Efficient Treatment of Oily Sludge via Fast Microwave-Assisted Pyrolysis, Followed by Thermal Plasma Vitrification
Abstract
1. Introduction
2. Results and Discussion
2.1. Fast MAP of Oily Sludge
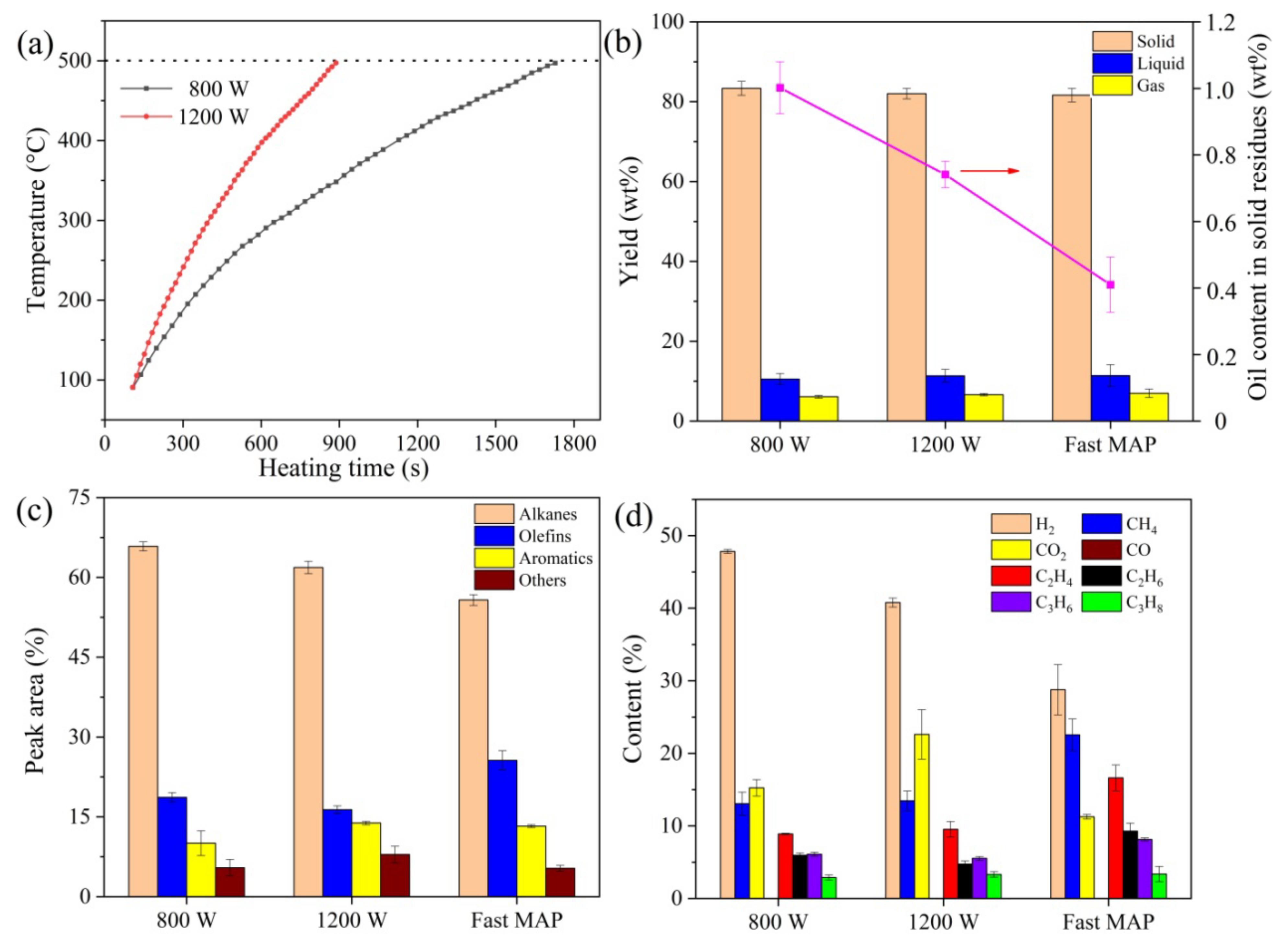
2.1.1. Comparison between Fast MAP and MAP While Using Premixing Mode
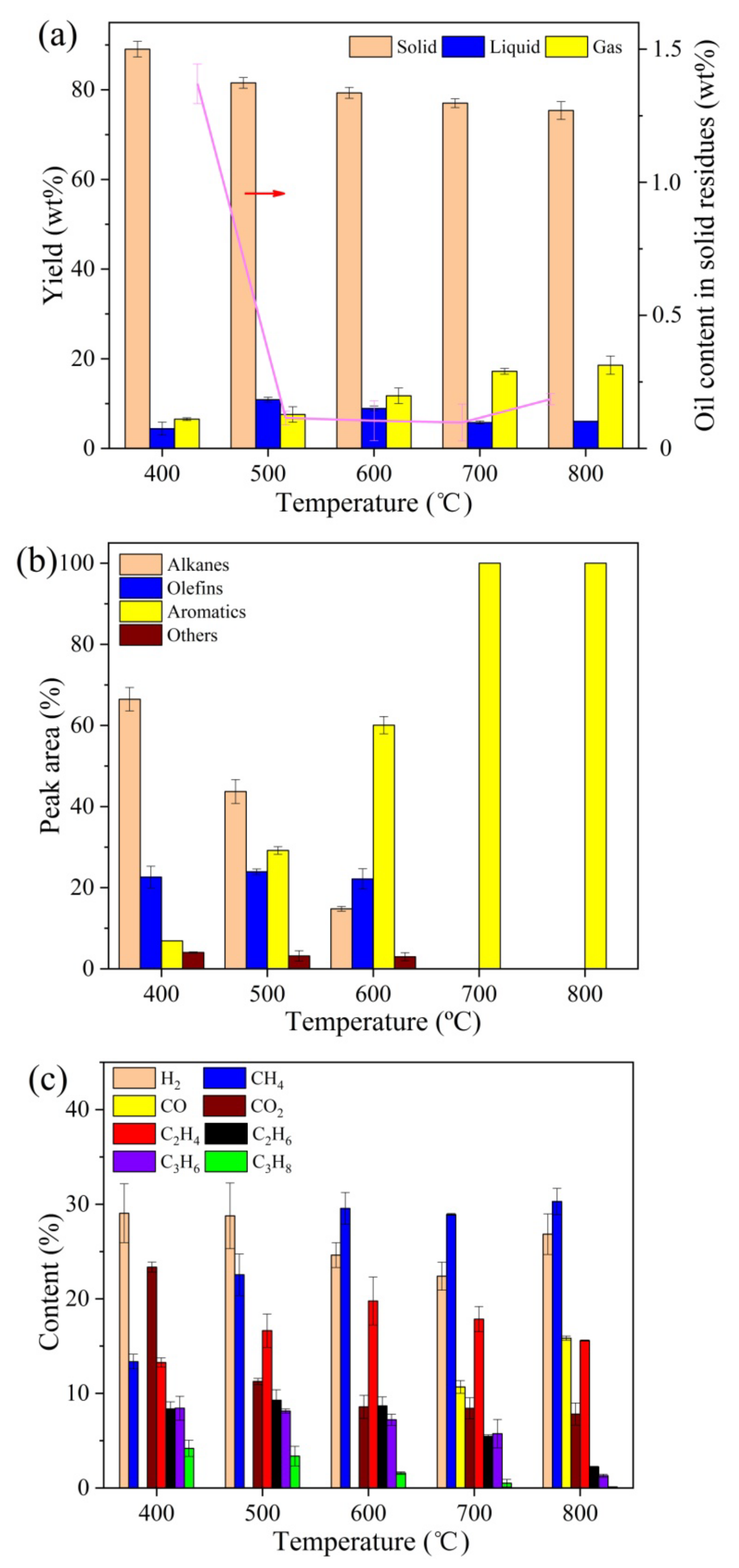
2.1.2. Effect of Temperature
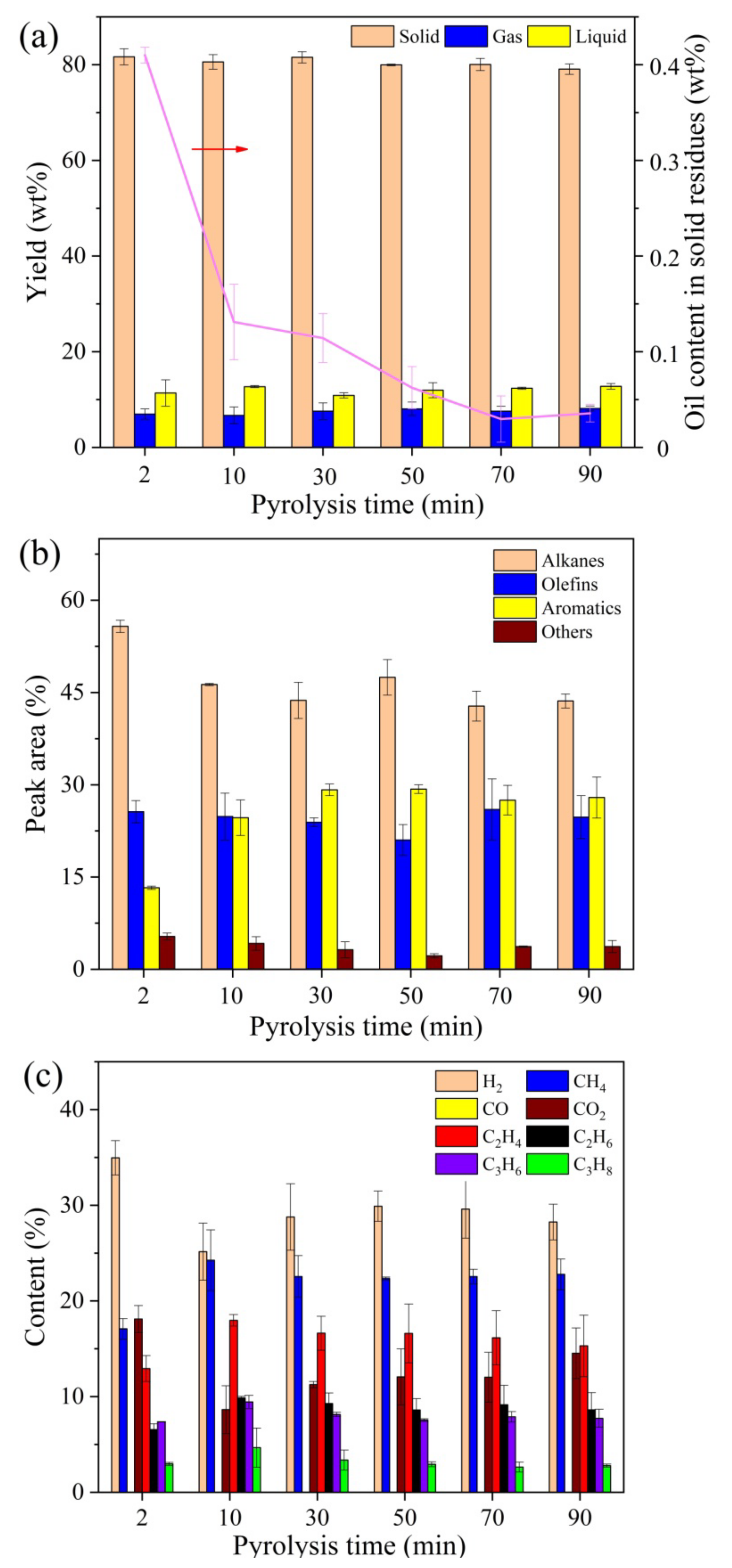
2.1.3. Effect of Pyrolysis Time
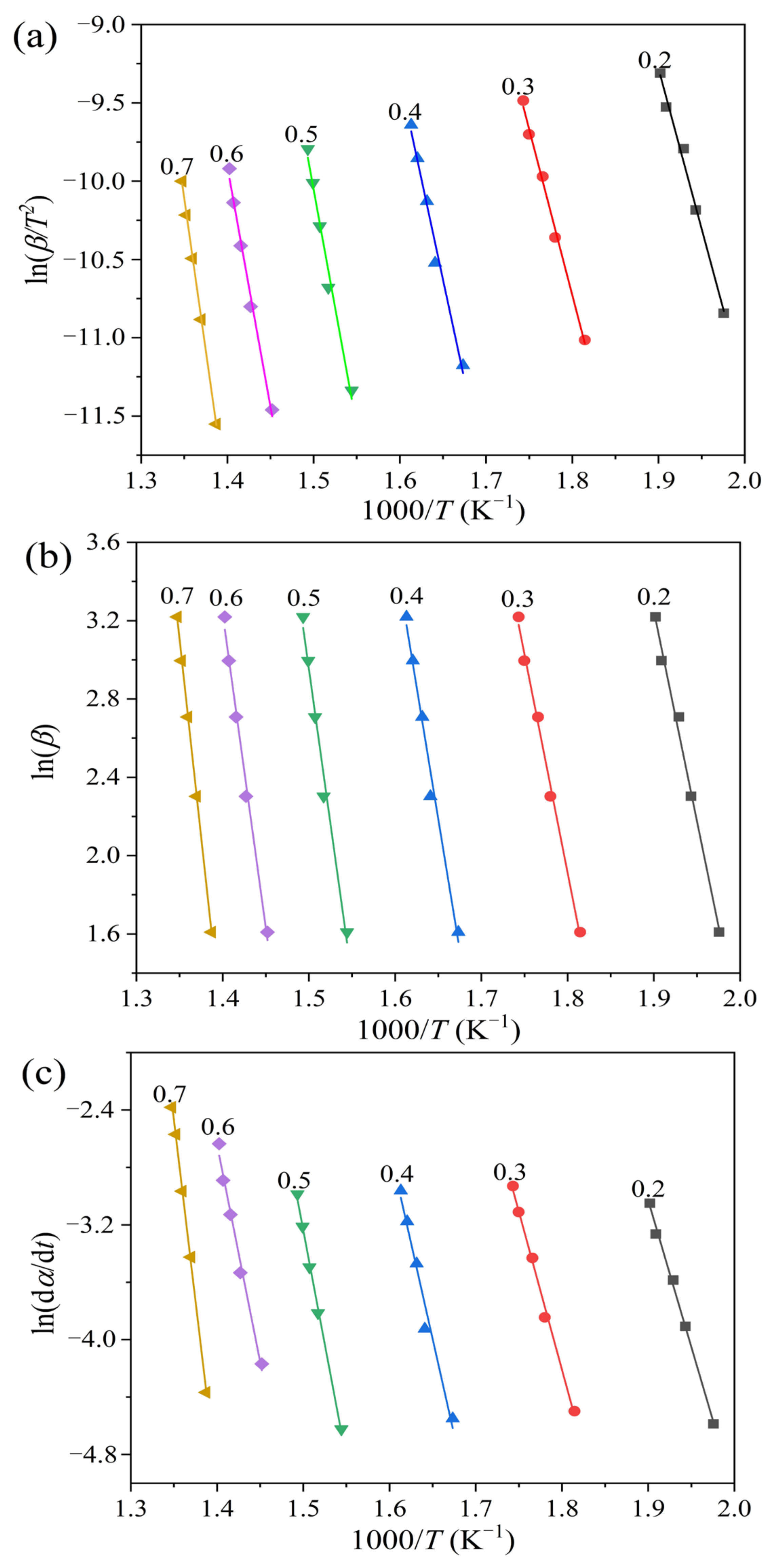
2.2. Pyrolysis Kinetics
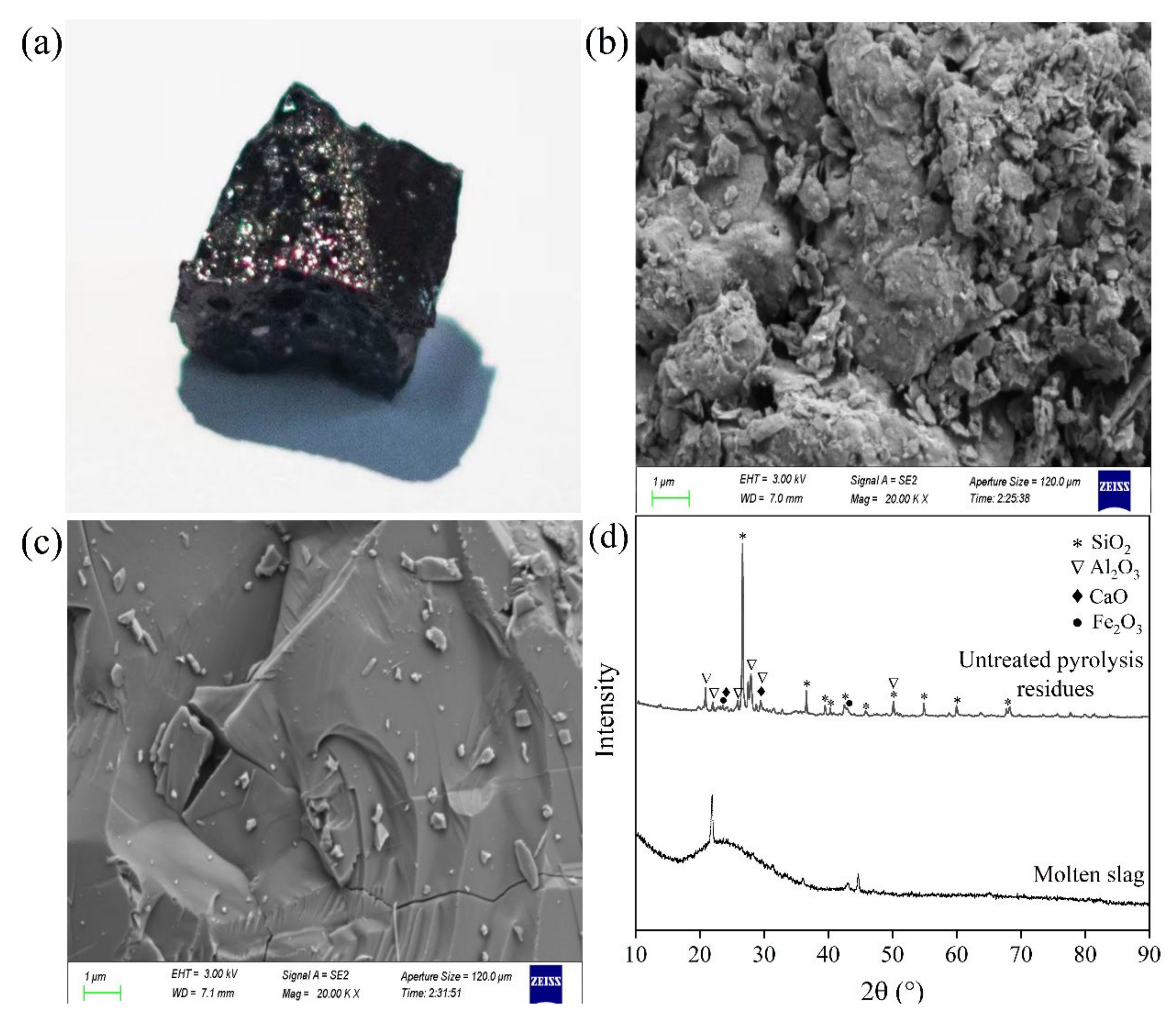
2.3. Thermal Plasma Vitrification of Pyrolysis Residues
2.3.1. Effect of Working Current
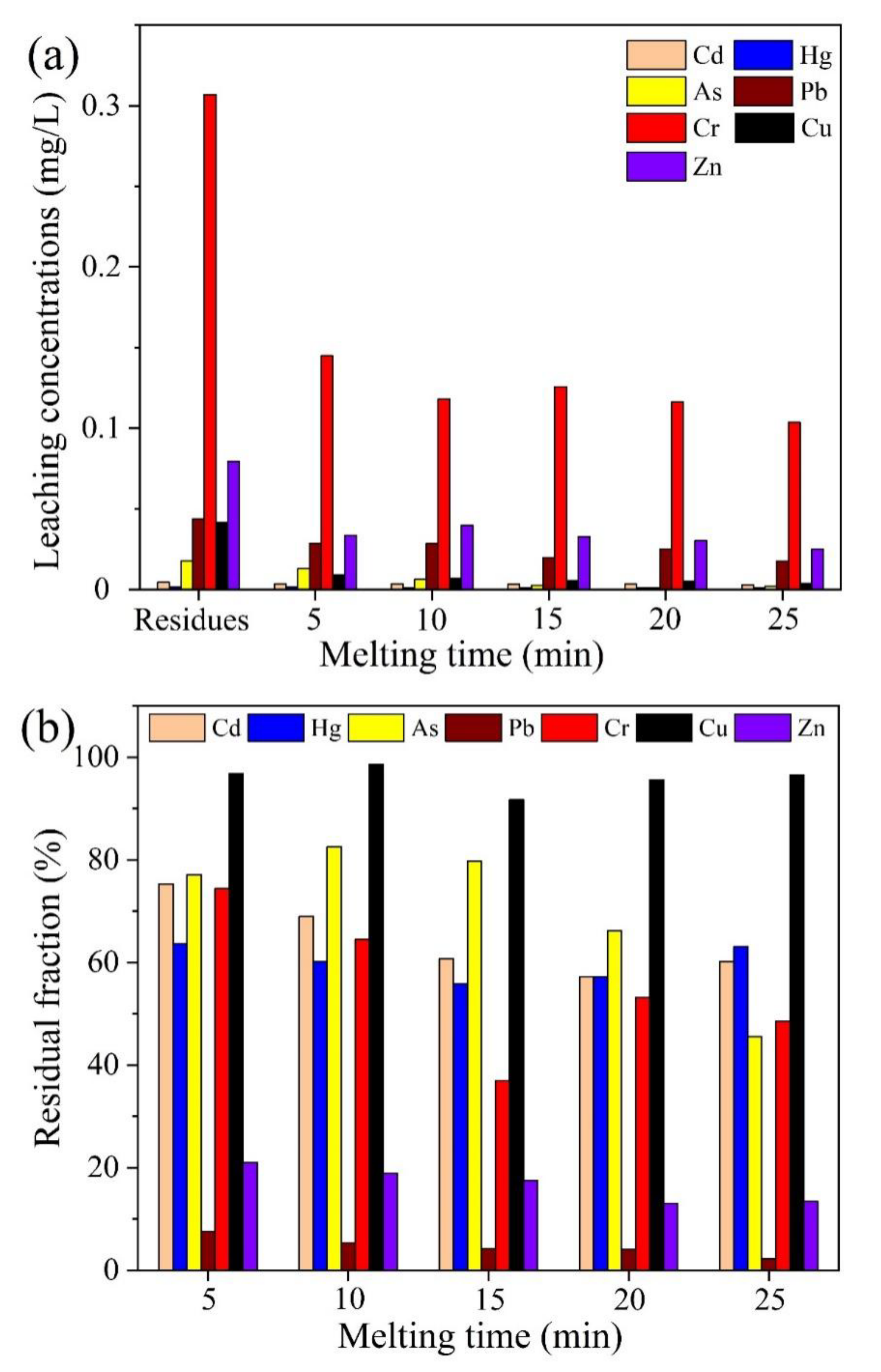
2.3.2. Effect of Melting Time
3. Materials and Methods
3.1. Materials Preparation
3.2. Experimental Apparatus
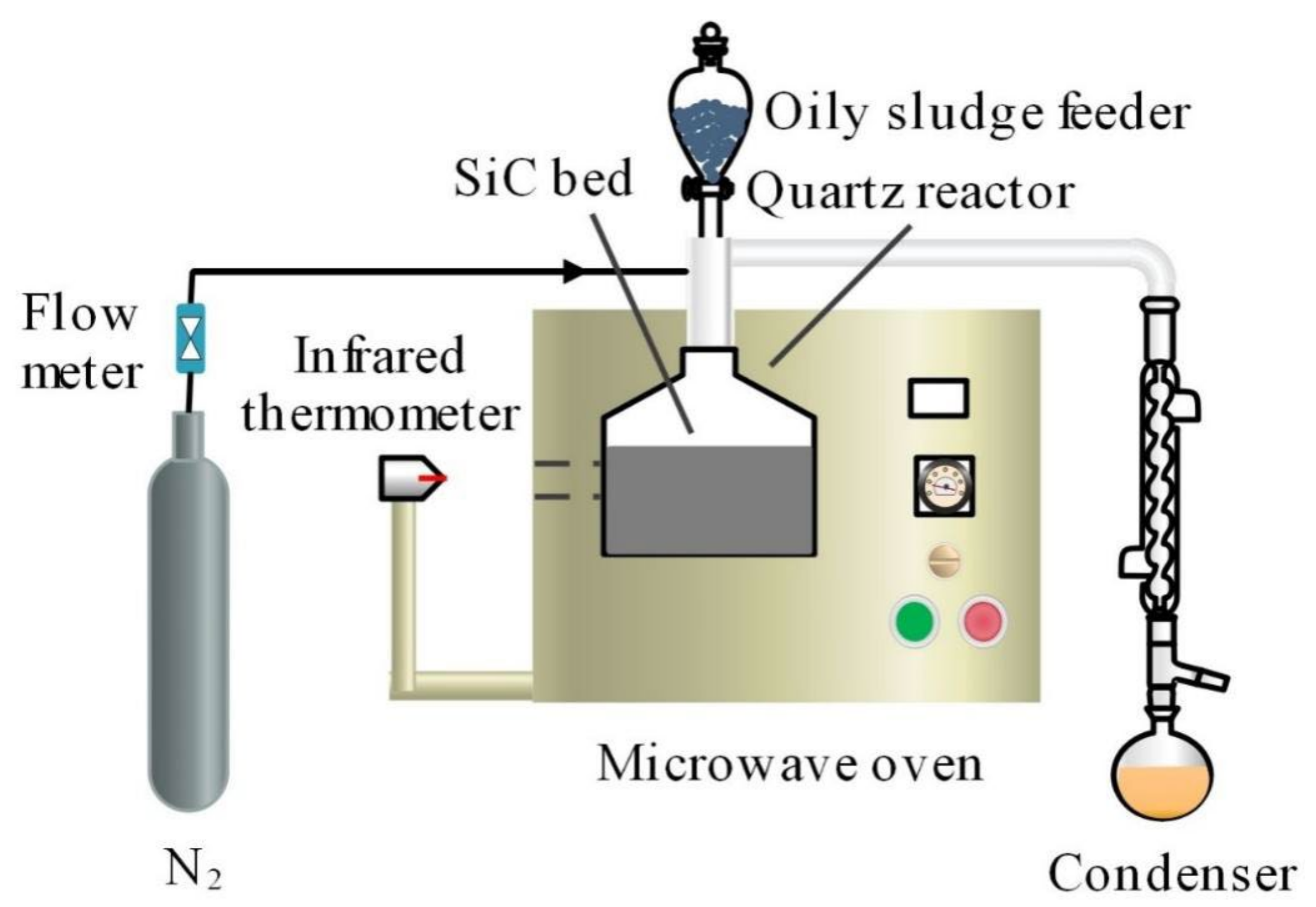
3.2.1. Fast MAP of Oily Sludge
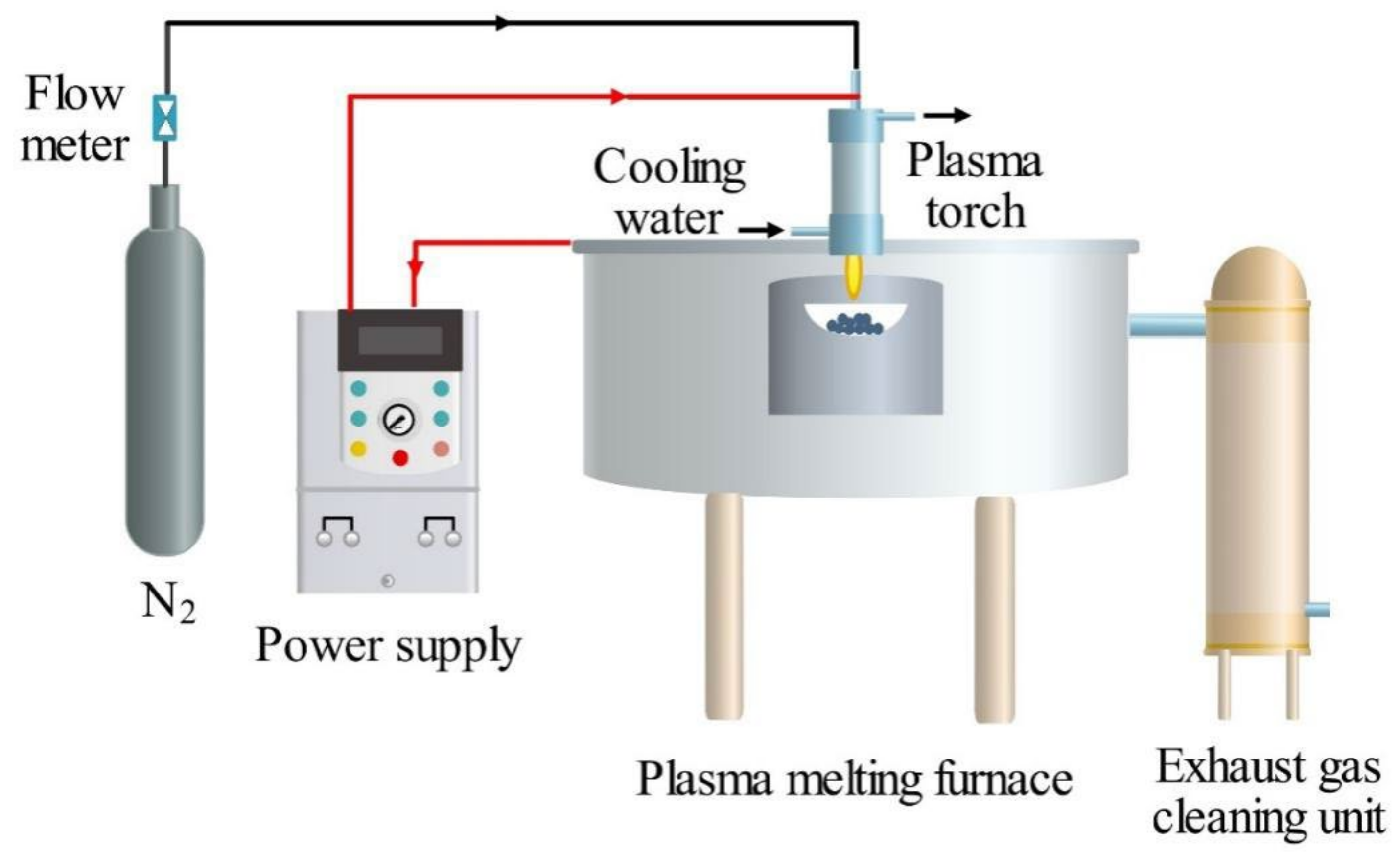
3.2.2. Thermal Plasma Vitrification of Pyrolysis Residues
3.3. Product Analysis
3.4. Kinetic Study of Oily Sludge Pyrolysis
3.4.1. The Kissinger-Akahira-Sunose (KAS) Method
3.4.2. The Flynn-Wall-Ozawa (FWO) Method
3.4.3. The Friedman Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Niu, A.; Sun, X.; Lin, C. Trend in research on characterization, environmental impacts and treatment of oily sludge: A systematic review. Molecules 2022, 27, 227795. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Li, J.; Quan, C.; Tan, H. Product property and environmental risk assessment of heavy metals during pyrolysis of oily sludge with fly ash additive. Fuel 2020, 266, 117090. [Google Scholar] [CrossRef]
- Hui, K.; Tang, J.; Lu, H.; Xi, B.; Qu, C.; Li, J. Status and prospect of oil recovery from oily sludge: A review. Arabian J. Chem. 2020, 13, 6523–6543. [Google Scholar] [CrossRef]
