Abstract
Biomass as a renewable energy resource is a major topic on a global scale. Several types of biomass heat treatment methods have been introduced to obtain useful byproducts via pyrolysis. Microwaves are a practical replacement for conventional stoves and ovens to perform pyrolysis of biomass. Their rapid heating rate and user-friendliness make them a good choice for the pyrolysis process over conventional methods. The current study reviewed research articles that used microwaves for the pyrolysis process on different types of biomass. This study primarily provides comprehensive details about the pyrolysis process, especially microwave-assisted pyrolysis (MAP) and its feasibility for treating biomass. A systematic literature review, according to the PRISMA guidelines, was performed to find research articles on biomass treatment using MAP technology. We analyzed various research studies (n = 32), retrieved from different databases, that used MAP for pyrolysis on various types of biomass, and we achieved good results. The main goal of this study was to examine the usefulness of the MAP technique, comparing its effects on distinguished types of biomass. We found MAP’s effective parameters, namely, temperature, concentration of microwave absorber, moisture percentage of starting material and flow rate, microwave power and residence time of the initial sweep gas that control the pyrolysis process, and effect quality of byproducts. The catalytic agent in MAP pyrolysis was found to be useful for treating biomass, and that it has great potential to increase (nearly double) the production yield. Although MAP could not be used for all types of materials due to some challenges, it produced good results compared to conventional heating (pyrolysis) methods. We concluded that MAP is an effective method for reducing pyrolysis reaction time and improving the quality of value-added products. Also, MAP eliminates the shredding requirement for biomass and improves heating quality. Therefore, it is a viable method for reducing pyrolysis processing costs and should be applied on a larger scale than lab scale for commercialization.
1. Introduction
Fossil fuels are consumed in increasingly large quantities as the global energy demand and population rise, which worsens the energy dilemma and leads to more significant environmental concerns. As a result, residents are paying attention to the deployment of renewable resources and are showing interest in novel environmentally friendly options. Biomass is a renewable energy source that has benefits over conventional fossil fuels owing to its environmental friendliness and simple accessibility [1]. Biomass is defined as material or residue retrieved from living organisms. The term is used in various fields according to the situation, e.g., living organisms (L.Os) in ecology; matter from L.Os in bioenergy and later biomass are considered residuals either from plants, combination of plants and algae, or plants/animals. Mostly, the term “biomass” is used for plant residuals to represent bioenergy that contributes to the mitigation of climate change, e.g., gases generated from the burning of plant wood or residuals [2]. Nowadays, the term “biomass” is used to define a waste residual collected from industrial, residential, and agricultural areas. Raw materials and effluent left behind from industrial processing, solid waste from households, and residuals left behind after harvesting crops or dry plants leaves, etc., are considered biomass waste. The combined effect of biomass waste processing generated from the aforesaid sources leads to the creation of significant issues related to human health and sustainable environmental conditions [3,4,5]. There are three methods for converting the energy contained in biomass into biopower: combustion, bacterial decomposition, and transformation into vapor or liquid fuel. The most common method for converting biomass into useful energy is direct combustion [6]. The thermochemical conversion of biomass comprises both pyrolysis and gasification [7,8]. Each method has its own limitations and advantages. Biomass could yield higher-value end-products by using thermochemical conversion processes such as microwave-assisted pyrolysis. The amount and quality of the world’s energy consumption are affected by a number of variables, including rising populations and expanding economies. The global population has been growing at a staggering pace [9,10,11,12,13], and the utilization of energy sources has significantly increased in recent decades [14,15]. The research community has expressed that the primary cause of global warming is the use of fossil fuels. Increasing carbon dioxide (CO2) emissions can be attributed to both the increasing carbon intensity of energy supply and increasing energy intensity of economic activity [16,17]. In light of the growing demand for energy as a result of an expanding economy and population, researchers, industrialists, and governments have been investigating alternative, less expensive energy sources to mitigate the threat of climate change and global warming [18,19,20].
The method used to treat biomass is of critical importance due to the indirect effect on human wellbeing by affecting the environmental ecosystem. An incinerator is used in the thermal treatment of biomass to perform the incineration process. Mostly, the industrial sector uses incinerators for the burning of biomass, which effects the atmosphere and has become a source of air pollution. Also, the high cost of incinerators, their maintenance, and the handling of residual leftovers after biomass burning creates difficulties in the use of incinerators. Therefore, an alternative method for treating biomass that also reduces the amount of residual leftovers after treatment is direly needed [21,22,23]. The process of incineration induces chemical changes that may produce harmful products that can escape through the stack, causing air pollution, or that can remain in the bottom ash, eventually finding its way into landfills. In developing countries, there are still concerns from experts and local communities about the environmental effect of incinerators. Currently, waste incinerators dispose of ash by sending it to landfills, which is both costly and takes up physical space. In some developed countries (USA, UK, Europe, China, Japan), incinerators built just a few decades ago often did not include a material separation process for removing hazardous, bulky, or recyclable materials before combustion. These facilities tended to risk the health of plant workers and the local environment due to inadequate levels of gas cleaning and combustion process control. Additionally, nearby residents often express displeasure with incinerators’ effects on their quality of life, including increased traffic, odors, and noise. People frequently have to cover their windows and stay inside throughout the summer since the odor becomes unbearable when the weather warms up [24]. To overcome these problems, the pyrolysis process was introduced and used to treat biomass. This process achieved its objectives by (1) reducing the volume of leftover residuals in the case of the incinerator and (2) generating useful byproducts. Also, the byproducts generated from the biomass pyrolysis process have a negligible effect on the atmosphere and even provide electricity and heating through the combustion of particulate emissions. Biomass pyrolysis, which occurs in an oxygen-depleted environment, uses heat to convert biomass feedstock into bio-oil, charcoal, and syngas [25]. The organic matter is thermally destroyed by breaking down chemical linkage bonds in an inert atmosphere [26]. It is an environment in which powder bed fusion may occur without the presence of reactive gases like oxygen and CO2, which are naturally present in the air but might potentially contaminate the reaction [25]. The final products of the pyrolysis process are useful products in gaseous form (synthesis gas—a mixture of hydrogen and carbon monoxide), liquid form (bio-oil), and solid form (bio-char), which increase system efficiency and decrease the effect of emissions on the environment.
An electric furnace was used for pyrolysis, and nitrogen (N2) was used as a cleaning agent. Various instruments are used to perform the pyrolysis process. Because of the advantages of microwave heating over more traditional heating methods, extensive research on microwave-assisted pyrolysis of biomass waste has been conducted over the last decade [26,27,28,29,30]. Various microwave systems may now be constructed, developed, and optimized to treat biomass into marketable end-products [31,32,33,34]. Many research studies have been conducted in recent years to investigate whether microwaves can be used to treat biomass during the pyrolysis process and transform biomass into useful products such as bio-fuel (liquids) and bio-char (solids) [35,36,37,38]. The following studies (oil palm shell [39], paper deinking residue [40], plastics [41], wood sawdust [42], oily sludge [43], sewage sludge [44], cellulose [45], gumwood [46], and rice straw [47]) are recently reported in literature which have used MAP technology to convert biomass into useable products.
Using microwave-assisted pyrolysis (MAP) to treat biomass has several obstacles that should be solved. Primarily, there are two main issues with microwave biomass pyrolysis processing: one is the microwaves themselves, and the other is the processing of biomass materials [48,49,50]. Biomass from different sources can be converted using various methods, but microwaves outperform traditional methods for treatment, pyrolysis, and heating processes [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71]. The ability of a material to convert into energy depends on its dielectric properties. Dielectric heating is a volumetric process because heat is generated inside the material by the selective absorption of electromagnetic energy. However, microwave pyrolysis cannot be used for all materials, e.g., transparent and dry materials, but it works efficiently in materials with a high moisture content. In addition, refined materials along with absorbing agents in the microwave assist in increasing the overall temperature of the reaction. The difficulty of monitoring the temperature in a microwave environment and the thermal damage to treated materials due to non-uniform heating behavior are challenging issues. Because the homogeneity of the microwave field may be improved by enlarging the cavity, a specific instrument design is required. Additionally, microwave leakage poses a risk to human health, necessitating safety measures and cautious handling. Therefore, the objectives of this research are (1) to study the MAP process for biomass treatment and (2) to examine factors that affect the quality of the final product using the MAP process.
2. Pyrolysis Process
Pyrolysis is a thermochemical process that can be used to treat any organic (carbon-based) product. It can be performed on both pure and mixture of materials. During the treatment process, the material is subjected to high temperatures and separated into distinct molecules through chemical and physical processes in the absence of oxygen [72]. Due to the poor thermal stability of chemical bonds in materials, breakdown occurs, and heat can dissolve them. New molecules are formed as a result of thermal breakdown. This allows products to be obtained that have a distinct, and often superior, quality to the original residue. Because of this property, pyrolysis is becoming great choice in today’s business and enabling considerably higher value to be extracted from ordinary materials and waste [73,74]. Thermal treatment is commonly related to pyrolysis. However, unlike combustion and gasification processes, which entail complete or partial oxidation of the material, pyrolysis is based on heating in the absence of air. This results in an endothermic process with significant energy content obtained in the products. Pyrolysis always results in solid (charcoal, bio-char), liquid (bio-oil), and non-condensable gases (hydrogen (H2), carbon monoxide (CO), and CO2). Because the liquid phase of pyrolysis gas is only removed after cooling, these two streams can be used together in certain applications when feeding hot syngas straight to the burner or oxidation chamber [75,76,77]. Pyrolysis can be classified as either slow, fast, or flash [78] based on the following variables: RT = residence time; HR = heating rate; Te = temperature; VR = vapor resentence time. Table 1 provides a categorization of the pyrolysis process using the numeric range of these variables.

Table 1.
Process conditions and pyrolysis modes.
Fast pyrolysis is preferable since it produces a higher percentage of liquid waste [79,80]. Numerous fast pyrolysis technologies have focused their commercial-scale applications on rapid catalytic pyrolysis, which has encouraged integrated process development initiatives [81,82]. Most volatiles develop in the biomass pyrolysis between 250 and 500 °C [83], giving pyrolysis a broad temperature range (400 to 1200 °C). Biomass pyrolysis at 400 °C recovers just a small amount of volatiles as bio-oil and syngas but produces a lot of bio-char. The final products of the biomass pyrolysis process are syngas, bio-oil, and bio-char. A slow pyrolysis temperature prevents the thermal cracking and water shift processes. There is a linear rise in H2 content between 500 and 900 °C, a more gradual increase in CO and H2 contents between 700 and 900 °C, and a minor drop in H2 contents between 800 and 900 °C. Because of these alterations, the value of gas products has increased. Thormann and de Oro [84] stated that when fast pyrolysis is performed in an optimal environment, high yields of liquid products may be effectively achieved.
