Biginelli Reaction Synthesis of Novel Multitarget-Directed Ligands with Ca2+ Channel Blocking Ability, Cholinesterase Inhibition, Antioxidant Capacity, and Nrf2 Activation
Abstract
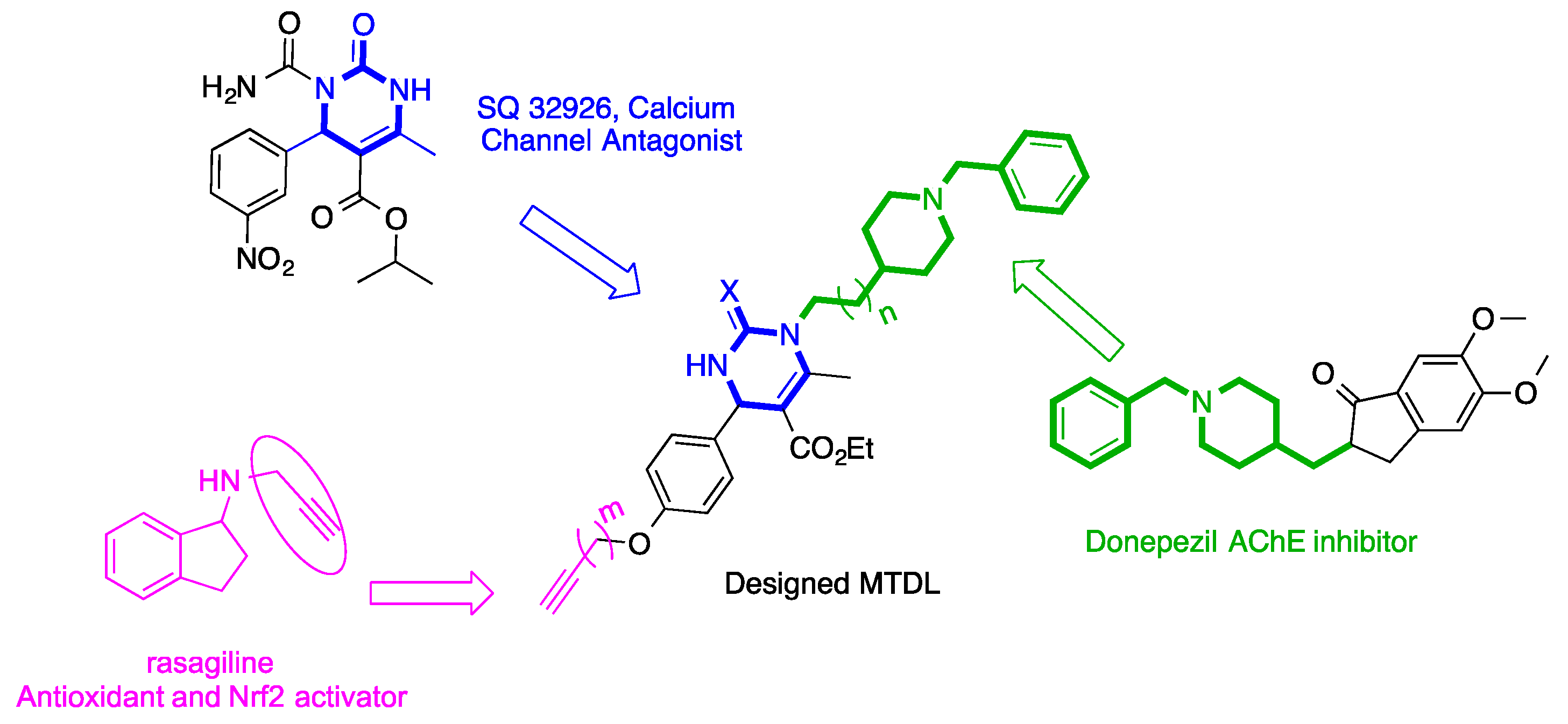
:1. Introduction
2. Results
2.1. Synthesis

2.2. Biological Evaluation
2.2.1. Inhibition of hAChE and eqBuChE
- Ca+2 channel blockade.
