Extraction of Bioactive Compounds from C. vulgaris Biomass Using Deep Eutectic Solvents
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Properties of DESs
2.2. Solvent Screening
2.3. Experimental Design Results
2.4. Statistical Analysis of Experimental Design Results
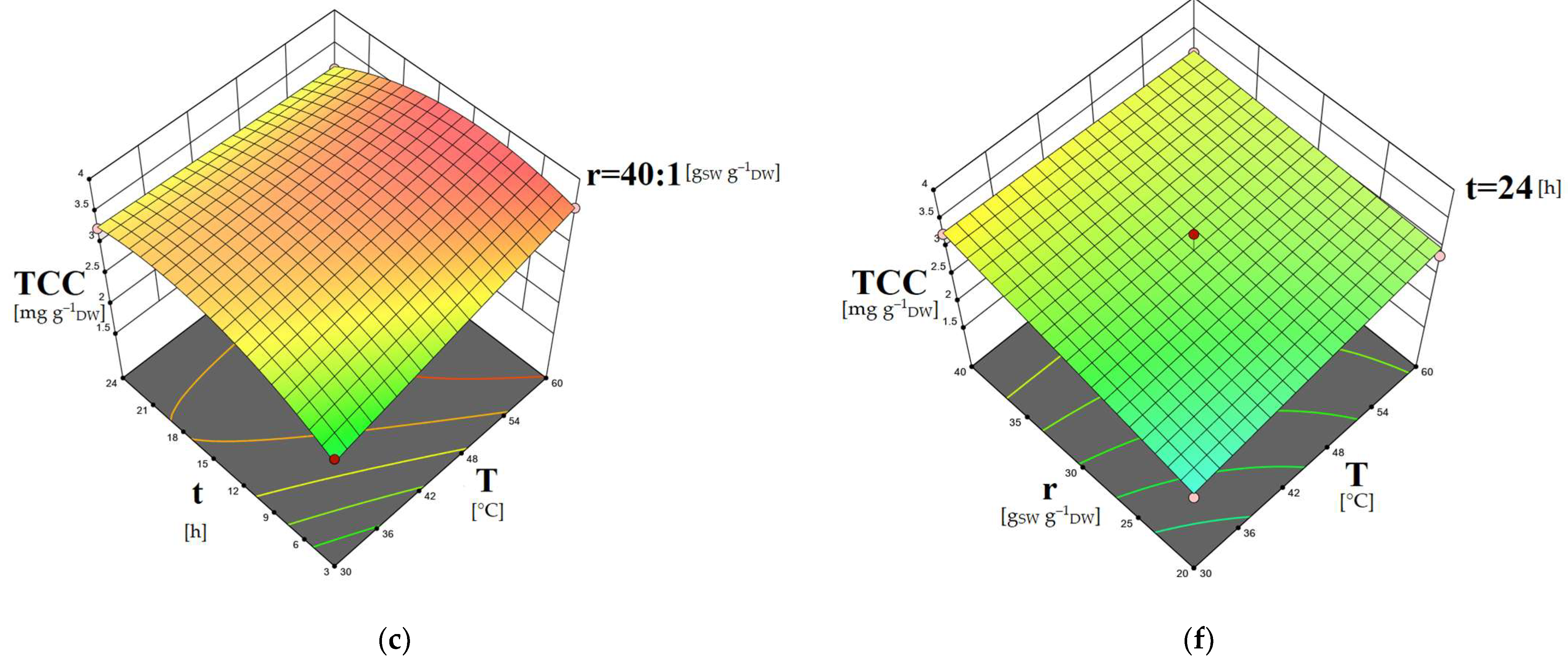
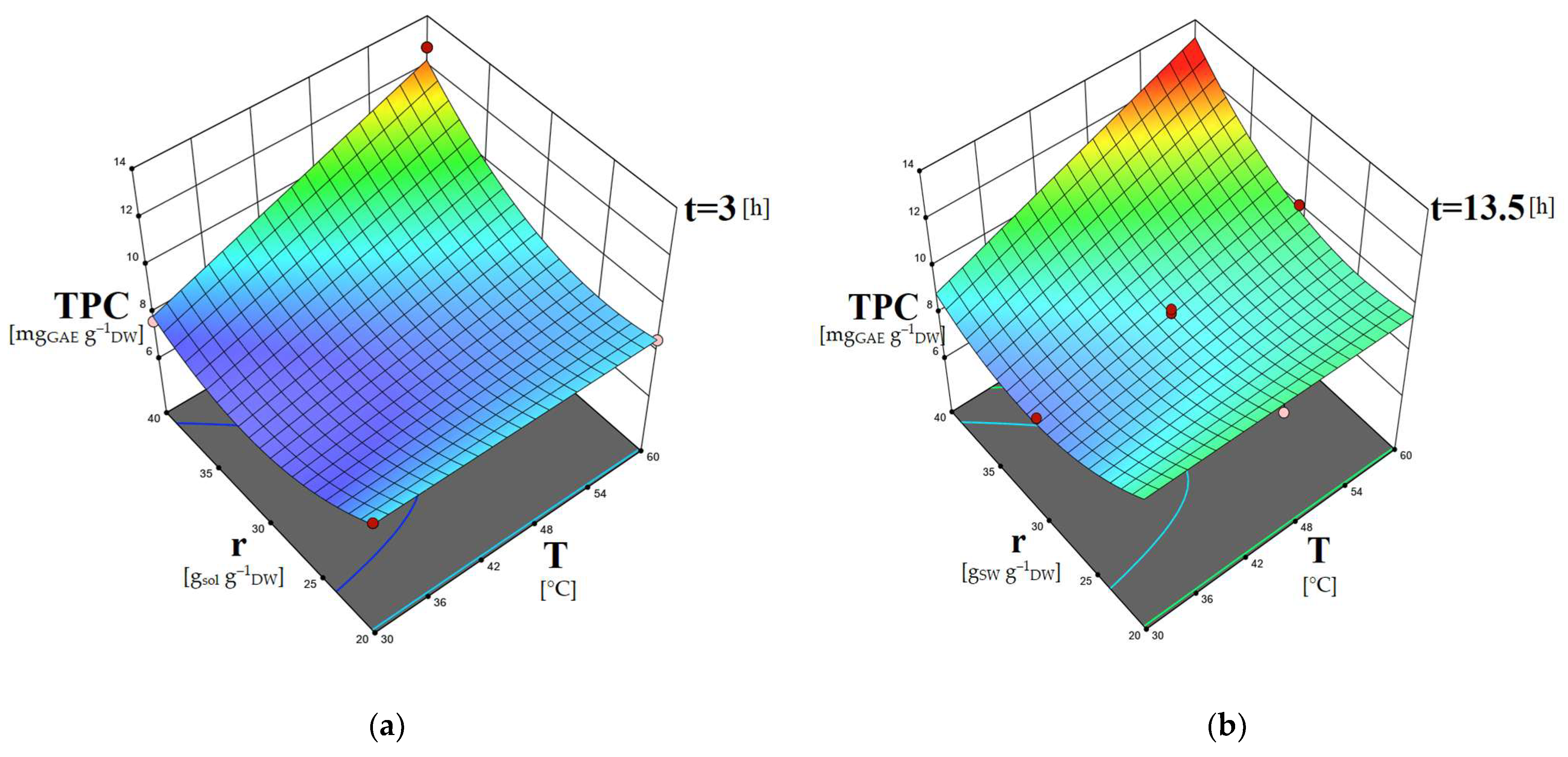
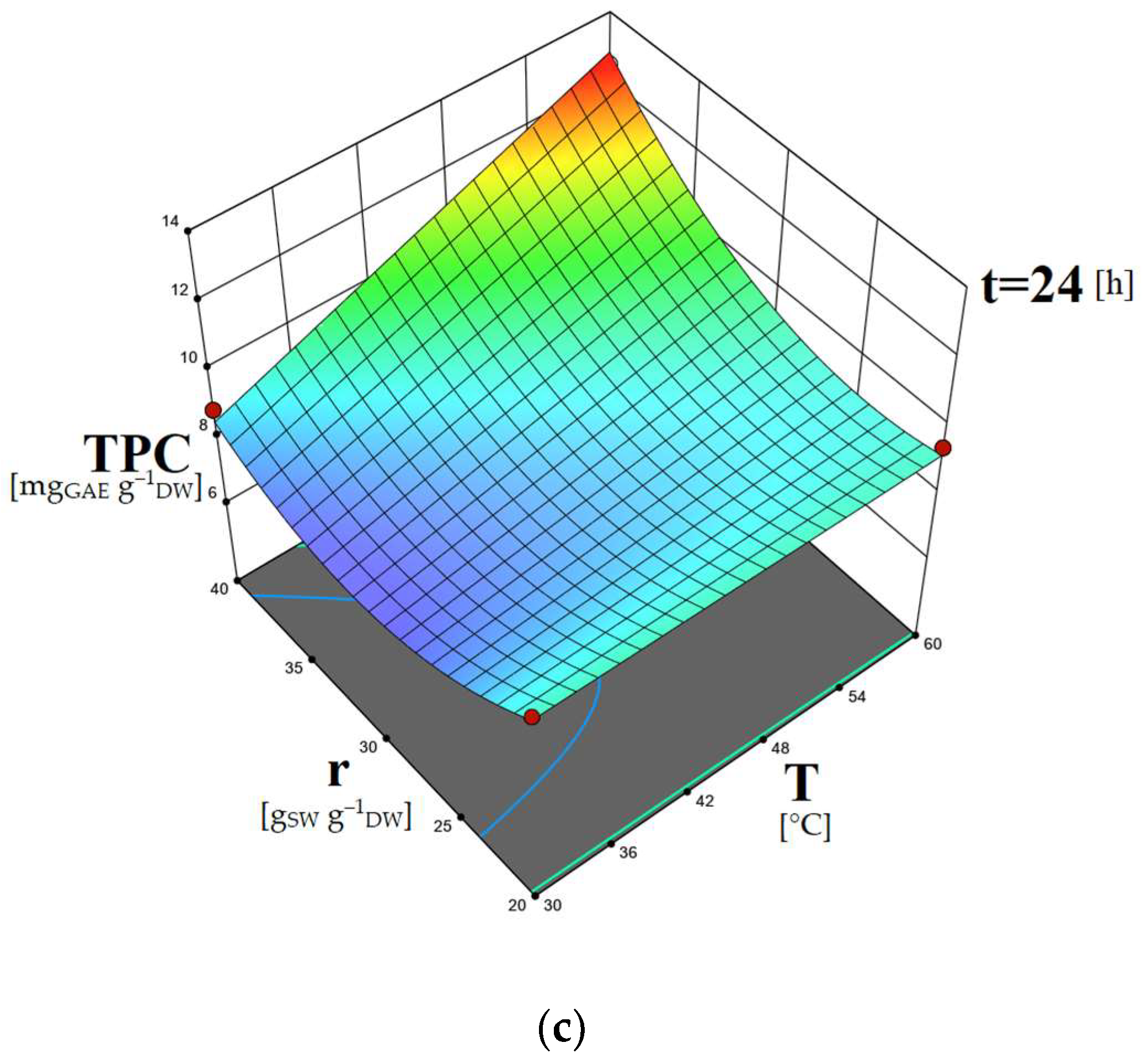
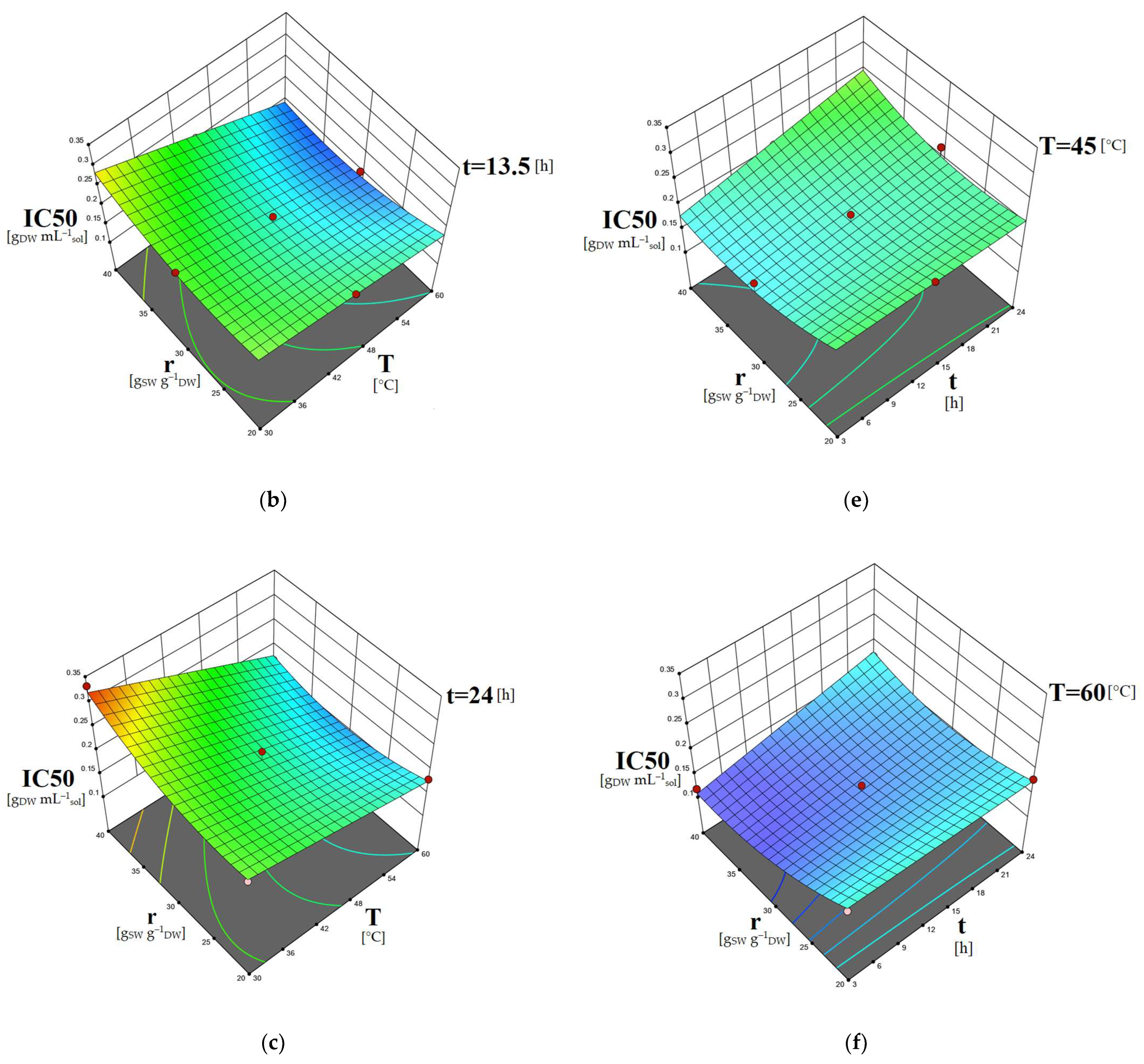
2.5. Study of the Factors’ Combined Effects
2.6. Experimental Validation of the Models
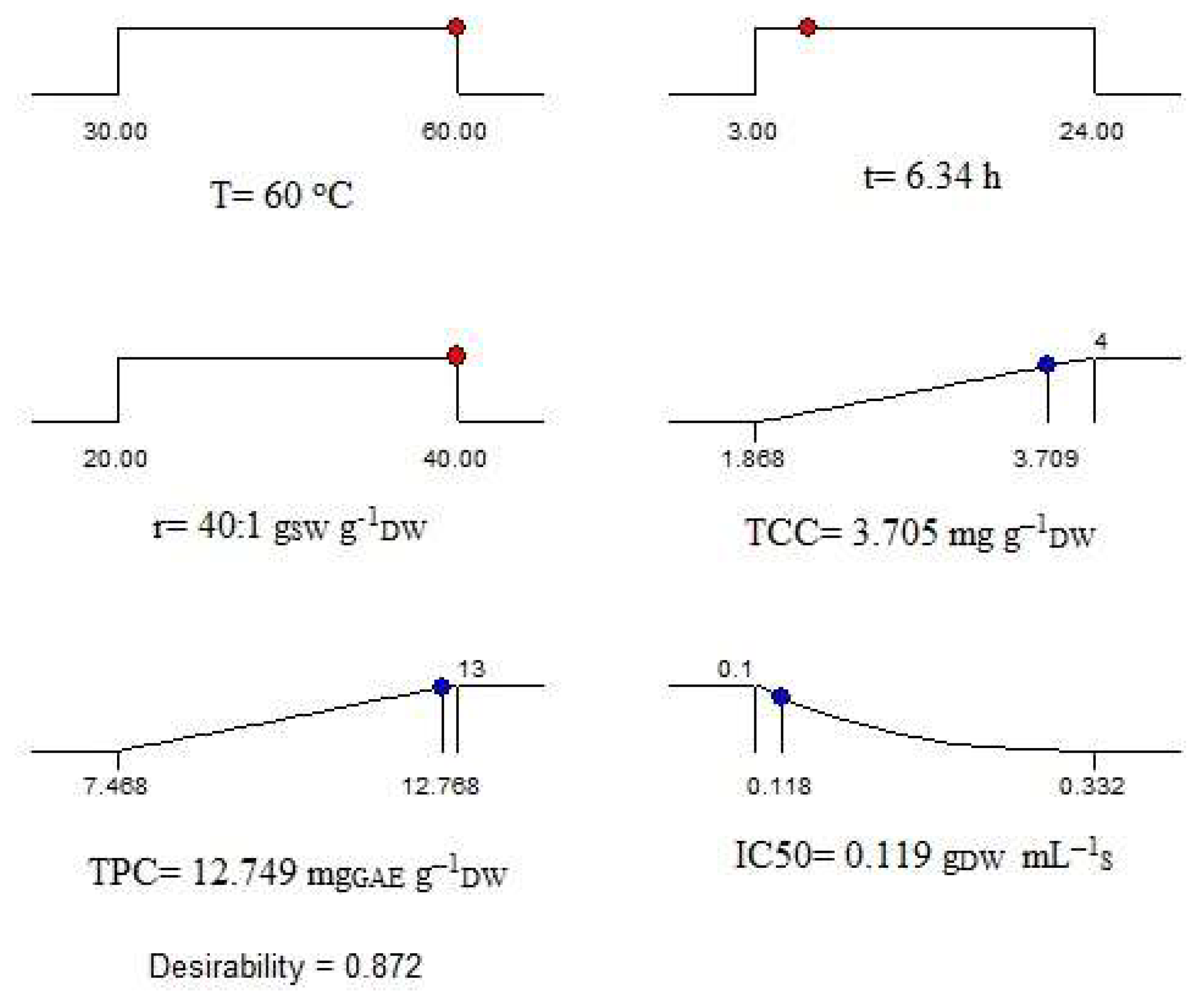
2.7. Optimization of Extraction Process
3. Materials and Methods
3.1. Chemicals
3.2. Microalgae Culture
3.3. Preparation of DESs
3.4. Measurement of DES Physical Properties
3.5. Extraction Process
3.6. Determination of the Extracts’ Total Phenolic and Carotenoid Contents
3.7. Determination of the Extracts’ Antioxidant Activity
3.8. Experimental Design and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yang, T.P.; Lee, H.J.; Ou, T.T.; Chang, Y.J.; Wang, C.J. Mulberry leaf polyphenol extract induced apoptosis involving regulation of adenosine monophosphate–activated protein kinase/fatty acid synthase in a p53–negative hepatocellular carcinoma cell. J. Agric. Food Chem. 2012, 60, 6891–6898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti–inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Caban, M.; Owczarek, K.; Chojnacka, K.; Lewandowska, U. Overview of polyphenols and polyphenol–rich extracts as modulators of IGF–1, IGF–1R, and IGFBP expression in cancer diseases. J. Funct. Foods 2019, 52, 389–407. [Google Scholar] [CrossRef]
- Hidalgo, G.I.; Almajano, M.P. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef]
- Gao, M.Z.; Cui, Q.; Wang, L.T.; Meng, Y.; Yu, L.; Li, Y.Y.; Fu, Y.J. A green and integrated strategy for enhanced phenolic compounds extraction from mulberry (Morus alba L.) leaves by deep eutectic solvent. Microchem. J. 2020, 154, 104598. [Google Scholar] [CrossRef]
- Serna–Vázquez, J.; Ahmad, M.Z.; Boczkaj, G.; Castro–Muñoz, R. Latest Insights on Novel Deep Eutectic Solvents (DES) for Sustainable Extraction of Phenolic Compounds from Natural Sources. Molecules 2021, 26, 5037. [Google Scholar] [CrossRef]
- Lasarte–Aragonés, G.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Use of switchable solvents in the microextraction context. Talanta 2015, 131, 645–649. [Google Scholar] [CrossRef]