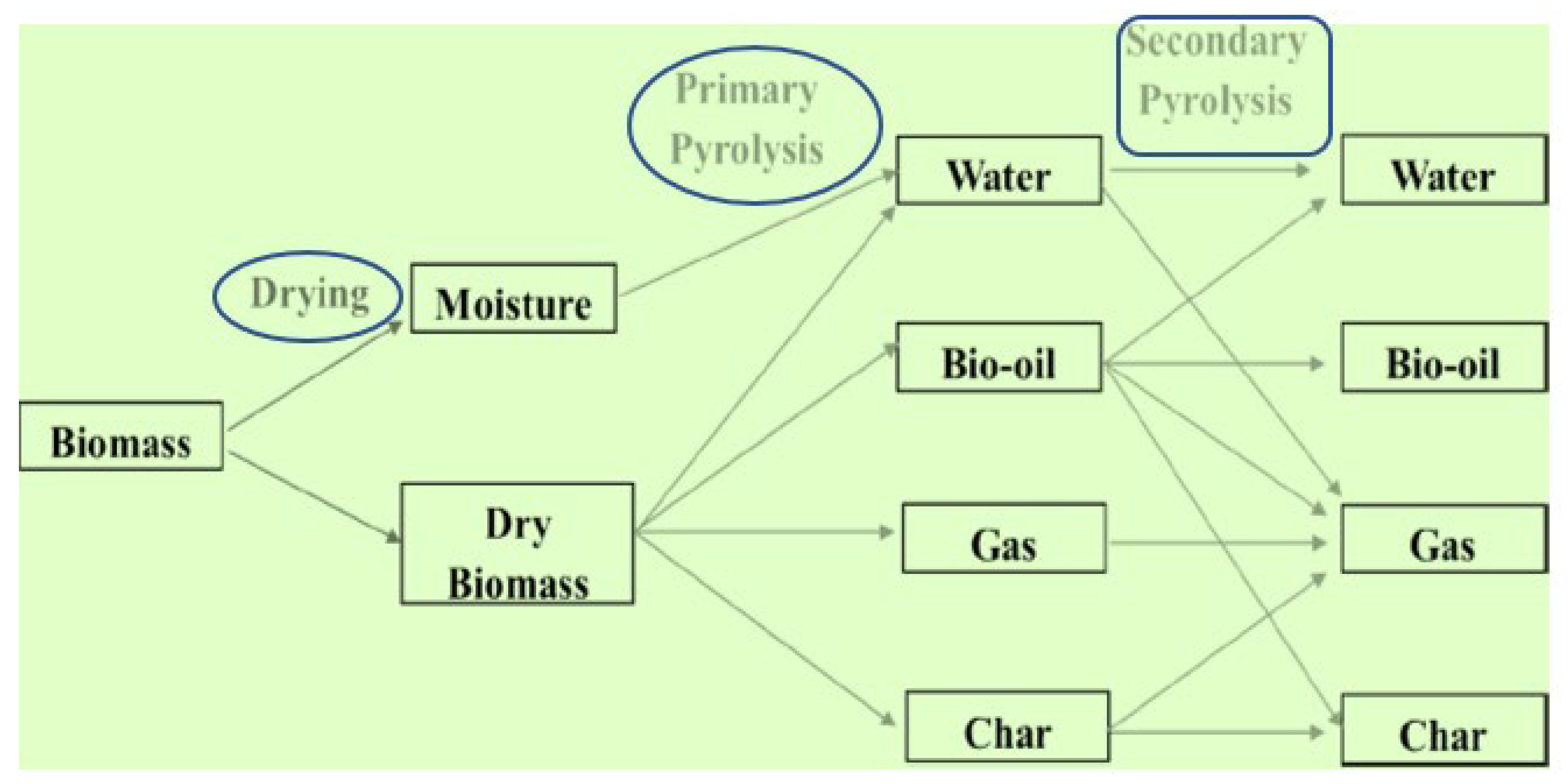
The mathematical formulation of biomass conversion can be found in previously published research articles [85,86,87]. However, the general formation of biomass into gaseous, liquid, and solid forms is shown in Figure 1. The graphic below clearly illustrates the main and secondary reactions. As shown in Figure 1, dry biomass can be converted into various products such as bio-char, biogas, bio-oil, and oily water during the primary reactions, whereas during the secondary reactions, the same products obtained during the primary reactions are converted into similar products; however, this time, the bio-char is converted into gaseous products as well as more refined bio-char. The bio-oil is changed into oily water, additional gaseous products are formed, bio-char is formed, and certain sections remain the same as the bio-oil. As the reaction progresses, the oily water is transformed into additional gaseous products, while the remaining water stays as oily water.
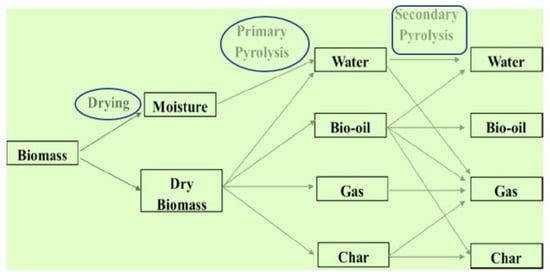
Figure 1.
General formation of biomass into gaseous, liquid, and solid state.
Biomass Pretreatment for Pyrolysis
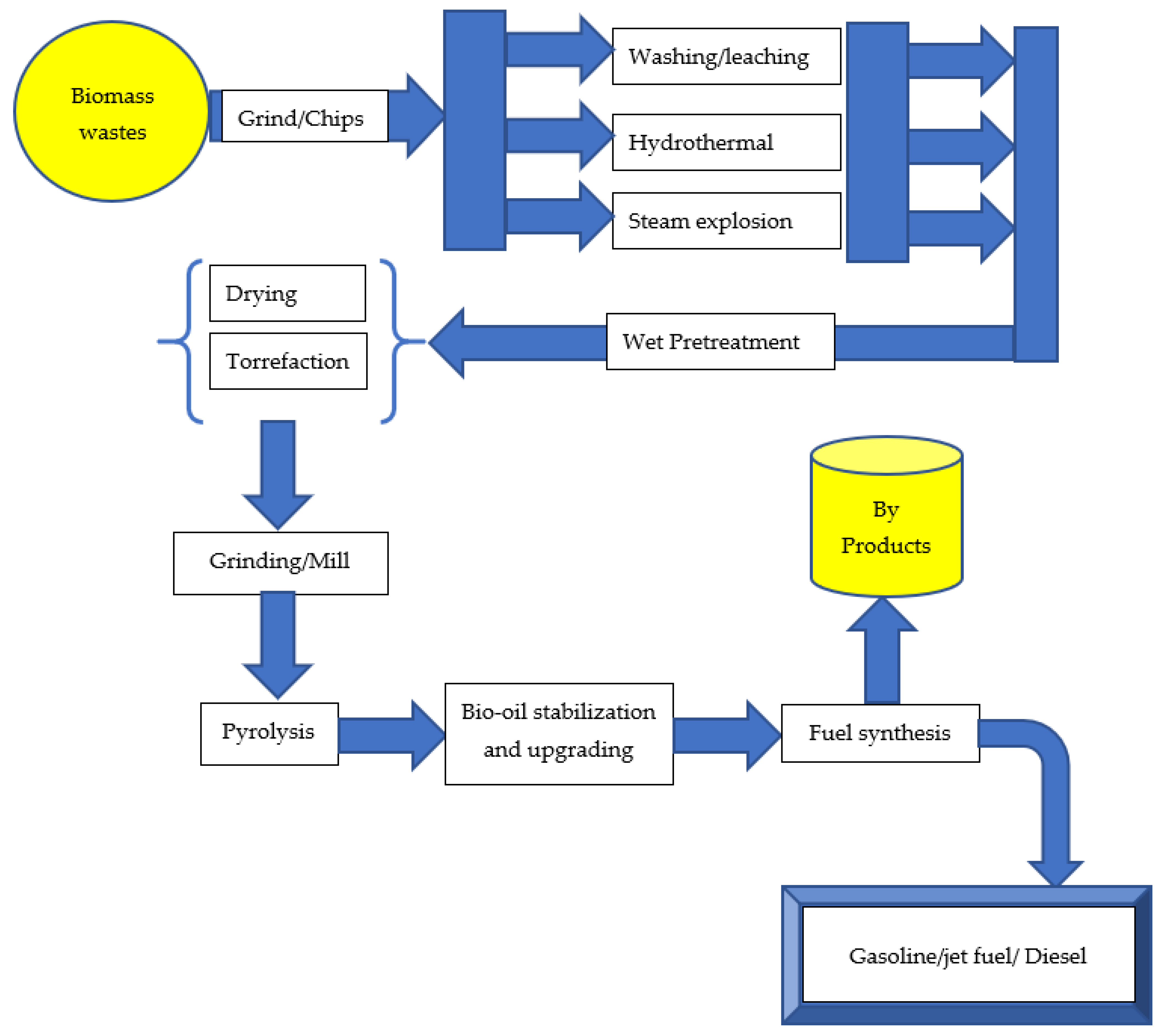
Biomass pretreatment can be performed before pyrolysis to improve the process’s selectivity for desired products. Cellulose, hemicellulose, and lignin are the three primary components of lignocellulosic biomass. Pretreatment modifies the lignocellulosic biomass material’s internal structure by decrystallizing the cellulose and removing the lignin and hemicellulose [4,28,29] to improve the efficiency of the subsequent biomass conversion. Biomass may be thermally broken down into liquid, solid, and gaseous phases. The conditions of biomass thermal decomposition have set limits on the yield of the three-phase products. Bio-oil produced through the pyrolysis process often represents the largest amount obtained compared to other processes. Higher heating rates may increase bio-oil yields; nevertheless, the increased oxygen and water content of bio-oil makes it less desirable for direct use as transportation fuel. The physicochemical characteristics of the biomass and the pyrolysis parameters (temperature, heating rate, particle size, residence duration) determine the bio-oil’s quality and yield. Biomass has a variety of physicochemical characteristics, including a high heating value, grindability, flowability, high volatile content, and low ash content. Wet and dry pretreatment techniques have the potential to enhance the physicochemical qualities of biomass. Wet pretreatment processes include washing and leaching, the steam explosion, and the hydrothermal method, whereas dry pretreatment processes include drying and torrefaction. These pretreatment methods may enhance the physicochemical qualities of biomass, resulting in a higher-quality and more abundant bio-oil after pyrolysis. The conversion of biomass into useful products via the pretreatment process is shown in Figure 2. These products can be used in a variety of sectors, including energy generation, chemical production, food production, and medicinal components. Additionally, physical, chemical, and biological pretreatment techniques could be used solely or in combined form to pretreat biomass [88]. Zadeh and colleagues [1] provided a comparative analysis on the pretreatment techniques and their impact on biomass pyrolysis.
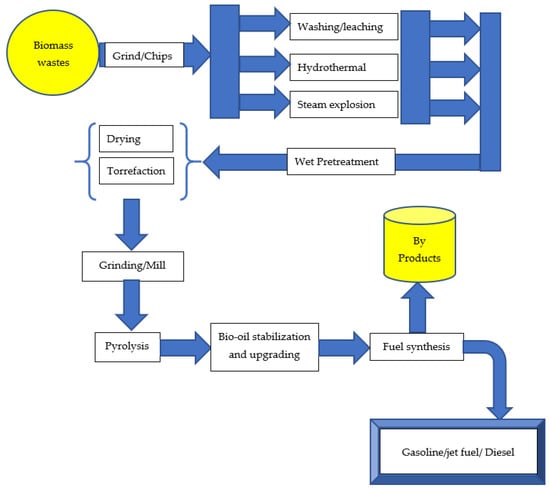
Figure 2.
Biomass pretreatment converts biomass into useful components.
