Ameliorating Phosphonic-Based Nonflammable Electrolytes Towards Safe and Stable Lithium Metal Batteries
Abstract
:1. Introduction
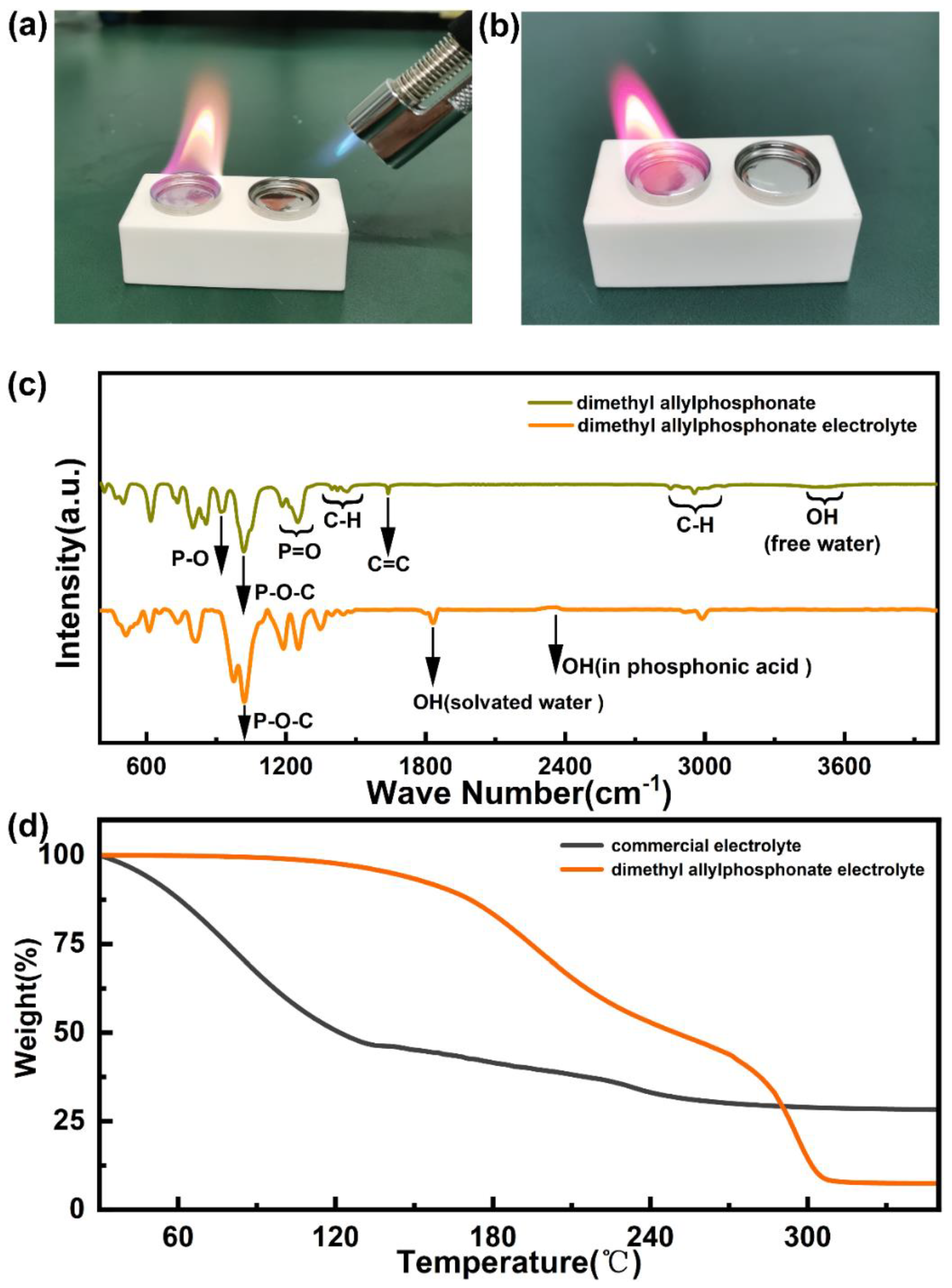
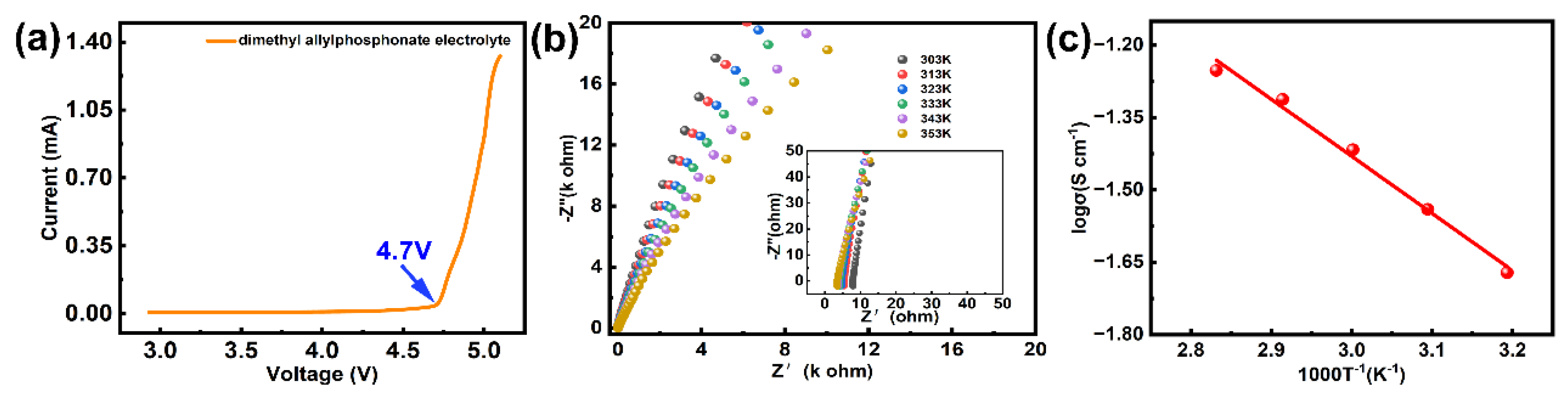
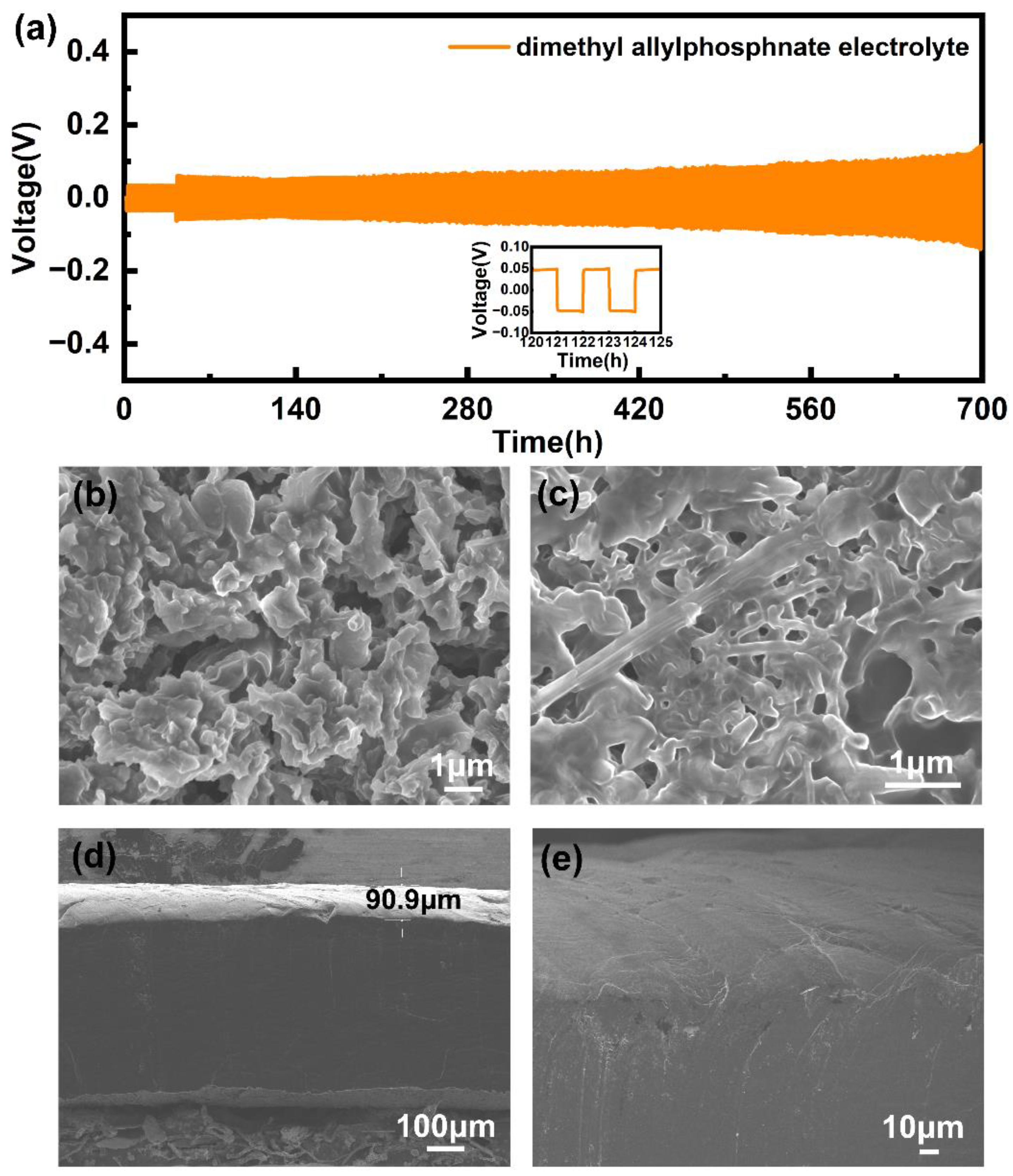
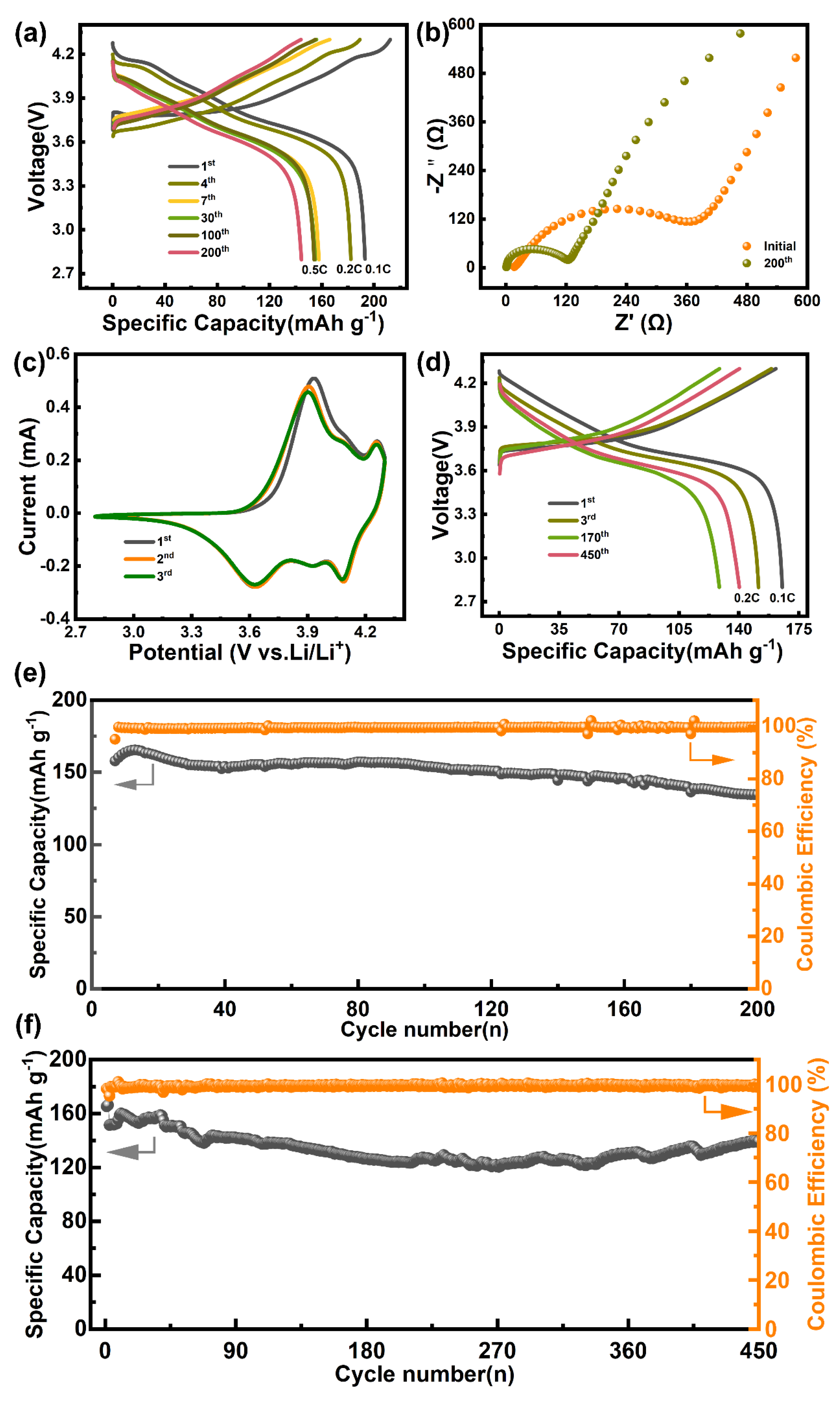
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation and Characterization of Phosphonate-Based Electrolyte
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Davide, D.; Piera, D.P.; Daniele, V.; Amici, J.; Francia, C.; Bodoardo, S.; Santarelli, M. Aging of a Lithium-Metal/LFP Cell: Predictive Model and Experimental Validation. Batteries 2023, 9, 146. [Google Scholar]
- Zhao, P.Y.; Li, Y.; Chen, S.J.; Fan, H.; Feng, Y.Y.; Hu, L.L.; Zhang, Y.H.; Nie, Q.N.; Pei, H.J.; Yang, C.; et al. Constructing Self-Adapting Electrostatic Interface on Lithium Metal Anode for Stable 400 Wh kg-1 Pouch Cells. Adv. Energy Mater. 2022, 12, 2200568. [Google Scholar] [CrossRef]
- Boroujeni, S.M.; Fill, A.; Ridder, A.; Birke, K.P. Influence of Temperature and Electrolyte Composition on the Performance of Lithium Metal Anodes. Batteries 2021, 7, 67. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, M.J.; Shen, C.L.; Ma, H.Q.; Cui, L.M.; Yang, W.; Zhao, T.H.; Zhao, Y.; Xu, X. Multifunctional Multilayer Nanospheres for Ion Regulation in Lithium Metal Batteries. Batteries 2023, 9, 149. [Google Scholar] [CrossRef]
- Tan, H.; Lin, X.Y. Electrolyte Design Strategies for Non-Aqueous High-Voltage Potassium-Based Batteries. Molecules 2023, 28, 823. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.M.; Zhang, Q.F.; Cui, Y. Designing High-Energy Lithium-Sulfur Batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Vandepaer, L.; Cloutier, J.; Amor, B. Environmental Impacts of Lithium Metal Polymer and Lithium-Ion Stationary Batteries. Renew. Sust. Energ. Rev. 2017, 78, 46–60. [Google Scholar] [CrossRef]