- Tang, W.; Row, K.H. Evaluation of CO2–induced azole–based switchable ionic liquid with hydrophobic/hydrophilic reversible transition as single solvent system for coupling lipid extraction and separation from wet microalgae. Bioresour. Technol. 2020, 296, 122309. [Google Scholar] [CrossRef]
- Tang, W.; Row, K.H. Design and evaluation of polarity controlled and recyclable deep eutectic solvent based biphasic system for the polarity driven extraction and separation of compounds. J. Clean. Prod. 2020, 268, 122306. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Comm. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of deep eutectic solvents in analytical chemistry. A review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Chan, Y.S.; Nandong, J. Extraction of antioxidant compounds from the wastes of Mangifera pajang fruit: A comparative study using aqueous ethanol and deep eutectic solvent. SN Appl. Sci. 2020, 2, 1–12. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart advanced solvents for bioactive compounds recovery from agri-food by-products: A review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Fernández, M.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel approaches mediated by tailor–made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Xu, P.; Yang, F.X.; Wu, H.; Zong, M.H.; Lou, W.Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Cui, Q.; Peng, X.; Yao, X.H.; Wei, Z.F.; Luo, M.; Wang, W.; Zu, Y.G. Deep eutectic solvent–based microwave–assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep. Purif. Technol. 2015, 150, 63–72. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Liu, C.; Lu, X.; Feng, X.; Ji, X. Thermodynamic Study of Choline Chloride–Based Deep Eutectic Solvents with Water and Methanol. Chem. Eng. Data 2020, 65, 2446–2457. [Google Scholar] [CrossRef]
- Coelho de Andrade, D.; Aquino Monteiro, S.; Merib, J. A review on recent applications of deep eutectic solvents in microextraction techniques for the analysis of biological matrices. Adv. Sample Prep. 2022, 1, 100007. [Google Scholar] [CrossRef]
- Rodríguez-Álvarez, M.J.; García-Garrido, S.E.; Perrone, S.; García-Álvarez, J.; Capriati, V. Deep Eutectic Solvents and Heterogeneous Catalysis with Metallic Nanoparticles: A Powerful Partnership in Sustainable Synthesis. Curr. Opin. Green Sustain. Chem. 2022, 39, 100723. [Google Scholar] [CrossRef]
