Dichloro(2,2′-bipyridine)copper/MAO: An Active and Stereospecific Catalyst for 1,3-Diene Polymerization
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization of CuCl2(bipy)
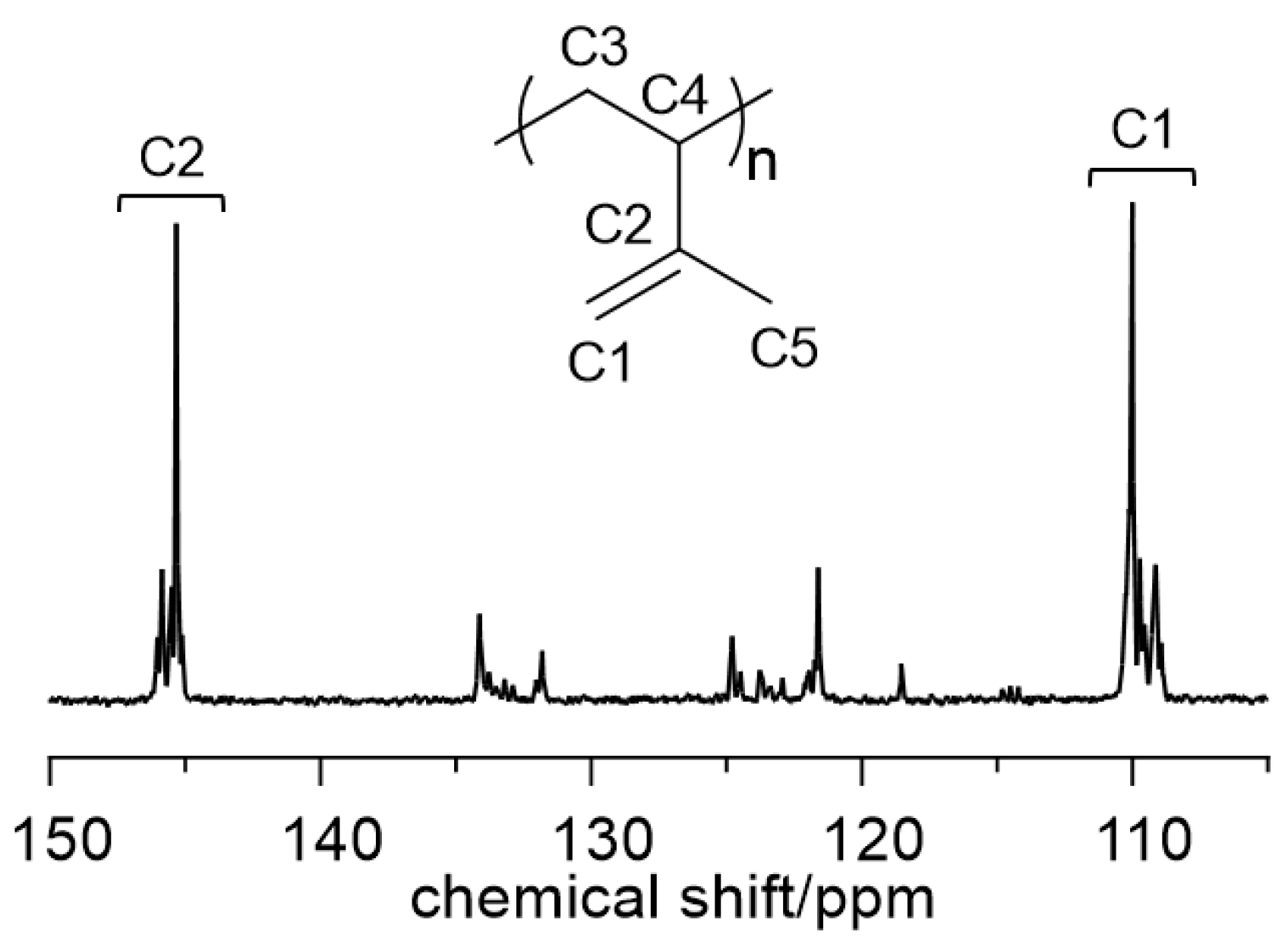
2.2. Polymerization of 1,3-Dienes
3. Discussion
4. Materials and Methods
4.1. General Procedures and Materials
4.2. Synthesis of CuCl2(bipy)
4.3. Polymerization of 1,3-Dienes
4.4. Polymer Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Porri, L.; Giarrusso, A. Conjugated Diene Polymerization. In Comprehensive Polymer Science; Eastmond, G., Edwith, A., Russo, S., Sigwalt, P., Eds.; Pergamon Press Ltd.: Oxford, UK, 1989; pp. 53–108. [Google Scholar]
- Thiele, S.K.H.; Wilson, D.R. Alternate Transition Metal Complex Based Diene Polymerization. J. Macromol. Sci. Polym. Rev. 2003, C43, 581–628. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-based Ziegler/Natta catalysts and their application in diene polymerization. Adv. Polym. Sci. 2006, 204, 1–154. [Google Scholar] [CrossRef]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well Defined Transition Metal Complexes with Phosphorus and Nitrogen Ligands for 1,3-Dienes Polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Ricci, G.; Pampaloni, G.; Sommazzi, A.; Masi, F. Dienes Polymerization: Where we are and what lies ahead. Macromolecules 2021, 54, 5879–5914. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Z.; Cui, D.; Liu, X. Precisely Controlled Polymerization of Styrene and Conjugated Dienes by Group 3 Single-Site Catalysts. ChemCatChem 2018, 10, 42–61. [Google Scholar] [CrossRef]
- Srivastava, V.K.; Maiti, M.; Basak, G.C.; Jasra, R.V. Role of catalysis in sustainable production of synthetic elastomers. J. Chem. Sci. 2014, 126, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Cui, D.; Wang, B.; Liu, B.; Yang, Y. Polymerization of 1,3-Conjugated Dienes with Rare-Earth Metal Precursors. Struct. Bond. 2010, 137, 49–108. [Google Scholar]
- Fischbach, A.; Anwander, R. Rare-Earth Metals and Aluminum Getting Close in Ziegler-type Organometallics. Adv. Polym. Sci. 2006, 204, 155–281. [Google Scholar]
- Jothieswaran, J.; Fadlallah, S.; Bonnet, F.; Visseaux, M. Recent Advances in Rare Earth Complexes Bearing Allyl Ligands and Their Reactivity towards Conjugated Dienes and Styrene Polymerization. Catalysts 2017, 7, 378. [Google Scholar] [CrossRef] [Green Version]
