Perspectives on the Lindman Hypothesis and Cellulose Interactions
Abstract
1. Introduction
2. The “History” of Cellulose in Science and Technology
2.1. Addressing Cellulose Intermolecular Interactions
2.1.1. In Cellulose Biosynthesis
2.1.2. In Dissolution and Regeneration of Cellulose
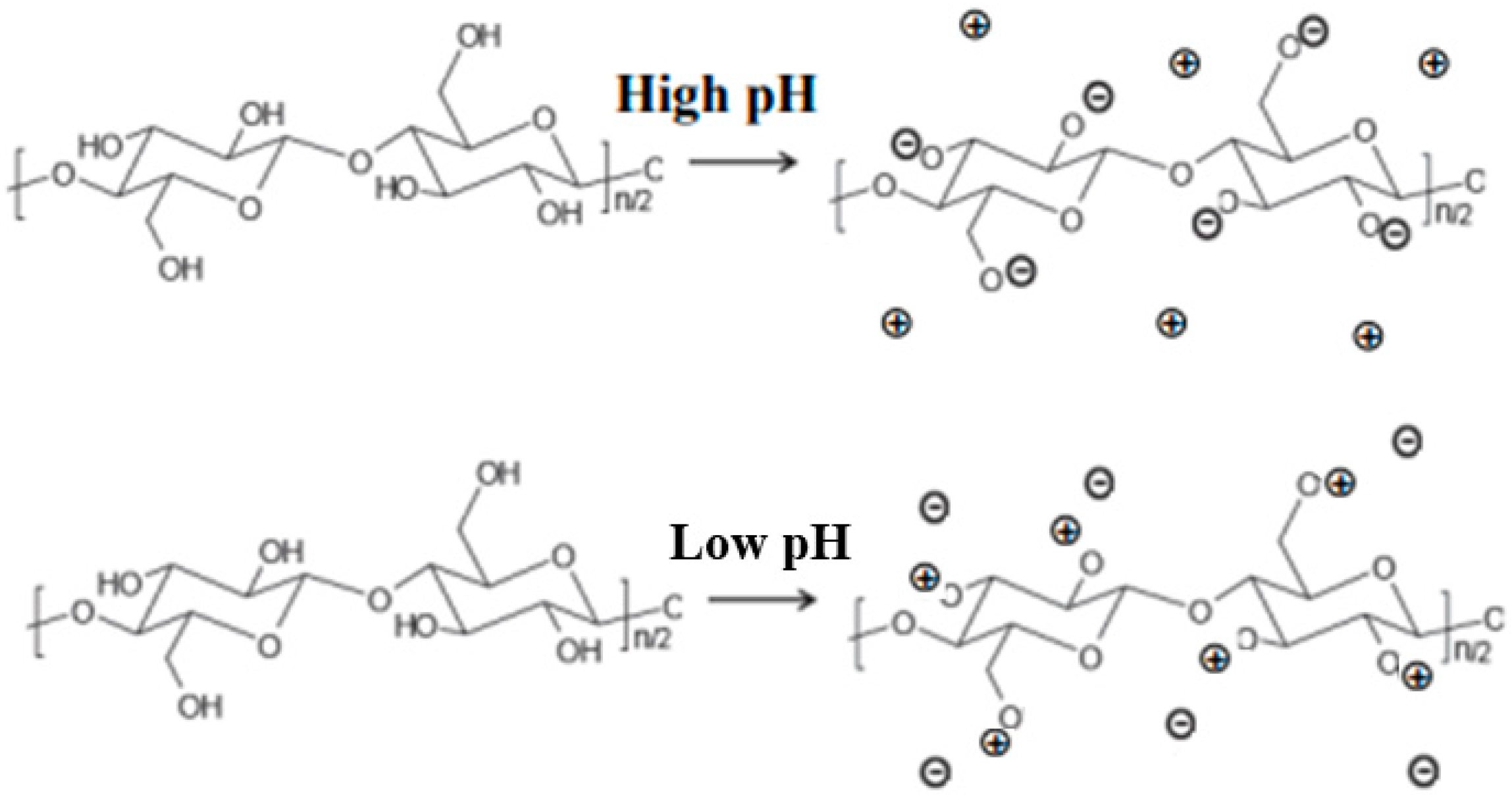
2.1.3. In Cellulose Swelling
2.1.4. In Partial Dissolution and Plasticization of Cellulose
2.1.5. In Cellulose Pulp Fiber and Papermaking
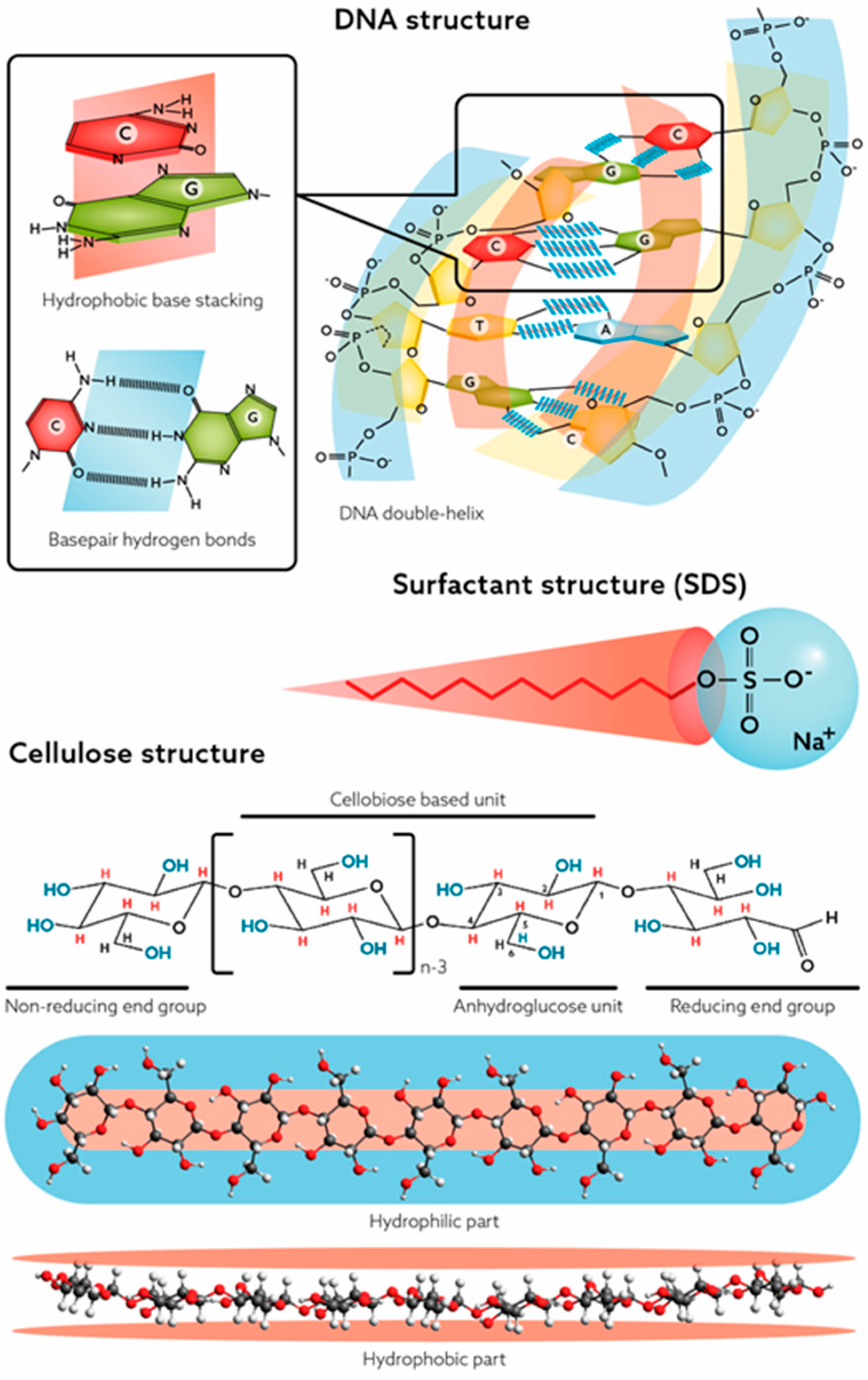
2.2. Revisiting Cellulose—The Lindman Hypothesis
2.2.1. Considerations and Implications of Cellulose’s Dual Properties
2.2.2. Response from the Scientific Community on Lindman’s Dispute