- Chang, X.X.; Mubarak, N.M.; Mazari, S.A.; Jatoi, A.S.; Ahmad, A.; Khalid, M.; Nizamuddin, S. A review on the properties and applications of chitosan, cellulose and deep eutectic solvent in green chemistry. J. Ind. Eng. Chem. 2021, 104, 362–380. [Google Scholar] [CrossRef]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep eutectic solvents as efficient solvents in biocatalysis. Trends Biotechnol. 2019, 37, 943–959. [Google Scholar] [CrossRef] [PubMed]
- . Tomé, L.I.; Baião, V.; Da Silva, W.; Brett, C.M. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Kalyniukova, A.; Holuša, J.; Musiolek, D.; Sedlakova–Kadukova, J.; Płotka–Wasylka, J.; Andruch, V. Application of deep eutectic solvents for separation and determination of bioactive compounds in medicinal plants. Ind. Crops. Prod. 2012, 172, 114047. [Google Scholar] [CrossRef]
- Orejuela-Escobar, L.M.; Landázuri, A.C.; Goodell, B. Second generation biorefining in Ecuador: Circular bioeconomy, zero waste technology, environment and sustainable development: The nexus. J. Bioresour. Bioprod. 2021, 6, 83–107. [Google Scholar] [CrossRef]
- Shao, J.; Ni, Y.; Yan, L. Oxidation of furfural to maleic acid and fumaric acid in deep eutectic solvent (DES) under vanadium pentoxide catalysis. J. Bioresour. Bioprod. 2021, 6, 39–44. [Google Scholar] [CrossRef]
- Barbieri, J.B.; Goltz, C.; Cavalheiro, F.B.; Toci, A.T.; Igarashi–Mafra, L.; Mafra, M.R. Deep eutectic solvents applied in the extraction and stabilization of rosemary (Rosmarinus officinalis L.) phenolic compounds. Ind. Crops Prod. 2020, 144, 112049. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor–made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Das, A.K.; Sharma, M.; Mondal, D.; Prasad, K. Deep eutectic solvents as efficient solvent system for the extraction of κ–carrageenan from Kappaphycus alvarezii. Carbohydr. Polym. 2016, 136, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Vilková, M.; Płotka-Wasylka, J.; Andruch, V. The role of water in deep eutectic solvent–base extraction. J. Mol. Liq. 2020, 304, 112747. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep eutectic solvents as extraction media for valuable flavonoids from natural sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef]
- Kafyra, M.S.G.; Papadaki, S.; Chronis, M.; Krokida, M. Microalgae based innovative animal fat and proteins replacers for application in functional baked products. Open Agric. 2018, 3, 427–436. [Google Scholar] [CrossRef]