- Lv, X.; Song, Z.; Yu, J.; Su, Y.; Zhao, X.; Sun, J.; Mao, Y.; Wang, W. Study on the demulsification of refinery oily sludge enhanced by microwave irradiation. Fuel 2020, 279, 118417. [Google Scholar] [CrossRef]
- Hu, C.; Fu, S.; Zhu, L.; Dang, W.; Zhang, T. Evaluation and prediction on the effect of ionic properties of solvent extraction performance of oily sludge using machine learning. Molecules 2021, 26, 247551. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Du, H.; Peng, X.; Tang, Y.; Zhou, Y.; Chen, X.; Fei, J.; Meng, Y.; Yuan, L. Degradation of several polycyclic aromatic hydrocarbons by laccase in reverse micelle system. Sci. Total Environ. 2020, 708, 134970. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Feng, H.; He, P.; Li, J.; Hewage, K.; Sadiq, R. Comparative life-cycle assessment of traditional and emerging oily sludge treatment approaches. J. Clean. Prod. 2020, 251, 119594. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, Z.; Wang, Z.; Li, X.; Chu, Z. Application and development of pyrolysis technology in petroleum oily sludge treatment. Environ. Eng. Res. 2021, 26, 190460. [Google Scholar] [CrossRef]
- Johnson, O.; Afam, A. Petroleum sludge treatment and disposal: A review. Environ. Eng. Res. 2019, 24, 191–201. [Google Scholar] [CrossRef]
- Khan, M.; Cahyadi, H.; Kim, S.; Kim, J. Efficient oil recovery from highly stable toxic oily sludge using supercritical water. Fuel 2019, 235, 460–472. [Google Scholar] [CrossRef]
- Teng, Q.; Zhang, D.; Yang, C. A review of the application of different treatment processes for oily sludge. Environ. Sci. Pollut. Res. 2020, 28, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.; Sikarwar, V.; He, J.; Dastyar, W.; Dionysiou, D.; Wang, W.; Zhao, M. Opportunities and challenges in sustainable treatment and resource reuse of sewage sludge: A review. Chem. Eng. J. 2018, 337, 616–641. [Google Scholar] [CrossRef]
