Biocytin-Labeling in Whole-Cell Recording: Electrophysiological and Morphological Properties of Pyramidal Neurons in CYLD-Deficient Mice
Abstract
:1. Introduction
2. Results
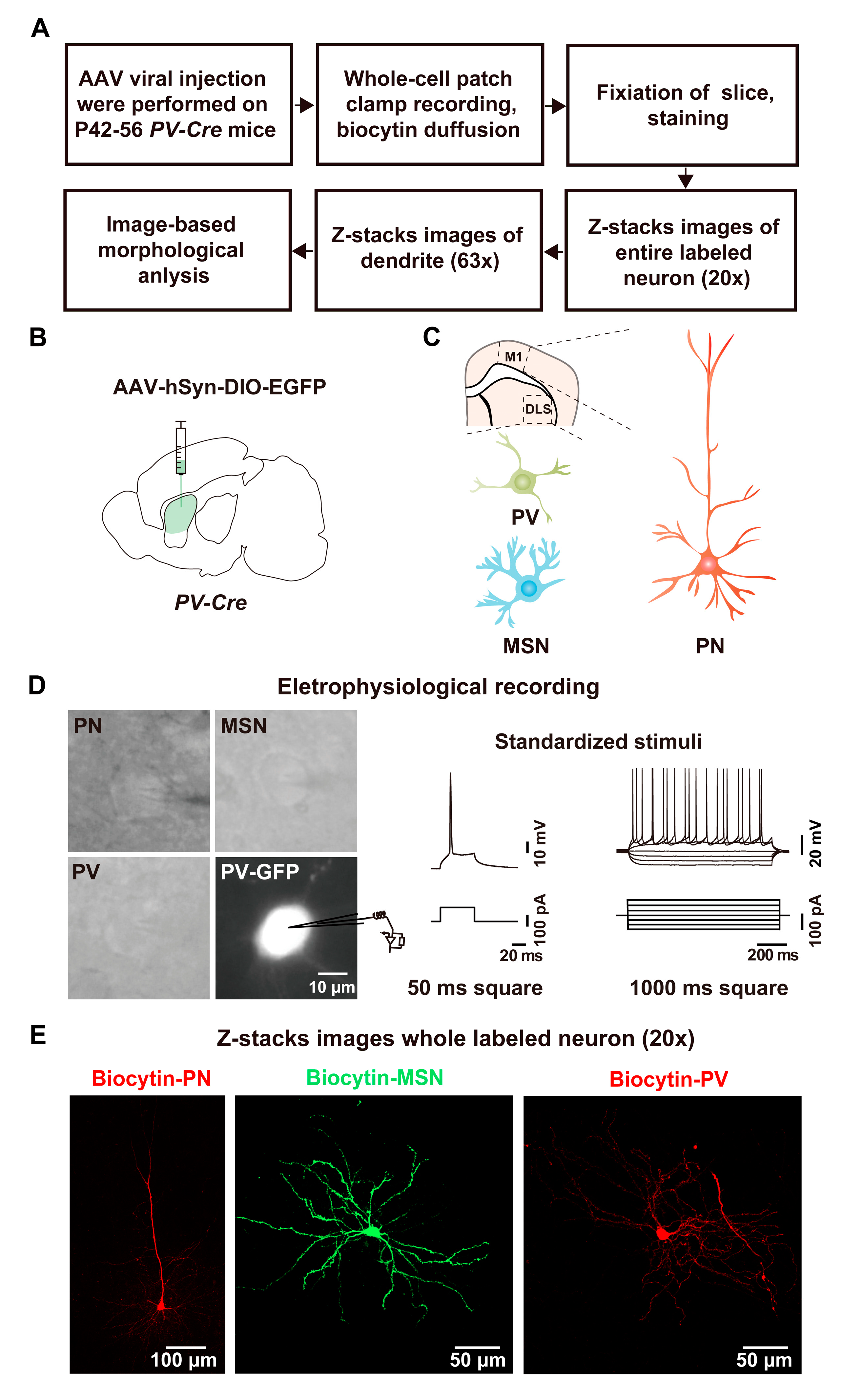
2.1. Whole-Cell Recording and Biocytin Labeling Procedure
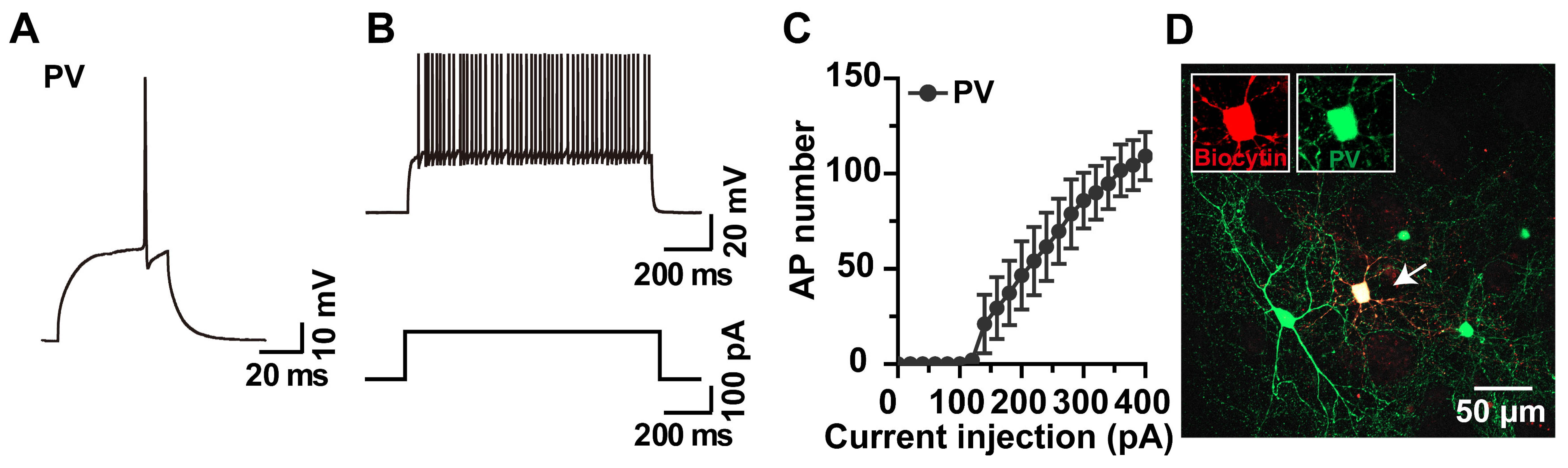
2.2. Whole-Cell Recording and Biocytin Labeling of PV Interneurons
2.3. Whole-Cell Recording and Biocytin Labeling of MSNs
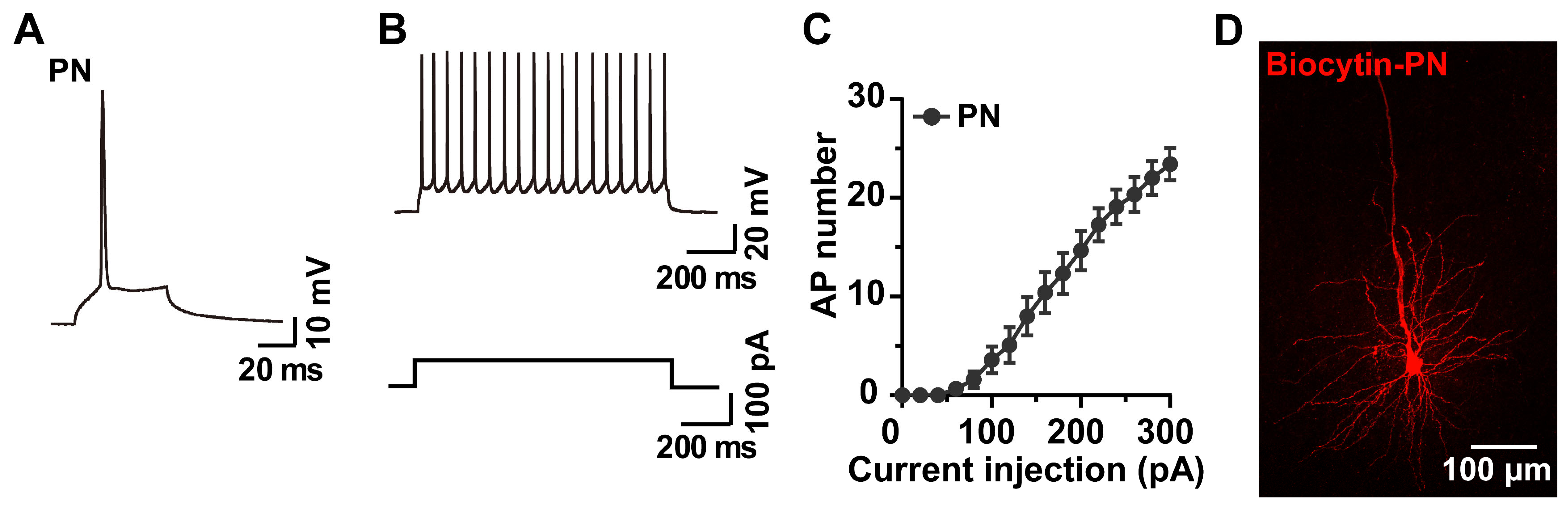
2.4. Whole-Cell Recording and Biocytin Labeling of PNs
2.5. Analysis of Biocytin-Labeled Neurons by ImageJ
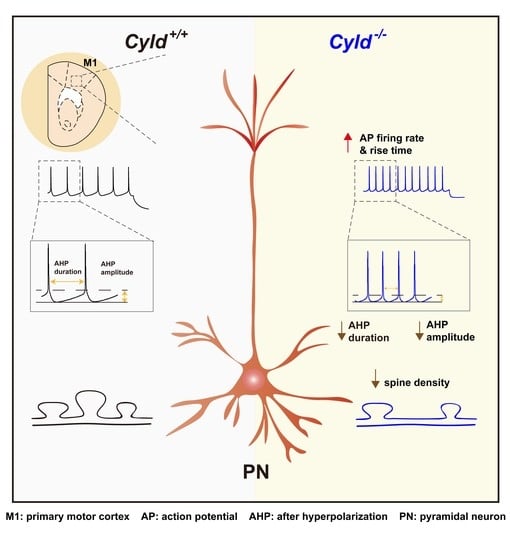
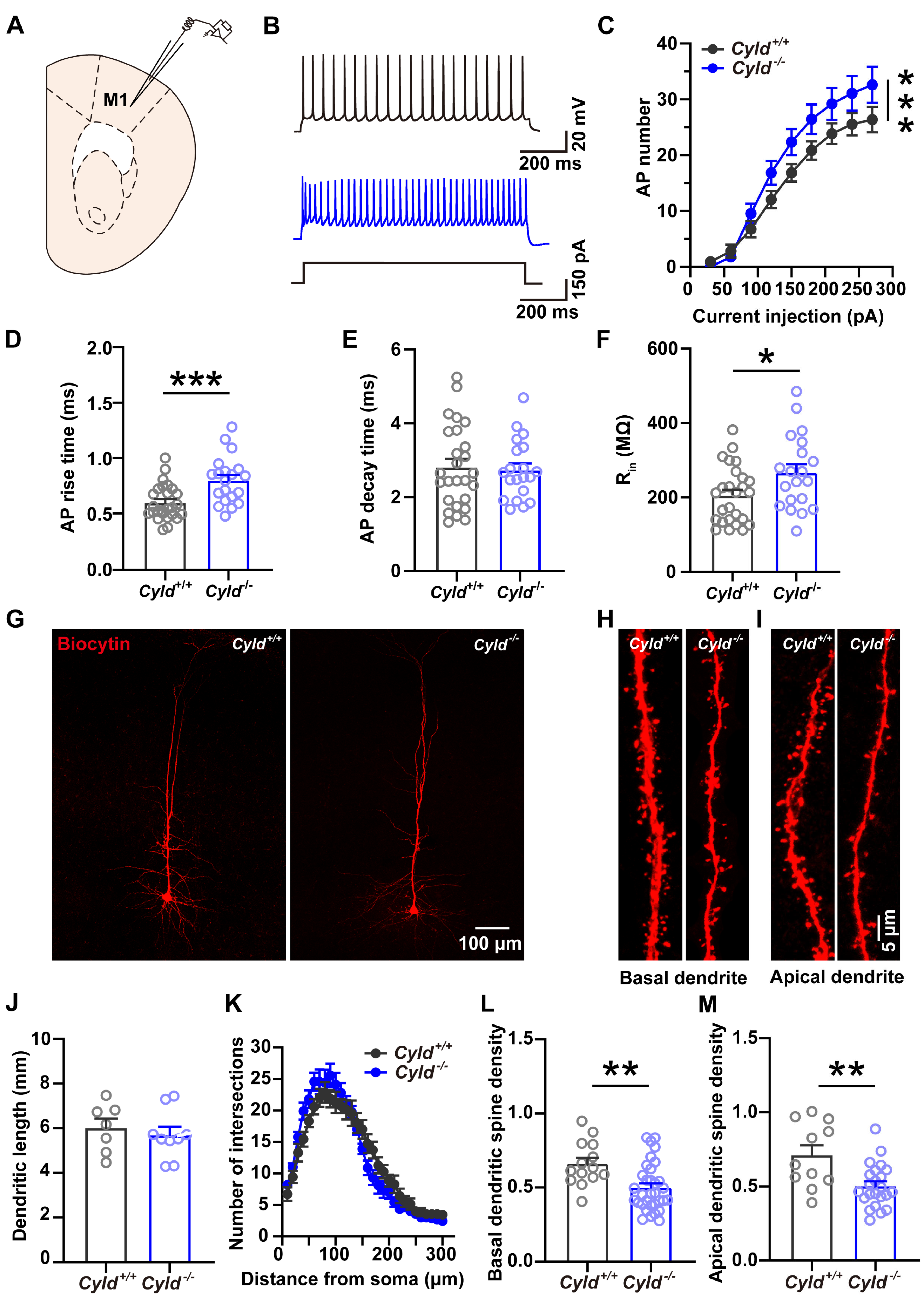
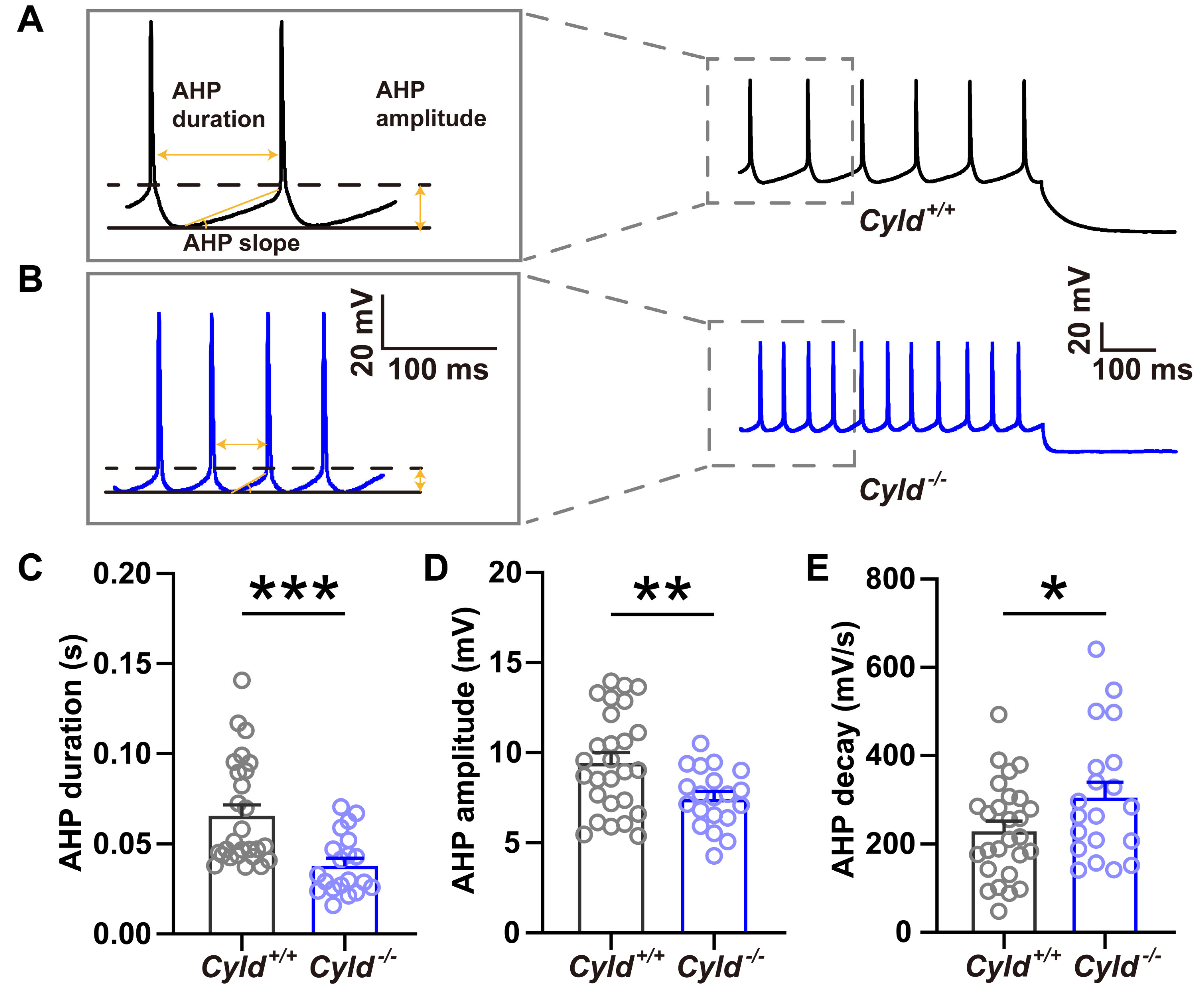
2.6. Altered APs and Spine Density in PNs of the M1 Cortical Region of Cyld−/− Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Stereotaxic Viral Injection
4.3. Internal and External Solutions for Patch-Clamp Recording
4.3.1. Artificial Cerebrospinal Fluids (aCSFs)
4.3.2. Intracellular Biocytin Solution
4.4. Slice Preparation for Whole-Cell Patch-Clamp Recording
4.5. Electrodes for Whole-Cell Recording and Biocytin Diffusion
4.6. Whole-Cell Recording of APs
4.6.1. Selection of Target Neurons for Whole-Cell Recording
4.6.2. Giga-Ohm (GΩ) Seal Formation and Establishment of Whole-Cell Mode in the Target Neuron
4.7. Biocytin Diffusion and Brain-Slice Fixation
4.8. Post-Hoc Immunohistochemistry
4.9. Image Processing
4.10. Morphological Analysis of Biocytin-Labeled Neurons
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hodapp, A.; Kaiser, M.E.; Thome, C.; Ding, L.; Rozov, A.; Klumpp, M.; Stevens, N.; Stingl, M.; Sackmann, T.; Lehmann, N.; et al. Dendritic axon origin enables information gating by perisomatic inhibition in pyramidal neurons. Science 2022, 377, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Ziv, N.E.; Okazaki, H.; Yagishita, S.; Toyoizumi, T. Spine dynamics in the brain, mental disorders and artificial neural networks. Nat. Rev. Neurosci. 2021, 22, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Peca, J.; Feliciano, C.; Ting, J.T.; Wang, W.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.; Feng, G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 2011, 472, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Booker, S.A.; Domanski, A.P.F.; Dando, O.R.; Jackson, A.D.; Isaac, J.T.R.; Hardingham, G.E.; Wyllie, D.J.A.; Kind, P.C. Altered dendritic spine function and integration in a mouse model of fragile X syndrome. Nat. Commun. 2019, 10, 4813. [Google Scholar] [CrossRef]