- Peng, X.; Duan, M.-H.; Yao, X.-H.; Zhang, Y.-H.; Zhao, C.-J.; Zu, Y.-G.; Fu, Y.-J. Green extraction of five target phenolic acids from Lonicerae japonicae Flos with deep eutectic solvent. Sep. Purif. Technol. 2016, 157, 249–257. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Dash, S.R.; Nayak, R.; Behera, C.; Jena, M. Evaluation of the anti–bacterial activity of methanolic extract of Chlorella vulgaris Beyerinck [Beijerinck] with special reference to antioxidant modulation. Future J. Pharm. Sci. 2021, 7, 1–11. [Google Scholar] [CrossRef]
- Georgiopoulou, I.; Tzima, S.; Pappa, G.D.; Louli, V.; Voutsas, E.; Magoulas, K. Experimental Design and Optimization of Recovering Bioactive Compounds from Chlorella vulgaris through Conventional Extraction. Molecules 2021, 27, 29. [Google Scholar] [CrossRef]
- Kulkarni, S.; Nikolov, Z. Process for selective extraction of pigments and functional proteins from Chlorella vulgaris. Algal Res. 2018, 35, 185–193. [Google Scholar] [CrossRef]
- Dimova, D.; Dobreva, D.; Panayotova, V.; Makedonski, L. DPPH antiradical activity and total phenolic content of methanol and ethanol extracts from macroalgae (Ulva rigida) and microalgae (Chlorella). Scr. Sci. Pharm. 2019, 6, 37–41. [Google Scholar] [CrossRef]
- Mahmood, W.; Lorwirachsutee, W.M.A.; Theodoropoulos, A.C.; Gonzalez-Miquel, M. Polyol–based deep eutectic solvents for extraction of natural polyphenolic antioxidants from Chlorella vulgaris. ACS Sustain. Chem. Eng. 2019, 7, 5018–5026. [Google Scholar] [CrossRef]
- Guo, W.; Hou, Y.; Ren, S.; Tian, S.; Wu, W. Formation of Deep Eutectic Solvents by Phenols and Choline Chloride and Their Physical Properties. J. Chem. Eng. Data 2013, 58, 866–872. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Van Osch, D.J.; Dietz, C.H.; Van Spronsen, J.; Kroon, M.C.; Gallucci, F.; van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Basaiahgari, A.; Panda, S.; Gardas, R.L. Effect of Ethylene, Diethylene, and Triethylene Glycols and Glycerol on the Physicochemical Properties and Phase Behavior of Benzyltrimethyl and Benzyltributylammonium Chloride Based Deep Eutectic Solvents at 283.15–343.15. J. Chem. Eng. Data 2018, 63, 2613–2627. [Google Scholar] [CrossRef]
- Abbott, A.P. Application of Hole Theory to the Viscosity of Ionic and Molecular Liquids. ChemPhysChem 2004, 5, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Ijardar, S.P. Deep eutectic solvents composed of tetrabutylammonium bromide and PEG: Density, speed of sound and viscosity as a function of temperature. J. Chem. Thermodyn. 2020, 140, 105897. [Google Scholar] [CrossRef]
