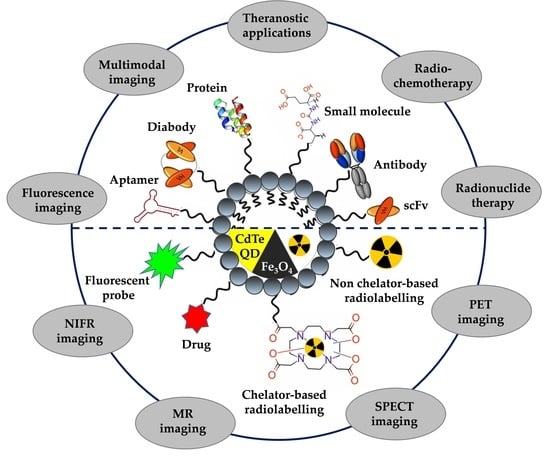
Nanoparticle-Based Radioconjugates for Targeted Imaging and Therapy of Prostate Cancer
Abstract
:1. Introduction
2. Selection Criteria of Suitable NPs, Ligands, Radionuclides, and Radiolabelling Strategies for Development of NP-Based Radioconjugates for Targeted Imaging and Therapy of PCa
2.1. Selection of a Suitable Type of NPs for Targeted Imaging and Therapy of PCa
| NPs | NPs Size | Radionuclide | Ligand | Final Compound | Modality | Cell Type/Animal Models | References |
|---|---|---|---|---|---|---|---|
| gadolinium vanadate NPs (GdVO4) | ∼150 nm | Copper-64 | Asp-Gly-Ala (DGEA) peptide | 64Cu-DOTA-GdVO 4 :4%Eu-DGEA | PET/MR | prostate cancer PC-3 cells, athymic nude mice bearing PC-3 xenograft | [46] |
| micellar NPs (LNP) | 12 nm | Copper-64 | single chain (scFv) | 64Cu-DOTA-scFv-LNP | PET | NOD/SCID mice bearing LNCaP xenograft | [42] |
| copper sulfide NPs (CuS) | 5 nm | Copper-64 | Bombesin (7–14) | Bom-PEG-[64Cu]CuS | PET | prostate cancer PC-3-KD1 cells Nu/Nu mice bearing PC-3-KD1 xenograft | [45] |
| iron oxide NPs (IO) | 11 nm | Gallium-68 | glutamate-urea-lysine ligand | 68Ga-DOTA-IO-GUL | PET/MR | prostate cancer 2Rv1, LNCaP, and PC-3 cells BALB/c nude mice bearing 2Rv1 and PC-3 xenograft | [47] |
| iron oxide NPs (mNP-S1/2) (mNP-N1/2) | 55–138 nm | Gallium-68 | glutamate-urea-lysine ligand Bombesin (7–14) | 68Ga-mNP-N1/2 68Ga-mNP-S1/2 | PET/MRI | prostate cancer LNCaP and PC-3 cells | [48] |
| quantum dots (QDs) | ~12 nm | Fluorine-18 | RGD peptide Bombesin (7–14) | 18F-FP-QD-RGD-BBN | PET/NIFR | prostate cancer PC-3 cells BALB/c nu/nu nude mice bearing PC-3 xenograft | [49] |
| melanin NPs (MNPs) | 13 nm | Iodine-124 | glutamate-urea-lysine ligand | 124I-MNPs-PEG-TL | PET | prostate cancer LNCaP and PC-3 cells athymic nude mice bearing LNCaP and 22RV1 xenograft | [44] |
| polymer PEG NPs (PEG-(DFB)1) (PEG-(DFB)3) | 15 nm | Zirconium-89 | ACUPA | 89Zr-PEG-(DFB)3(ACUPA)1 89Zr-PEG-(DFB)1(ACUPA)3 | PET | prostate cancer PC3 and PC3-Flu cells nu/nu athymic mice bearing PC3 and PC3-Flu xenograft | [40] |
| micellar NPs (CCPM) | ~22 nm | Indium-111 | TNYL-RAW | 111In-TNYL-RAW-CCPM(Cy-7) | SPECT/NIRF | prostate cancer PC3-MM2 cells nude mice bearing PC3-MM2 xenograft | [41] |
| poly(lactic acid)− polyethyene glycol NPs (PLA−PEG) | ~100 nm | Indium-111 | ACUPA | 111In-DOTA-RDye680RD-PEG-PLA-ACUPA RDye680RD-PEG-PLA-ACUPA | SPECT/NIFR | prostate cancer PC-3 and PC-3 flu cells athymic mice bearing PC-3 and PC-3 flu dual xenograft | [39] |
| gold NPs (AuNP) | ~20 nm | Technetium-99m | Bombesin (7–14) | 99mTc-EDDA/HYNIC-GGC-AuNP-Lys3-bombesin | SPECT | prostate cancer PC-3 cells nude mice bearing PC-3 xenograft | [31] |
| quantum dots (QDs) gold NPs (AuNP) | ~6 nm 7 and 14 nm | Technetium-99m | glutamate-urea-lysine ligand | 99mTh-DAP-HS-PEG (12)-AuNPs-HS-PEG-DAP-TF | SPECT | prostate cancer LNCaP cells nude NMR mice bearing LNCaP xenograft | [33] |
| gold NPs (DTDTPA-AuNP) | 113 nm | Gallium-67 | Bombesin (7–14) | 67Ga-DTDTPA-AuNP-BBN | SPECT | prostate cancer PC-3 cells athymic nude mice bearing PC3 xenograft | [32] |
| liposomal NPs (LNP) | 107 nm | Actinium-225 | antibody J591aptamer (A10) | 225Ac-LNP-PEG-J591 225Ac-LNP-PEG-A10 | therapy | prostate cancer LNCaP and Mat-Lu cells, endothelial HUVEC, BT474, and breast cancer MDA-MB-231 cells | [34] |
| liposomal NPs (LNP) | 107 nm | Actinium-225 | antibody J591 glutamate-urea-lysine ligand | 225Ac-LNP-PEG-anti PSMA mAb 225Ac-LNP-PEG-GUL | therapy | endothelial HUVEC (PSMA+), HUVEC (PSMA-) and breast cancer MDA-MB-231 cells | [35] |
| zeolite NPs | ~120 nm | Radium-223 | antibody D2B | 223RaA-silane-PEG-D2B | therapy | prostate cancer LNCaP C4-2, DU145 and prostate normal RWPE-1, HPrEC cells BALB/c nude mice bearing LNCaP C4-2 xenograft | [50,59] |