3. Microwave-Assisted Pyrolysis
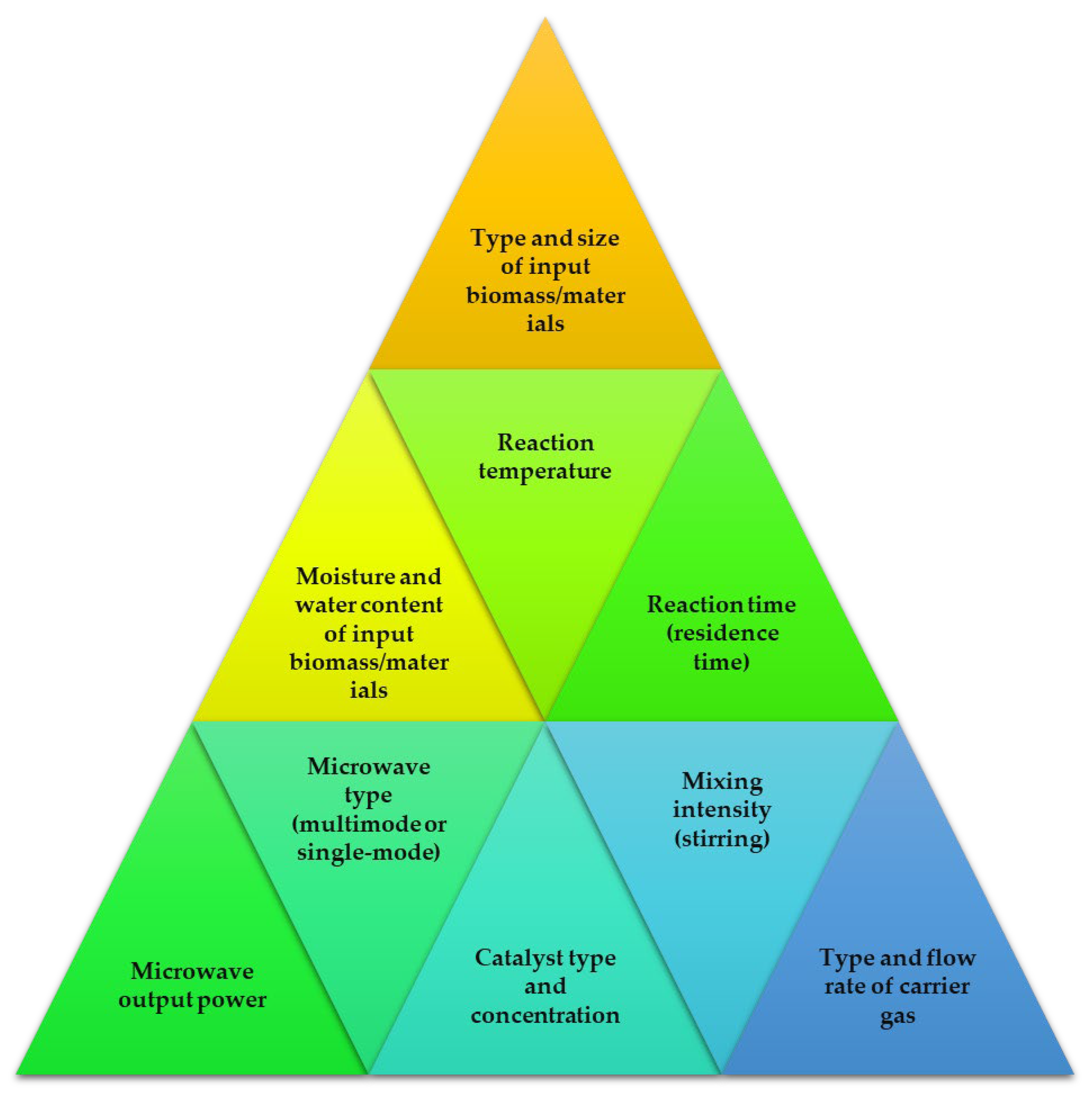
When it comes to improving and speeding up chemical processes, the microwave method is great choice. Due to excellent heat transfer patterns, the reactions can be performed in less time compared to conventional methods [89]. It has quickly become one of the most promising new technologies because of the time and energy saved by shortening the residence time and speeding up the chemical processes. Electromagnetic radiation, known as microwaves, has wavelengths between one millimeter and one meter. The microwave portion of the electromagnetic spectrum spans from 300 MHz to 30 GHz, lying between the infrared and radio frequency portions of the spectrum [90]. To avoid interfering with RADAR signals and telecommunications wavelength bands, most microwave systems run at 2.45 GHz or 900 MHz [91]. Several crucial elements in the MAP process have an impact on the final product’s yield and quality. Optimal results in terms of quality and conversion rate may be achieved by adjusting these factors, as shown in Figure 3.
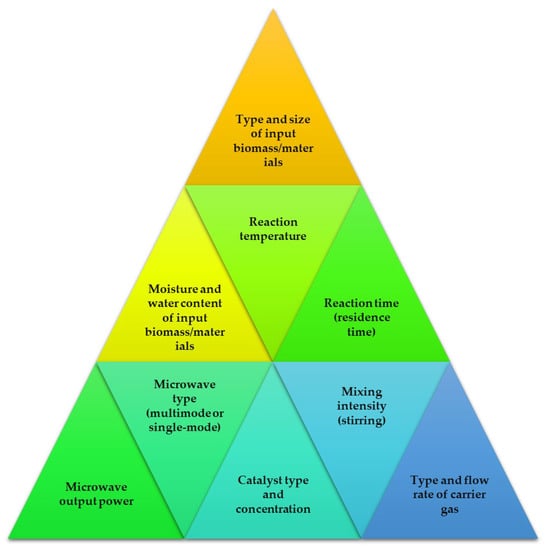
Figure 3.
Factors contributing to the microwave-assisted pyrolysis reaction processes.
Energy and money savings may be possible using MAP technology. It has been shown to be an effective method of recycling unwanted materials and creating useful products from biomass and bio-waste. Despite the method’s promise for future industrialization, it has not yet been implemented at a commercial scale. The benefits of MAP technology as a viable alternative to traditional methods (incinerators) are outlined below.
The primary benefits of this technology are its speed, selectivity, and uniformity in heating, which facilitate the treatment and usage of inhomogeneous wastes and biomass of significant size. Forest and agricultural wastes and residues, municipal solid waste (MSW), and MSW sludge are all examples of high-moisture biomass which can be treated easily with MAP technology. The effectiveness of the product, the chemical processes, and the process as a whole improved using MAP. On other hand, the adaptability of process, and the mobility of the necessary equipment made easy to use MAP technology for biomass treatment.
Gro Harlem Brundtland presided over the United Nations’ World Commission on Environment and Development, which was established to determine solutions for human activities. This research led to the publication of a book titled “Our Common Future” [92], which serves as a roadmap for environmentally responsible growth [93]. In this book, sustainable development is explained as “meeting the requirements of the present generation without compromising the needs for future generations”. The worldwide demand for bio-products, including bio-oil, bio-char, and syngas, has increased as a result of the need to meet these objectives and criteria. The adaptation of MAP technology offers many developing countries a way out of poverty by creating new opportunities for research and development (R&D), generating income for farmers, reducing greenhouse gas emissions, increasing crop yields and land productivity, and fostering agricultural sustainability [94].
Application and Limitation
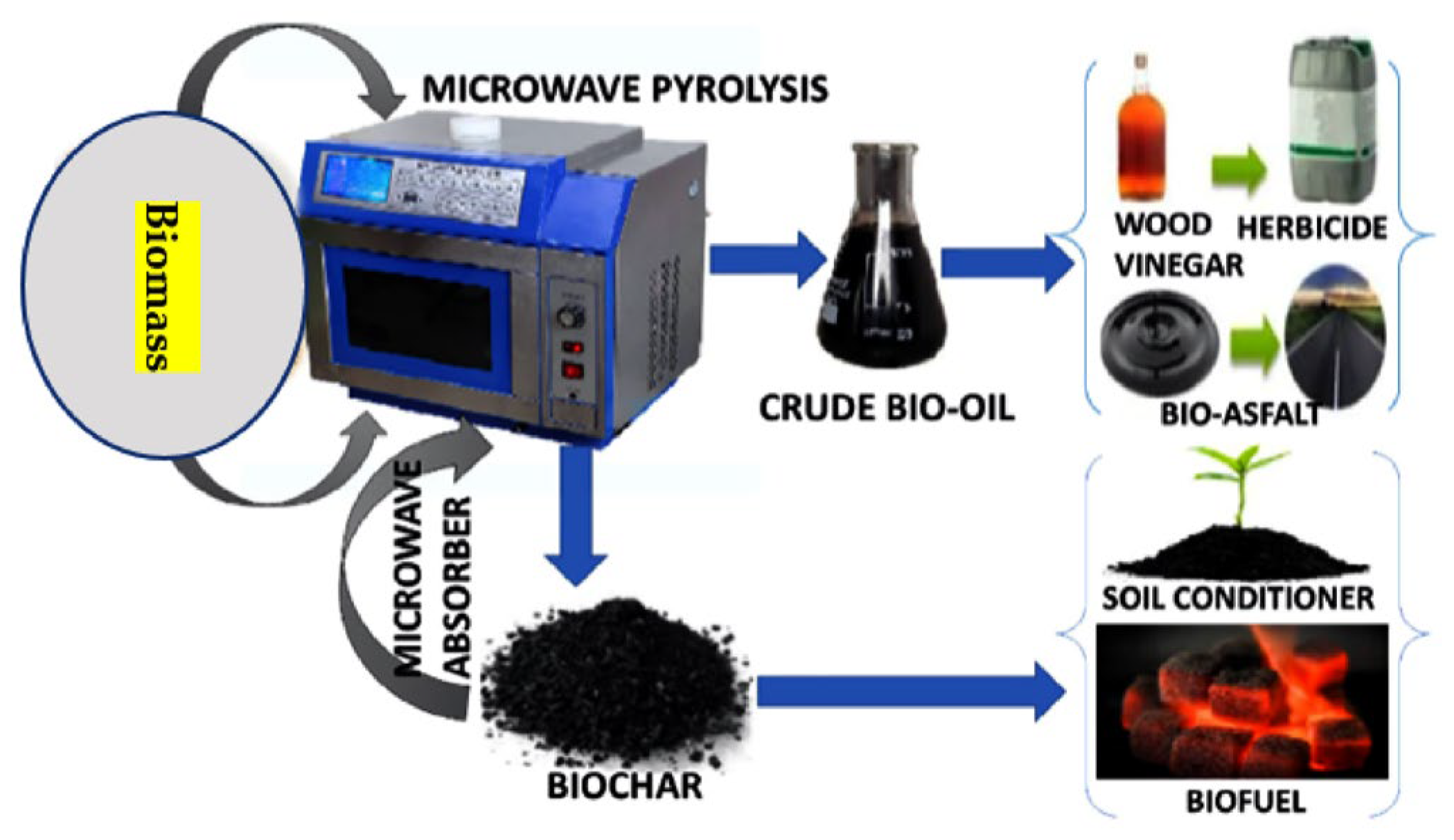
MAP for biomass treatment is a novel thermochemical conversion method. The oxygen content and heating value of the bio-oils produced are both greater than those produced by traditional biomass pyrolysis. Because dielectric characteristics influence microwave absorption, they play a significant role in the MAP process. By using exogenous microwave absorbents and reaction catalysts, it is possible, via MAP, to increase bio-oil output and quality via rapid heating, high pyrolysis temperatures, and catalytic breaking of big molecules. However, the bio-oil production may be reduced if the pyrolysis temperature is too high, since this will trigger secondary processes that convert the volatiles to gases that cannot be condensed. MAP technology has significant benefits over traditional pyrolysis techniques and, hence, a promising future. Microwave heating, in particular, is a well-established method that can be quickly and accurately applied. Cost-effectively producing high-quality bio-oils and commercializing the concept requires more work in the areas of catalyst screening and applications, co-pyrolysis of biomass with complimentary microwave heating properties, pilot research, and equipment development. The potential applications of the MAP process for creating useful products from biomass are shown in Figure 4.
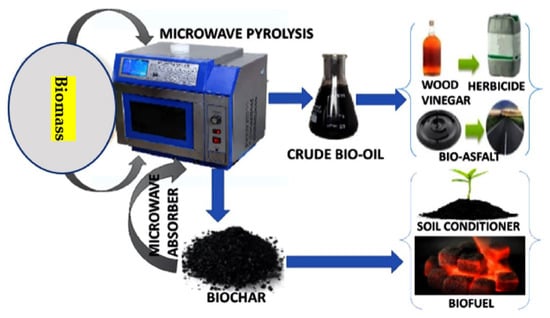
Figure 4.
Microwave-assisted pyrolysis creating useful products out of biomass.