- Gouwens, N.W.; Sorensen, S.A.; Berg, J.; Lee, C.; Jarsky, T.; Ting, J.; Sunkin, S.M.; Feng, D.; Anastassiou, C.A.; Barkan, E.; et al. Classification of electrophysiological and morphological neuron types in the mouse visual cortex. Nat. Neurosci. 2019, 22, 1182–1195. [Google Scholar] [CrossRef]
- Jiang, J.; Tang, B.; Wang, L.; Huo, Q.; Tan, S.; Misrani, A.; Han, Y.; Li, H.; Hu, H.; Wang, J.; et al. Systemic LPS-induced microglial activation results in increased GABAergic tone: A mechanism of protection against neuroinflammation in the medial prefrontal cortex in mice. Brain Behav. Immun. 2022, 99, 53–69. [Google Scholar] [CrossRef]
- Mishra, A.; Dhingra, K.; Schuz, A.; Logothetis, N.K.; Canals, S. Improved neuronal tract tracing with stable biocytin-derived neuroimaging agents. ACS Chem. Neurosci. 2010, 1, 129–138. [Google Scholar] [CrossRef]
- Horikawa, K.; Armstrong, W.E. A versatile means of intracellular labeling: Injection of biocytin and its detection with avidin conjugates. J. Neurosci. Methods 1988, 25, 1–11. [Google Scholar] [CrossRef]
- Scala, F.; Kobak, D.; Bernabucci, M.; Bernaerts, Y.; Cadwell, C.R.; Castro, J.R.; Hartmanis, L.; Jiang, X.; Laturnus, S.; Miranda, E.; et al. Phenotypic variation of transcriptomic cell types in mouse motor cortex. Nature 2021, 598, 144–150. [Google Scholar] [CrossRef]
- Ma, Q.; Ruan, H.; Peng, L.; Zhang, M.; Gack, M.U.; Yao, W.D. Proteasome-independent polyubiquitin linkage regulates synapse scaffolding, efficacy, and plasticity. Proc. Natl. Acad. Sci. USA 2017, 114, E8760–E8769. [Google Scholar] [CrossRef]
- Sato, Y.; Goto, E.; Shibata, Y.; Kubota, Y.; Yamagata, A.; Goto-Ito, S.; Kubota, K.; Inoue, J.; Takekawa, M.; Tokunaga, F.; et al. Structures of CYLD USP with Met1- or Lys63-linked diubiquitin reveal mechanisms for dual specificity. Nat. Struct. Mol. Biol. 2015, 22, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, A.S.; Ruan, H.; Dai, H.; Skolfield, M.C.; Phillips, H.L.; Burnette, W.J.; Javidfar, B.; Sun, S.C.; Akbarian, S.; Yao, W.D. Cylindromatosis drives synapse pruning and weakening by promoting macroautophagy through Akt-mTOR signaling. Mol. Psychiatry 2022, 27, 2414–2424. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sekine-Aizawa, Y.; Ebrahimi, S.; Tanaka, S.; Okabe, S. Tumor suppressor protein CYLD regulates morphogenesis of dendrites and spines. Eur. J. Neurosci. 2019, 50, 2722–2739. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ma, N.; Wu, X.; Ni, H.; Gao, S.; Sun, L.; Zhou, P.; Tala; Ran, J.; Zhou, J.; et al. CYLD deficiency causes auditory neuropathy due to reduced neurite outgrowth. J. Clin. Lab. Anal. 2021, 35, e23783. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Horta, G.; Roesler, M.K.; Ihbe, N.; Chhabra, S.; Radyushkin, K.; Liberto, G.M.; Kreutzfeldt, M.; Schumann, S.; Engelhardt, J.V.; et al. The K63 deubiquitinase CYLD modulates autism-like behaviors and hippocampal plasticity by regulating autophagy and mTOR signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e110755118. [Google Scholar] [CrossRef]
- Lee, J.; Wang, W.; Sabatini, B.L. Anatomically segregated basal ganglia pathways allow parallel behavioral modulation. Nat. Neurosci. 2020, 23, 1388–1398. [Google Scholar] [CrossRef]
- Li, N.; Chen, T.W.; Guo, Z.V.; Gerfen, C.R.; Svoboda, K. A motor cortex circuit for motor planning and movement. Nature 2015, 519, 51–56. [Google Scholar] [CrossRef]
- Feng, X.Y.; Hu, H.D.; Chen, J.; Long, C.; Yang, L.; Wang, L. Acute neuroinflammation increases excitability of prefrontal parvalbumin interneurons and their functional recruitment during novel object recognition. Brain Behav. Immun. 2021, 98, 48–58. [Google Scholar] [CrossRef]
- Klug, J.R.; Engelhardt, M.D.; Cadman, C.N.; Li, H.; Smith, J.B.; Ayala, S.; Williams, E.W.; Hoffman, H.; Jin, X. Differential inputs to striatal cholinergic and parvalbumin interneurons imply functional distinctions. Elife 2018, 7, e35657. [Google Scholar] [CrossRef]
- Lee, K.; Holley, S.M.; Shobe, J.L.; Chong, N.C.; Cepeda, C.; Levine, M.S.; Masmanidis, S.C. Parvalbumin Interneurons Modulate Striatal Output and Enhance Performance during Associative Learning. Neuron 2017, 93, 1451–1463.e4. [Google Scholar] [CrossRef]