- Yadav, A.; Trivedi, S.; Rai, R.; Pandey, S. Densities and dynamic viscosities of (choline chloride + glycerol) deep eutectic solvent and its aqueous mixtures in the temperature range (283.15–363.15) K. Fluid. Phase Equilib. 2014, 367, 135–142. [Google Scholar] [CrossRef]
- Pappou, S.; Dardavila, M.M.; Savvidou, M.G.; Louli, V.; Magoulas, K.; Voutsas, E. Extraction of Bioactive Compounds from Ulva lactuca. Appl. Sci. 2022, 12, 2117. [Google Scholar] [CrossRef]
- Bulut, O.; Akın, D.; Sönmez, Ç.; Öktem, A.; Yücel, M.; Öktem, H.A. Phenolic compounds, carotenoids, and antioxidant capacities of a thermo–tolerant Scenedesmus sp. (Chlorophyta) extracted with different solvents. J. Appl. Phycol. 2019, 31, 1675–1683. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Psychol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Corrêa, P.S.; Morais Júnior, W.G.; Martins, A.A.; Caetano, N.S.; Mata, T.M. Microalgae Biomolecules: Extraction, Separation and Purification Methods. Processes 2021, 9, 10. [Google Scholar] [CrossRef]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents–based ultrasound–assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Strati, I.F.; Oreopoulou, V. Effect of extraction parameters on the carotenoid recovery from tomato waste. Int. J. Food Sci. 2011, 46, 23–29. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M.; Harun, M.R.; Omar, R.; Siajam, S.I. Subcritical Water Technology for Extraction of Phenolic Compounds from Chlorella sp. Microalgae and Assessment on Its Antioxidant Activity. Molecules 2017, 22, 1105. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Pereira, C.V.; Leonardo, I.C.; Fernández, N.; Gaspar, F.B.; Silva, J.M.; Reis, R.L.; Duarte, A.R.C.; Paiva, A.; Matias, A.A. Terpene-Based Natural Deep Eutectic Systems as Efficient Solvents To Recover Astaxanthin from Brown Crab Shell Residues. ACS Sustain. Chem. Eng. 2020, 8, 2246–2259. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez–Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef]
- Yao, X.H.; Zhang, D.Y.; Duan, M.H.; Cui, Q.; Xu, W.J.; Luo, M.; Li, C.Y.; Zu, Y.G.; Fu, Y.J. Preparation and determination of phenolic compounds from Pyrola incarnata Fisch. with a green polyols based–deep eutectic solvent. Sep. Purif. Technol. 2015, 149, 116–123. [Google Scholar] [CrossRef]