3. Recent Progress in the Scientific Understanding of Cellulose
3.1. Theoretical Considerations of Molecular Cellulose
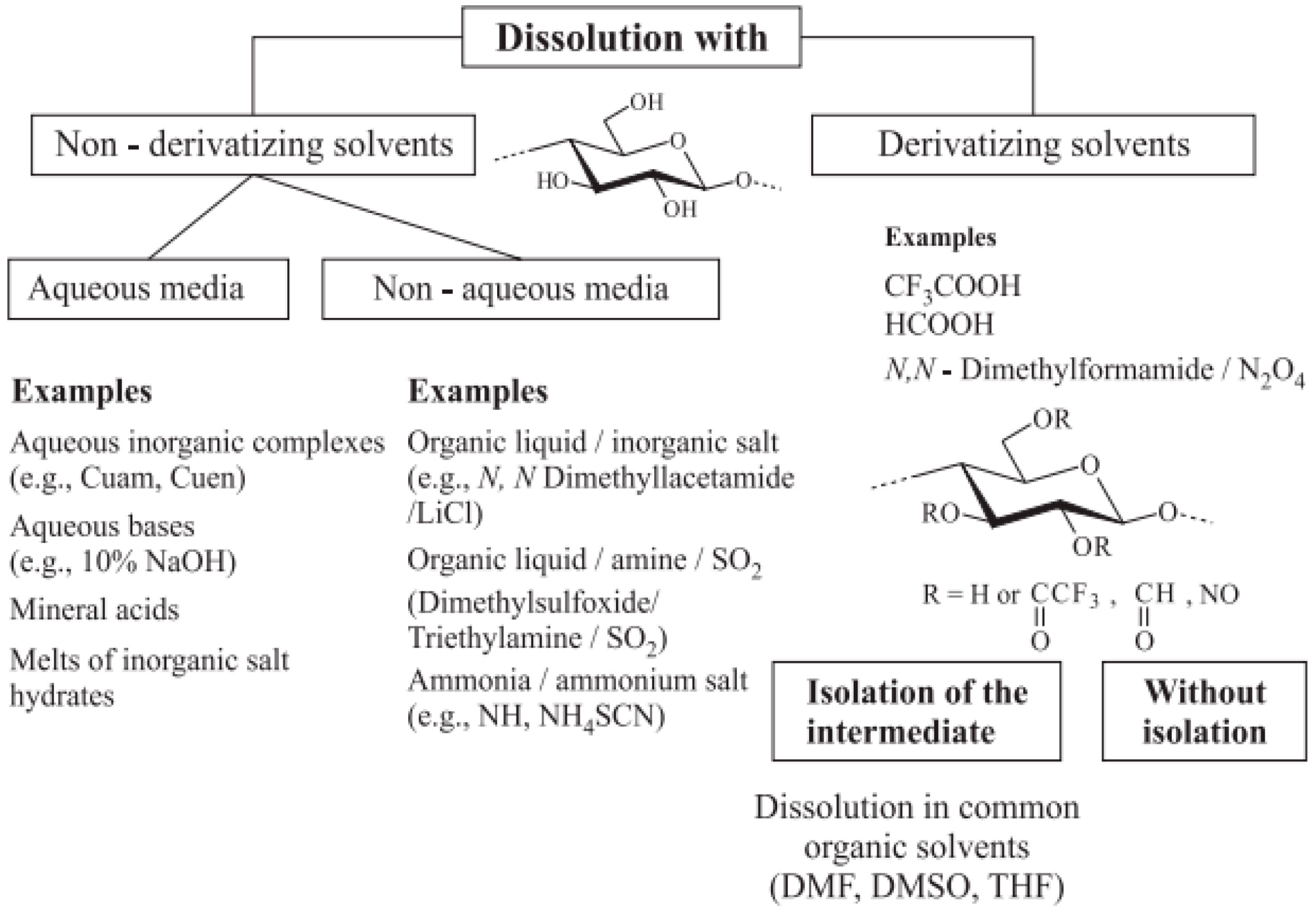
3.2. Dissolution of Cellulose
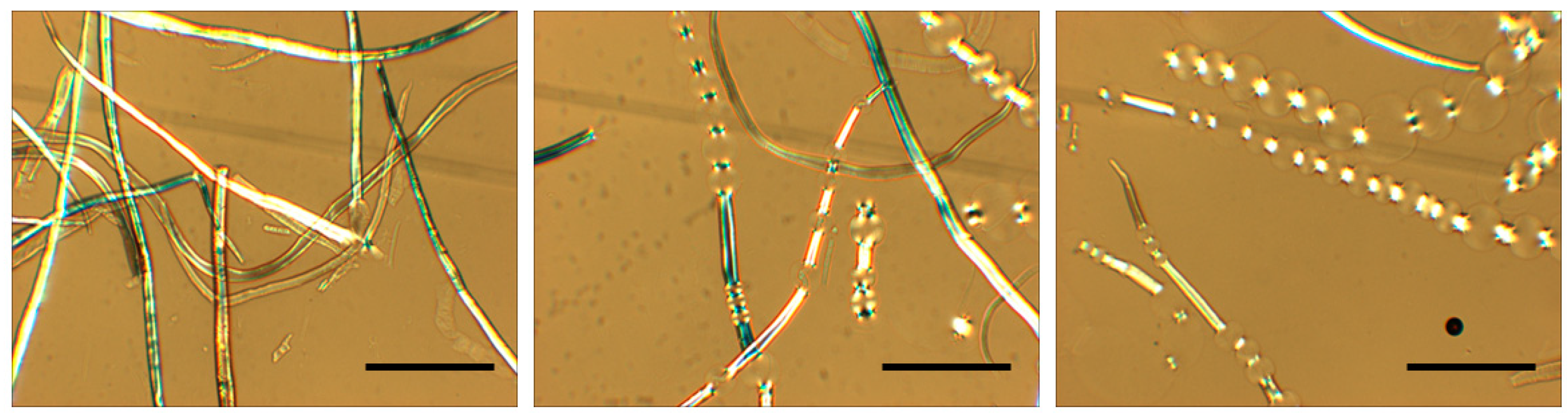
3.3. Cellulose in Emulsions
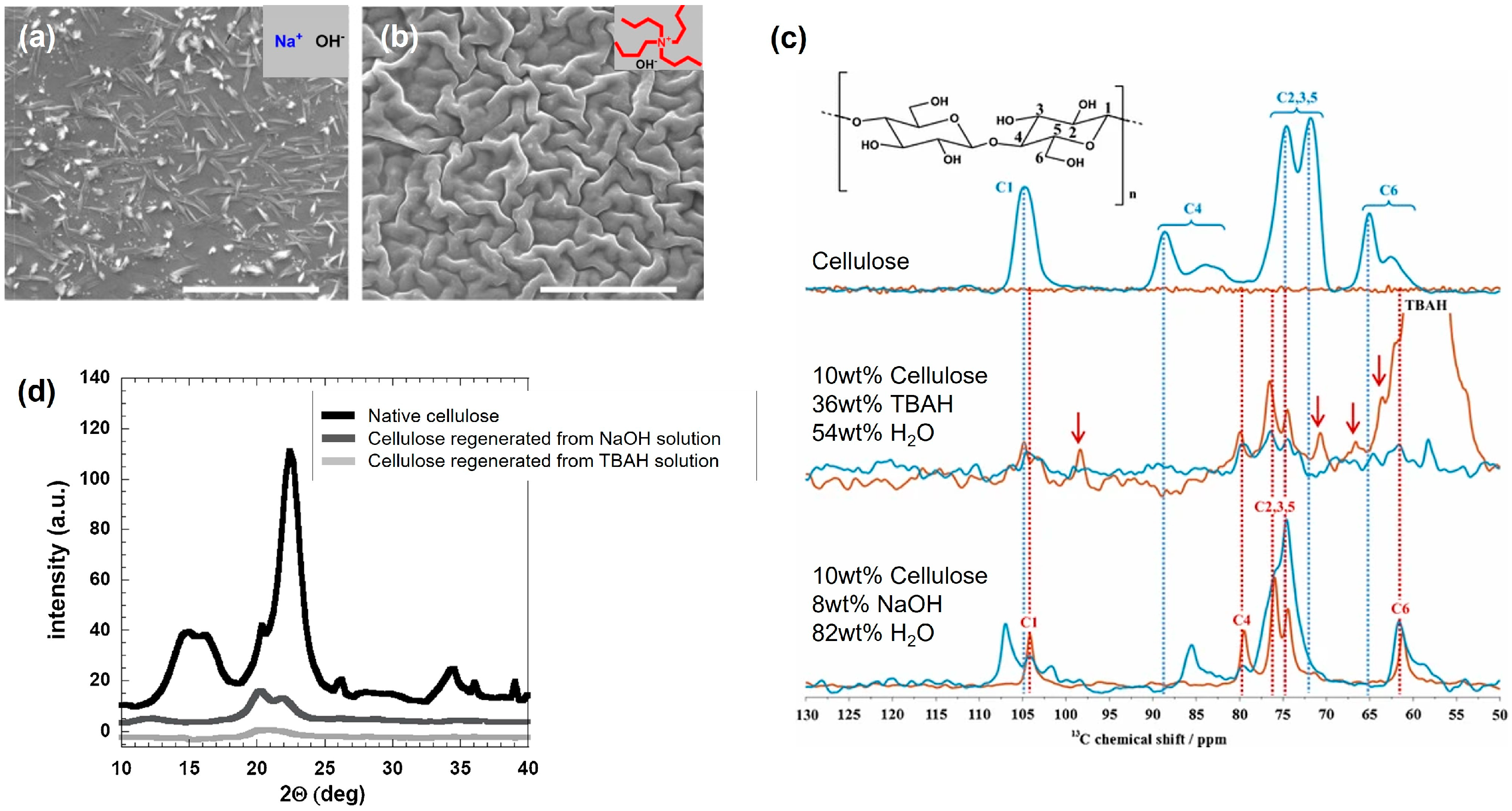
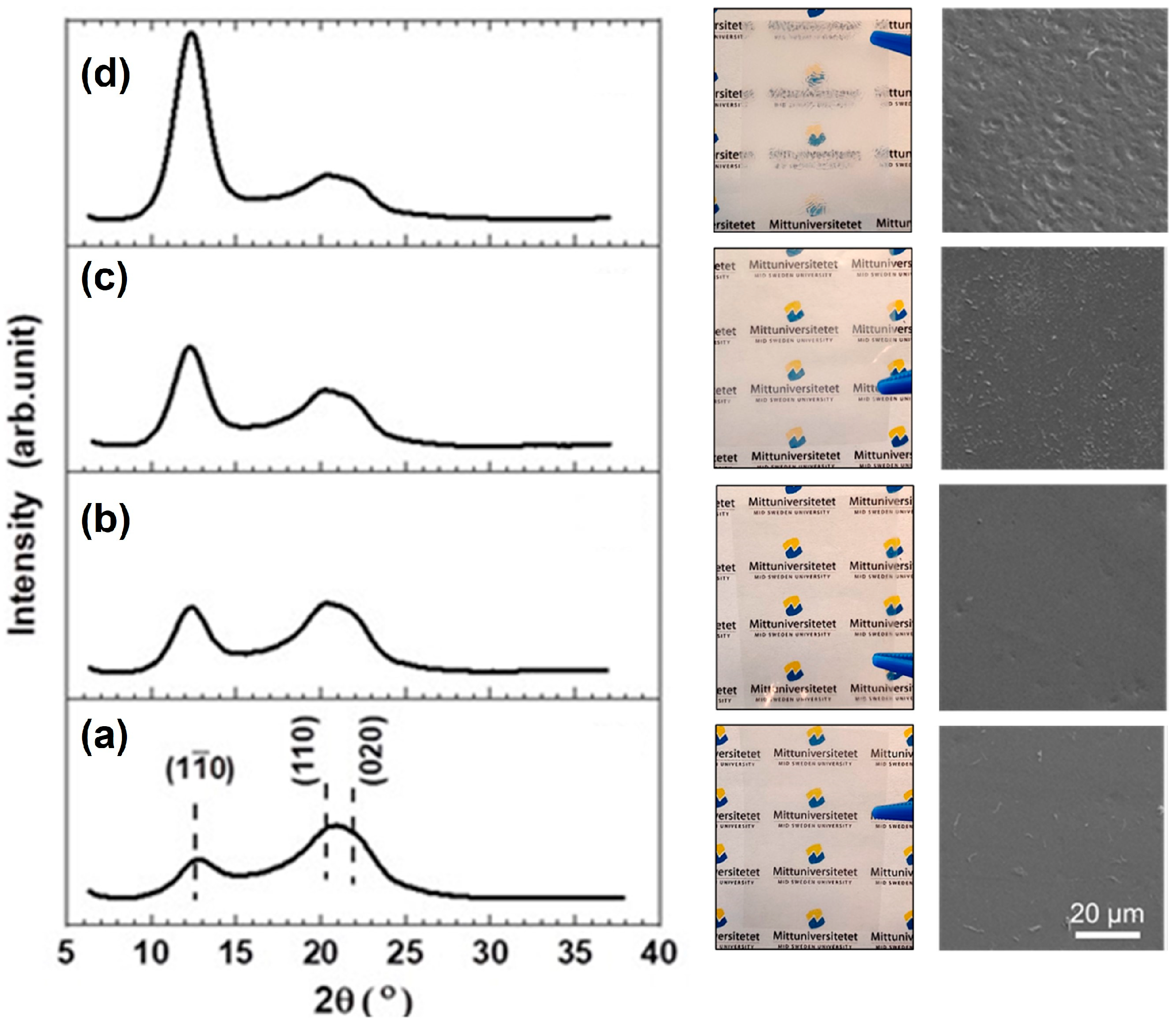
3.4. Regeneration of Cellulose
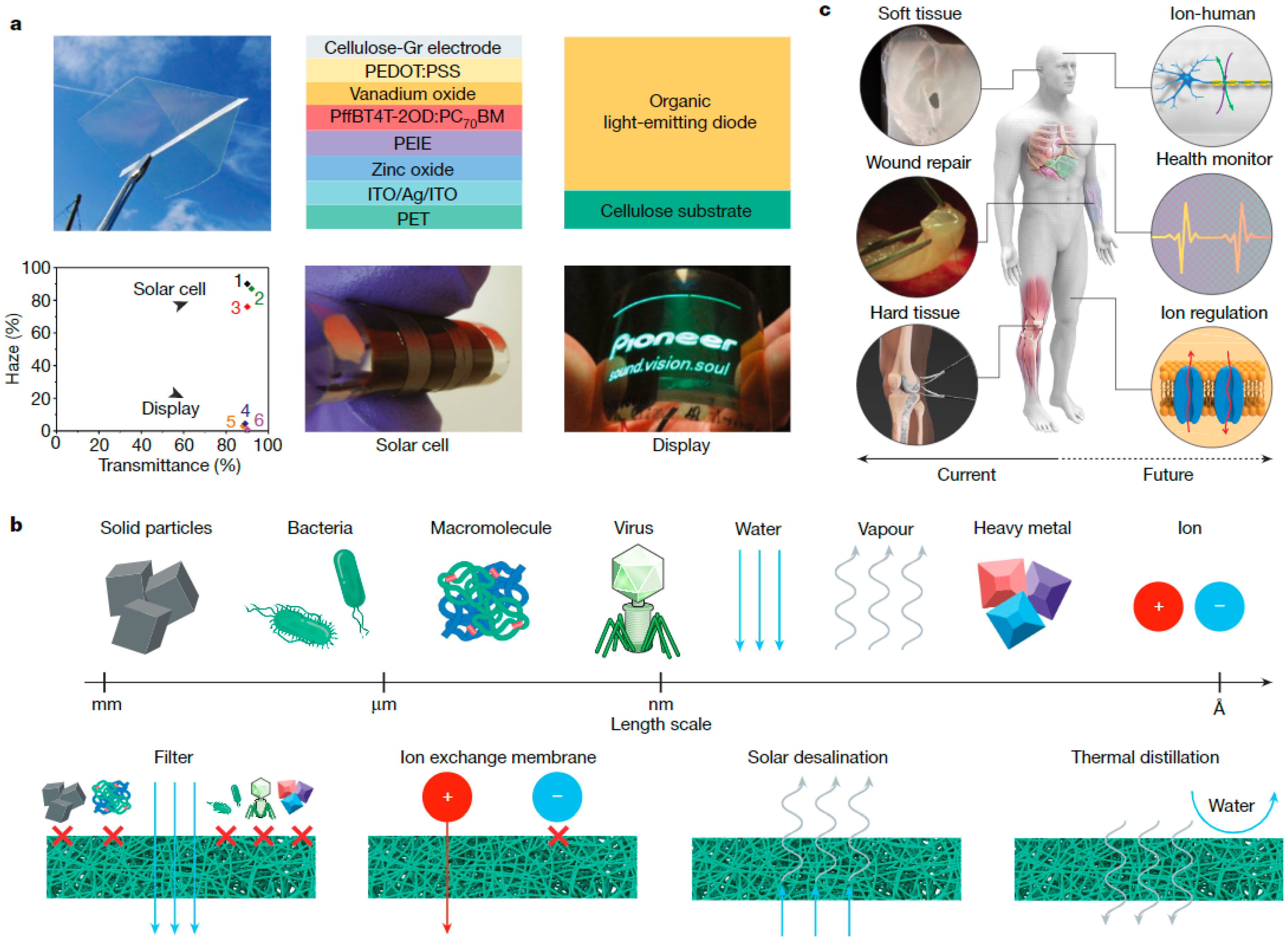
4. Emerging Applications and Future Development
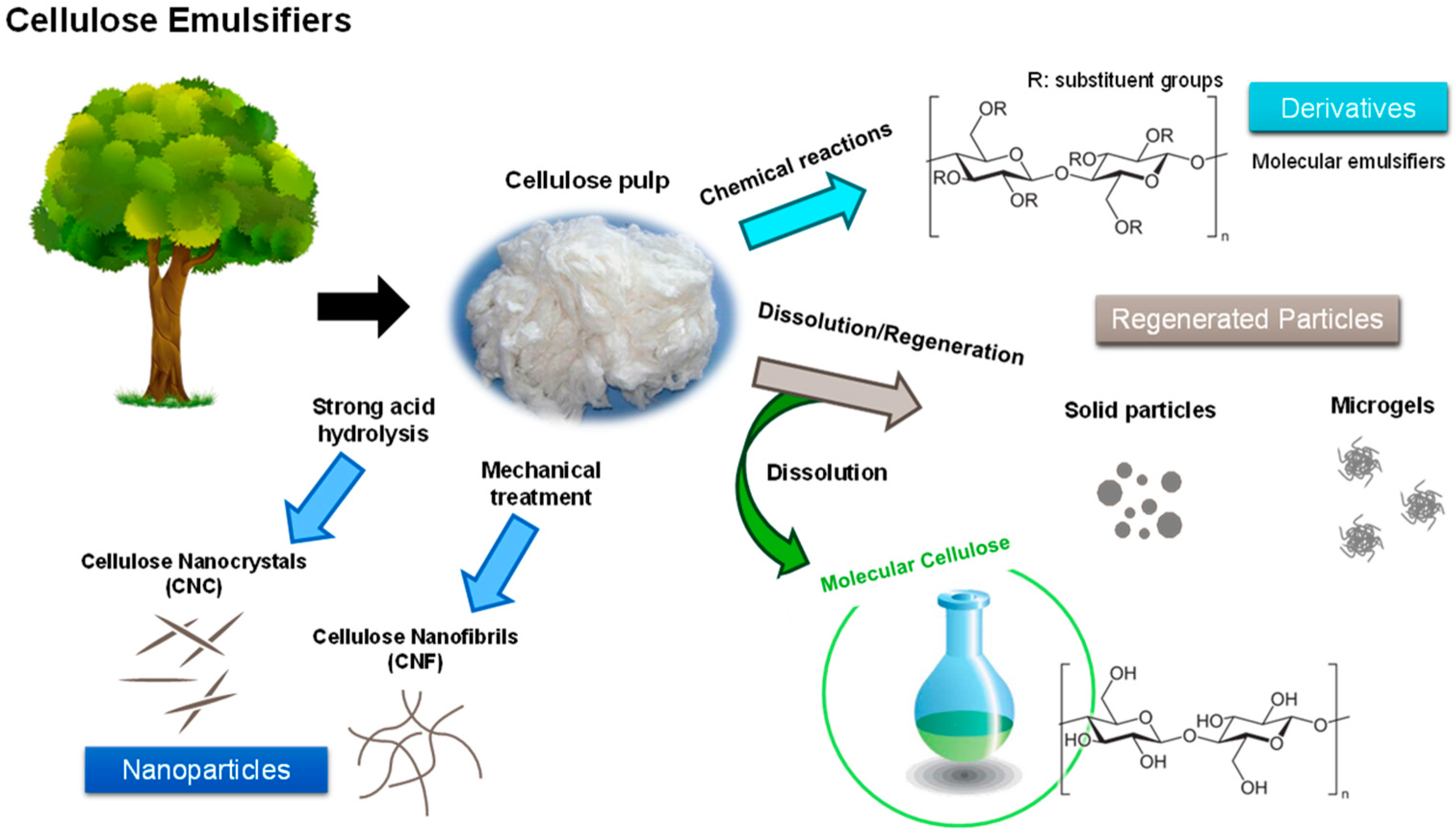
4.1. Nanocellulose in Material Design
4.2. Regenerated Cellulose with Added Functionalities
4.3. Cellulose in Electrical Applications