- Tepper, J.M.; Wilson, C.J.; Koos, T. Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Res. Rev. 2008, 58, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Nair, A.; Augustine, G.J. A Novel Type of Neuron Within the Dorsal Striatum. Front. Neural Circuits 2019, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Gertler, T.S.; Chan, C.S.; Surmeier, D.J. Dichotomous anatomical properties of adult striatal medium spiny neurons. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 10814–10824. [Google Scholar] [CrossRef] [PubMed]
- Nisenbaum, E.S.; Wilson, C.J. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 4449–4463. [Google Scholar] [CrossRef] [PubMed]
- Tjia, M.; Yu, X.; Jammu, L.S.; Lu, J.; Zuo, Y. Pyramidal Neurons in Different Cortical Layers Exhibit Distinct Dynamics and Plasticity of Apical Dendritic Spines. Front. Neural Circuits 2017, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Albarran, E.; Raissi, A.; Jaidar, O.; Shatz, C.J.; Ding, J.B. Enhancing motor learning by increasing the stability of newly formed dendritic spines in the motor cortex. Neuron 2021, 109, 3298–3311.e4. [Google Scholar] [CrossRef]
- Pape, A.A.; Siegel, M. Motor cortex activity predicts response alternation during sensorimotor decisions. Nat. Commun. 2016, 7, 13098. [Google Scholar] [CrossRef]
- Oswald, M.J.; Tantirigama, M.L.; Sonntag, I.; Hughes, S.M.; Empson, R.M. Diversity of layer 5 projection neurons in the mouse motor cortex. Front. Cell. Neurosci. 2013, 7, 174. [Google Scholar] [CrossRef]
- Bekkers, J.M. Pyramidal neurons. Curr. Biol. 2011, 21, R975. [Google Scholar] [CrossRef]
- Saba, L.; Viscomi, M.T.; Caioli, S.; Pignataro, A.; Bisicchia, E.; Pieri, M.; Molinari, M.; Ammassari-Teule, M.; Zona, C. Altered Functionality, Morphology, and Vesicular Glutamate Transporter Expression of Cortical Motor Neurons from a Presymptomatic Mouse Model of Amyotrophic Lateral Sclerosis. Cereb. Cortex 2016, 26, 1512–1528. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Ilavazhagan, G.; Rossignol, J.; Dunbar, G.L. Molecular regulation of dendritic spine dynamics and their potential impact on synaptic plasticity and neurological diseases. Neurosci. Biobehav. Rev. 2015, 59, 208–237. [Google Scholar] [CrossRef] [PubMed]
- Dosemeci, A.; Thein, S.; Yang, Y.; Reese, T.S.; Tao-Cheng, J.H. CYLD, a deubiquitinase specific for lysine63-linked polyubiquitins, accumulates at the postsynaptic density in an activity-dependent manner. Biochem. Biophys. Res. Commun. 2013, 430, 245–249. [Google Scholar] [CrossRef]