4. Systematic Literature Review
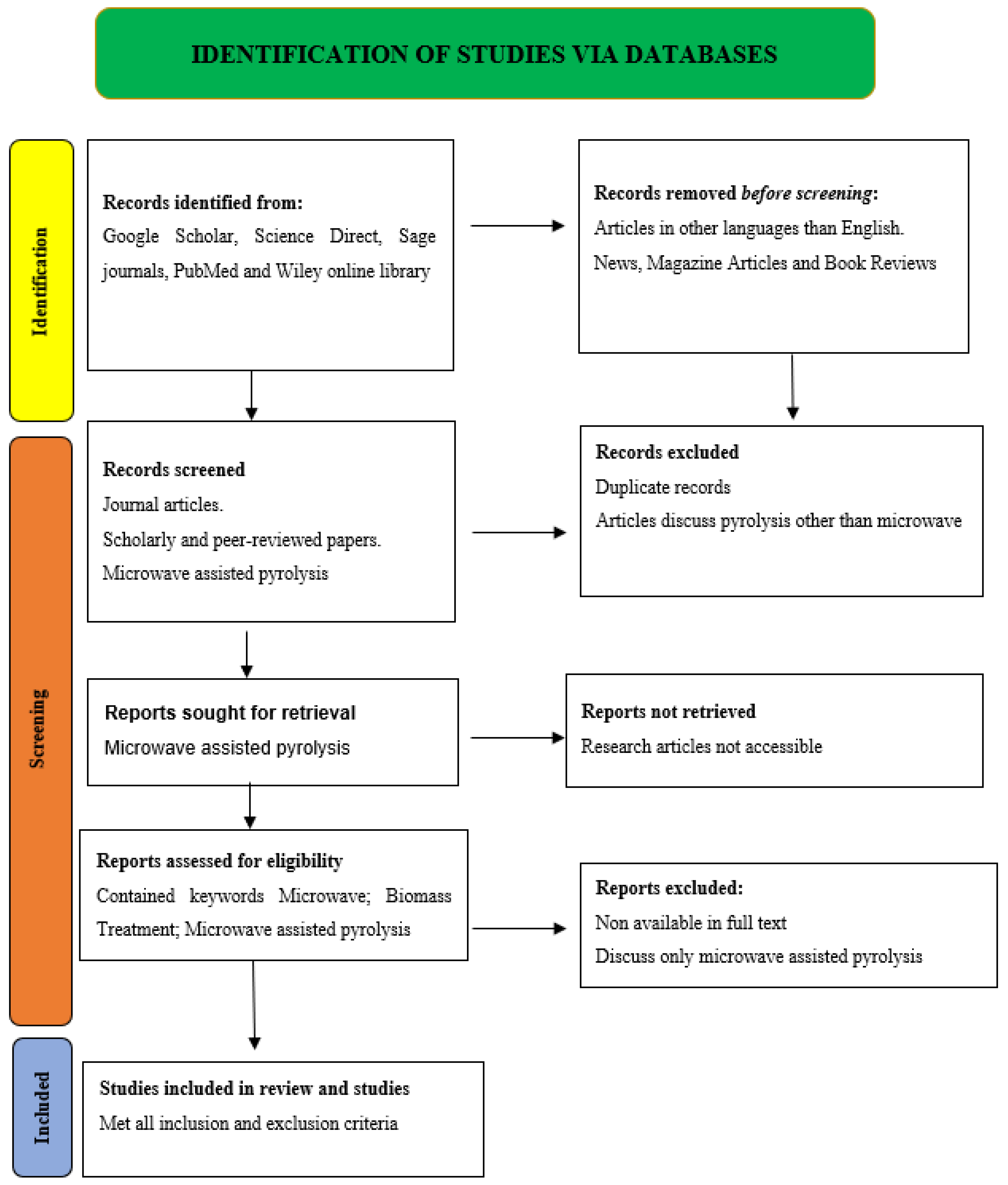
This systematic review followed the PRISMA guidelines for systematic reviews and meta-analyses. The PRISMA schematic diagram is prepared according to Figure 5 standards. Considerations for the PRISMA flow diagram understanding included the following:
- (1)
- Data sources: Google Scholar, Science Direct, Sage journals, PubMed, and Wiley online library databases are used with the following titles and keywords: microwave-assisted pyrolysis, biomass, pyrolysis processes, microwave processes, etc.
- (2)
- Article screening: the chosen databases include duplicate, irrelevant, full-text unavailable, and non-English entries. These are deleted from identifiable records.
- (3)
- Article inclusion: The investigation included papers on microwave-assisted pyrolysis, biomass, pyrolysis processes, and microwave processes.
As of 5 May 2023, the last Google Scholar, Science Direct, Sage journals, PubMed, and Wiley online library search for “microwave assisted pyrolysis” yielded 35,277 publications. Google Scholar returned 15,000 results, Sage Journals 128 results, Science Direct 14,838 results, PubMed 250 results, and Wiley Online Library 5061 results for research studies. The review excluded local language articles, hearings, and non-peer-reviewed publications. Finally, the 32 research articles relevant to MAP technology for the treatment of biomass included in this review examine its effectiveness and affirm that it produces good-quality products.
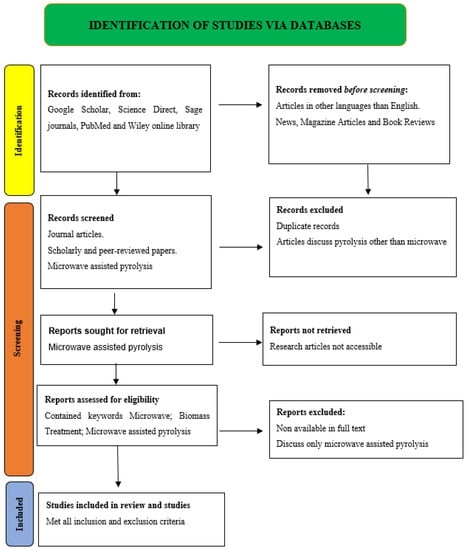
Figure 5.
PRISMA diagram of current study.
Figure 5.
PRISMA diagram of current study.
As previously stated, the MAP process is one of the most promising ways to boost and speed up chemical processes for biomass conversion. Due to a good heat transfer mechanism, the reactions may be performed in less time compared to more traditional heating processes [94]. Since it shortens the residence time and speeds up the chemical processes, both of which result in significant savings of energy as well as money. The following studies affirmed the use of MAP technology to convert different types of biomass into useful products.
Zhao et al. [95] examined the impact of temperature on microwave-induced pyrolysis of wheat straw as a biomass input. A domestic microwave with a frequency of 2.45 GHz and a power output of 3000 W was used. A thermocouple was installed to monitor the pyrolysis temperature in the microwave reactor. The effectiveness of MAP technology was found to be highly temperature dependent. The yield of gas products increased from 17.69 to 22.27 wt% when the temperature was increased from 400 °C to 600 °C. The average pore size decreased from 282.16 to 46.64 nm, while the specific surface area of the solid fraction rose from 0.89 to 9.81 m2/g, and the pore volume rose from 0.006 to 0.012 cm3/g. Hu et al. [96] examined the impact of microwave power level and catalysts, including activated carbon (Cac), CaO, and SiC, on the metabolites of Chlorella vulgaris. Guangzhou Wancheng Microwave Equipment Co., Ltd., Gaungzhou, China, utilized a microwave (2.45 GHz, 3750 W) in the experiment. The microwave oven’s carrier gas was 300 mL/min (mins) of N2 gas from a canister connected to the reactor’s three apertures. The maximum volume of the reactor was 500 mL. The gases escaped through the bottom neck of the container and were captured in flasks equipped with thermocouples (TCs). When microwave power was increased from 750 W to 2250 W and temperature increased from 200–800 °C, the gas product output increased, while the solid fraction production decreased. The results showed that a microwave exit power of 1500 W produced the highest bio-oil yields (35.83 wt%), while a microwave exit power of 2250 W produced the highest gas yields (52.37 wt%). At higher microwave power levels and higher concentrations of catalysts, Cac was found to be the most efficient at diminishing solid residue and enhancing gas production. Using the optimal amount of Cac (5%), maximized biofuel production (87.47%) and increased gas output (75.66%) could be achieved.
Ren et al. [97] examined how reaction time and temperature affect the production of syngas, bio-oil, and charcoal from Douglas fir sawdust particles with the help of a microwave. The synthesis of volatiles and charcoal was optimized using central composite design (CCD) and response surface analysis (RSA). In this research, a Sineo MAS-II bulk microwave oven with a maximum power output of 1000 watts was utilized. Each mixture of 400 g of pelletized Douglas fir sawdust was microwaved for one minute in a one-liter quartz reactor. The greatest concentrations of volatiles (bio-oil and syngas) were observed after 15 min at 471 °C when the reaction parameters (time and temperature) were altered. Depending on the pyrolysis parameters, volatiles and bio-oil yields varied between 39.3–68.8 wt% and 33.8–57.8 wt%, respectively. Depending on the reaction parameters, the yields of charcoal and syngas achieved were 31.2% to 60.7% and 7.9% to 15.0%, respectively. In the study of Chen et al. [98], NaOH, Na2CO3, Na2SiO3, NaCl, TiO2, HZSM-5, H3PO4, and Fe2(SO4)3 were employed as additives, and microwave irradiation at 470 °C with Silicon carbide (SiC) as an absorber was used to conduct microwave heating. For 15 min, a 0.2 m3/h N2 discharge was used to purge the reactor of air. All eight additives increased the solid fraction production, while the gaseous product yield decreased, and the liquid product yield remained relatively constant. These additives resulted in the production of H2, CH4, CO, and CO2 during pyrolysis, with sodium being the most efficient. NaCl, TiO2, and Fe2(SO4)3 were the only three reagents that did not reduce CO2 and CH4 while increasing H2 production. Except for HZSM-5 and Na2SiO3, all of the additions were successful in reducing CO levels. The most effective at increasing acetol production were the alkaline sodium compounds (NaOH, Na2CO3, and Na2SiO3). HZSM-5 had no effect, and TiO2 caused acetol production to decrease.
Omar et al. [99] investigated the use of microwave irradiation to characterize the empty fruit bundle (EFB) of oil palm for pyrolysis. In addition to demonstrating that a potassium concentration of 12.8% influenced microwave absorption positively, the dielectric properties and moisture content were observed to be proportional up to 60% at 2.45 GHz. Hussain et al. [100] used the microwave–copper interaction method to co-pyrolyze waste polystyrene and Makarwal coal at high temperatures. Co-pyrolysis yields better results than single pyrolysis, with a 6 percent gas component, 10 percent water (including sulfide), and 66 percent oil. In order to remove bio-oil from sewage sludge, Tian et al. [101] used MAP technology. Bio-oil was produced from wastewater by using the optimum microwave exit power and product mass balance. Between 400 and 600 watts of electricity was ideal for bio-oil production. Between 600 and 800 W of departing power, gas output rose, while bio-oil production decreased. Beyond 800 W, gas creation rose, but the solid fraction reduced, while bio-oil production remained unchanged. Bio-oil output was maximized at 49.8% when microwaves were used at 400 W for 6 min at 35.5 MJ/kg and 929 kg/m3. When the microwave departure power was raised from 200 W to 1200 W, the production of biogas rose from 4.4% to 60.21%, while the output of solid fraction dropped from 77.3% to 32.6%. Du et al. [102] used MAP technology on rice chaff and sawdust. The results of varying the exit power of the microwave oven (160–800 W), the heating duration in the microwave oven (0–25 min), and the concentration of the catalyst (from 0 to 0.5 catalyst to biomass) were studied. Two types of ionic liquids (IL-C and IL-B) were used as catalysts. At an exit microwave power of 640 W, bio-oil production from rice straw was 38%, and at an IL-C/straw ratio of 0.15, it was 42%. The bio-oil yield was 34% after 20 min in the microwave at a ratio of 34% IL-B/sawdust. IL-C and IL-B stimulated bio-oil synthesis in 2 and 8 min, respectively. The production of bio-oil from rice straw stabilized after 20 min of microwave irradiation (both catalysts).