- Yu, S.; Duan, Y.; Zhou, X.; Xie, Q.; Zeng, G.; Mao, X.; Liang, X.; Lu, M.; Nie, Y.; Ji, J. Three-dimensional simulation of a novel microwave-assisted heating device for methyl ricinoleate pyrolysis. Appl. Therm. Eng. 2019, 153, 341–351. [Google Scholar] [CrossRef]
- Risco, A.; Sucunza, D.; Gonzalez-Egido, S. Chemical recovery of waste electrical and electronic equipment by microwave-assisted pyrolysis: A review. J. Anal. Appl. Pyrolysis 2021, 159, 105323. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Y.; Zhang, T.; Jiang, Z.; Siyal, A.; Dai, J.; Fu, J.; Zhou, C.; Wang, L.; Li, X.; et al. Microwave-assisted pyrolysis of oily sludge from offshore oilfield for recovery of high-quality products. J. Hazard. Mater. 2021, 420, 126578. [Google Scholar] [CrossRef]
- Chen, Y. Microwave pyrolysis of oily sludge with activated carbon. Environ. Technol. 2016, 37, 3139–3145. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Q.; Yang, S.; Yang, Q.; Wu, J.; Ma, Z.; Jiang, L.; Yu, Z.; Dai, L.; Liu, Y.; et al. Microwave-assisted catalytic fast pyrolysis coupled with microwave-absorbent of soapstock for bio-oil in a downdraft reactor. Energy Convers. Manage 2019, 185, 11–20. [Google Scholar] [CrossRef]
- Tian, Y.; Zuo, W.; Ren, Z.; Chen, D. Estimation of a novel method to produce bio-oil from sewage sludge by microwave pyrolysis with the consideration of efficiency and safety. Bioresour. Technol. 2011, 102, 2053–2061. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Liu, S.; Fan, L.; Zhou, N.; Peng, P.; Wang, Y.; Guo, F.; Min, M.; Cheng, Y. Fast microwave-assisted pyrolysis of wastes for biofuels production-A review. Bioresour. Technol. 2020, 297, 122480. [Google Scholar] [CrossRef]