- Porri, L.; Giarrusso, A.; Ricci, G. Recent views on the mechanism of diolefin polymerization with transition metal initiator systems. Prog. Polym. Sci. 1991, 16, 405–441. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Pan, Y.; Lin, F.; Wu, C.; Qu, J.; Luo, Y.; Cui, D. Unprecedented 3,4-isoprene and cis-1,4-butadiene copolymers with controlled sequence distribution by single Yttrium cationic species. Macromolecules 2014, 47, 8524–8530. [Google Scholar] [CrossRef]
- Tanaka, R.; Shinto, Y.; Nakayama, Y.; Shiono, T. Synthesis of stereodiblock polybutadiene using Cp*Nd(BH4)2(thf)2 as a catalyst. Catalysts 2017, 7, 284. [Google Scholar] [CrossRef]
- Dong, B.; Liu, H.; Peng, C.; Zhao, W.; Zheng, W.; Zhang, C.; Bi, J.; Hu, Y.; Zhang, X. Synthesis of stereoblock polybutadiene possessing cis-1,4 and syndiotactic-1,2 segments by imino-pyridine cobalt complex-based catalyst through one-pot polymerization process. Eur. Polym. J. 2018, 108, 116–123. [Google Scholar] [CrossRef]
- Gong, D.; Ying, W.; Zhao, J.; Li, W.; Xu, Y.; Luo, Y.; Zhang, X.; Capacchione, C.; Grassi, A. Controlling external diphenylcyclohexylphosphine feeding to achieve cis-1,4-syn-1,2 sequence controlled polybutadienes via cobalt catalyzed 1,3-butadiene polymerization. J. Catal. 2019, 377, 367–372. [Google Scholar] [CrossRef]
- Bazzini, C.; Giarrusso, A.; Porri, L. Diethylbis (2,2′-bipyridine) iron/MAO. A Very Active and Stereospecific Catalyst for 1,3-Diene Polymerization. Macromol. Rapid Commun. 2002, 23, 922–927. [Google Scholar] [CrossRef]
- Ricci, G.; Morganti, D.; Sommazzi, A.; Santi, R.; Masi, F. Polymerization of 1, 3-dienes with iron complexes based catalysts: Influence of the ligand on catalyst activity and stereospecificity. J. Mol. Cat. A Chem. 2003, 204/205, 287–293. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Boglia, A.; Bertini, F.; Boccia, A.C.; Zetta, L. Synthesis and Characterization of Isotactic 1,2-Poly(E-3-methyl-1,3-pentadiene). Some Remarks about the Influence of Monomer Structure on Polymerization Stereoselectivity. Macromolecules 2009, 42, 3048–3056. [Google Scholar] [CrossRef]
- Ricci, G.; Battistella, M.; Porri, L. Chemoselectivity and stereospecificity of chromium(II) catalysts for 1,3-diene polymerization. Macromolecules 2001, 34, 5766–5769. [Google Scholar] [CrossRef]
- Ricci, G.; Forni, A.; Boglia, A.; Sonzogni, M. New chromium(II) bidentate phosphine complexes: Synthesis, characterization, and behavior in the polymerization of 1,3-butadiene. Organometallics 2004, 23, 3727–3732. [Google Scholar] [CrossRef]