| curcumin-containing poly(lactic-co-glycolic acid) NPs (PLGA-CUR) | 76 nm | Iodine-131 | antibody J591 | 131I-PSMA-PLGA-CUR | theranostic SPECT/ chemotherapy/ radiotherapy | prostate LNCaP C4-2, DU145 and PC-3 cells athymic nude mice bearing LNCaP C4-2 xenograft | [38] |
| sorafenib-containing silica NPs (PSi) | ~10 nm | Indium-111 | iRGD peptide | 111In-PSi-Alexa488-DBCO-DOTA-iRGD | theranostic SPECT/ IF/ chemotherapy | prostate cancer PC3-MM2 cells Hsd:NMRI-Foxnlnu/nu nude mice bearing PC3-MM2 xenograft | [30] |
| micellar NPs (LNP) | 20 nm | Copper-64 | diabody (cys-DB) based on the J591 antibody | 64Cu-DOTA-cysDB-LNP Dox-DOTA-LNP | theranostic PET/ chemotherapy | NOD/SCID mice bearing LNCaP xenograft | [43] |
| texaphyrin NPs (texaphyrin) | ~100 nm | Indium-111 Lutetium-175 | glutamate-urea-lysine ligand | 111In/175Lu-texaphyrin-TL | theranostic SPECT/NIRF/ PDT therapy | prostate cancer PC3 luc6 cells athymic nude mice bearing PC-3 or PC3 flu or PC3 luc6 xenograft | [37] |
| doxorubicin-containing liposomal NPs modified with P3-liposomes) | ~180 nm | Technetium-99m | glutamate-urea-lysine ligand | 99mTc-P3-Liposomes | theranostic SPECT/ chemotherapy | prostate cancer LNCaP and PC-3 cells | [36] |
2.2. Selection of a Suitable Type of Ligand for Targeted Imaging and Therapy of PCa
2.3. Selection of a Suitable Type of Radionuclide for Targeted Imaging and Therapy of PCa
| Radionuclide | Half-Life | Decay Energy [MeV] | Decay Mode | Production Mode | Reference |
|---|---|---|---|---|---|
| Positron-emitting radionuclides | |||||
| Copper-64 | 12.7 h | β+ 0.653; β− 0.579 | β+/β−/EC | 64Ni(p,n)64Cu | [71] |
| Gallium-68 | 67.6 min | β+ 1.899 | β+/EC | 68Ge/68Ga | [72] |
| Fluorine-18 | 110 min | β+ 0.634 | β+/EC | 18O(p,n)18F | [73] |
| Iodine-124 | 4.17 d | β+ 0.819; γ 0.603 | β+/EC | 124Te(p,n)124I | [74] |
| Zirconium-89 | 78.4 h | β+ 0.511; 0.902; 0.909 | β+/EC | 89Y(p,n)89Zr | [75] |
| Gamma-emitting radionuclides | |||||
| Indium-111 | 2.8 h | γ 0.171; 0.245 | EC | 111Cd(p,n)111m.gIn 112Cd(p,2n)111m.gIn | [76] |
| Technetium-99m | 6.0 h | γ 0.141 | γ/IT | 99Mo/99mTc | [77] |
| Gallium-67 | 78.26 h | γ 0.093; 0.185; 0.288; 0.394 | EC | natZn(p,x)67Ga 68Zn(p,2n)67Ga | [78] |
| Iodine-131 | 8.02 d | β− 0.606; γ 0.364; 0. 637; 0.284 | β− | natTe(n,γ)131I | [79] |
| Therapeutic radionuclides | |||||
| Radium-223 | 11.43 d | α 5.979 | α | 227Ac/223Ra | [80] |
| Iodine-125 | 59.4 d | γ 0.035 | EC | 124Xe(n,γ)125Xe/125I | [81] |
| Actinium-225 | 10.0 d | α 5.935 | α | 229Th/225Ra/225Ac 226Ra(p,2n)225Ac | [82] |
2.4. Selection of a Suitable Radiolabelling Strategy for NPs
3. Nanoparticle-Based Radioconjugates for Targeted Prostate Cancer Imaging
3.1. Nanoparticle-Based Radioconjugates for Targeted PET, PET/MR, and PET/NIRF Imaging of PCa
3.2. NP-Based Radioconjugates for PCa Targeted SPECT, SPECT/IF, and SPECT/NIRF Imaging
3.3. NP-Based Radioconjugates for PCa-Targeted Therapy
3.4. NP-Based Radioconjugates for Theranostic Applications in PCa
3.5. Clinical Studies on NP-Based Radioconjugates for Targeted Imaging and Therapy of PCa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| A549 | human lung cancer cell line |
| acetBr | acetamidobromomide |
| ACUPA | urea-bearing PSMA-targeted ligand |
| Alexa Fluor 488 | fluorescent dye |
| AuNPs | gold nanoparticles |
| BALB/c nu/nu | atymic mice |
| BBN | bombesin |
| BS | bisphosphonate bone scintigraphy |
| CCPM | polymeric micellar nanoparticles |
| CdTeQDs | cadmium telluride quantum dots |
| CT | computed tomography |
| CUR | curcumin |
| CuS | copper sulphide |
| Cy7 | indocyanine 7 |
| D2B | anty-PSMA antibody |
| cys-DB | diabody |
| DBCO | dibenzo cyclooctyne |
| DFO-B | desferrioxamine B |
| DGEA | Asp-Gly-Ala peptide |
| DOTA | 1,4,7,10-tetraacetic acid |
| DT | dithiol |
| DTPA | diethylene triamine penta-acetic acid |
| DU-145 | human prostate cancer cell line |
| EC | energy capture |
| EphB4 | ephrin receptor B4 |
| FDA | Food and Drug Administration |
| GGC | Gly-Gly-Cys peptide peptide |
| GRPR | gastrin-releasing peptide receptor |