- Li, J.; Lin, F.; Li, K.; Zheng, F.; Yan, B.; Che, L.; Tian, W.; Chen, G.; Yoshikawa, K. A critical review on energy recovery and non-hazardous disposal of oily sludge from petroleum industry by pyrolysis. J. Hazard. Mater. 2021, 406, 124706. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, L.; Liu, F.; Liu, H.; Zhang, N.; Zhu, Y. Energy, environment and economy assessment of medical waste disposal technologies in China. Sci. Total Environ. 2021, 796, 148964. [Google Scholar] [CrossRef] [PubMed]
- Sikarwar, V.; Hrabovský, M.; Oost, G.; Pohořelý, M.; Jeremiáš, M. Progress in waste utilization via thermal plasma. Prog. Energy Combust. Sci. 2020, 81, 100873. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Chi, Y.; Li, X.; Lu, S. Application of thermal plasma to vitrify fly ash from municipal solid waste incinerators. Chemosphere 2010, 78, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Al Arni, S. Comparison of slow and fast pyrolysis for converting biomass into fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Bok, J.; Choi, H.; Choi, Y.; Park, H.; Kim, S. Fast pyrolysis of coffee grounds: Characteristics of product yields and biocrude oil quality. Energy 2012, 47, 17–24. [Google Scholar] [CrossRef]
- Gautam, R.; Shyam, S.; Reddy, B.; Govindaraju, K.; Vinu, R. Microwave-assisted pyrolysis and analytical fast pyrolysis of macroalgae: Product analysis and effect of heating mechanism. Sustain. Energy Fuels 2019, 3, 3009–3020. [Google Scholar] [CrossRef]
- Quan, C.; Zhang, G.; Xu, L.; Wang, J.; Gao, N. Improvement of the pyrolysis products of oily sludge: Catalysts and catalytic process. J. Energy Inst. 2022, 104, 67–79. [Google Scholar] [CrossRef]