- Bazzini, C.; Giarrusso, A.; Porri, L.; Pirozzi, B.; Napolitano, R. Synthesis and characterization of syndiotactic 3,4-polyisoprene prepared with diethylbis(2,2’-bipyridine)iron−MAO. Polymer 2004, 45, 2871–2875. [Google Scholar] [CrossRef]
- Raynaud, J.; Wu, J.Y.; Ritter, T. Iron-catalyzed polymerization of isoprene and other 1,3-dienes. Angew. Chem. 2012, 51, 11805–11808. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Jing, X.; Xiong, S.; Liu, Y.; Liu, Z.; Chen, C. Influences of alkyl and aryl substituents on iminopyridine Fe(II)- and Co(II)-catalyzed isoprene polymerization. Polymers 2016, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Champouret, Y.; Hashmi, O.H.; Visseaux, M. Discrete iron-based complexes: Applications in homogeneous coordination-insertion polymerization catalysis. Coord. Chem. Rev. 2019, 390, 127–170. [Google Scholar] [CrossRef]
- Hashmi, O.H.; Champouret, Y.; Visseaux, M. Highly active iminopyridyl iron-based catalysts for the polymerization of isoprene. Molecules 2019, 24, 3024. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Wang, L.; Mahmood, Q.; Jing, C.; Zhu, G.; Zhang, X.; Wang, X.; Wang, Q. Controlled isoprene polymerization mediated by iminopyridine-iron (II) acetylacetonate pre-catalysts. Appl. Organomet. Chem. 2019, 33, e4836. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Zanchin, G.; Palucci, B.; Boccia, A.C.; Sommazzi, A.; Masi, F.; Zacchini, S.; Guelfi, M.; Pampaloni, G. Highly Stereoregular 1,3-Butadiene and Isoprene Polymers through Monoalkyl−N−Aryl Substituted Iminopyridine Iron Complex-Based Catalysts: Synthesis and Characterization. Macromolecules 2021, 54, 9947–9959. [Google Scholar] [CrossRef]
- Eremina, J.A.; Lider, E.V.; Samsonenko, D.G.; Sheludyakova, L.A.; Berezin, A.S.; Klyushova, L.S.; Ostrovskii, V.A.; Trifonov, R.E. Mixed-ligand copper(II) complexes with tetrazole derivatives and 2,2′- bipyridine, 1,10-phenanthroline: Synthesis, structure and cytotoxic activity. Inorg. Chim. Acta 2019, 487, 138–144. [Google Scholar] [CrossRef]
- Hernandez-Molina, M.; Gonzalez-Platas, J.; Ruiz-Perez, C.; Lloret, F.; Julve, M. Crystal structure and magnetic properties of the single-m-chloro copper(II) chain [Cu(bipy)Cl2] (bipy = 2,2’-bipyridine). Inorg. Chim. Acta 1999, 284, 258–265. [Google Scholar] [CrossRef]
- Ricci, G.; Italia, S.; Giarrusso, A.; Porri, L. Polymerization of 1,3-dienes with the soluble catalyst system methylaluminoxanes-[CpTiCl3]. Influence of monomer structure on polymerization stereospecificity. J. Organomet. Chem. 1993, 451, 67–72. [Google Scholar] [CrossRef]