| GUL | glutamate-ureido-lysine moiety |
| Hsd:NMRI-Foxnl nu/nu | nude mice |
| HS-PEG-DAP | bifunctional ligand |
| HS-PEG-DAP-TF | bifunctional ligand |
| HUVEC | human umbilical vein endothelial cells |
| HYNIC | 2-hydrazinonicotinic acid |
| ID | injected dose |
| ID/g | injected dose per gram |
| IF | immunofluorescence imaging |
| IO | iron oxide |
| IRDye 680RD | infrared dye |
| IT | isomeric transition |
| J591 | anty-PSMA antibody |
| Kd | dissociation equilibrium constant |
| LNCap | human prostate cancer cell line |
| LNCaP C4-2 | human prostate cancer cell line |
| Lys3-bombesin | bombesin analogue |
| mal | maleimide |
| mCRPC | metastatic castration-resistant prostate cancer |
| microPET | micro positron emission tomography |
| mNP-N1/2 | silica layer carrying NH2 groups |
| mNP-N1/2 | silica layer carrying SH groups |
| mpMRI | multiparametric MRI |
| MRI | magnetic resonance imaging |
| NIFR | near-infrared fluorescence imaging |
| nm | nanometre |
| nmCRPC | non-metastatic castration-resistant PCa |
| NOD/SCID | atymic mice |
| NODAGA | 1,4,7-triazaciclononane, 1-glutaric-4,7-acetic acid |
| NOTA | 1,4,7-triazacyclononane-1,4,7-triacetic acid |
| NPs | nanoparticles |
| nu/nu | atymic mice |
| P3 | lipopolymer |
| PC3 | human prostate cancer cell line |
| PC3-Flu | human prostate cancer cell line |
| PC-3-KD1 | human prostate cancer cell line |
| PC3-MM2 | human prostate cancer cell line |
| PC3-Pip | human prostate cancer cell line |
| PCa | prostate cancer |
| PDT | photodynamic therapy |
| PEG | polyethylene glycol |
| PEG3 | tripolyethylene glycol |
| PET | positron emission tomography |
| PLA–PEG | poly(lactic acid)-polyethyene glycol |
| PSMA | prostate-specific membrane antigen |
| PSMAL | small molecule PSMA inhibitor |
| PSMA-SH | small molecule inhibitor |
| QDs | quantum dots |
| RGD | tripeptide L-arginine-glycine-L-aspartic acid |
| scFv | single-chain fragment |
| SPECT | single-photon emission computed tomography |
| starPEG40kDa | four-armed nanocarrier |
| TETA | triethylenetetramine |
| TNYL-RAW | TNYLFSPNGPIARAW peptide |
| TRUS | trans-rectal ultrasound-guided biopsy |
| UnTHCPSi | undecylenic acid-modified silicon NPs |
| USI | ultrasound imaging |
| YC-XII-35 | PSMA-targeting moiety |
| αVβ3 | integrin receptor |
| β-Glu | β-glutamate |
| 22Rv1 | human prostate cancer cell line |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed]
- Tourinho-Barbosa, R.; Srougi, V.; Nunes-Silva, I.; Baghdadi, M.; Rembeyo, G.; Eiffel, S.S.; Barret, E.; Rozet, F.; Galiano, M.; Cathelineau, X.; et al. Biochemical Recurrence after Radical Prostatectomy: What Does It Mean? Int. Braz. J. Urol. 2018, 44, 14–21. [Google Scholar] [CrossRef]
- Mehtälä, J.; Zong, J.; Vassilev, Z.; Brobert, G.; Gabarró, M.S.; Stattin, P.; Khanfir, H. Overall Survival and Second Primary Malignancies in Men with Metastatic Prostate Cancer. PLoS ONE 2020, 15, e0227552. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Oki, R.; Sekine, Y.; Arai, S.; Miyazawa, Y.; Shibata, Y.; Suzuki, K.; Kurosawa, I. Screening for Prostate Cancer: History, Evidence, Controversies and Future Perspectives toward Individualized Screening. Int. J. Urol. 2019, 26, 956–970. [Google Scholar] [CrossRef]
- Abdelrazek, A.; Mahmoud, A.M.; Joshi, V.B.; Habeeb, M.; Ahmed, M.E.; Ghoniem, K.; Delgado, A.; Khater, N.; Kwon, E.; Kendi, A.T. Recent Advances in Prostate Cancer (PCa) Diagnostics. Uro 2022, 2, 109–121. [Google Scholar] [CrossRef]
- Tanaka, T.; Yang, M.; Froemming, A.T.; Bryce, A.H.; Inai, R.; Kanazawa, S.; Kawashima, A. Current Imaging Techniques for and Imaging Spectrum of Prostate Cancer Recurrence and Metastasis: A Pictorial Review. RadioGraphics 2020, 40, 709–726. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, S. A Review of Imaging Methods for Prostate Cancer Detection: Supplementary Issue: Image and Video Acquisition and Processing for Clinical Applications. Biomed. Eng. Comput. Biol. 2016, 7s1, BECB.S34255. [Google Scholar] [CrossRef]