- Rozas, S.; Benito, C.; Alcalde, R.; Atilhan, M.; Aparicio, S. Insights on the water effect on deep eutectic solvents properties and structuring: The archetypical case of choline chloride + ethylene glycol. J. Mol. Liq. 2021, 344, 117717. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists International, 16th ed.; The Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Brand–Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]


| Solvent | Viscosity,
[cP] | Density,
[g cm−3] |
|---|---|---|
| DES1 | 15.01 ± 0.04 | 1.0120 ± 0.0001 |
| DES2 | 53.07 ± 0.22 | 1.1725 ± 0.0001 |
| DES3 | 13.49 ± 0.11 | 1.0965 ± 0.0001 |
| DES1/w | 3.54 ± 0.01 | 1.0145 ± 0.0001 |
| DES2/w | 4.34 ± 0.02 | 1.1179 ± 0.0001 |
| DES3/w | 2.78 ± 0.05 | 1.0678 ± 0.0001 |
| Solvent | TCC [mg g–1DW] | TPC [mgGAE g–1DW] | IC50 [gDW mL–1sol] |
|---|---|---|---|
| DES1/w | 3.462 ± 0.121 | 8.553 ± 0.213 | 0.180 ± 0.011 |
| DES2/w | 0.218 ± 0.011 | 4.407 ± 0.128 | 0.260 ± 0.014 |
| DES3/w | 0.293 ± 0.014 | 4.687 ± 0.131 | 0.360 ± 0.018 |
| EtOH/w | 8.436 ± 0.211 | 7.686 ± 0.219 | 0.139 ± 0.010 |
| Run | Experimental Design Conditions | Experimental Results | ||||
|---|---|---|---|---|---|---|
| X1: T [°C] | X2: t [h] | X3: r [gSW g−1DW] | Y1: TCC [mg g–1DW] | Y2: TPC [mgGAE g–1DW] | Y3: IC50 [gDW mL–1sol] | |
| 1 | 45 | 13.5 | 20:1 | 3.102 | 9.257 | 0.215 |
| 2 | 45 | 13.5 | 40:1 | 3.517 | 10.787 | 0.207 |
| 3 | 30 | 3 | 20:1 | 1.868 | 8.696 | 0.255 |
| 4 | 30 | 24 | 40:1 | 3.257 | 8.897 | 0.332 |
| 5 | 60 | 24 | 20:1 | 2.872 | 9.438 | 0.181 |
| 6 | 45 | 13.5 | 30:1 | 3.268 | 8.904 | 0.201 |
| 7 | 30 | 24 | 20:1 | 2.332 | 9.383 | 0.237 |
| 8 | 30 | 3 | 40:1 | 2.721 | 7.667 | 0.241 |
| 9 | 45 | 24 | 30:1 | 3.256 | 8.058 | 0.220 |
| 10 | 60 | 3 | 20:1 | 3.462 | 8.553 | 0.180 |
| 11 | 45 | 13.5 | 30:1 | 3.152 | 8.643 | 0.166 |
| 12 | 45 | 13.5 | 30:1 | 3.415 | 9.074 | 0.164 |
| 13 | 45 | 3 | 30:1 | 2.693 | 7.468 | 0.189 |
| 14 | 60 | 24 | 40:1 | 3.137 | 12.586 | 0.170 |
| 15 | 30 | 13.5 | 30:1 | 2.818 | 8.062 | 0.241 |
| 16 | 60 | 13.5 | 30:1 | 3.709 | 10.131 | 0.147 |
| 17 | 45 | 13.5 | 30:1 | 3.277 | 8.894 | 0.164 |
| 18 | 60 | 3 | 40:1 | 3.571 | 12.768 | 0.118 |
| RESPONSE Y1-TCC | |||||
|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
| Prob > F | |||||
| Model | 3.45 | 6 | 0.5750 | 31.55 | <0.0001 |
| -T | 1.41 | 1 | 1.41 | 77.36 | <0.0001 |
| -t | 0.0291 | 1 | 0.0291 | 1.59 | 0.2329 |