4.3.1. Energy Storage—Supercapacitors and Batteries
4.3.2. Triboelectric Nanogenerators
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindman, B.; Karlström, G.; Stigsson, L. On the Mechanism of Dissolution of Cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing Cellulose (in) Solubility: Reviewing Basic Physicochemical Aspects and Role of Hydrophobic Interactions. Cellulose 2012, 19, 581–587. [Google Scholar] [CrossRef]
- Glasser, W.G.; Atalla, R.H.; Blackwell, J.; Malcolm Brown, R.; Burchard, W.; French, A.D.; Klemm, D.O.; Nishiyama, Y. About the Structure of Cellulose: Debating the Lindman Hypothesis. Cellulose 2012, 19, 589–598. [Google Scholar] [CrossRef]
- Brown, R.M.; Montezinos, D. Cellulose Microfibrils: Visualization of Biosynthetic and Orienting Complexes in Association with the Plasma Membrane. Proc. Natl. Acad. Sci. USA 1976, 73, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Giddings, T.H.; Brower, D.L.; Staehelin, L.A. Visualization of Particle Complexes in the Plasma Membrane of Micrasterias Denticulata Associated with the Formation of Cellulose Fibrils in Primary and Secondary Cell Walls. J. Cell Biol. 1980, 84, 327–339. [Google Scholar] [CrossRef]
- Doblin, M.S.; Kurek, I.; Jacob-Wilk, D.; Delmer, D.P. Cellulose Biosynthesis in Plants: From Genes to Rosettes. Plant Cell Physiol. 2002, 43, 1407–1420. [Google Scholar] [CrossRef]