- Antioxidant assay.
| Compounds | hAChE IC50 (nM) ± SD a | eqBuChE IC50 (μM) ± SD a | Calcium Antagonism (% Inhibition at 10 μM) ± SD | ORAC b |
|---|---|---|---|---|
| BIGI 4a | - | 31.2 ± 1.70 | 45 ± 11 | 1.44 ± 0.06 |
| BIGI 4b | 342 ± 64 | 4.78 ± 0.58 | 67 ± 10 | 1.60 ± 0.15 |
| BIGI 4c | - | - | 40± 13 | 0.98 ± 0.03 |
| BIGI 4d | 462 ± 40 | 6.63 ± 0.71 | 50 ± 13 | 1.85 ± 0.16 |
| BIGI 5a | - | 7.64 ± 0.79 | 46 ± 18 | 1.35 ± 0.04 |
| BIGI 5b | 352 ± 15 | 5.43 ± 0.24 | 74 ± 16 | 1.41 ± 0.06 |
| BIGI 5c | - | 33.8 ± 7.89 | 63 ± 18 | 1.75 ± 0.06 |
| BIGI 5d | 1271 ± 5 | 8.54 ± 0.71 | 68 ± 12 | 1.57 ± 0.13 |
| Donepezil | 12.7 ± 0.9 | nd | nd | nd |
| Tacrine | nd | 2.2 ± 0.1 nM | nd | nd |
| Nimodipine | nd | nd | 50± 10 | nd |
| Melatonin | nd | nd | nd | 2.45 ± 0.09 |
- Nrf2 transcriptional activation potencies of compounds BIGI 4b, BIGI 4d, BIGI 5b, and BIGI 5d
2.2.2. ADME Studies
3. Materials and Methods
3.1. General Procedure for the Synthesis of Ureas and Thioureas
3.1.1. 1-((1-Benzylpiperidin-4-yl)methyl)urea (2a)
3.1.2. 1-(2-(1-Benzylpiperidin-4-yl)ethyl)urea (2b)
3.1.3. 1-((1-Benzylpiperidin-4-yl)methyl)thiourea (2c)
3.1.4. 1-(2-(1-Benzylpiperidin-4-yl)ethyl)urea (2d)
3.1.5. Synthesis of 4-(prop-2-yn-1-yloxy)benzaldehyde (3a)
3.1.6. Synthesis of 4-(but-3-yn-1-yloxy)benzaldehyde (3b)
3.2. Synthesis of the Biginelli Products
3.2.1. General Procedure for the Synthesis of Biginelli Products
3.2.2. Ethyl 1-((1-benzylpiperidin-4-yl)methyl)-6-methyl-2-oxo-4-(4-(prop-2-yn-1-yloxy)phenyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (BIGI 4a)
3.2.3. Ethyl 1-(2-(1-benzylpiperidin-4-yl)ethyl)-6-methyl-2-oxo-4-(4-(prop-2-yn-1-yloxy)phenyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (BIGI 4b)
3.2.4. Ethyl 1-((1-benzylpiperidin-4-yl)methyl)-4-(4-(but-3-yn-1-yloxy)phenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (BIGI 4c)
3.2.5. Ethyl 1-(2-(1-benzylpiperidin-4-yl)ethyl)-4-(4-(but-3-yn-1-yloxy)phenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (BIGI 4d)
3.2.6. Ethyl 1-((1-benzylpiperidin-4-yl)methyl)-6-methyl-4-(4-(prop-2-yn-1-yloxy)phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (BIGI 5a)
3.2.7. Ethyl 1-(2-(1-benzylpiperidin-4-yl)ethyl)-6-methyl-4-(4-(prop-2-yn-1-yloxy)phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (BIGI 5b)
3.2.8. Ethyl 1-((1-benzylpiperidin-4-yl)methyl)-4-(4-(but-3-yn-1-yloxy)phenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (BIGI 5c)
3.2.9. Ethyl 1-(2-(1-benzylpiperidin-4-yl)ethyl)-4-(4-(but-3-yn-1-yloxy)phenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (BIGI 5d)
3.3. Biological Evaluation
3.3.1. hAChE and eqBuChE
3.3.2. Calcium Channel Blockade
3.3.3. Oxygen Radical Absorbance Capacity Assay
3.3.4. Nrf2 Transcriptional Activation Potencies of Compounds BIGI 4b, BIGI 4d, BIGI 5b, and BIGI 5d
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 11 April 2022).