| -r | 0.6589 | 1 | 0.6589 | 36.15 | <0.0001 |
| 0.5121 | 1 | 0.5121 | 28.10 | 0.0003 | |
| 0.2464 | 1 | 0.2464 | 13.52 | 0.0036 | |
| 0.5932 | 1 | 0.5932 | 32.55 | 0.0001 | |
| Residual | 0.2005 | 11 | 0.0182 | ||
| Lack of fit | 0.1657 | 8 | 0.0207 | 1.79 | 0.3432 |
| Std. Dev. | 0.1350 | R2 | 0.9451 | ||
| Mean | 3.08 | Adj R2 | 0.9151 | ||
| C.V. % | 4.38 | Pred R2 | 0.8464 | ||
| Adeq Precision | 22.0389 | ||||
| RESPONSE Y2-TPC | |||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
| Prob > F | |||||
| Model | 35.39 | 6 | 5.90 | 47.85 | <0.0001 |
| -T | 11.61 | 1 | 11.61 | 94.16 | <0.0001 |
| -t | 1.03 | 1 | 1.03 | 8.37 | 0.0146 |
| -r | 5.45 | 1 | 5.45 | 44.19 | <0.0001 |
| 9.85 | 1 | 9.85 | 79.87 | <0.0001 | |
| 1.59 | 1 | 1.59 | 12.90 | 0.0042 | |
| 7.38 | 1 | 7.38 | 59.85 | <0.0001 | |
| Residual | 1.36 | 11 | 0.1233 | ||
| Lack of fit | 1.26 | 8 | 0.1577 | 5.00 | 0.1064 |
| Std. Dev. | 0.3511 | R2 | 0.9631 | ||
| Mean | 9.29 | Adj R2 | 0.9430 | ||
| C.V. % | 3.78 | Pred R2 | 0.8731 | ||
| Adeq Precision | 23.5207 | ||||
| RESPONSE Y3-IC50 | |||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
| Prob > F | |||||
| Model | 0.0379 | 6 | 0.0063 | 23.39 | <0.0001 |
| -T | 0.0260 | 1 | 0.0260 | 96.30 | <0.0001 |
| -t | 0.0025 | 1 | 0.0025 | 9.13 | 0.0116 |
| -r | 0.0000 | 1 | 0.0000 | 0.0000 | 1.0000 |
| 0.0030 | 1 | 0.0030 | 10.98 | 0.0069 | |
| 0.0032 | 1 | 0.0032 | 11.85 | 0.0055 | |
| 0.0033 | 1 | 0.0033 | 12.08 | 0.0052 | |
| Residual | 0.0030 | 11 | 0.0003 | ||
| Lack of fit | 0.0020 | 8 | 0.0002 | 0.7473 | 0.6714 |
| Std. Dev. | 0.0164 | R2 | 0.9273 | ||
| Mean | 0.2016 | Adj R2 | 0.8877 | ||
| C.V. % | 8.15 | Pred R2 | 0.8156 | ||
| Adeq Precision | 20.676 | ||||
| Extraction | Experimental Values | Calculated Values | ||||
|---|---|---|---|---|---|---|
| TCC [mg g–1DW] | TPC [mgGAE g–1DW] | IC50 [gDW mL–1sol] | TCC [mg g–1DW] | TPC [mgGAE g–1DW] | IC50 [gDW mL–1sol] | |
| 1 (30 °C, 6 h, 20:1 gSW g−1DW) | 1.927 | 9.103 | 0.238 | 2.069 | 9.075 | 0.249 |
| 2 (45 °C, 24 h, 20:1 gSW g−1DW) | 2.706 | 8.256 | 0.194 | 2.714 | 9.243 | 0.209 |
| Chemical Reagents | Provider | Purity |
|---|---|---|
| 2,2–Diphenyl–1–picrylhydrazyl | Alfa Aesar | 95% |
| Folin Ciocalteu’s reagent | Carlo Erba reagents | Special grade |
| Methanol | Fisher Scientific | ≥99.8% |
| Ethanol | Fisher Scientific | ≥99.8% |
| Water | Fisher Scientific | HPLC grade |
| Choline chloride | Sigma Aldrich | ≥98% |
