152 | |
| RO-S | MRSA USA300 | −0.841 | 0.185 | 0.144 | −2.492 | 0.309 | 0.003 |
| Acinetobacter baumannii AB5075 | −0.833 | 0.139 | 0.147 | −1.092 | 0.180 | 0.081 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, N.B.; El-Shiekh, R.A.; Ashour, R.M.; El-Gayed, S.H.; Abdel-Sattar, E.; Hassan, M. In Vitro and In Vivo Antibiofilm Activity of Red Onion Scales: An Agro-Food Waste. Molecules 2023, 28, 355. https://doi.org/10.3390/molecules28010355
Ali NB, El-Shiekh RA, Ashour RM, El-Gayed SH, Abdel-Sattar E, Hassan M. In Vitro and In Vivo Antibiofilm Activity of Red Onion Scales: An Agro-Food Waste. Molecules. 2023; 28(1):355. https://doi.org/10.3390/molecules28010355
Chicago/Turabian StyleAli, Nermeen B., Riham A. El-Shiekh, Rehab M. Ashour, Sabah H. El-Gayed, Essam Abdel-Sattar, and Mariam Hassan. 2023. "In Vitro and In Vivo Antibiofilm Activity of Red Onion Scales: An Agro-Food Waste" Molecules 28, no. 1: 355. https://doi.org/10.3390/molecules28010355
APA StyleAli, N. B., El-Shiekh, R. A., Ashour, R. M., El-Gayed, S. H., Abdel-Sattar, E., & Hassan, M. (2023). In Vitro and In Vivo Antibiofilm Activity of Red Onion Scales: An Agro-Food Waste. Molecules, 28(1), 355. https://doi.org/10.3390/molecules28010355