- Ludwig, B.S.; Kessler, H.; Kossatz, S.; Reuning, U. RGD-Binding Integrins Revisited: How Recently Discovered Functions and Novel Synthetic Ligands (Re-)Shape an Ever-Evolving Field. Cancers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, J.; Zhou, C.; Li, J.; Hu, S. False-Positive MpMRI and True-Negative 68Ga-PSMA PET/CT Xanthogranulomatous Prostatitis: A Case Report. Transl. Androl. Urol. 2022, 11, 561–566. [Google Scholar] [CrossRef]
- Chang, S.S. Overview of Prostate-Specific Membrane Antigen. Rev. Urol. 2004, 6 (Suppl. 10), S13–S18. [Google Scholar] [PubMed]
- Kiess, A.P.; Banerjee, S.R.; Mease, R.C.; Rowe, S.P.; Rao, A.; Foss, C.A.; Chen, Y.; Yang, X.; Cho, S.Y.; Nimmagadda, S.; et al. Prostate-specific membrane antigen as a target for cancer imaging and therapy. Q. J. Nucl. Med. Mol. Imaging. 2015, 59, 241–268. [Google Scholar] [PubMed]
- Czerwińska, M.; Bilewicz, A.; Kruszewski, M.; Wegierek-Ciuk, A.; Lankoff, A. Targeted Radionuclide Therapy of Prostate Cancer—From Basic Research to Clinical Perspectives. Molecules 2020, 25, 1743. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Zhou, N.; McLaughlin, M.; Rice, S.; Pillai, A.K.; Hao, G.; Sun, X. PSMA-Targeting Imaging and Theranostic Agents—Current Status and Future Perspective. Int. J. Mol. Sci. 2022, 23, 1158. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Combes, A.D.; Palma, C.A.; Calopedos, R.; Wen, L.; Woo, H.; Fulham, M.; Leslie, S. PSMA PET-CT in the Diagnosis and Staging of Prostate Cancer. Diagnostics 2022, 12, 2594. [Google Scholar] [CrossRef]
- Bodar, Y.J.L.; Luining, W.I.; Keizer, B.; Meijer, D.; Vellekoop, A.; Schaaf, M.; Hendrikse, N.H.; Van Moorselaar, R.J.A.; Oprea-Lager, D.E.; Vis, A.N. A Prospective, Multicenter Head-to-Head Comparative Study in Patients with Primary High-Risk Prostate Cancer Investigating the Bone Lesion Detection of Conventional Imaging and 18F-PSMA-PET/CT. Urol. Oncol. 2023, 41, 205.e17–205.e24. [Google Scholar] [CrossRef]
- Brunello, S.; Salvarese, N.; Carpanese, D.; Gobbi, C.; Melendez-Alafort, L.; Bolzati, C. A Review on the Current State and Future Perspectives of [99mTc]Tc-Housed PSMA-i in Prostate Cancer. Molecules 2022, 27, 2617. [Google Scholar] [CrossRef]
- Maurin, M.; Wyczółkowska, M.; Sawicka, A.; Sikora, A.E.; Karczmarczyk, U.; Janota, B.; Radzik, M.; Kłudkiewicz, D.; Pijarowska-Kruszyna, J.; Jaroń, A.; et al. [99mTc]Tc-PSMA-T4—Novel SPECT Tracer for Metastatic PCa: From Bench to Clinic. Molecules 2022, 27, 7216. [Google Scholar] [CrossRef]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef]
- Miyahira, A.K.; Pienta, K.J.; Morris, M.J.; Bander, N.H.; Baum, R.P.; Fendler, W.P.; Goeckeler, W.; Gorin, M.A.; Hennekes, H.; Pomper, M.G.; et al. Meeting Report from the Prostate Cancer Foundation PSMA-Directed Radionuclide Scientific Working Group. Prostate 2018, 78, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Eder, M. [177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals 2022, 15, 1292. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Dwivedi, S.N.; Bal, C. Radioligand Therapy With 177 Lu-PSMA for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2019, 213, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Kostos, L.; Buteau, J.P.; Yeung, T.; Iulio, J.D.; Xie, J.; Cardin, A.; Chin, K.Y.; Emmerson, B.; Owen, K.L.; Parker, B.S.; et al. AlphaBet: Combination of Radium-223 and [177Lu]Lu-PSMA-I&T in Men with Metastatic Castration-Resistant Prostate Cancer (Clinical Trial Protocol). Front. Med. 2022, 9, 1059122. [Google Scholar] [CrossRef]