Zeng et al. [103] studied the morphology and production of carbon nanostructures (nanospheres and CNTs) during catalyst-free microwave-assisted methane pyrolysis. The experiment made use of a 2.45 GHz home microwave oven with a vertical quartz tube. There was no need for additional minerals to encourage the production of nanostructures in the carbon/carbon composite microwave absorber (MA) material utilized in the pipe. Flushing N2 through the hole removed oxygen prior to testing; the quartz wall material underwent further testing after being exposed to a CH4/N2 (1:4) mixed gas flow reaction for 60 min. Some of the experimental conditions that impacted the resultant nanostructures were the methane-to-N2 ratio, the reaction temperature, and the overall gas flow rate. Huang et al. [104] used MAP to turn rice chaff into hydrogen-rich propellant gas. A 2-KW single-mode microwave was used to perform experimental analysis. N2 was used as the pyrolysis carrier gas, and the chamber was outfitted with a quartz reaction tube and a sample receptacle. Hydrogen (H2), carbon dioxide (CO2), carbon monoxide (CO), and methane (CH4) were the primary gases released, with an estimated 60% of the output usable as bioenergy. Fu et al. [105] used the MAP of methane to construct SiC nanowires on a quartz plate without using a catalyst. Tests were conducted using a 2.465 GHz microwave pyrolysis reactor with three 1 kW magnetrons in a laboratory environment. The effect of reaction gas concentration and temperature on the shape of SiC nanowires was investigated. The nanowires made using this cutting-edge technique were very pure. After sieving, four dry fractions and two wet fractions ranging in size from fine dust to 7–12 mm were achieved. Andersson et al. [106] analyzed six distinct fractions of discarded electrical and electronic equipment waste to generate bio-oil and syngas using MAP technology. The results exhibited that MAP is an effective method for transforming waste into valuable products. Chemat and Poux [107] investigated the pyrolysis of urea into cyanuric acid using MAP technology in a heterogeneous environment. The results indicated that graphite enhanced product yield from 15.2% to 61.2% at 300 °C, and from 4.6% to 9.6% at 200 °C. Heterogeneous pyrolysis was responsible for the faster response times.
In order to investigate MAP effectiveness on oil palm shell and fiber (OPS, OPF) biomass, Salema and Ani [108] used char as an MA. The research was conducted using a modified household microwave oven set at 2.45 GHz and 1 kW. A steel distribution plate, a quartz glass reactor, and two k-type metallic TCs were used to monitor both the outside and inner temperatures of the furnace. At a constant microwave exit power of 450 W and a reaction time of 25 min, the impact of raising the OPS/OPF-to-MA ratio from 1:0.25 to 1:1 was investigated. Increasing the biomass-to-MA ratio from 1:0.25 to 1:0.5 is suitable for maximizing bio-oil and gas production in OPS and OPF while avoiding char formation. Changing the biomass-to-MA ratio from 1:0.5 to 1:1 boosts char production while lowering bio-oil output and maintaining gas yield. MAP technology on distiller’s dried grain with soluble (DDGS) was studied by Lei et al. for its potential to provide bio-char, bio-oil, and syngas [109]. In this investigation, the effects of reaction time, temperature, and microwave power were examined by means of response surface methodology (RSM). The recovery rates for bio-char, bio-oil, and syngas all varied between 22.6% and 62.2% depending on the pyrolysis conditions. Increasing the reaction temperature and time enhanced bio-oil and syngas yields while decreasing charcoal yields. Wan et al. [110] investigated the catalyst effects on the MAP of dry maize stover and aspen wood pellets. MAP was performed on a mixture including 100 g of dried biomass and 8 g of powders/crystals containing K2Cr2O7, Al2O3, KAc, H3BO3, Na2HPO4, MgCl2, AlCl3, CoCl2, and ZnCl2. Each test lasted for 20 min and was performed in a microwave reactor operating at 450–550 °C and 2450 MHz, with 875 W continuous input power. Charcoal and gas production were both reduced, while bio-oil production was increased by the addition of these substances (KAc, Al2O4, MgCl2, H3BO3, and NaHPO3). When absorbed by a catalyst, microwaves may accelerate the heating process. The MAP of dried maize stover (4 g/100 g sample) and aspen wood pellets (8 g/100 g sample) resulted in bio-oil yields of 42.70 to 71.11% and 41.12 to 71.4%, respectively; catalysts Na2HPO4, K2Cr2O7, and Al2O3 generated the highest yields of bio-oil (44%), syngas (37%), and charcoal (30%). Bio-oil generation from biomass (Chlorella sp.) was studied by Du et al. [111] utilizing MAP technology. All of the pyrolysis took place in a microwave oven (Panasonic, NN-SD787S) set to 2.45 GHz at an exit power of 1.25 kW. For these analyses, a 500 mL quartz flask was filled with 500 mL/min of N2. Biomass pyrolysis char, with an effective ratio of 1:5 (char to biomass), was utilized as the MA to improve heating quality. The production of bio-oil was 28.6% at an exit microwave power of 750 W. Adding more fuel reduced performance. The gas production was enhanced by 12% when the power was raised from 500 to 1250 W.
For the pyrolysis of used motor oil, Lam et al. [36] used MAP technology. An oil waste reactor made of carbon particles and quartz, heated in a microwave oven at 5 kW, was utilized. The pyrolysis temperatures ranged from 250 °C to 700 °C, and the carrier gas was N2 at a flow rate of 0.1 to 0.75 L/min, with a heating rate of 60 °C/min. The temperature of the reaction was monitored by two TCs placed at the top of the carbon bed and the bottom of the stainless-steel stirrer shaft. At 250 mL/min N2 and 5.0 kg/h waste oil input rates, 88 wt% pyrolysis oil was produced. When raising the waste oil feed rate from 0.4 kg/h to 5.0 kg/h and maintaining a N2 purge rate of 250 mL/min, the yield was maximized. MAP with Cac was used by Bu et al. [112] to extract phenol and phenolics from Douglas fir (lignocellulosic bio-mass). The reaction temperature, duration, and Cac-to-biomass ratio varied from 1.32 to 4.86, and the microwave input power was 700 W with a heating rate of 60 kelvin (K)/min. Phenol (38.9%) and phenolic (66.9%) concentrations were both high while using a catalytic ratio of 3:1, a reaction temperature of 589 K, and a retention time of 8 min. The MAP of automobile engine oil created incondensable gases, which were examined by Lam et al. [113]. The identical apparatus was utilized for the experiment as in Ref. [36], but a membrane filter was installed to clean the pyrolyzed volatiles of any metallic solid residue before they entered the condensation apparatus. The by-product gases included H2 and H2CO concentrations of 19–35 vol%.
MAP was used by Quan et al. [114] to investigate high-phenol biofuels. In this investigation, phenolic-rich pyrolysis oil was generated using the microwave pyrolysis system described in Ref. [112]. The effects of time, temperature, and catalyst-to-feedstock ratio were investigated. Because Cac decomposes lignin, MAP has promise for the bioconversion of Douglas fir (biomass feed-stock). Phenol (at 38.9%) and phenolics (at 66.9%) were found in quite high proportions in the biofuels. Volatile yields were significantly boosted by using Cac. The gas output was reduced, while the liquid yield increased from 32.3% (315.85 °C) to 38.8% (399.85 °C). Lam et al. [115] examined the MAP of vintage vehicle engines. The reaction temperature was increased from 400 to 700 °C, while a quartz reactor containing 1 kg of carbon was heated in a microwave oven with a carrier gas flow rate of 0.2 L/mins of N2. The most lucrative products were obtained at 600 °C. The yield for liquids was 75% at this temperature, whereas the yields for gas and solids were the lowest. The metal pollutants in the recovered liquid oils were drastically cut down on, with Cd reductions of 46%, Cr reductions of 32%, and Cu, Ni, Pb, Zn, and Fe reductions of 93% to 97%. Bio-oil was extracted from sewage sludge by Lin et al. [116] using MAP technology. The quality and quantity of bio-oil were investigated, along with the effects of different reaction conditions and chemical additions. In each test, 5–20 L/mins of N2 was added to 3.5 kg of dry sewage sludge. The effects of additions on bio-oil composition were studied by checking the reactions between the oil and catalyst (KOH, H2SO4, H3BO3, ZnCl2, and FeSO4). All catalysts enhanced calorific value, density, viscosity, and carbon content, while they reduced bio-oil production. Product quality was diminished by ZnCl2. KOH favored alkanes and monoaromatics, whereas H2SO4 and H3BO3 favored cyclics, ketones, alcohols, and nitriles but repressed amides and esters.
The MAP of rice straw was studied by Huang et al. [117]. The pyrolysis process was carried out in a single-mode microwave oven operating at a frequency of 2.45 GHz with a maximum power output of 2 kW. The pyrolysis efficiency was impacted by the particle size and the microwave exit power. Less microwave energy is needed for the pyrolysis of rice straw when the particles are tiny. Water and waste water treatment (metallic pollutant removal) showed promise in the solid fraction study (specific surface area, seta potential, and Cu2 adsorption). Half of the rice straw sample was converted into a rich flue gas consisting of 55 vol% H2, 17 vol% CO2, 13 vol% CO, and 10 vol% CH4. OPS biomass conversion was performed by Salema and Ani [118] using a microwave-assisted pyrolysis reactor with an overhead stirrer. Similar microwave apparatus and temperature measurements [108] were utilized in this study, but a three-necked glass cover was placed above the reactor in order to accommodate an overhead stirrer. In order to produce an inert environment, N2 was employed at a rate of 10 L/min for 5 min before the experiment and at a rate of 5 L/min during the operation. Both 300 W and 450 W exit outputs from the microwave were employed in these tests, with a reaction time of 25 min and 200 revolutions per minute (rpm). At 180 W and 450 W, the OPS did not pyrolyze without Cac. Cac at 10% yielded the least bio-oil but the most bio-char. Bio-char production was greatest at 900 °C, whereas it was lowest at 400 °C and highest at 700 °C. Finally, mechanical stirrers have the potential to raise the temperature and heat transfer of microwave pyrolysis.
MAP for biomass (aspen hardwood pellets) treatment was studied by Moen et al. [119]. Catalysts such as chlorides, nitrates, metal oxides, and magnesium were used in a Sineo MAS-II batch microwave oven at 800 W continuous power. The production of liquid, pyrolysis gas, and heavy oil all rose in response to the addition of chlorides, nitrates, and metal oxides, but light oil production remained stable. Chlorides were used alone to boost bio-mass input liquid production from 35% to 41%. It was found that nitrates, metal oxides, and chlorides produced the highest percentages of gas (35.57%), char (32.01%), heavy oil (12.93%), and light oil (10.68%), respectively. Synthesis gas made up 69% of the pyrolysis gas volume, whereas CH4 only made up 18%. The MAP of maize stover (stalks, leaves, cobs, and husks) was performed by Yu et al. [120]. The input power ranged from 300 W to 900 W, and the response time was 60 min. This research examined the catalytic effects of NaOH and the MA properties of charcoal. Increases in gas and bio-oil production were accompanied by decreases in charcoal output when the microwave input power was raised from 300 W to 900 W. Without any additions, 900 W produced the most bio-oil (30.2%) and gas (46.7%), whereas 300 W created the most charcoal (69.3%). Increasing the power to 600 W raised the amount of bio-oil and gas produced to 29.5 and 45.7%, respectively, while decreasing the amount of charcoal produced.