- Porri, L.; Ricci, G.; Giarrusso, A.; Shubin, N.; Lu, Z. Recent developments in Lanthanide catalysts for 1,3-diene polymerization. In ACS Symposium Series 749—Olefin Polymerization: Emerging Frontiers; Arjunan, P., McGrath, J.C., Hanlon, T., Eds.; Oxford University Press: Oxford, UK, 2000; pp. 15–30. [Google Scholar]
- Mochel, V.D. Carbon-13 NMR of polybutadiene. J. Polym. Sci. Part A-1 Polym. Chem. 1972, 10, 1009–1018. [Google Scholar] [CrossRef]
- Elgert, K.F.; Quack, G.; Stutzel, B. Zur struktur des polybutadiens, 2. Das 13C-NMR-Spektrum des 1,2-polybutadiens. Makromol. Chem. 1974, 175, 1955–1960. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sato, H. Sequence distribution of cis-1,4-and trans-1,4-units in polyisoprenes. Polymer 1976, 17, 113–116. [Google Scholar] [CrossRef]
- Sato, H.; Ono, A.; Tanaka, Y. Distribution of isomeric structures in polyisoprenes. Polymer 1977, 18, 580–586. [Google Scholar] [CrossRef]
- Beebe, D.H. Structure of 3,4-(cis-1,4-)trans-1,4-polyisoprene by 13C n.m.r. Polymer 1978, 19, 231–233. [Google Scholar] [CrossRef]
- Annunziata, L.; Pragliola, S.; Pappalardo, D.; Tedesco, C.; Pellecchia, C. New (Anilidomethyl)pyridine Titanium(IV) and Zirconium(IV) Catalyst Precursors for the Highly Chemo- and Stereoselective cis-1,4-Polymerization of 1,3-Butadiene. Macromolecules 2011, 44, 1934–1941. [Google Scholar] [CrossRef]
- Pampaloni, G.; Guelfi, M.; Sommazzi, A.; Leone, G.; Masi, F.; Zacchini, S.; Ricci, G. Synthesis and spectroscopic characterization of titanium pyridylanilido complexes as catalysts for the polymerization of 1,3-butadiene and isoprene. Inorg. Chim. Acta 2019, 487, 331–338. [Google Scholar] [CrossRef]
- Colamarco, E.; Milione, S.; Cuomo, C.; Grassi, A. Homo- and copolymerization of butadiene catalyzed by a bis(imino)pyridyl vanadium compolex. Macromol. Rapid Commun. 2004, 25, 450–454. [Google Scholar] [CrossRef]
- Leone, G.; Zanchin, G.; Pierro, I.; Sommazzi, A.; Forni, A.; Ricci, G. Synthesis, structure and 1,3-butadiene polymerization behavior of vanadium (III) phosphine complexes. Catalysts 2017, 7, 369. [Google Scholar] [CrossRef] [Green Version]
- Ricci, G.; Forni, A.; Boglia, A.; Sommazzi, A.; Masi, F. Synthesis, structure and butadiene polymerization behavior of CoCl2(PRxPh3-x)2 (R = methyl, ethyl, propyl, allyl, isopropyl, cyclohexyl; x = 1,2). Influence of the phosphorous ligand on polymerization stereoselectivity. J. Organomet. Chem. 2005, 690, 1845–1854. [Google Scholar] [CrossRef]