- Lee, H. Relative Efficacy of 225Ac-PSMA-617 and 177Lu-PSMA-617 in Prostate Cancer Based on Subcellular Dosimetry. Mol. Imaging Radionucl. Ther. 2022, 31, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Seitzer, K.E.; Seifert, R.; Kessel, K.; Roll, W.; Schlack, K.; Boegemann, M.; Rahbar, K. Lutetium-177 Labelled PSMA Targeted Therapy in Advanced Prostate Cancer: Current Status and Future Perspectives. Cancers 2021, 13, 3715. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.R.; Singh, S.B.; Thapaliya, S.; Shrestha, S.; Deo, S.; Khanal, K. A Review of 177Lutetium-PSMA and 225Actinium-PSMA as Emerging Theranostic Agents in Prostate Cancer. Cureus 2022, 14, e29369. [Google Scholar] [CrossRef]
- Calatayud, D.G.; Neophytou, S.; Nicodemou, E.; Giuffrida, S.G.; Ge, H.; Pascu, S.I. Nano-Theranostics for the Sensing, Imaging and Therapy of Prostate Cancers. Front. Chem. 2022, 10, 830133. [Google Scholar] [CrossRef]
- Choksi, A.U.; Khan, A.I.; Lokeshwar, S.D.; Segal, D.; Weiss, R.M.; Martin, D.T. Functionalized Nanoparticles Targeting Biomarkers for Prostate Cancer Imaging and Therapy. Am. J. Clin. Exp. Urol. 2022, 10, 142–153. [Google Scholar]
- Wang, C.-F.; Sarparanta, M.P.; Mäkilä, E.M.; Hyvönen, M.L.K.; Laakkonen, P.M.; Salonen, J.J.; Hirvonen, J.T.; Airaksinen, A.J.; Santos, H.A. Multifunctional Porous Silicon Nanoparticles for Cancer Theranostics. Biomaterials 2015, 48, 108–118. [Google Scholar] [CrossRef]
- Mendoza-Sánchez, A.N.; Ferro-Flores, G.; Ocampo-García, B.E.; Morales-Avila, E.; Ramírez, F.D.M.; De León-Rodríguez, L.M.; Santos-Cuevas, C.L.; Medina, L.A.; Rojas-Calderón, E.L.; Camacho-López, M.A. Lys3-bombesin conjugated to 99mTc-labelled gold nanoparticles for in vivo gastrin releasing peptide-receptor imaging. J. Biomed. Nanotechnol. 2010, 6, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Zambre, A.; Silva, F.; Upendran, A.; Gano, L.; Paulo, A.; Raghuraman, K. Evaluation of Tumor Targeting Efficacy of 67Ga-Labeled Bombesin Peptide Functionalized Gold Nanoparticle in Mice Model. J. Nucl. Med. 2014, 55 (Suppl. 1), 1386. [Google Scholar]
- Felber, M.; Bauwens, M.; Mateos, J.M.; Imstepf, S.; Mottaghy, F.M.; Alberto, R. 99m Tc Radiolabeling and Biological Evaluation of Nanoparticles Functionalized with a Versatile Coating Ligand. Chem. Eur. J. 2015, 21, 6090–6099. [Google Scholar] [CrossRef] [PubMed]
- Bandekar, A.; Zhu, C.; Jindal, R.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Anti–Prostate-Specific Membrane Antigen Liposomes Loaded with 225 Ac for Potential Targeted Antivascular α-Particle Therapy of Cancer. J. Nucl. Med. 2014, 55, 107–114. [Google Scholar] [CrossRef]
- Zhu, C.; Bandekar, A.; Sempkowski, M.; Banerjee, S.R.; Pomper, M.G.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Nanoconjugation of PSMA-Targeting Ligands Enhances Perinuclear Localization and Improves Efficacy of Delivered Alpha-Particle Emitters against Tumor Endothelial Analogues. Mol. Cancer Ther. 2016, 15, 106–113. [Google Scholar] [CrossRef]
- Yari, H.; Nkepang, G.; Awasthi, V. Surface Modification of Liposomes by a Lipopolymer Targeting Prostate Specific Membrane Antigen for Theranostic Delivery in Prostate Cancer. Materials 2019, 12, 756. [Google Scholar] [CrossRef]
- Cheng, M.H.Y.; Overchuk, M.; Rajora, M.A.; Lou, J.W.H.; Chen, Y.; Pomper, M.G.; Chen, J.; Zheng, G. Targeted Theranostic 111 In/Lu-Nanotexaphyrin for SPECT Imaging and Photodynamic Therapy. Mol. Pharm. 2022, 19, 1803–1813. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Khan, S.; Maher, D.M.; Ebeling, M.C.; Sundram, V.; Chauhan, N.; Ganju, A.; Balakrishna, S.; Gupta, B.K.; Zafar, N.; et al. Anti-Cancer Activity of Curcumin Loaded Nanoparticles in Prostate Cancer. Biomaterials 2014, 35, 8635–8648. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Foss, C.A.; Horhota, A.; Pullambhatla, M.; McDonnell, K.; Zale, S.; Pomper, M.G. 111 In- and IRDye800CW-Labeled PLA–PEG Nanoparticle for Imaging Prostate-Specific Membrane Antigen-Expressing Tissues. Biomacromolecules 2017, 18, 201–209. [Google Scholar] [CrossRef]
- Meher, N.; Ashley, G.W.; Bidkar, A.P.; Dhrona, S.; Fong, C.; Fontaine, S.D.; Beckford Vera, D.R.; Wilson, D.M.; Seo, Y.; Santi, D.V.; et al. Prostate-Specific Membrane Antigen Targeted Deep Tumor Penetration of Polymer Nanocarriers. ACS Appl. Mater. Interfaces 2022, 14, 50569–50582. [Google Scholar] [CrossRef]