- Yan, M.; Xu, D.; Wu, B.; Yang, Y.; Li, Y. Insight into the performance of different Pt/KL catalysts for n-alkane (C6–C8) aromatization: Catalytic role of zeolite channels. Catal. Sci. Technol. 2022, 12, 1610–1618. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Q.; Liu, X.; Cao, C. Low temperature pyrolysis characteristics of oil sludge under various heating conditions. Energy Fuels 2007, 21, 957–962. [Google Scholar] [CrossRef]
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef]
- Huang, X.; Cao, J.; Shi, P.; Zhao, X.; Feng, X.; Zhao, Y.; Fan, X.; Wei, X.; Takarada, T. Influences of pyrolysis conditions in the production and chemical composition of the bio-oils from fast pyrolysis of sewage sludge. J. Anal. Appl. Pyrolysis 2014, 110, 353–362. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y.; Wang, X.; Wan, Y.; Chen, Q.; Wang, C.; Lin, X.; Liu, Y.; Chen, P.; Ruan, R. Microwave-assisted pyrolysis of microalgae for biofuel production. Bioresour. Technol. 2011, 102, 4890–4896. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Sun, A.; Wu, X.; Hai, G.; Hu, J.; Li, T.; Li, G. The preparation of nano-sized H[Zn, Al]ZSM-5 zeolite and its application in the aromatization of methanol. Microporous Mesoporous Mater. 2011, 143, 435–442. [Google Scholar] [CrossRef]
- Fan, S.; Wang, D.; Li, H.; Tuo, J.; Zhang, X.; Gao, X.; Zhao, T. Enhancing stability and coaromatization of n-hexane and methanol over [Zn,Cr]/HZSM-5. Appl. Catal. A Gen. 2020, 599, 117602. [Google Scholar] [CrossRef]
- Fanchiang, W.; Lin, Y. Catalytic fast pyrolysis of furfural over H-ZSM-5 and Zn/H-ZSM-5 catalysts. Appl. Catal. A Gen. 2012, 419, 102–110. [Google Scholar] [CrossRef]
- Gabrienko, A.; Arzumanov, S.; Freude, D.; Stepanov, A. Propane aromatization on Zn-modified zeolite BEA studied by solid-state NMR in situ. J. Phys. Chem. C 2010, 114, 12681–12688. [Google Scholar] [CrossRef]