- Saxena, I.M.; Brown, R.M. Cellulose Biosynthesis: Current Views and Evolving Concepts. Ann. Bot. 2005, 96, 9–21. [Google Scholar] [CrossRef]
- Haigler, C.H.; Brown, R.M.; Benziman, M. Calcofluor White ST Alters the in Vivo Assembly of Cellulose Microfibrils. Science 1980, 210, 903–906. [Google Scholar] [CrossRef]
- Cousins, S.K.; Brown, R.M. Cellulose I Microfibril Assembly: Computational Molecular Mechanics Energy Analysis Favours Bonding by van Der Waals Forces as the Initial Step in Crystallization. Polymer 1995, 36, 3885–3888. [Google Scholar] [CrossRef]
- Cousins, S.K.; Brown, R.M. X-Ray Diffraction and Ultrastructural Analyses of Dye-Altered Celluloses Support van Der Waals Forces as the Initial Step in Cellulose Crystallization. Polymer 1997, 38, 897–902. [Google Scholar] [CrossRef]
- Cousins, S.K.; Brown, R.M. Photoisomerization of a Dye-Altered β-1,4 Glucan Sheet Induces the Crystallization of a Cellulose-Composite. Polymer 1997, 38, 903–912. [Google Scholar] [CrossRef]
- McCorsley, C.C. Process for Shaped Cellulose Article Prepared from a Solution Containing Cellulose Dissolved in a Tertiary Amine N-Oxide Solvent. U.S. Patent nº 4246221, 20 January 1981. [Google Scholar]
- Medronho, B.; Lindman, B. Competing Forces during Cellulose Dissolution: From Solvents to Mechanisms. Curr. Opin. Colloid Interface Sci. 2014, 19, 32–40. [Google Scholar] [CrossRef]