- Zhang, R.; Xiong, C.; Huang, M.; Zhou, M.; Huang, Q.; Wen, X.; Liang, D.; Li, C. Peptide-Conjugated Polymeric Micellar Nanoparticles for Dual SPECT and Optical Imaging of EphB4 Receptors in Prostate Cancer Xenografts. Biomaterials 2011, 32, 5872–5879. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Li, L.; Chea, J.; Delgado, M.K.; Crow, D.; Poku, E.; Szpikowska, B.; Bowles, N.; Channappa, D.; Colcher, D.; et al. PET Imaging of 64Cu-DOTA-ScFv-Anti-PSMA Lipid Nanoparticles (LNPs): Enhanced Tumor Targeting over Anti-PSMA ScFv or Untargeted LNPs. Nucl. Med. Biol. 2017, 47, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Li, L.; Chea, J.; Delgado, M.K.; Poku, E.; Szpikowska, B.; Bowles, N.; Minnix, M.; Colcher, D.; Wong, J.Y.C.; et al. Synthesis, Positron Emission Tomography Imaging, and Therapy of Diabody Targeted Drug Lipid Nanoparticles in a Prostate Cancer Murine Model. Cancer Biother. Radiopharm. 2017, 32, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Wen, L.; Meng, X.; Zhou, N.; Guo, X.; Liu, T.; Xu, X.; Wang, F.; Zhu, H.; Yang, Z. Application Analysis of 124I-PPMN for Enhanced Retention in Tumors of Prostate Cancer Xenograft Mice. Int. J. Nanomed. 2021, 16, 7685–7695. [Google Scholar] [CrossRef]
- Cai, H.; Xie, F.; Mulgaonkar, A.; Chen, L.; Sun, X.; Hsieh, J.-T.; Peng, F.; Tian, R.; Li, L.; Wu, C.; et al. Bombesin Functionalized 64 Cu-Copper Sulfide Nanoparticles for Targeted Imaging of Orthotopic Prostate Cancer. Nanomedicine 2018, 13, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, D.; Liu, S.; Wang, M.; Moats, R.; Conti, P.S.; Li, Z. Integrin α2β1 targeted GdVO4:Eu ultrathin nanosheet for multimodal PET/MR imaging. Biomaterials 2014, 35, 8649–8658. [Google Scholar] [CrossRef]
- Moon, S.-H.; Yang, B.Y.; Kim, Y.J.; Hong, M.K.; Lee, Y.-S.; Lee, D.S.; Chung, J.-K.; Jeong, J.M. Development of a Complementary PET/MR Dual-Modal Imaging Probe for Targeting Prostate-Specific Membrane Antigen (PSMA). Nanomed. Nanotechnol. Biol. Med. 2016, 12, 871–879. [Google Scholar] [CrossRef]
- Liolios, C.; Koutsikou, T.S.; Salvanou, E.-A.; Kapiris, F.; Machairas, E.; Stampolaki, M.; Kolocouris, A.; Efthimiadou, E.Κ.; Bouziotis, P. Synthesis and in Vitro Proof-of-Concept Studies on Bispecific Iron Oxide Magnetic Nanoparticles Targeting PSMA and GRP Receptors for PET/MR Imaging of Prostate Cancer. Int. J. Pharm. 2022, 624, 122008. [Google Scholar] [CrossRef]
- Hu, K.; Wang, H.; Tang, G.; Huang, T.; Tang, X.; Liang, X.; Yao, S.; Nie, D. In Vivo Cancer Dual-Targeting and Dual-Modality Imaging with Functionalized Quantum Dots. J. Nucl. Med. 2015, 56, 1278–1284. [Google Scholar] [CrossRef]
- Czerwińska, M.; Fracasso, G.; Pruszyński, M.; Bilewicz, A.; Kruszewski, M.; Majkowska-Pilip, A.; Lankoff, A. Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy–Part I. Materials 2020, 13, 3875. [Google Scholar] [CrossRef]
- Yagublu, V.; Karimova, A.; Hajibabazadeh, J.; Reissfelder, C.; Muradov, M.; Bellucci, S.; Allahverdiyev, A. Overview of Physicochemical Properties of Nanoparticles as Drug Carriers for Targeted Cancer Therapy. J. Funct. Biomater. 2022, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kröger, M.; Liu, W.K. Shape Effect in Cellular Uptake of PEGylated Nanoparticles: Comparison between Sphere, Rod, Cube and Disk. Nanoscale 2015, 7, 16631–16646. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Niculescu, V.-C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold Nanoparticles (GNPs) in Biomedical and Clinical Applications: A Review. Nano Sel. 2022, 3, 792–828. [Google Scholar] [CrossRef]
- Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Almatroudi, A.; Rahmani, A.H. Recent Strategies towards the Surface Modification of Liposomes: An Innovative Approach for Different Clinical Applications. 3 Biotech 2020, 10, 163. [Google Scholar] [CrossRef]