- Abd-Elghany, M.; Klapotke, T.; Elbeih, A. Thermal behavior and decomposition kinetics of bis(2,2,2-trinitroethyl)-oxalate as a high energy dense oxidizer and its mixture with nitrocellulose. Propellants Explos. Pyrotech. 2017, 42, 1373–1381. [Google Scholar] [CrossRef]
- Cai, J.; Xu, D.; Dong, Z.; Yu, X.; Yang, Y.; Banks, S.; Bridgwater, A. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: Case study of corn stalk. Renew. Sustain. Energy Rev. 2018, 82, 2705–2715. [Google Scholar] [CrossRef]
- Dhyani, V.; Kumar, J.; Bhaskar, T. Thermal decomposition kinetics of sorghum straw via thermogravimetric analysis. Bioresour. Technol. 2017, 245, 1122–1129. [Google Scholar] [CrossRef]
- Popescu, C. Integral method to analyze the kinetics of heterogeneous reactionsunder non-isothermal conditions: A variant on the Ozawa-Flynn-Wall method. Thermochim. Acta 1996, 285, 309–323. [Google Scholar] [CrossRef]
- Chong, C.; Mong, G.; Ng, J.; Chong, W.; Ani, F.; Lam, S.; Ong, H. Pyrolysis characteristics and kinetic studies of horse manure using thermogravimetric analysis. Energy Convers. Manag. 2019, 180, 1260–1267. [Google Scholar] [CrossRef]
- Deng, L.; Yao, B.; Lu, W.; Zhang, M.; Li, H.; Chen, H.; Zhao, M.; Du, Y.; Zhang, M.; Ma, Y. Effect of SiO2/Al2O3 ratio on the crystallization and heavy metal immobilization of glass ceramics derived from stainless steel slag. J. Non-Cryst. Solids 2022, 593, 121770. [Google Scholar] [CrossRef]
- Vu, D.; Wang, K.; Chen, J.; Nam, B.; Bac, B. Glass-ceramic from mixtures of bottom ash and fly ash. Waste Manag. 2012, 32, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Liu, L.; Shen, B. Preparation and characterization of glass-ceramics via co-sintering of coal fly ash and oil shale ash-derived amorphous slag. Ceram. Int. 2019, 45, 20058–20065. [Google Scholar] [CrossRef]