- Cacabelos, R. Have there been improvements in Alzheimer’s disease drug discovery over the past 5 years? Expert Opin. Drug Discov. 2018, 13, 523–538. [Google Scholar] [CrossRef]
- Greenough, M.A.; Camakaris, J.; Bush, A.I. Metal dyshomeostasis and oxidative stress in Alzheimer’s disease. Neurochem. Int. 2012, 62, 540–555. [Google Scholar] [CrossRef]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-Target-Directed Ligands to Combat Neurodegenerative Diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- Albertini, C.; Salerno, A.; Pinheiro, P.D.S.M.; Bolognesi, M.L. From combinations to multitarget-directed ligands: A continuum in Alzheimer’s disease polypharmacology. Med. Res. Rev. 2020, 41, 2606–2633. [Google Scholar] [CrossRef]
- Prati, F.; Cavalli, A.; Bolognesi, M.L. Navigating the Chemical Space of Multitarget-Directed Ligands: From Hybrids to Fragments in Alzheimer’s Disease. Molecules 2016, 21, 466. [Google Scholar] [CrossRef] [Green Version]
- Guzior, N.; Wieckowska, A.; Panek, D.; Malawska, B. Recent Development of Multifunctional Agents as Potential Drug Candidates for the Treatment of Alzheimer’s Disease. Curr. Med. Chem. 2014, 22, 373–404. [Google Scholar] [CrossRef]
- Codony, S.; Pont, C.; Griñán-Ferré, C.; Di Pede-Mattatelli, A.; Calvó-Tusell, C.; Feixas, F.; Osuna, S.; Jarné-Ferrer, J.; Naldi, M.; Bartolini, M.; et al. Discovery and In Vivo Proof of Concept of a Highly Potent Dual Inhibitor of Soluble Epoxide Hydrolase and Acetylcholinesterase for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2022, 65, 4909–4925. [Google Scholar] [CrossRef]
- Oset-Gasque, M.J.; Marco-Contelles, J. Alzheimer’s Disease, the “One-Molecule, One-Target” Paradigm, and the Multitarget Directed Ligand Approach. ACS Chem. Neurosci. 2018, 9, 401–403. [Google Scholar] [CrossRef] [Green Version]
- Ismaili, L. Multicomponent Reactions for Multitargeted Compounds for Alzheimer’s Disease. Curr. Top. Med. Chem. 2018, 17, 3319–3327. [Google Scholar] [CrossRef]
- Ismaili, L.; Monnin, J.; Etievant, A.; Arribas, R.L.; Viejo, L.; Refouvelet, B.; Soukup, O.; Janockova, J.; Hepnarova, V.; Korabecny, J.; et al. (±)-BIGI-3h: Pentatarget-Directed Ligand Combining Cholinesterase, Monoamine Oxidase, and Glycogen Synthase Kinase 3β Inhibition with Calcium Channel Antagonism and Antiaggregating Properties for Alzheimer’s Disease. ACS Chem. Neurosci. 2021, 12, 1328–1342. [Google Scholar] [CrossRef]
- Dgachi, Y.; Martin, H.; Malek, R.; Jun, D.; Janockova, J.; Sepsova, V.; Soukup, O.; Iriepa, I.; Moraleda, I.; Maalej, E.; et al. Synthesis and biological assessment of KojoTacrines as new agents for Alzheimer’s disease therapy. J. Enzym. Inhib. Med. Chem. 2018, 34, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Malek, R.; Arribas, R.L.; Palomino-Antolin, A.; Totoson, P.; Demougeot, C.; Kobrlova, T.; Soukup, O.; Iriepa, I.; Moraleda, I.; Diez-Iriepa, D.; et al. New Dual Small Molecules for Alzheimer’s Disease Therapy Combining Histamine H3 Receptor (H3R) Antagonism and Calcium Channels Blockade with Additional Cholinesterase Inhibition. J. Med. Chem. 2019, 62, 11416–11422. [Google Scholar] [CrossRef]
- Dömling, A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef]
- Wang, Z.; Dömling, A. Multicomponent Reactions in Medicinal Chemistry. In Multicomponent Reactions towards Heterocycles; Van der Eycken, E., Sharma, U.K., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 91–137. ISBN 978-3-527-34908-1. [Google Scholar]
- Matos, L.H.S.; Masson, F.T.; Simeoni, L.A.; Homem-De-Mello, M. Biological activity of dihydropyrimidinone (DHPM) derivatives: A systematic review. Eur. J. Med. Chem. 2018, 143, 1779–1789. [Google Scholar] [CrossRef]