- Oh, J.K. Surface Modification of Colloidal CdX-Based Quantum Dots for Biomedical Applications. J. Mater. Chem. 2010, 20, 8433. [Google Scholar] [CrossRef]
- Lankoff, A.; Czerwińska, M.; Walczak, R.; Karczmarczyk, U.; Tomczyk, K.; Brzóska, K.; Fracasso, G.; Garnuszek, P.; Mikołajczak, R.; Kruszewski, M. Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy—Part II. Toxicity, Pharmacokinetics and Biodistribution. Int. J. Mol. Sci. 2021, 22, 5702. [Google Scholar] [CrossRef]
- De Visser, M.; Van Weerden, W.M.; De Ridder, C.M.A.; Reneman, S.; Melis, M.; Krenning, E.P.; De Jong, M. Androgen-dependent expression of the gastrin-releasing peptide receptor in human prostate tumor xenografts. J. Nucl. Med. 2007, 48, 88–93. [Google Scholar]
- Manrique-Arias, J.C.; Pitalua-Cortes, Q.; Pedrero-Piedras, R.; Rodríguez-Mena, G.; López, T.; Cabezas-Ortiz, C.; García-Pérez, O. Synthesis and Radiation Dosimetry of [68Ga]-Ga-Lys1, Lys3-DOTA-Bombesin (1,14) Antagonist for PET-Imaging, as a Potential Theragnostic Tracer in Oncology. J. Encapsulation Adsorpt. Sci. 2020, 10, 29–41. [Google Scholar] [CrossRef]
- Huynh, T.T.; van Dam, E.M.; Sreekumar, S.; Mpoy, C.; Blyth, B.J.; Muntz, F.; Harris, M.J.; Rogers, B.E. Copper-67-Labeled Bombesin Peptide for Targeted Radionuclide Therapy of Prostate Cancer. Pharmaceuticals 2022, 15, 728. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, X.; Yu, J.; Yuan, S. Preliminary Clinical Application of RGD-Containing Peptides as PET Radiotracers for Imaging Tumors. Front. Oncol. 2022, 12, 837952. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Guo, H.; Tang, T.; Wang, Y.; Wei, Y.; Seth, P.; Li, Y.; Dehm, S.M.; Ruoslahti, E.; Pang, H. IRGD-Liposomes Enhance Tumor Delivery and Therapeutic Efficacy of Antisense Oligonucleotide Drugs against Primary Prostate Cancer and Bone Metastasis. Adv. Funct. Mater. 2021, 31, 2100478. [Google Scholar] [CrossRef]
- VanderWeele, D.J.; Kocherginsky, M.; Munir, S.; Martone, B.; Sagar, V.; Morgans, A.; Stadler, W.M.; Abdulkadir, S.; Hussain, M. A Phase II Study of SEphB4-HSA in Metastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2022, 20, 575–580. [Google Scholar] [CrossRef]
- Chrencik, J.E.; Brooun, A.; Recht, M.I.; Kraus, M.L.; Koolpe, M.; Kolatkar, A.R.; Bruce, R.H.; Martiny-Baron, G.; Widmer, H.; Pasquale, E.B.; et al. Structure and Thermodynamic Characterization of the EphB4/Ephrin-B2 Antagonist Peptide Complex Reveals the Determinants for Receptor Specificity. Structure 2006, 14, 321–330. [Google Scholar] [CrossRef]
- Pimlott, S.L.; Sutherland, A. Molecular Tracers for the PET and SPECT Imaging of Disease. Chem. Soc. Rev. 2011, 40, 149–162. [Google Scholar] [CrossRef]
- Lau, J.; Rousseau, E.; Kwon, D.; Lin, K.-S.; Bénard, F.; Chen, X. Insight into the Development of PET Radiopharmaceuticals for Oncology. Cancers 2020, 12, 1312. [Google Scholar] [CrossRef]
- Okoye, N.C.; Baumeister, J.E.; Najafi Khosroshahi, F.; Hennkens, H.M.; Jurisson, S.S. Chelators and Metal Complex Stability for Radiopharmaceutical Applications. Radiochimica Acta 2019, 107, 1087–1120. [Google Scholar] [CrossRef]
- Stokke, C.; Kvassheim, M.; Blakkisrud, J. Radionuclides for Targeted Therapy: Physical Properties. Molecules 2022, 27, 5429. [Google Scholar] [CrossRef]
- Anderson, C.J.; Ferdani, R. Copper-64 Radiopharmaceuticals for PET Imaging of Cancer: Advances in Preclinical and Clinical Research. Cancer Biother. Radiopharm. 2009, 24, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Rodnick, M.E.; Sollert, C.; Stark, D.; Clark, M.; Katsifis, A.; Hockley, B.G.; Parr, D.C.; Frigell, J.; Henderson, B.D.; Abghari-Gerst, M.; et al. Cyclotron-Based Production of 68Ga, [68Ga]GaCl3, and [68Ga]Ga-PSMA-11 from a Liquid Target. EJNMMI Radiopharm. Chem. 2020, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Q.; Shi, H.; Cheng, D. Fluorine-18: Radiochemistry and Target-Specific PET Molecular Probes Design. Front. Chem. 2022, 10, 884517. [Google Scholar] [CrossRef]