- Ma, W.; Shi, W.; Shi, Y.; Chen, D.; Liu, B.; Chu, C.; Li, D.; Li, Y.; Chen, G. Plasma vitrification and heavy metals solidification of MSW and sewage sludge incineration fly ash. J. Hazard. Mater. 2021, 408, 124809. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, J.; Sun, F.; Wu, S.; Pan, Y.; Zhou, J.; Qian, G. Heavy metal leaching and distribution in glass products from the co-melting treatment of electroplating sludge and MSWI fly ash. J. Environ. Manag. 2019, 232, 226–235. [Google Scholar] [CrossRef]
- Mao, L.; Wu, Y.; Hu, L.; Huang, Q.; Dai, Z.; Zhang, W. Activity calculation and phase transformation predication for heavy metals with slag activity calculation model during using electroplating sludge in production of bricks. J. Environ. Chem. Eng. 2019, 7, 103242. [Google Scholar] [CrossRef]
- Xie, Q.; Li, S.; Gong, R.; Zheng, G.; Wang, Y.; Xu, P.; Duan, Y.; Yu, S.; Lu, M.; Ji, W.; et al. Microwave-assisted catalytic dehydration of glycerol for sustainable production of acrolein over a microwave absorbing catalyst. Appl. Catal. B Environ. 2019, 243, 455–463. [Google Scholar] [CrossRef]
- Yu, S.; Duan, Y.; Mao, X.; Xie, Q.; Zeng, G.; Lu, M.; Nie, Y.; Ji, J. Pyrolysis of methyl ricinoleate by microwave-assisted heating coupled with atomization feeding. J. Anal. Appl. Pyrolysis 2018, 135, 176–183. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Sunol, J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Chen, W.; Ye, M.; Li, M.; Xi, B.; Hou, J.; Qi, X.; Zhang, J.; Wei, Y.; Meng, F. Characteristics, kinetics and product distribution on pyrolysis process for waste wind turbine blades. J. Anal. Appl. Pyrolysis 2023, 169, 105859. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Thermogravimetric analysis and kinetic modeling of the AAEM-catalyzed pyrolysis of woody biomass. Molecules 2022, 27, 227662. [Google Scholar] [CrossRef] [PubMed]