- Ricci, G.; Sommazzi, A.; Leone, G.; Boglia, A.; Masi, F. Bis- imine complex of lanthanide, catalytic system comprising said bis- imine complex and process for the (co)polymerization of conjugated dienes. Patent WO2013037910 A1 to Versalis, 21 March 2013. [Google Scholar]




| M b | Entry | Al/Cu | Time (h) | Yield (%) | cis-1,4 c (%) | 1,2 c (%) | 3,4 c (%) | [rrrr] d (%) | Tm e (°C) | Tc e (°C) | Tg e (°C) | Mw f (g/mol) | Mw/Mn f |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 1 | 1000 | 4 | 10 | 34.2 | 65.8 | 31.3 | 22 | 5 | −46 | 1,319,100 | 1.3 | |
| 2 | 1000 | 20 | 42 | 33.8 | 66.2 | 32.6 | 30 | 7 | −47 | 1,900,000 | 1.7 | ||
| 3 g,h | 500 | 4 | 35 | 34.9 | 65.1 | 25.8 | nd | nd | nd | 633,800 | 4.6 | ||
| 4 g | 500 | 24 | 52 | 35.1 | 64.9 | 30.9 | 24 | 6 | −46 | 1,442,600 | 2.3 | ||
| IP | 5 | 1000 | 0.5 | 8 | 24.1 | 75.9 | 49.5 | 113.8 | 78 | 5.4 | 910,000 | 1.6 | |
| 6 | 1000 | 5 | 57 | 25.7 | 74.3 | 56.9 | 114.9 | 76.5 | 9.4 | 1,668,750 | 2.0 | ||
| 7 h | 1000 | 5 | 35 | 31.8 | 68.2 | 40.5 | 45.4 | −1.2 | 457,970 | 3.7 | |||
| 8 g | 500 | 4 | 50 | 24.4 | 75.6 | 57.6 | 113 | 77 | 8.1 | 2,473,140 | 1.4 | ||
| 9 i | 100 | 2 | 35 | 25.1 | 74.9 | 54.4 | 103 | 64 | 9.6 | 2,922,670 | 1.6 | ||
| DMB | 10 | 1000 | 4 | 76 | ≥99 | 197.5 | 170 | −4.1 | nd | nd | |||
| 3MP | 11 | 1000 | 24 | 19 | ≥99 | 241 | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, G.; Leone, G.; Zanchin, G.; Masi, F.; Guelfi, M.; Pampaloni, G. Dichloro(2,2′-bipyridine)copper/MAO: An Active and Stereospecific Catalyst for 1,3-Diene Polymerization. Molecules 2023, 28, 374. https://doi.org/10.3390/molecules28010374
Ricci G, Leone G, Zanchin G, Masi F, Guelfi M, Pampaloni G. Dichloro(2,2′-bipyridine)copper/MAO: An Active and Stereospecific Catalyst for 1,3-Diene Polymerization. Molecules. 2023; 28(1):374. https://doi.org/10.3390/molecules28010374
Chicago/Turabian StyleRicci, Giovanni, Giuseppe Leone, Giorgia Zanchin, Francesco Masi, Massimo Guelfi, and Guido Pampaloni. 2023. "Dichloro(2,2′-bipyridine)copper/MAO: An Active and Stereospecific Catalyst for 1,3-Diene Polymerization" Molecules 28, no. 1: 374. https://doi.org/10.3390/molecules28010374
APA StyleRicci, G., Leone, G., Zanchin, G., Masi, F., Guelfi, M., & Pampaloni, G. (2023). Dichloro(2,2′-bipyridine)copper/MAO: An Active and Stereospecific Catalyst for 1,3-Diene Polymerization. Molecules, 28(1), 374. https://doi.org/10.3390/molecules28010374