- Liang, H.P.; Zarrabeitia, M.; Chen, Z.; Jovanovic, S.; Merz, S.; Granwehr, J.; Passerini, S.; Bresser, D. Polysiloxane-Based Single-Ion Conducting Polymer Blend Electrolyte Comprising Small-Molecule Organic Carbonates for High-Energy and High-Power Lithium-Metal Batteries. Adv. Energy Mater. 2022, 12, 2200013. [Google Scholar] [CrossRef]
- Um, J.H.; Kim, K.; Park, J.; Sung, Y.E.; Yu, S.H. Revisiting the Strategies for Stabilizing Lithium Metal Anodes. J. Mater. Chem. A 2020, 8, 13874–13895. [Google Scholar] [CrossRef]
- Sun, J.X.; Ye, L.Q.; Zhao, X.R.; Zhang, P.P.; Yang, J. Electronic Modulation and Structural Engineering of Carbon-Based Anodes for Low-Temperature Lithium-Ion Batteries: A Review. Molecules 2023, 28, 2108. [Google Scholar] [CrossRef]
- Chen, S.C.; Wang, Z.R.; Yan, W. Identification and Characteristic Analysis of Powder Ejected from a Lithium Ion Battery During Thermal Runaway at Elevated Temperatures. J. Hazard. Mater. 2020, 400, 123169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.Y.; Sun, T.; Xu, Y.W.; Zheng, Y.J.; Zhou, L. Identification of Internal Short-Circuit Faults in Lithium-Ion Batteries Based on a Multi-Machine Learning Fusion. Batteries 2023, 9, 154. [Google Scholar] [CrossRef]
- Jiang, F.N.; Yang, S.J.; Cheng, X.B.; Shi, P.; Ding, J.F.; Chen, X.; Yuan, H.; Liu, L.; Huang, J.Q.; Zhang, Q. Thermal Safety of Dendritic Lithium Against Non-Aqueous Electrolyte in Pouch-Type Lithium Metal Batteries. J. Energy Chem. 2022, 72, 158–165. [Google Scholar] [CrossRef]
- Hou, J.X.; Feng, X.N.; Wang, L.; Liu, X.; Ohma, A.; Lu, L.G.; Ren, D.S.; Huang, W.S.; Li, Y.; Yi, M.C.; et al. Unlocking the Self-Supported Thermal Runaway of High-Energy Lithium-Ion Batteries. Energy Storage Mater. 2021, 39, 395–402. [Google Scholar] [CrossRef]