Bio-oil was obtained from OPS using the MAP technology described by Salema and Ani [121]. In this study, char was used as an MA at weight ratios of 0:1, 0.25:1, 0.5:1, and 1:1 (biomass to char). The reaction was run at 450 W of microwave radiation for 25 min using N2 as the carrier gas at a flow rate of 20 L/min. The optimal ratio of biomass to MA was 1:0.5, which resulted in high bio-oil and gas production with little char formation. High-density polyethylene and aluminum/polymer laminates were studied by Palafox and Chase [122] using semi-batch MAP. They were studying the degradation of high-density polyethylene and aluminum/polymer laminates in a semi-batch bench-scale apparatus. Toothpaste tubing was used as an example of a laminated material to be treated with the novel process. Clean aluminum was recovered together with hydrocarbons, and the trial proved that the process has excellent potential for the treatment of plastic waste. MAP for the treatment of maize stover for bio-oil, syngas, and bio-char production was studied by Hanwu Lei et al. [123] by using particle size (0.5–4 mm), reaction time (4–22 min), and temperature (515–685 °C). A 1 L quartz flask was heated in a 700 W Sineo MAS II batch microwave oven. After 6 min of running at 50 and 160 °C per minute, the stage had heated to the proper level. The greatest volatile output was 76% (34% bio-oil and 42% biogas) during an 8 min reaction at 650 °C with 4 mm particles.
Using Cac, Fernandez et al. [124] compared conventional and microwave-assisted glycerol-to-syngas production. Gas output from glycerol conversion rose significantly with high syngas and H2/Co ratios after being heated in a microwave oven. MAP technology showed promising results in the breakdown process by decreasing the solid fraction yield. Syngas production was enhanced, and CO2 emissions were reduced by using carbonaceous catalysts. Activating Jatropha shell carbon was studied by Xin-hui et al. [125] by comparing microwave and traditional heating techniques. The microwave oven was a 3-kilowatt (2.45 GHz) model with a quartz pipe in its center. The experimental analysis was carried out using the statistical response surface (RSM) method. In this experiment, pyrolysis was performed using steam and CO2. The activation temperature, duration, and CO2 flow rate were all reduced by microwave heating, while the output yield was increased from 18.02% to 36.60%. Despite the carbon having the same porosity, this method reduced costs. The production of Cac was unaffected by the use of a microwave-assisted heating method for steam activation, which quadrupled the pore volume and surface area.
Table 2 summarizes the reviewed articles that use MAP technology on different types of biomass and obtain useful products, e.g., bio-oil, bio-char, etc. The research target and main findings of the review can be found in Table 2. It can be perceived from Table 2 that mostly MAP technology is used for treatment of agricultural crop residue or virgin biomass resources; however, a limited number of studies have tested sewage sludge as a biomass input for treatment. The research target, biomass input, product output, microwave power and frequency and findings of reviewed articles are listed in Table 2. One of the biggest difficulties in microwave pyrolysis is measuring temperature accurately since it impacts the conditions and effectiveness of the reaction. In order to minimize measurement oversights, selecting the right temperature sensor appears essential. The results from grounded thermocouple probes will not be as reliable since they sometimes interact with electromagnetic fields. In an effort to address this issue and improve the accuracy of temperature monitoring systems, grounded probe TCs may be used in conjunction with infrared optical pyrometers. Most microwave pyrolysis systems analyzed in the above-mentioned research, have on–off switching systems. However, having a temperature feedback control system is one of the crucial instruments for achieving a constant pyrolysis reaction state. Reaction conditions may be stabilized to create bio-products with decreased solidity and retention time. The viability of microwave pyrolysis has only been evaluated in laboratories. The viability of the system is determined by pyrolyzing only 3–400 g of biomass leftovers. Before commercialization, the technology has to be scaled up, but there has been no pilot testing. A continuous microwave pyrolysis system should be able to overcome many issues that other processes have, as pyrolysis is one of the finest ways to recover energy and chemicals from biomass residuals. Microwave pyrolysis cannot be used for all materials, e.g., transparent and dry materials, but it works efficiently in materials with high moisture content. In addition, transparent materials with added absorbers in the microwave assist in increasing overall temperature of the reaction. Another issue is the difficulty of monitoring temperature in a microwave environment, and thermal damage to treated materials is caused by the microwave’s non-uniform heating behavior. Because the homogeneity of the microwave field may be improved by enlarging the cavity, a specific instrument design is required. Additionally, microwave leakage poses a risk to human health, necessitating safety measures as well as cautious handling.

Table 2.
Summary of reviewed articles to apply microwave pyrolysis for different biomass.
5. Conclusions and Future Work Suggestions
Microwave-assisted pyrolysis is a revolutionary approach for the effective in situ processing of biomass. Microwave heating is a practical option for recovering energy from biomass and turning it into usable goods. When compared to oils obtained through traditional pyrolysis, those obtained through this method yield fewer harmful substances and more chemicals of industrial importance. Microwave-assisted pyrolysis offers a promising opportunity to divert biomass from environmentally detrimental disposal techniques like landfilling and incineration while simultaneously providing a practical means of recovering marketable commodities from leftover ash. Parameters and operational variables that control this process have been the subject of some attention and may yet benefit from optimization. By defining the right process parameters (RT = residence time; HR = heating rate; Te = temperature; VR = vapor resentence time) through optimization, the microwave-assisted pyrolysis process may be taken to a larger scale.
The primary factor contributing to the price tag is the expense of the pyrolysis procedure. As a result, one way to lower product prices is to advance pyrolysis technology. The use of microwave technology in thermochemical processes like pyrolysis has been shown to save energy and money. Time and energy savings are only two of the many benefits of the microwave-assisted pyrolysis approach that cannot be achieved with more traditional heating methods. The type of feedstock utilized and the reaction time greatly influence the features of the pyrolysis process and the yield of the products it generates. The current study reviewed research articles that applied microwave-assisted pyrolysis techniques and yielded some useful products, e.g., bio-oil, bio-char, and charcoal, without negatively impacting the environment.
The use of MAP technology is mostly tested on a lab scale. There is currently limited pilot-scale testing using MAP technology for biomass conversion, which must be scaled up for commercialization. Also, various types of biomass belong to various categories that should be explored by using MAP technology. Future studies should pay attention to and work on these suggestions to investigate the performance of MAP both at the lab scale and on a large scale.
Author Contributions
B.Z.: data curation, conceptualization, visualization, funding acquisition, writing—original draft; N.R.S.: data curation, investigation, writing—original draft; S.M.: formal analysis, writing—review and editing; A.S.A.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Fund Program of China (No. 52306043), the Science and Technology Project of Jiangsu Province (No. BE2022604 & BE2021701), the Youth Talent Promotion Project of Jiangsu Provincial Association for Science and Technology (No. JSTJ-2022-046), the Inner Mongolia Major Science and Technology Project (No. 2021ZD0022), and the State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering (China) (No. 2022-K25 & 2022-K37).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on reasonable request from the first author.
Acknowledgments
We are thankful to the Editor and reviewers for their helpful and constructive suggestions to improve the quality of this manuscript. All the authors and co-authors agreed to publish final version of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Zadeh, Z.E.; Abdulkhani, A.O.; Saha, B. Recent Insights into lignocellulosic biomass pyrolysis: A critical review on pretreatment, characterization, and products upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, S.M.; Biak, D.A. Microwave-assisted pretreatment of lignocellulosic biomass: A review. J. Eng. Sci. Technol. 2015, 2, 97–109. [Google Scholar]
- Ethaib, S. Solid waste situation in Thi-Qar governorate. IOP Conf. Ser. Mater. Sci. Eng. 2019, 584, 012023. [Google Scholar]
- Ethaib, S.; Omar, R.; Mustapa Kamal, S.M.; Awang Biak, D.R. Comparison of sodium hydroxide and sodium bicarbonate pretreatment methods for characteristic and enzymatic hydrolysis of sago palm bark. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Erabee, I.K.; Ethaib, S. Performane of Activated Carbon Adsorption in Removing of Organic Pollutants from River Water. Int. J. Eng. Technol. 2018, 7, 356–358. [Google Scholar] [CrossRef]
- Jung, W.; Savithri, D.; Sharma-Shivappa, R.; Kolar, P. Changes in lignin chemistry of switchgrass due to delignification by sodium hydroxide pretreatment. Energies 2018, 11, 376. [Google Scholar] [CrossRef]
- Gupta, R.; Yadav, G.; Kumar, G.; Yadav, A.; Saini, J.K.; Kuhad, R.C. Second generation bioethanol production: The state of art. In Sustainable Approaches for Biofuels Production Technologies; Springer: Cham, Switzerland, 2019; pp. 121–146. [Google Scholar]
- Maj, G.; Najda, A.; Klimek, K.; Balant, S. Estimation of Energy and Emissions Properties of Waste from Various Species of Mint in the Herbal Products Industry. Energies 2020, 13, 55. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Atabani, A.E.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Mekhilef, S. A review on prospect of Jatropha curcas for biodiesel in Indonesia. Renew. Sustain. Energy Rev. 2011, 15, 3733–3756. [Google Scholar] [CrossRef]
- Enweremadu, C.C.; Mbarawa, M.M. Technical aspects of production and analysis of biodiesel from used cooking oil—A review. Renew. Sustain. Energy Rev. 2009, 13, 2205–2224. [Google Scholar] [CrossRef]
- Demirbas, A. Recent advances in biomass conversion technologies. Energy Educ. Sci. Technol. 2000, 6, 19–40. [Google Scholar]
- International Energy Agency. World Energy Outlook 2010; International Energy Agency: Paris, France, 2010. [Google Scholar]
- U.S. Energy Information Administration (EIA). Annual Energy Outlook 2011; U.S. Energy Information Administration (EIA): Washington, DC, USA, 2011.
- Davis, S.C.; Diegel, S.W.; Boundy, R.G. Transportation Energy Data Book, 30th ed.; Noyes Pubns: Norwich, NY, USA, 2011. [Google Scholar]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. International Energy Outlook 2011; U.S. Energy Information Administration: Washington, DC, USA, 2011.
- Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H. A review on emissions and mitigation strategies for road transport in Malaysia. Renew. Sustain. Energy Rev. 2011, 15, 3516–3522. [Google Scholar] [CrossRef]
- Fridleifsson, I.B. Geothermal energy for the benefit of the people. Renew. Sustain. Energy Rev. 2001, 5, 299–312. [Google Scholar] [CrossRef]
- Thirugnanasambandam, M.; Iniyan, S.; Goic, R. A review of solar thermal technologies. Renew. Sustain. Energy Rev. 2010, 14, 312–322. [Google Scholar] [CrossRef]
- Joselin Herbert, G.M.; Iniyan, S.; Sreevalsan, E.; Rajapandian, S. A review of wind energy technologies. Renew. Sustain. Energy Rev. 2007, 11, 1117–1145. [Google Scholar] [CrossRef]
- Montes, G.M.; Del Mar Serrano López, M.; Del Carmen Rubio Gámez, M.; Ondina, A.M. An overview of renewable energy in Spain. The small hydro-power case. Renew. Sustain. Energy Rev. 2005, 9, 521–534. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Renewable Energy. Available online: http://www.eere.energy.gov/topics/renewable_energy.html (accessed on 20 July 2012).