- Kuker, R.; Sztejnberg, M.; Gulec, S. I-124 Imaging and Dosimetry. Mol. Imaging Radionucl. Ther. 2017, 26 (Suppl. 1), 66–73. [Google Scholar] [CrossRef] [PubMed]
- De Feo, M.S.; Pontico, M.; Frantellizzi, V.; Corica, F.; De Cristofaro, F.; De Vincentis, G. 89Zr-PET Imaging in Humans: A Systematic Review. Clin. Transl. Imaging 2022, 10, 23–36. [Google Scholar] [CrossRef]
- Lahiri, S.; Maiti, M.; Ghosh, K. Production and Separation of 111In: An Important Radionuclide in Life Sciences: A Mini Review. J. Radioanal. Nucl. Chem. 2013, 297, 309–318. [Google Scholar] [CrossRef]
- Boschi, A.; Uccelli, L.; Martini, P. A Picture of Modern Tc-99m Radiopharmaceuticals: Production, Chemistry, and Applications in Molecular Imaging. Appl. Sci. 2019, 9, 2526. [Google Scholar] [CrossRef]
- Bailey, D.L.; Sabanathan, D.; Aslani, A.; Walsh, B.J.; Lengkeek, N.A. RetroSPECT: Gallium-67 as a Long-Lived Imaging Agent for Theranostics. Asia Ocean J. Nucl. Med. Biol. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Ramdhani, D.; Widyasari, E.M.; Sriyani, M.E.; Arnanda, Q.P.; Watabe, H. Iodine-131 Labeled Genistein as a Potential Radiotracer for Breast Cancer. Heliyon 2020, 6, e04780. [Google Scholar] [CrossRef]
- Mokhodoeva, O.; Guseva, L.; Dogadkin, N. Isolation of generator-produced 223Ra in 0.9-% NaCl solutions containing EDTA for direct radiotherapeutic studies. J. Radioanal. Nucl. Chem. 2015, 304, 449–453. [Google Scholar] [CrossRef]
- Bodei, L.; Kassis, A.I.; Adelstein, S.J.; Mariani, G. Radionuclide Therapy with Iodine-125 and Other Auger–Electron-Emitting Radionuclides: Experimental Models and Clinical Applications. Cancer Biother. Radiopharm. 2003, 18, 861–877. [Google Scholar] [CrossRef] [PubMed]
- Kozempel, J.; Mokhodoeva, O.; Vlk, M. Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators. Molecules 2018, 23, 581. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Zhang, J.; Wang, Y.; Jiao, C.; Song, Z.; Ma, Y.; Ding, Y.; Zhang, Z.; He, X. Radiolabeling of Nanomaterials: Advantages and Challenges. Front. Toxicol. 2021, 3, 753316. [Google Scholar] [CrossRef]
- Pellico, J.; Gawne, P.J.; de Rosales, R.T. Radiolabelling of Nanomaterials for Medical Imaging and Therapy. Chem. Soc. Rev. 2021, 50, 3355–3423. [Google Scholar] [CrossRef] [PubMed]
- Gutfilen, B.; Souza, S.; Valentini, G. Copper-64: A Real Theranostic Agent. Drug Des. Devel. Ther. 2018, 12, 3235–3245. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chae, J.H.; Hur, M.G.; Yang, S.D.; Kong, Y.B.; Lee, J.; Ju, J.S.; Choi, P.S.; Park, J.H. Theragnostic 64Cu/67Cu Radioisotopes Production With RFT-30 Cyclotron. Front. Med. 2022, 9, 889640. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Xu, X.; Zhao, M.; Zhang, B.; Deng, S.; Wu, Y. 64 Cu-Based Radiopharmaceuticals in Molecular Imaging. Technol. Cancer Res. Treat. 2019, 18, 153303381983075. [Google Scholar] [CrossRef]
- Elgqvist, J. Nanoparticles as Theranostic Vehicles in Experimental and Clinical Applications—Focus on Prostate and Breast Cancer. Int. J. Mol. Sci. 2017, 18, 1102. [Google Scholar] [CrossRef]
- Hao, B.; Wei, L.; Cheng, Y.; Ma, Z.; Wang, J. Advanced Nanomaterial for Prostate Cancer Theranostics. Front. Bioeng. Biotechnol. 2022, 10, 1046234. [Google Scholar] [CrossRef]





















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lankoff, A.; Czerwińska, M.; Kruszewski, M. Nanoparticle-Based Radioconjugates for Targeted Imaging and Therapy of Prostate Cancer. Molecules 2023, 28, 4122. https://doi.org/10.3390/molecules28104122
Lankoff A, Czerwińska M, Kruszewski M. Nanoparticle-Based Radioconjugates for Targeted Imaging and Therapy of Prostate Cancer. Molecules. 2023; 28(10):4122. https://doi.org/10.3390/molecules28104122
Chicago/Turabian StyleLankoff, Anna, Malwina Czerwińska, and Marcin Kruszewski. 2023. "Nanoparticle-Based Radioconjugates for Targeted Imaging and Therapy of Prostate Cancer" Molecules 28, no. 10: 4122. https://doi.org/10.3390/molecules28104122