- Huang, B.; Chu, B.B.; Huang, T.; Yu, A.S. Nitrogen-Doped Carbon-Coating Disproportionated SiO Materials as Long Cycling Stable Anode for Lithium Ion Batteries. Molecules 2021, 26, 1536. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Yue, J.P.; Fan, M.; Tan, S.J.; Zhang, J.; Wang, W.P.; Liu, Y.; Tian, Y.F.; Xu, Q.; Yin, Y.X.; et al. Formulating the Electrolyte Towards High-Energy and Safe Rechargeable Lithium–Metal Batteries. Angew. Chem. Int. Ed. 2021, 60, 16690–16696. [Google Scholar] [CrossRef]
- ŁEbkowski, A. Electric Vehicle Fire Extinguishing System. Prz. Elektrotechniczny 2017, 1, 329–332. [Google Scholar] [CrossRef]
- Kang, S.; Kwon, M.; Choi, J.Y.; Choi, S. Full-Scale Fire Testing of Battery Electric Vehicles. Appl. Energy 2023, 332, 120497. [Google Scholar] [CrossRef]
- Sun, P.Y.; Bisschop, R.; Niu, H.C.; Huang, X.Y. A Review of Battery Fires in Electric Vehicles. Fire Technol. 2020, 56, 1361–1410. [Google Scholar] [CrossRef]
- Zhang, C.M.; Li, F.; Zhu, X.Q.; Yu, J.G. Triallyl Isocyanurate as an Efficient Electrolyte Additive for Layered Oxide Cathode Material-Based Lithium-Ion Batteries with Improved Stability under High-Voltage. Molecules 2022, 27, 3107. [Google Scholar] [CrossRef]
- Xu, J.J.; Ji, X.; Zhang, J.X.; Yang, C.Y.; Wang, P.F.; Liu, S.F.; Ludwig, K.; Chen, F.; Kofinas, P.; Wang, C.S. Aqueous Electrolyte Design for Super-Stable 2.5 V LiMn2O4 || Li4Ti5O12 Pouch Cells. Nat. Energy 2022, 7, 186–193. [Google Scholar] [CrossRef]
- Kim, J.J.; Yoon, K.; Park, I.; Kang, K. Progress in the Development of Sodium-Ion Solid Electrolytes. Small Methods 2017, 1, 1700219. [Google Scholar] [CrossRef]
- Sun, H.H.; Liu, J.D.; He, J.W.; Ping, H.; Jiang, G.X.; Qi, S.H.; Ma, J.M. Stabilizing the Cycling Stability of Rechargeable Lithium Metal Batteries with Tris(hexafluoroisopropyl)phosphate Additive. Sci. Bull. 2022, 67, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, H.S.; Kim, M.P.; Lee, Y.G.; Hong, S.Y.; Kim, K.M. A Trade-Off-Free Fluorosulfate-Based Flame-Retardant Electrolyte Additive for High-Energy Lithium Batteries. J. Mater. Chem. A 2022, 10, 21933–21940. [Google Scholar] [CrossRef]