| α | KAS Method | FWO Method | Friedman Method | |||
|---|---|---|---|---|---|---|
| Eα (kJ/mol) | R2 | Eα (kJ/mol) | R2 | Eα (kJ/mol) | R2 | |
| 0.2 | 170.0 | 0.9919 | 169.7 | 0.9927 | 169.8 | 0.9966 |
| 0.3 | 176.2 | 0.9948 | 176.3 | 0.9953 | 183.3 | 0.9949 |
| 0.4 | 213.4 | 0.9857 | 212.4 | 0.9869 | 221.9 | 0.9762 |
| 0.5 | 251.8 | 0.9871 | 249.7 | 0.9881 | 265.0 | 0.9975 |
| 0.6 | 255.0 | 0.9924 | 253.5 | 0.9930 | 287.2 | 0.9890 |
| 0.7 | 318.5 | 0.9996 | 319.1 | 0.9996 | 419.0 | 0.9987 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Q.; Chen, Z.; Zhou, Y.; Pan, T.; Duan, Y.; Yu, S.; Liang, X.; Wu, Z.; Ji, W.; Nie, Y. Efficient Treatment of Oily Sludge via Fast Microwave-Assisted Pyrolysis, Followed by Thermal Plasma Vitrification. Molecules 2023, 28, 4036. https://doi.org/10.3390/molecules28104036
Xie Q, Chen Z, Zhou Y, Pan T, Duan Y, Yu S, Liang X, Wu Z, Ji W, Nie Y. Efficient Treatment of Oily Sludge via Fast Microwave-Assisted Pyrolysis, Followed by Thermal Plasma Vitrification. Molecules. 2023; 28(10):4036. https://doi.org/10.3390/molecules28104036
Chicago/Turabian StyleXie, Qinglong, Zhen Chen, Yuqiang Zhou, Tongbo Pan, Ying Duan, Shangzhi Yu, Xiaojiang Liang, Zhenyu Wu, Weirong Ji, and Yong Nie. 2023. "Efficient Treatment of Oily Sludge via Fast Microwave-Assisted Pyrolysis, Followed by Thermal Plasma Vitrification" Molecules 28, no. 10: 4036. https://doi.org/10.3390/molecules28104036
APA StyleXie, Q., Chen, Z., Zhou, Y., Pan, T., Duan, Y., Yu, S., Liang, X., Wu, Z., Ji, W., & Nie, Y. (2023). Efficient Treatment of Oily Sludge via Fast Microwave-Assisted Pyrolysis, Followed by Thermal Plasma Vitrification. Molecules, 28(10), 4036. https://doi.org/10.3390/molecules28104036