- Grover, G.J.; Dzwonczyk, S.; McMullen, D.M.; Normandin, D.E.; Parham, C.S.; Sleph, P.G.; Moreland, S. Pharmacologic Profile of the Dihydropyrimidine Calcium Channel Blockers SQ 32,547 and SQ 32,926 [Correction of SQ 32,946]. J. Cardiovasc. Pharmacol. 1995, 26, 289–294. [Google Scholar] [CrossRef]
- Irer, S.V.; Alper, G.E.; Sezer, E.D.; Duman, E.; Saatcioglu, F.; Yilmaz, C. The effect of l-deprenyl on tissue mRNA expressions of NOS isoforms and NO levels in an experimental diabetes mellitus model. J. Neural Transm. 2007, 114, 811–815. [Google Scholar] [CrossRef]
- Czerniczyniec, A.; Bustamante, J.; Lores-Arnaiz, S. Modulation of brain mitochondrial function by deprenyl. Neurochem. Int. 2006, 48, 235–241. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Neuroprotective Function of Rasagiline and Selegiline, Inhibitors of Type B Monoamine Oxidase, and Role of Monoamine Oxidases in Synucleinopathies. Int. J. Mol. Sci. 2022, 23, 11059. [Google Scholar] [CrossRef]
- Nakaso, K.; Nakamura, C.; Sato, H.; Imamura, K.; Takeshima, T.; Nakashima, K. Novel cytoprotective mechanism of anti-parkinsonian drug deprenyl: PI3K and Nrf2-derived induction of antioxidative proteins. Biochem. Biophys. Res. Commun. 2006, 339, 915–922. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Prim. 2015, 1, 15056. [Google Scholar] [CrossRef]
- Chakroborty, S.; Stutzmann, G.E. Calcium channelopathies and Alzheimer’s disease: Insight into therapeutic success and failures. Eur. J. Pharmacol. 2014, 739, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. The Keap1-Nrf2-antioxidant response element pathway: A review of its regulation by melatonin and the proteasome. Mol. Cell. Endocrinol. 2014, 401, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Mertens, M.D.; Hinz, S.; Müller, C.E.; Gütschow, M. Alkynyl–coumarinyl ethers as MAO-B inhibitors. Bioorganic Med. Chem. 2014, 22, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Carling, W.R.; Moore, K.W. Imidazolone and Oxazolone Derivatives as Dopamine Antagonists. U.S. Patent No. 5,698,573, 16 December 1997. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef]
- Chioua, M.; Buzzi, E.; Moraleda, I.; Iriepa, I.; Maj, M.; Wnorowski, A.; Giovannini, C.; Tramarin, A.; Portali, F.; Ismaili, L.; et al. Tacripyrimidines, the first tacrine-dihydropyrimidine hybrids, as multi-target-directed ligands for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 155, 839–846. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, A.C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC−Fluorescein) Assay. J. Agric. Food Chem. 2003, 52, 48–54. [Google Scholar] [CrossRef]
- Benchekroun, M.; Ismaili, L.; Pudlo, M.; Luzet, V.; Gharbi, T.; Refouvelet, B.; Marco-Contelles, J. Donepezil–ferulic acid hybrids as anti-Alzheimer drugs. Futur. Med. Chem. 2015, 7, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J.; Hayes, J.D.; Wolf, C.R. Generation of a Stable Antioxidant Response Element–Driven Reporter Gene Cell Line and Its Use to Show Redox-Dependent Activation of Nrf2 by Cancer Chemotherapeutic Agents. Cancer Res. 2006, 66, 10983–10994. [Google Scholar] [CrossRef] [Green Version]
- Pachón-Angona, I.; Martin, H.; Chhor, S.; Oset-Gasque, M.J.; Refouvelet, B.; Marco-Contelles, J.; Ismaili, L. Synthesis of new ferulic/lipoic/comenic acid-melatonin hybrids as antioxidants and Nrf2 activators via Ugi reaction. Futur. Med. Chem. 2019, 11, 3097–3108. [Google Scholar] [CrossRef]
- Parada, E.; Buendia, I.; León, R.; Negredo, P.; Romero, A.; Cuadrado, A.; López, M.G.; Egea, J. Neuroprotective effect of melatonin against ischemia is partially mediated by alpha-7 nicotinic receptor modulation and HO-1 overexpression. J. Pineal Res. 2014, 56, 204–212. [Google Scholar] [CrossRef]