- Lenus, S.; Thakur, P.; Samantaray, S.S.; Narayanan, T.N.; Dai, Z.F. Two-Dimensional Iron Phosphorus Trisulfide as a High-Capacity Cathode for Lithium Primary Battery. Molecules 2023, 28, 537. [Google Scholar] [CrossRef]
- Zhang, S.C.; Li, S.Y.; Lu, Y.Y. Designing Safer Lithium-Based Batteries with Nonflammable Electrolytes: A Review. eScience 2021, 1, 163–177. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, G.Z.; Zhu, Y.M.; Lin, M.C.; Chen, H.; Li, Y.Y.; Hung, W.H.; Zhou, B.; Wang, X.; Bai, Y.X.; et al. High-Safety and High-Energy-Density Lithium Metal Batteries in A Novel Ionic-Liquid Electrolyte. Adv. Mater. 2020, 32, 2001741. [Google Scholar] [CrossRef]
- Xiao, L.F.; Zeng, Z.Q.; Liu, X.W.; Fang, Y.J.; Jiang, X.Y.; Shao, Y.Y.; Zhuang, L.; Ai, X.P.; Yang, H.X.; Cao, Y.L.; et al. Stable Li Metal Anode with “Ion–Solvent-Coordinated” Nonflammable Electrolyte for Safe Li Metal Batteries. ACS Energy Lett. 2019, 4, 483–488. [Google Scholar] [CrossRef]
- Fan, X.L.; Ji, X.; Chen, L.; Chen, J.; Deng, T.; Han, F.D.; Yue, J.; Piao, N.; Wang, R.X.; Zhou, X.Q.; et al. All-Temperature Batteries Enabled by Fluorinated Electrolytes with Non-Polar Solvents. Nat. Energy 2019, 4, 882–890. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, T.H.; Ashirov, T.; Kazzi, M.E.; Cancellieri, C.; Jeurgens, L.P.H.; Choi, J.W.; Coskun, A. Fluorinated Ether Electrolyte with Controlled Solvation Structure for High Voltage Lithium Metal Batteries. Nat. Commun. 2022, 13, 2575. [Google Scholar] [CrossRef]