- Li, H.D.; Li, D.N.; Yang, L.; Long, C. Deficiency of the CYLD Impairs Fear Memory of Mice and Disrupts Neuronal Activity and Synaptic Transmission in the Basolateral Amygdala. Front. Cell. Neurosci. 2021, 15, 740165. [Google Scholar] [CrossRef]
- Tan, S.Y.; Jiang, J.X.; Huang, H.X.; Mo, X.P.; Feng, J.R.; Chen, Y.; Yang, L.; Long, C. Neural mechanism underlies CYLD modulation of morphology and synaptic function of medium spiny neurons in dorsolateral striatum. Front. Mol. Neurosci. 2023, 16, 1107355. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Y.; Jin, K.; Pan, Q.S.; Li, B.; Wu, Z.Q.; Gan, L.; Yang, L.; Long, C. Microglial activation in the dorsal striatum participates in anxiety-like behavior in Cyld knockout mice. Brain Behav. Immun. 2020, 89, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Rane, M.; Manchanda, R. Effect of Spine Density on Excitability in Accumbal Medium Spiny Neurons-A Computational Approach. J. Addict. Res. Ther. 2017, 8, 337. [Google Scholar] [CrossRef]
- Popescu, I.R.; Le, K.Q.; Ducote, A.L.; Li, J.E.; Leland, A.E.; Mostany, R. Increased intrinsic excitability and decreased synaptic inhibition in aged somatosensory cortex pyramidal neurons. Neurobiol. Aging 2021, 98, 88–98. [Google Scholar] [CrossRef]
- Niday, Z.; Hawkins, V.E.; Soh, H.; Mulkey, D.K.; Tzingounis, A.V. Epilepsy-Associated KCNQ2 Channels Regulate Multiple Intrinsic Properties of Layer 2/3 Pyramidal Neurons. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 576–586. [Google Scholar] [CrossRef]
- Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef]
- Dumenieu, M.; Fourcaud-Trocme, N.; Garcia, S.; Kuczewski, N. Afterhyperpolarization (AHP) regulates the frequency and timing of action potentials in the mitral cells of the olfactory bulb: Role of olfactory experience. Physiol. Rep. 2015, 3, e12344. [Google Scholar] [CrossRef]
- Haber, S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016, 18, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Pascoli, V.; Hiver, A.; Flakowski, J.; Luscher, C. Corticostriatal Activity Driving Compulsive Reward Seeking. Biol. Psychiatry 2021, 90, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Farfan, A.K.; Baez-Cordero, A.S.; Pena-Rangel, T.M.; Rueda-Orozco, P.E. Cortico-striatal circuits for bilaterally coordinated movements. Sci. Adv. 2022, 8, eabk2241. [Google Scholar] [CrossRef] [PubMed]