| 1,2 Butanediol | Sigma Aldrich | 98% |
| Glycerol | Sigma Aldrich | ≥99.0% |
| Ethylene glycol | Sigma Aldrich | 99.8% |
| β–carotene | Alfa Aesar | 99% |
| Gallic acid | Acros Organics | 98% |
| DES | HBA | HBD | HBA:HBD Ratio |
|---|---|---|---|
| DES1 | choline chloride | 1,2 butanediol | 1:4 |
| DES2 | glycerol | 1:2 | |
| DES3 | ethylene glycol | 1:2 |
| Run | Space Type | Factor 1/Level | Factor 2/Level | Factor 3/Level |
|---|---|---|---|---|
| X1: T [°C] | X2: t [h] | X3: r [gsol g–1DW] | ||
| 1 | Axial | 45/0 | 13.5/0 | 20/–1 |
| 2 | Axial | 45/0 | 13.5/0 | 40/+1 |
| 3 | Factorial | 30/–1 | 3/–1 | 20/–1 |
| 4 | Factorial | 30/–1 | 24/+1 | 40/+1 |
| 5 | Factorial | 60/+1 | 24/+1 | 20/–1 |
| 6 | Center | 45/0 | 13.5/0 | 30/0 |
| 7 | Factorial | 30/–1 | 24/+1 | 20/–1 |
| 8 | Factorial | 30/–1 | 3/–1 | 40/+1 |
| 9 | Axial | 45/0 | 24/+1 | 30/0 |
| 10 | Factorial | 60/+1 | 3/–1 | 20/–1 |
| 11 | Center | 45/0 | 13.5/0 | 30/0 |
| 12 | Center | 45/0 | 13.5/0 | 30/0 |
| 13 | Axial | 45/0 | 3/–1 | 30/0 |
| 14 | Factorial | 60/+1 | 24/+1 | 40/+1 |
| 15 | Axial | 30/–1 | 13.5/0 | 30/0 |
| 16 | Axial | 60/+1 | 13.5/0 | 30/0 |
| 17 | Center | 45/0 | 13.5/0 | 30/0 |
| 18 | Factorial | 60/+1 | 3/–1 | 40/+1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dardavila, M.M.; Pappou, S.; Savvidou, M.G.; Louli, V.; Katapodis, P.; Stamatis, H.; Magoulas, K.; Voutsas, E. Extraction of Bioactive Compounds from C. vulgaris Biomass Using Deep Eutectic Solvents. Molecules 2023, 28, 415. https://doi.org/10.3390/molecules28010415
Dardavila MM, Pappou S, Savvidou MG, Louli V, Katapodis P, Stamatis H, Magoulas K, Voutsas E. Extraction of Bioactive Compounds from C. vulgaris Biomass Using Deep Eutectic Solvents. Molecules. 2023; 28(1):415. https://doi.org/10.3390/molecules28010415
Chicago/Turabian StyleDardavila, Maria Myrto, Sofia Pappou, Maria G. Savvidou, Vasiliki Louli, Petros Katapodis, Haralambos Stamatis, Kostis Magoulas, and Epaminondas Voutsas. 2023. "Extraction of Bioactive Compounds from C. vulgaris Biomass Using Deep Eutectic Solvents" Molecules 28, no. 1: 415. https://doi.org/10.3390/molecules28010415
APA StyleDardavila, M. M., Pappou, S., Savvidou, M. G., Louli, V., Katapodis, P., Stamatis, H., Magoulas, K., & Voutsas, E. (2023). Extraction of Bioactive Compounds from C. vulgaris Biomass Using Deep Eutectic Solvents. Molecules, 28(1), 415. https://doi.org/10.3390/molecules28010415








_Stamatis.png)