- Fan, X.L.; Chen, L.; Borodin, O.; Ji, X.; Chen, J.; Hou, S.; Deng, T.; Zheng, J.; Yang, C.Y.; Liou, S.C.; et al. Non-Flammable Electrolyte Enables Li-Metal Batteries with Aggressive Cathode Chemistries. Nat. Nanotech. 2018, 13, 715–722. [Google Scholar] [CrossRef]
- Yu, Z.A.; Yu, W.L.; Chen, Y.L.; Mondonico, L.; Xiao, X.; Zheng, Y.; Liu, F.; Hung, S.T.; Cui, Y.; Bao, Z.N. Tuning Fluorination of Linear Carbonate for Lithium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 040555. [Google Scholar] [CrossRef]
- Zheng, Q.F.; Yamada, Y.; Shang, R.; Ko, S.; Lee, Y.Y.; Kim, K.; Nakamura, E.; Yamada, A. A Cyclic Phosphate-Based Battery Electrolyte for High Voltage and Safe Operation. Nat. Energy 2020, 5, 291–298. [Google Scholar] [CrossRef]
- Tan, S.J.; Yue, J.P.; Hu, X.C.; Shen, Z.Z.; Wang, W.P.; Li, J.Y.; Zuo, T.T.; Duan, H.; Xiao, Y.; Yin, Y.X.; et al. Nitriding-Interface-Regulated Lithium Plating Enables Flame-Retardant Electrolytes for High-Voltage Lithium Metal Batteries. Angew. Chem. Int. Ed. 2019, 58, 7802–7807. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.J.; Tian, Y.F.; Zhao, Y.; Feng, X.X.; Zhang, J.; Zhang, C.H.; Fan, M.; Guo, J.C.; Yin, Y.X.; Wang, F.Y.; et al. Noncoordinating Flame-Retardant Functional Electrolyte Solvents for Rechargeable Lithium-Ion Batteries. J. Am. Chem. Soc. 2022, 144, 18240–18245. [Google Scholar] [CrossRef]
- Zeng, Z.Q.; Murugesan, V.; Han, K.S.; Jiang, X.Y.; Cao, Y.L.; Xiao, L.F.; Ai, X.P.; Yang, H.X.; Zhang, J.G.; Sushko, M.L.; et al. Non-Flammable Electrolytes with High Salt-to-Solvent Ratios for Li-Ion and Li-Metal Batteries. Nat. Energy 2018, 3, 674–681. [Google Scholar] [CrossRef]
- Ling, W.; Wang, Z.A.; Ma, Q.; Qi, D.; Tang, J.F.; Deng, L.; Zhu, L.H.; Wu, X.W.; Yue, J.P.; Guo, Y.G. Phosphorus and Oxygen Co-Doped Composite Electrode with Hierarchical Electronic and Ionic Mixed Conducting Networks for Vanadium Redox Flow Batteries. Chem. Comm. 2019, 55, 11515–11518. [Google Scholar] [CrossRef]