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Psaltis, P.; Komilis, D. Environmental and economic assessment of the use of biodrying before thermal treatment of municipal solid waste. Waste Manag. 2019, 83, 95–103. [Google Scholar] [CrossRef]
- Kar, T.; Keles, S. Environmental impacts of biomass combustion for heating and electricity generation. J. Eng. Res. Appl. Sci. 2016, 5, 458–465. [Google Scholar]
- Andrade, L.A.; Batista, F.R.X.; Lira, T.S.; Barrozo, M.A.S.; Vieira, L.G.M. haracterization and product formation during the catalytic and non-catalytic pyrolysis of the green microalgae Chlamydomonas reinhardtii. Renew. Energy 2018, 119, 731–740. [Google Scholar] [CrossRef]
- Wu, C.; Budarin, V.L.; Gronnow, M.J.; De Bruyn, M.; Onwudili, J.A.; Clark, J.H.; Williams, P.T. Conventional and microwave-assisted pyrolysis of biomass under different heating rates. J. Anal. Appl. Pyrolysis 2014, 107, 276–283. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Mazlina, M.; Radiah, A.; Syafiie, S.; Harun, M.Y. Effect of microwave-assisted acid or alkali pretreatment on sugar release from Dragon fruit foliage. Int. Food Res. J. 2016, 23, S149–S154. [Google Scholar]
- Ethaib, S.; Omar, R.; Mazlina, M.K.S.; Radiah, A.B.D.; Syafiie, S. Microwave-assisted dilute acid pretreatment and enzymatic hydrolysis of sago palm bark. BioResources 2016, 11, 5687–5702. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Geng, C.; El Mashad, H.; Li, H.; Yin, W. An economic analysis of rice straw microwave pyrolysis for hydrogen-rich fuel gas. RSC Adv. 2017, 7, 53396–53400. [Google Scholar] [CrossRef]
- Rahimi, M.A.; Omar, R.; Ethaib, S.; Mazlina, M.S.; Biak, D.A.; Aisyah, R.N. Microwave-assisted extraction of lipid from fish waste. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012096. [Google Scholar]
- Ethaib, S.; Omar, R.; Mazlina, M.K.S.; Radiah, A.B.D.; Syafiie, S. Development of a hybrid PSO–ANN model for estimating glucose and xylose yields for microwave-assisted pretreatment and the enzymatic hydrolysis of lignocellulosic biomass. Neural Comput. Appl. 2018, 30, 1111–1121. [Google Scholar] [CrossRef]
- Fernández Díez, Y.; Arenillas de la Puente, A.; Menéndez Díaz, J.Á. Microwave heating applied to pyrolysis. In Advances in Induction and Microwave Heating of Mineral and Organic Materials; InTech: London, UK, 2011. [Google Scholar]
- Ravikumar, C.; Kumar, P.S.; Subhashni, S.K.; Tejaswini, P.V.; Varshini, V. Microwave assisted fast pyrolysis of corn cob, corn stover, saw dust and rice straw: Experimental investigation on bio-oil yield and high heating values. Sustain. Mater. Technol. 2017, 11, 19–27. [Google Scholar] [CrossRef]
- Jones, D.A.; Lelyveld, T.P.; Mavrofidis, S.D.; Kingman, S.W.; Miles, N.J. Microwave heating applications in environmental engineering—A review. Resour. Conserv. Recycl. 2002, 34, 75–90. [Google Scholar] [CrossRef]
- Lam, S.S.; Russell, A.D.; Lee, C.L.; Chase, H.A. Microwave-heated pyrolysis of waste automotive engine oil: Influence of operation parameters on the yield, composition, and fuel properties of pyrolysis oil. Fuel 2012, 92, 327–339. [Google Scholar] [CrossRef]
- Huang, Y.F.; Shih, C.H.; Chiueh, P.T.; Lo, S.L. Microwave co-pyrolysis of sewage sludge and rice straw. Energy 2015, 87, 638–644. [Google Scholar] [CrossRef]
- Shang, H.; Lu, R.R.; Shang, L.; Zhang, W.H. Effect of additives on the microwave-assisted pyrolysis of sawdust. Fuel Process. Technol. 2015, 131, 167–174. [Google Scholar] [CrossRef]
- Beneroso, D.; Bermúdez, J.M.; Arenillas, A.; Menéndez, J.A. Influence of the microwave absorbent and moisture content on the microwave pyrolysis of an organic municipal solid waste. J. Anal. Appl. Pyrolysis 2014, 105, 234–240. [Google Scholar] [CrossRef]
- Kong, S.H.; Lam, S.S.; Yek, P.N.Y.; Liew, R.K.; Ma, N.L.; Osman, M.S.; Wong, C.C. Self-purging microwave pyrolysis: An innovative approach to convert oil palm shell into carbon-rich biochar for methylene blue adsorption. J. Chem. Technol. Biotechnol. 2019, 94, 1397–1405. [Google Scholar] [CrossRef]
- Luo, H.; Bao, L.; Kong, L.; Sun, Y. Low temperature microwave-assisted pyrolysis of wood sawdust for phenolic rich compounds: Kinetics and dielectric properties analysis. Bioresour. Technol. 2017, 238, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Matharu, A.S. Thermochemical Valorization of Paper Deinking Residue through Microwave-Assisted Pyrolysis. In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 671–692. [Google Scholar]
- Rosi, L.; Bartoli, M.; Frediani, M. Microwave assisted pyrolysis of halogenated plastics recovered from waste computers. Waste Manag. 2018, 73, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, N.M.; Ethaib, S.; Omar, R. Effects of microwave absorbers on the products of microwave pyrolysis of oily sludge. J. Eng. Sci. Technol. 2018, 13, 3313–3330. [Google Scholar]
- Zhou, J.; Liu, S.; Zhou, N.; Fan, L.; Zhang, Y.; Peng, P.; Chen, P. Development and application of a continuous fast microwave pyrolysis system for sewage sludge utilization. Bioresour. Technol. 2018, 256, 295–301. [Google Scholar] [CrossRef]
- Fan, J.; Shuttleworth, P.S.; Gronnow, M.; Breeden, S.W.; Clark, J.H.; Macquarrie, D.J.; Budarin, V.L. Influence of density on microwave pyrolysis of cellulose. ACS Sustain. Chem. Eng. 2018, 6, 2916–2920. [Google Scholar] [CrossRef]
- Parvez, A.M.; Wu, T.; Afzal, M.T.; Mareta, S.; He, T.; Zhai, M. Conventional and microwave-assisted pyrolysis of gumwood: A comparison study using thermodynamic evaluation and hydrogen production. Fuel Process. Technol. 2019, 184, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Z.; Tian, X.; Dai, L.; Jiang, L.; Zhang, S.; Ruan, R. Production of bio-oil from agricultural waste by using a continuous fast microwave pyrolysis system. Bioresour. Technol. 2018, 269, 162–168. [Google Scholar] [CrossRef]
- Hasan, M.H.; Mahlia, T.M.I.; Nur, H. A review on energy scenario and sustainable energy in Indonesia. Renew. Sustain. Energy Rev. 2012, 16, 2316–2328. [Google Scholar] [CrossRef]
- Yuksel, I.; Kaygusuz, K. Renewable energy sources for clean and sustainable energy policies in Turkey. Renew. Sustain. Energy Rev. 2011, 15, 4132–4144. [Google Scholar] [CrossRef]
- Berndes, G.; Hoogwijk, M.; van den Broek, R. The contribution of biomass in the future global energy supply: A review of 17 studies. Biomass Bioenergy 2003, 25, 1–28. [Google Scholar] [CrossRef]
- Hall, D.O. Biomass energy in industrialised countries—A view of the future. For. Ecol. Manag. 1997, 91, 17–45. [Google Scholar] [CrossRef]
- Guehenneux, G.; Baussand, P.; Brothier, M.; Poletiko, C.; Boissonnet, G. Energy production from biomass pyrolysis: A new coefficient of pyrolytic valorisation. Fuel 2005, 84, 733–739. [Google Scholar] [CrossRef]
- Börjesson, P.; Berglund, M. Environmental systems analysis of biogas systemsPart I: Fuel-cycle emissions. Biomass Bioenergy 2006, 30, 469–485. [Google Scholar] [CrossRef]
- Chung, K.H.; Kim, J.; Lee, K.Y. Biodiesel production by transesterification of duck tallow with methanol on alkali catalysts. Biomass Bioenergy 2009, 33, 155–158. [Google Scholar] [CrossRef]
- Bilgen, S.; Keleş, S.; Kaygusuz, A.; Sarı, A.; Kaygusuz, K. Global warming and renewable energy sources for sustainable development: A case study in Turkey. Renew. Sustain. Energy Rev. 2008, 12, 372–396. [Google Scholar] [CrossRef]
- Hashim, H.; Ho, W.S. Renewable energy policies and initiatives for a sustainable energy future in Malaysia. Renew. Sustain. Energy Rev. 2011, 15, 4780–4787. [Google Scholar] [CrossRef]
- Martinot, E.; Chaurey, A.; Lew, D.; Moreira, J.R.; Wamukonya, N. Renewable energy markets in developing countries. Annu. Rev. Energy Environ. 2002, 27, 309–348. [Google Scholar] [CrossRef]
- Dodic, S.N.; Vasiljevic, T.Z.; Maric, R.M.; Kosanovic, A.J.R.; Dodic, J.M.; Popov, S.D. Possibilities of application of waste wood biomass as an energy source in Vojvodina. Renew. Sustain. Energy Rev. 2012, 16, 2355–2360. [Google Scholar] [CrossRef]
- Dominguez, A.; Menéndez, J.A.; Fernandez, Y.; Pis, J.J.; Nabais, J.V.; Carrott, P.J.M.; Carrott, M.R. Conventional and microwave induced pyrolysis of coffee hulls for the production of a hydrogen rich fuel gas. J. Anal. Appl. Pyrolysis 2007, 79, 128–135. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lee, M.K.; Chang, Y.M. Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J. Anal. Appl. Pyrolysis 2006, 76, 230–237. [Google Scholar] [CrossRef]
- Shie, J.-L.; Tsou, F.-J.; Lin, K.-L.; Chang, C.-Y. Bioenergy and products from thermal pyrolysis of rice straw using plasma torch. Bioresour. Technol. 2010, 101, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Uzun, B.B.; Apaydin-Varol, E.; Ateş, F.; Ozbay, N.; Putun, A.E. Synthetic fuel production from tea waste: Characterisation of bio-oil and bio-char. Fuel 2010, 89, 176–184. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, B.; Chen, X.; Bai, Z.; Zhang, H. Studies on pyrolysis of wheat straw residues from ethanol production by solid-state fermentation. J. Anal. Appl. Pyrolysis 2008, 81, 243–246. [Google Scholar] [CrossRef]
- De Wild, P.J.; Huijgen, W.J.J.; Heeres, H.J. Pyrolysis of wheat straw-derived organosolv lignin. J. Anal. Appl. Pyrolysis 2012, 93, 95–103. [Google Scholar] [CrossRef]
- Rumphorst, M.P.; Ringel, H.D. Pyrolysis of sewage sludge and use of pyrolysis coke. J. Anal. Appl. Pyrolysis 1994, 28, 137–155. [Google Scholar] [CrossRef]
- Fonts, I.; Azuara, M.; Gea, G.; Murillo, M.B. Study of the pyrolysis liquids obtained from different sewage sludge. J. Anal. Appl. Pyrolysis 2009, 85, 184–191. [Google Scholar] [CrossRef]
- Fonts, I.; Gea, G.; Azuara, M.; Ábrego, J.; Arauzo, J. Sewage sludge pyrolysis for liquid production: A review. Renew. Sustain. Energy Rev. 2012, 16, 2781–2805. [Google Scholar] [CrossRef]
- Nourreddine, M. Recycling of auto shredder residue. J. Hazard. Mater. 2007, 139, 481–490. [Google Scholar] [CrossRef]
- Decker, S.R.; Sheehan, J.; Dayton, D.C.; Bozell, J.J.; Adney, W.S.; Hames, B.; Thomas, S.R.; Bain, R.L.; Czernik, S.; Zhang, M.; et al. Biomass conversion. In Kent and Riegel’s Handbook of Industrial Chemistry and Biotechnology; Kent, J.A., Ed.; Springer: Boston, MA, USA, 2007; pp. 1499–1548. [Google Scholar]
- Faaij, A. Modern biomass conversion technologies. Mitig. Adapt. Strateg. Glob. Change 2006, 11, 335–367. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Siti Mazlina, M.K.; Dayang Radiah, A.B. Evaluation of the interactive effect pretreatment parameters via three types of microwave-assisted pretreatment and enzymatic hydrolysis on sugar yield. Processes 2020, 8, 787. [Google Scholar] [CrossRef]