- Nahar, L.; Delacroix, B.M.; Nam, H.W. The Role of Parvalbumin Interneurons in Neurotransmitter Balance and Neurological Disease. Front. Psychiatry 2021, 12, 679960. [Google Scholar] [CrossRef]
- Szydlowski, S.N.; Pollak Dorocic, I.; Planert, H.; Carlen, M.; Meletis, K.; Silberberg, G. Target selectivity of feedforward inhibition by striatal fast-spiking interneurons. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 1678–1683. [Google Scholar] [CrossRef]
- McColgan, P.; Joubert, J.; Tabrizi, S.J.; Rees, G. The human motor cortex microcircuit: Insights for neurodegenerative disease. Nat. Rev. Neurosci. 2020, 21, 401–415. [Google Scholar] [CrossRef]
- Peters, A.J.; Fabre, J.M.J.; Steinmetz, N.A.; Harris, K.D.; Carandini, M. Striatal activity topographically reflects cortical activity. Nature 2021, 591, 420–425. [Google Scholar] [CrossRef]
- Jiang, X.; Shen, S.; Cadwell, C.R.; Berens, P.; Sinz, F.; Ecker, A.S.; Patel, S.; Tolias, A.S. Principles of connectivity among morphologically defined cell types in adult neocortex. Science 2015, 350, aac9462. [Google Scholar] [CrossRef]
- Shepherd, G.M. Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci. 2013, 14, 278–291. [Google Scholar] [CrossRef]
- Nair, A.; Razi, A.; Gregory, S.; Rutledge, R.B.; Rees, G.; Tabrizi, S.J. Imbalanced basal ganglia connectivity is associated with motor deficits and apathy in Huntington’s disease. Brain A J. Neurol. 2022, 145, 991–1000. [Google Scholar] [CrossRef]
- Gittis, A.H.; Kreitzer, A.C. Striatal microcircuitry and movement disorders. Trends Neurosci. 2012, 35, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Dobson-Stone, C.; Luty, A.A.; Thompson, E.M.; Blumbergs, P.; Brooks, W.S.; Short, C.L.; Field, C.D.; Panegyres, P.K.; Hecker, J.; Solski, J.A.; et al. Frontotemporal dementia-amyotrophic lateral sclerosis syndrome locus on chromosome 16p12.1-q12.2: Genetic, clinical and neuropathological analysis. Acta Neuropathol. 2013, 125, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Dobson-Stone, C.; Hallupp, M.; Shahheydari, H.; Ragagnin, A.M.G.; Chatterton, Z.; Carew-Jones, F.; Shepherd, C.E.; Stefen, H.; Paric, E.; Fath, T.; et al. CYLD is a causative gene for frontotemporal dementia–Amyotrophic lateral sclerosis. Brain A J. Neurol. 2020, 143, 783–799. [Google Scholar] [CrossRef] [PubMed]