- Cui, Y.; Wan, J.Y.; Ye, Y.S.; Liu, K.; Chou, L.Y.; Cui, Y. A Fireproof, Lightweight, Polymer–Polymer Solid-State Electrolyte for Safe Lithium Batteries. Nano Lett. 2020, 20, 1686–1692. [Google Scholar] [CrossRef]
- Zhou, L.D.; Zuo, T.T.; Kwok, C.Y.; Kim, S.Y.; Assoud, A.; Zhang, Q.; Janek, J.; Nazar, L.F. High Areal Capacity, long Cycle Life 4 V Ceramic All-Solid-State Li-Ion Batteries Enabled by Chloride Solid Electrolytes. Nat. Energy 2022, 7, 83–93. [Google Scholar] [CrossRef]
- Yan, Y.G.; Kühnel, R.S.; Remhof, A.; Duchêne, L.; Reyes, E.C.; Rentsch, D.; Łodziana, Z.; Battaglia, C. A Lithium Amide-Borohydride Solid-State Electrolyte with Lithium-Ion Conductivities Comparable to Liquid Electrolytes. Adv. Energy Mater. 2017, 7, 1700294. [Google Scholar] [CrossRef]
- Mu, X.W.; Li, X.J.; Liao, C.; Yu, H.; Jin, Y.; Yu, B.; Han, L.F.; Chen, L.K.; Kan, Y.C.; Song, L.; et al. Phosphorus-Fixed Stable Interfacial Nonflammable Gel Polymer Electrolyte for Safe Flexible Lithium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2203006. [Google Scholar] [CrossRef]
- Wang, J.Y.; Chen, R.S.; Yang, L.F.; Zan, M.W.; Chen, P.H.; Li, Y.; Li, W.J.; Yu, H.G.; Yu, X.-Q.; Huang, X.J.; et al. Raising the Intrinsic Safety of Layered Oxide Cathodes by Surface Re-Lithiation with LLZTO Garnet-Type Solid Electrolytes. Adv. Mater. 2022, 34, 2200655. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Shu, J.; Xu, L.; Xia, Y.Y.; Du, L.L.; Zhang, G.; Mai, L.Q. Flexible Nanowire Cathode Membrane with Gradient Interfaces and Rapid Electron/Ion Transport Channels for Solid-State Lithium Batteries. Adv. Energy Mater. 2021, 11, 2100026. [Google Scholar] [CrossRef]
- Huang, Y.F.; Gu, T.; Rui, G.C.; Shi, P.R.; Fu, W.B.; Chen, L.; Liu, X.T.; Zeng, J.P.; Kang, B.H.; Yan, Z.C.; et al. A Relaxor Ferroelectric Polymer with An Ultrahigh Dielectric Constant Largely Promotes the Dissociation of Lithium Salts to Achieve High Ionic Conductivity. Energy Environ. Sci. 2021, 14, 6021–6029. [Google Scholar] [CrossRef]