- Saadi, W.; Rodríguez-Sánchez, S.; Ruiz, B.; Souissi-Najar, S.; Ouederni, A.; Fuente, E. Pyrolysis technologies for pomegranate (Punica granatum L.) peel wastes. Prospects in the bioenergy sector. Renew. Energy 2019, 136, 373–382. [Google Scholar] [CrossRef]
- Demirbas, A. Partial hydrogenation effect of moisture contents on the combustion oils from biomass pyrolysis. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 30, 508–515. [Google Scholar] [CrossRef]
- Staš, M.; Chudoba, J.; Kubička, D.; Blažek, J.; Pospíšil, M. Petroleomic characterization of pyrolysis bio-oils: A review. Energy Fuels 2017, 31, 10283–10299. [Google Scholar] [CrossRef]
- Vitasari, C.R.; Meindersma, G.W.; De Haan, A.B. Water extraction of pyrolysis oil: The first step for the recovery of renewable chemicals. Bioresour. Technol. 2011, 102, 7204–7210. [Google Scholar] [CrossRef]
- Kappler, G.; de Souza, D.M.; Moraes, C.A.M.; Modolo, R.C.E.; Brehm, F.A.; Wander, P.R.; da Cruz Tarelho, L.A. Conversion of Lignocellulosic Biomass through Pyrolysis to Promote a Sustainable Value Chain for brazilian agribusiness. In Lignocellulosic Biorefining Technologies; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 265–283. [Google Scholar]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Mahari, W.A.W.; Aghbashlo, M. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.J.; Saravanan, A. Advances in production and application of biochar from lignocellulosic feedstocks for remediation of environmental pollutants. Bioresour. Technol. 2019, 292, 122030. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Khole, P.R.; Shukla, S. Bio-oil production through biomass pyrolysis and upgrading research. Int. J. Agric. Eng. 2018, 11, 257–263. [Google Scholar] [CrossRef]
- Qureshi, K.M.; Lup, A.N.K.; Khan, S.; Abnisa, F.; Daud, W.M.A.W. A technical review on semi-continuous and continuous pyrolysis process of biomass to bio-oil. J. Anal. Appl. Pyrolysis 2018, 131, 52–75. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zhu, X.F.; Zhang, L.Q.; Zhu, X.F. Preparation and characterization of microemulsion fuels from diesel and model compound of walnut shell pyrolysis oil. Fuel 2019, 243, 478–484. [Google Scholar] [CrossRef]
- Yan, R.; Yang, H.; Chin, T.; Liang, D.T.; Chen, H.; Zheng, C. Influence of temperature on the distribution of gaseous products from pyrolyzing palm oil wastes. Combust. Flame 2005, 142, 24–32. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Liang, D.T.; Zheng, C. Pyrolysis of palm oil wastes for enhanced production of hydrogen rich gases. Fuel Process. Technol. 2006, 87, 935–942. [Google Scholar] [CrossRef]
- Klass, D. Biomass for Renewable Energy, Fuel and Chemicals; Academic Press: London, UK, 1998. [Google Scholar]
- Thormann, L.; de Oro, P.P. Fuels from Pyrolysis. In Biokerosene; Springer: Berlin/Heidelberg, Germany, 2018; pp. 575–605. [Google Scholar]
- Cerone, N.; Zimbardi, F.; Villone, A.; Strjiugas, N.; Kiyikci, E.G. Gasification of wood and torrefied wood with air, oxygen, and steam in a fixed-bed pilot plant. Energy Fuels 2016, 30, 4034–4043. [Google Scholar] [CrossRef]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A.; Dallinger, D. Microwaves in Organic and Medicinal Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Nascimento, U.M.; Azevedo, E.B. Microwaves and their coupling to advanced oxidation processes: Enhanced performance in pollutants degradation. J. Environ. Sci. Health Part A 2013, 48, 1056–1072. [Google Scholar] [CrossRef]
- World Commission on Environment and Development. Our Common Future; Oxford University Press: Melbourne, Australia, 1990. [Google Scholar]
- Kreith, F.; Krumdieck, S.; Kreider, J.F. Principles of Sustainable Energy; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- International Biochar Initiative (IBI). Available online: http://www.biochar-international.org/ (accessed on 14 February 2012).
- Zhao, X.; Wang, M.; Liu, H.; Li, L.; Ma, C.; Song, Z. A microwave reactor for characterization of pyrolyzed biomass. Bioresour. Technol. 2012, 104, 673–678. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, X.; Chen, C. A study on experimental characteristic of microwaveassisted pyrolysis of microalgae. Bioresour. Technol. 2012, 107, 487–493. [Google Scholar] [CrossRef]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J.; Julson, J.; Ruan, R. Biofuel production and kinetics analysis for microwave pyrolysis of Douglas fir sawdust pellet. J. Anal. Appl. Pyrolysis 2012, 94, 163–169. [Google Scholar] [CrossRef]
- Chen, M.Q.; Wang, J.; Zhang, M.X.; Chen, M.G.; Zhu, X.F.; Min, F.F.; Tan, Z.C. Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J. Anal. Appl. Pyrolysis 2008, 82, 145–150. [Google Scholar] [CrossRef]
- Omar, R.; Idris, A.; Yunus, R.; Khalid, K.; Aida Isma, M.I. Characterization of empty fruit bunch for microwave-assisted pyrolysis. Fuel 2011, 90, 1536–1544. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, K.M.; Basheer, N.; Hussain, K. Co-liquefaction of Makarwal coal and waste polystyrene by microwave–metal interaction pyrolysis in copper coil reactor. J. Anal. Appl. Pyrolysis 2011, 90, 53–55. [Google Scholar] [CrossRef]
- Tian, Y.; Zuo, W.; Ren, Z.; Chen, D. Estimation of a novel method to produce biooil from sewage sludge by microwave pyrolysis with the consideration of efficiency and safety. Bioresour. Technol. 2011, 102, 2053–2061. [Google Scholar] [CrossRef]
- Du, J.; Liu, P.; Liu, Z.-H.; Sun, D.-G.; Tao, C.-Y. Fast pyrolysis of biomass for bio-oil with ionic liquid and microwave irradiation. J. Fuel Chem. Technol. 2010, 38, 554–559. [Google Scholar] [CrossRef]
- Zeng, X.; Fu, D.; Sheng, H.; Xie, S.; Li, X.; Hu, Q.; Zou, J. Growth and morphology of carbon nanostructures by microwave-assisted pyrolysis of methane. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 42, 2103–2108. [Google Scholar] [CrossRef]
- Huang, Y.F.; Kuan, W.H.; Lo, S.L.; Lin, C.F. Hydrogen-rich fuel gas from rice straw via microwave-induced pyrolysis. Bioresour. Technol. 2010, 101, 1968–1973. [Google Scholar] [CrossRef]
- Fu, D.; Zeng, X.; Zou, J.; Li, L.; Li, X.; Deng, F. In situ synthesis and photoluminescence of SiC nanowires by microwave-assisted pyrolysis of methane. J. Alloys Compd. 2009, 486, 406–409. [Google Scholar] [CrossRef]
- Andersson, M.; Knutson Wedel, M.; Forsgren, C.; Christéen, J. Microwave assisted pyrolysis of residual fractions of waste electrical and electronics equipment. Miner. Eng. 2012, 29, 105–111. [Google Scholar] [CrossRef]
- Chemat, F.; Poux, M. Microwave assisted pyrolysis of urea supported on graphite under solvent-free conditions. Tetrahedron Lett. 2001, 42, 3693–3695. [Google Scholar] [CrossRef]
- Salema, A.A.; Ani, F.N. Microwave induced pyrolysis of oil palm biomass. Bioresour. Technol. 2011, 102, 3388–3395. [Google Scholar] [CrossRef]
- Lei, H.; Ren, S.; Wang, L.; Bu, Q.; Julson, J.; Holladay, J.; Ruan, R. Microwave pyrolysis of distillers dried grain with solubles (DDGS) for biofuel production. Bioresour. Technol. 2011, 102, 6208–6213. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Chen, P.; Zhang, B.; Yang, C.; Liu, Y.; Lin, X.; Ruan, R. Microwave-assisted pyrolysis of biomass: Catalysts to improve product selectivity. J. Anal. Appl. Pyrolysis 2009, 86, 161–167. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y.; Wang, X.; Wan, Y.; Chen, Q.; Wang, C.; Lin, X.; Liu, Y.; Chen, P.; Ruan, R. Microwave-assisted pyrolysis of microalgae for biofuel production. Bioresour. Technol. 2011, 102, 4890–4896. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Lei, H.; Ren, S.; Wang, L.; Holladay, J.; Zhang, Q.; Tang, J.; Ruan, R. Phenol and phenolics from lignocellulosic biomass by catalytic microwave pyrolysis. Bioresour. Technol. 2011, 102, 7004–7007. [Google Scholar] [CrossRef]
- Lam, S.S.; Russell, A.D.; Lee, C.L.; Lam, S.K.; Chase, H.A. Production of hydrogen and light hydrocarbons as a potential gaseous fuel from microwave-heated pyrolysis of waste automotive engine oil. Int. J. Hydrogen Energy 2012, 37, 5011–5021. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Ren, S.; Wang, L.; Zhang, Q.; Tang, J.; Ruan, R. Production of phenols and biofuels by catalytic microwave pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2012, 108, 274–279. [Google Scholar] [CrossRef]
- Lam, S.S.; Russell, A.D.; Chase, H.A. Pyrolysis using microwave heating: A sustainable process for recycling used car engine oil. Ind. Eng. Chem. Res. 2010, 49, 10845–10851. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, G.; Liu, Y. Scale-up of microwave heating process for the production of bio-oil from sewage sludge. J. Anal. Appl. Pyrolysis 2012, 94, 114–119. [Google Scholar] [CrossRef]
- Huang, Y.F.; Kuan, W.H.; Lo, S.L.; Lin, C.F. Total recovery of resources and energy from rice straw using microwave-induced pyrolysis. Bioresour. Technol. 2008, 99, 8252–8258. [Google Scholar] [CrossRef]
- Salema, A.A.; Ani, F.N. Microwave-assisted pyrolysis of oil palm shell biomass using an overhead stirrer. J. Anal. Appl. Pyrolysis 2012, 96, 162–172. [Google Scholar] [CrossRef]
- Moen, J.; Yang, C.; Zhang, B.; Lei, H.; Hennessy, K.; Wan, Y.; Le, Z.; Liu, Y.; Chen, P.; Ruan, R. Catalytic microwave assisted pyrolysis of aspen. Int. J. Agric. Biol. Eng. 2009, 2, 70–75. [Google Scholar]
- Yu, F.; Ruan, R.; Steele, P. Microwave pyrolysis of corn stover. Trans. Am. Soc. Agric. Biol. Eng. 2009, 52, 1595–1601. [Google Scholar]
- Salema, A.A.; Ani, F.N. Microwave pyrolysis of oil palm fibers. J. Mek. 2010, 30, 77–86. [Google Scholar]
- Ludlow-Palafox, C.; Chase, H.A. Microwave-induced pyrolysis of plastic wastes. Ind. Eng. Chem. Res. 2001, 40, 4749–4756. [Google Scholar] [CrossRef]
- Lei, H.; Ren, S.; Julson, J. The effects of reaction temperature and time and particle size of corn stover on microwave pyrolysis. Energy Fuels 2009, 23, 3254–3261. [Google Scholar] [CrossRef]
- Fernandez, Y.; Arenillas, A.; Bermúdez, J.M.; Menendez, J.A. Comparative study of conventional and microwave-assisted pyrolysis, steam and dry reforming of glycerol for syngas production, using a carbonaceous catalyst. J. Anal. Appl. Pyrolysis 2010, 88, 155–159. [Google Scholar] [CrossRef]
- Duan, X.-H.; Srinivasakannan, C.; Peng, J.-H.; Zhang, L.-B.; Zhang, Z.-Y. Comparison of activated carbon prepared from Jatropha hull by conventional heating and microwave heating. Biomass Bioenergy 2011, 35, 3920–3926. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).