- González-Olvera, R.; Román-Rodríguez, V.; Negrón-Silva, G.E.; Espinoza-Vázquez, A.; Rodríguez-Gómez, F.J.; Santillan, R. Multicomponent Synthesis and Evaluation of New 1,2,3-Triazole Derivatives of Dihydropyrimidinones as Acidic Corrosion Inhibitors for Steel. Molecules 2016, 21, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pachòn Angona, I.; Daniel, S.; Martin, H.; Bonet, A.; Wnorowski, A.; Maj, M.; Jóźwiak, K.; Silva, T.B.; Refouvelet, B.; Borges, F.; et al. Design, Synthesis and Biological Evaluation of New Antioxidant and Neuroprotective Multitarget Directed Ligands Able to Block Calcium Channels. Molecules 2020, 25, 1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angona, I.P.; Martin, H.; Daniel, S.; Moraleda, I.; Bonet, A.; Wnorowski, A.; Maj, M.; Jozwiak, K.; Iriepa, I.; Refouvelet, B.; et al. Synthesis of Hantzsch Adducts as Cholinesterases and Calcium Flux inhibitors, Antioxidants and Neuroprotectives. Int. J. Mol. Sci. 2020, 21, 7652. [Google Scholar] [CrossRef] [PubMed]



| Compound | CD (µM) |
|---|---|
| BIGI 4b | 7.1 ± 0.8 |
| BIGI 4d | 7.7 ± 2.9 |
| BIGI 5b | 13.5 ± 9.1 |
| BIGI 5d | 33.4 ± 4.8 |
| TBHQ | 0.8 ± 0.2 |
| Name | Molweight (g/mol) | CLogP | CLogS | H-Donors | H-Acceptors | Druglikeness | TPSA (Ų) |
|---|---|---|---|---|---|---|---|
| BIGI 4a | 501.625 | 4.0324 | −4.489 | 1 | 7 | 3.8852 | 71.11 |
| BIGI 4b | 515.652 | 4.4868 | −4.759 | 1 | 7 | 3.5578 | 71.11 |
| BIGI 4c | 515.652 | 4.4868 | −4.759 | 1 | 7 | 2.3145 | 71.11 |
| BIGI 4d | 529.679 | 4.9412 | −5.029 | 1 | 7 | 1.9887 | 71.11 |
| BIGI 5a | 517.692 | 4.3929 | −4.573 | 1 | 6 | 2.6429 | 86.13 |
| BIGI 5b | 531.719 | 4.8473 | −4.843 | 1 | 6 | 2.2966 | 86.13 |
| BIGI 5c | 531.719 | 4.8473 | −4.843 | 1 | 6 | 1.0706 | 86.13 |
| BIGI 5d | 531.719 | 4.8473 | −4.843 | 1 | 6 | 1.0706 | 86.13 |
| Donepezil | 379.498 | 4.2149 | −4.347 | 0 | 4 | 5.6409 | 38.77 |
| Selegiline | 173.258 | 1.8326 | −2.606 | 1 | 1 | 4.7279 | 12.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malek, R.; Simakov, A.; Davis, A.; Maj, M.; Bernard, P.J.; Wnorowski, A.; Martin, H.; Marco-Contelles, J.; Chabchoub, F.; Dallemagne, P.; et al. Biginelli Reaction Synthesis of Novel Multitarget-Directed Ligands with Ca2+ Channel Blocking Ability, Cholinesterase Inhibition, Antioxidant Capacity, and Nrf2 Activation. Molecules 2023, 28, 71. https://doi.org/10.3390/molecules28010071
Malek R, Simakov A, Davis A, Maj M, Bernard PJ, Wnorowski A, Martin H, Marco-Contelles J, Chabchoub F, Dallemagne P, et al. Biginelli Reaction Synthesis of Novel Multitarget-Directed Ligands with Ca2+ Channel Blocking Ability, Cholinesterase Inhibition, Antioxidant Capacity, and Nrf2 Activation. Molecules. 2023; 28(1):71. https://doi.org/10.3390/molecules28010071
Chicago/Turabian StyleMalek, Rim, Alexey Simakov, Audrey Davis, Maciej Maj, Paul J. Bernard, Artur Wnorowski, Helene Martin, José Marco-Contelles, Fakher Chabchoub, Patrick Dallemagne, and et al. 2023. "Biginelli Reaction Synthesis of Novel Multitarget-Directed Ligands with Ca2+ Channel Blocking Ability, Cholinesterase Inhibition, Antioxidant Capacity, and Nrf2 Activation" Molecules 28, no. 1: 71. https://doi.org/10.3390/molecules28010071
APA StyleMalek, R., Simakov, A., Davis, A., Maj, M., Bernard, P. J., Wnorowski, A., Martin, H., Marco-Contelles, J., Chabchoub, F., Dallemagne, P., Rochais, C., Jozwiak, K., & Ismaili, L. (2023). Biginelli Reaction Synthesis of Novel Multitarget-Directed Ligands with Ca2+ Channel Blocking Ability, Cholinesterase Inhibition, Antioxidant Capacity, and Nrf2 Activation. Molecules, 28(1), 71. https://doi.org/10.3390/molecules28010071