- Liu, Y.J.; Tao, X.Y.; Wang, Y.; Jiang, C.; Ma, C.; Sheng, O.W.; Lu, G.X.; Lou, X.W.D. Self-Assembled Monolayers Direct A LiF-Rich Interphase Toward Long-Life Lithium Metal Batteries. Science 2022, 375, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.P.; Sun, Q.; Yu, C.; Zhang, S.M.; Adair, K.; Wang, S.Z.; Liu, Y.L.; Zhao, Y.; Liang, J.W.; Wang, C.H.; et al. Ultrastable Anode Interface Achieved by Fluorinating Electrolytes for All-Solid-State Li Metal Batteries. ACS Energy Lett. 2020, 5, 1035–1043. [Google Scholar] [CrossRef]
- Abramowicz, M.; Osial, M.; Urbańska, W.; Walicki, M.; Wilczewski, S.; Pregowska, A.; Skórczewska, K.; Jenczyk, P.; Warczak, M.; Pisarek, M.; et al. Upcycling of Acid-Leaching Solutions from Li-Ion Battery Waste Treatment through the Facile Synthesis of Magnetorheological Fluid. Molecules 2023, 28, 2558. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, K.; Ahn, S.; Liu, H.K. Allyl-Substituted Triazines as Additives for Enhancing the Thermal Stability of Li-Ion Batteries. J. Power Sources 2011, 196, 1483–1487. [Google Scholar] [CrossRef]
- Xu, K.; Ding, M.S.; Zhang, S.S.; Allen, J.L.; Jow, T.R. An Attempt to Formulate Nonflammable Lithium Ion Electrolytes with Alkyl Phosphates and Phosphazenes. J. Electrochem. Soc. 2002, 149, A622–A626. [Google Scholar] [CrossRef]
- Lindsay, C.I.; Hill, S.B.; Hearn, M.; Manton, G.; Everall, N.; Bunn, A.; Heron, J.; Fletcher, I. Mechanisms of Action of Phosphorus Based Flame Retardants in Acrylic Polymers. Polym. Int. 2000, 49, 1183–1192. [Google Scholar] [CrossRef]