- Tabuas-Pereira, M.; Santana, I.; Kun-Rodrigues, C.; Bras, J.; Guerreiro, R. CYLD variants in frontotemporal dementia associated with severe memory impairment in a Portuguese cohort. Brain A J. Neurol. 2020, 143, e67. [Google Scholar] [CrossRef]
- Yousefian-Jazi, A.; Seol, Y.; Kim, J.; Ryu, H.L.; Lee, J.; Ryu, H. Pathogenic Genome Signatures That Damage Motor Neurons in Amyotrophic Lateral Sclerosis. Cells 2020, 9, 2687. [Google Scholar] [CrossRef]
- Mazarei, G.; Neal, S.J.; Becanovic, K.; Luthi-Carter, R.; Simpson, E.M.; Leavitt, B.R. Expression analysis of novel striatal-enriched genes in Huntington disease. Hum. Mol. Genet. 2010, 19, 609–622. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Noakes, P.G.; Bellingham, M.C. Motor cortex layer V pyramidal neurons exhibit dendritic regression, spine loss, and increased synaptic excitation in the presymptomatic hSOD1(G93A) mouse model of amyotrophic lateral sclerosis. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 643–647. [Google Scholar] [CrossRef]
- Toro-Fernandez, L.F.; Zuluaga-Monares, J.C.; Saldarriaga-Cartagena, A.M.; Cardona-Gomez, G.P.; Posada-Duque, R. Targeting CDK5 in Astrocytes Promotes Calcium Homeostasis Under Excitotoxic Conditions. Front. Cell. Neurosci. 2021, 15, 643717. [Google Scholar] [CrossRef]
- Honig, M.G.; Hume, R.I. Dil and diO: Versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 1989, 12, 333–341. [Google Scholar] [CrossRef]
- Veldman, M.B.; Park, C.S.; Eyermann, C.M.; Zhang, J.Y.; Zuniga-Sanchez, E.; Hirano, A.A.; Daigle, T.L.; Foster, N.N.; Zhu, M.; Langfelder, P.; et al. Brainwide Genetic Sparse Cell Labeling to Illuminate the Morphology of Neurons and Glia with Cre-Dependent MORF Mice. Neuron 2020, 108, 111–127.e6. [Google Scholar] [CrossRef]
- Lu, X.H.; Yang, X.W. Genetically-directed Sparse Neuronal Labeling in BAC Transgenic Mice through Mononucleotide Repeat Frameshift. Sci. Rep. 2017, 7, 43915. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M.; Ermilov, L.G.; Schmalz, P.F.; Louzon, V.; Miller, S.M.; Szurszewski, J.H. The three-dimensional structure of myenteric neurons in the guinea-pig ileum. J. Auton. Nerv. Syst. 1998, 71, 1–9. [Google Scholar] [CrossRef]