- Liang, H.J.; Gu, Z.Y.; Zhao, X.X.; Guo, J.Z.; Yang, J.L.; Li, W.H.; Li, B.; Liu, Z.M.; Sun, Z.H.; Zhang, J.P.; et al. Advanced Flame-Retardant Electrolyte for Highly Stabilized K-Ion Storage in Graphite Anode. Sci. Bull. 2022, 67, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.J.; Gu, Z.Y.; Zhao, X.X.; Guo, J.Z.; Yang, J.L.; Li, W.H.; Li, B.; Liu, Z.M.; Li, W.L.; Wu, X.L. Ether-Based Electrolyte Chemistry Towards High-Voltage and Long-Life Na-Ion Full Batteries. Angew. Chem. Int. Ed. Engl. 2021, 60, 26837–26846. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.H.; Liu, J.D.; He, J.; Wang, H.P.; Wu, M.G.; Wu, D.X.; Huang, J.D.; Li, F.; Li, X.; Ren, Y.R.; et al. Structurally Tunable Characteristics of Ionic Liquids for Optimizing Lithium Plating/Stripping via Electrolyte Engineering. J. Energy Chem. 2021, 63, 270–277. [Google Scholar] [CrossRef]
- He, J.; Wang, H.P.; Zhou, Q.; Qi, S.H.; Wu, M.G.; Li, F.; Hu, W.; Ma, J.M. Unveiling the Role of Li+ Solvation Structures with Commercial Carbonates in the Formation of Solid Electrolyte Interphase for Lithium Metal Batteries. Small Methods 2021, 5, e2100441. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.; Xie, X.; Huangyang, X.; Yang, L.; Zeng, X.; Ma, Q.; Wu, X.; Xiao, M.; Wu, Y. Ameliorating Phosphonic-Based Nonflammable Electrolytes Towards Safe and Stable Lithium Metal Batteries. Molecules 2023, 28, 4106. https://doi.org/10.3390/molecules28104106
Fu S, Xie X, Huangyang X, Yang L, Zeng X, Ma Q, Wu X, Xiao M, Wu Y. Ameliorating Phosphonic-Based Nonflammable Electrolytes Towards Safe and Stable Lithium Metal Batteries. Molecules. 2023; 28(10):4106. https://doi.org/10.3390/molecules28104106
Chicago/Turabian StyleFu, Sha, Xuanzhi Xie, Xiaoyi Huangyang, Longxi Yang, Xianxiang Zeng, Qiang Ma, Xiongwei Wu, Mingtao Xiao, and Yuping Wu. 2023. "Ameliorating Phosphonic-Based Nonflammable Electrolytes Towards Safe and Stable Lithium Metal Batteries" Molecules 28, no. 10: 4106. https://doi.org/10.3390/molecules28104106