- Hanani, M. Lucifer yellow–An angel rather than the devil. J. Cell Mol. Med. 2012, 16, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Sando, R.; Jiang, X.; Sudhof, T.C. Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins. Science 2019, 363, eaav7969. [Google Scholar] [CrossRef]
- Boxer, E.E.; Seng, C.; Lukacsovich, D.; Kim, J.; Schwartz, S.; Kennedy, M.J.; Foldy, C.; Aoto, J. Neurexin-3 defines synapse- and sex-dependent diversity of GABAergic inhibition in ventral subiculum. Cell Rep. 2021, 37, 110098. [Google Scholar] [CrossRef]
- Reiley, W.W.; Jin, W.; Lee, A.J.; Wright, A.; Wu, X.; Tewalt, E.F.; Leonard, T.O.; Norbury, C.C.; Fitzpatrick, L.; Zhang, M.; et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J. Exp. Med. 2007, 204, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, Y.; Huo, Q.; Wang, L.; Tan, S.; Misrani, A.; Jiang, J.; Chen, J.; Chen, S.; Zhang, J.; et al. Enhancing GABAergic signaling ameliorates aberrant gamma oscillations of olfactory bulb in AD mouse models. Mol. Neurodegener. 2021, 16, 14. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Blackman, A.V.; Oyrer, J.; Jayabal, S.; Chung, A.J.; Watt, A.J.; Sjostrom, P.J.; van Meyel, D.J. Neuronal morphometry directly from bitmap images. Nat. Methods 2014, 11, 982–984. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.; Mo, X.; Qin, H.; Dong, B.; Zhou, J.; Long, C.; Yang, L. Biocytin-Labeling in Whole-Cell Recording: Electrophysiological and Morphological Properties of Pyramidal Neurons in CYLD-Deficient Mice. Molecules 2023, 28, 4092. https://doi.org/10.3390/molecules28104092
Tan S, Mo X, Qin H, Dong B, Zhou J, Long C, Yang L. Biocytin-Labeling in Whole-Cell Recording: Electrophysiological and Morphological Properties of Pyramidal Neurons in CYLD-Deficient Mice. Molecules. 2023; 28(10):4092. https://doi.org/10.3390/molecules28104092
Chicago/Turabian StyleTan, Shuyi, Xiuping Mo, Huihui Qin, Binbin Dong, Jiankui Zhou, Cheng Long, and Li Yang. 2023. "Biocytin-Labeling in Whole-Cell Recording: Electrophysiological and Morphological Properties of Pyramidal Neurons in CYLD-Deficient Mice" Molecules 28, no. 10: 4092. https://doi.org/10.3390/molecules28104092