- Medronho, B.; Lindman, B. Brief Overview on Cellulose Dissolution/Regeneration Interactions and Mechanisms. Adv. Colloid Interface Sci. 2015, 222, 502–508. [Google Scholar] [CrossRef]
- Heinze, T.; Koschella, A. Solvents Applied in the Field of Cellulose Chemistry: A Mini Review. Polímeros 2005, 15, 84–90. [Google Scholar] [CrossRef]
- Budtova, T.; Navard, P. Cellulose in NaOH–Water Based Solvents: A Review. Cellulose 2016, 23, 5–55. [Google Scholar] [CrossRef]
- Nägeli, C. Über Den Inneren Bau Der Vegetabilischen Zellmembranen. Sitzber Bay Akad. Wiss. München 1984, 1, 282–323. [Google Scholar]
- Pennetier, G. Note Micrographique Sur Les Altérations Du Cotton. Bull. Soc. Ind. Rouen. 1883, 11, 235–237. [Google Scholar]
- Fleming, N.; Thaysen, A.C. On the Deterioration of Cotton on Wet Storage. Biochem. J. 1921, 15, 407–414.1. [Google Scholar] [CrossRef] [PubMed]
- Fleming, N.; Thaysen, A.C. On the Deterioration of Cotton on Wet Storage. Biochem. J. 1920, 14, 25–28.1. [Google Scholar] [CrossRef]
- Tripp, V.W.; Rollins, M.L. Morphology and Chemical Composition of Certain Components of Cotton Fiber Cell Wall. Anal. Chem. 1952, 24, 1721–1728. [Google Scholar] [CrossRef]
- Hock, C.W. Microscopic structure. In Cellulose and Cellulose Derivatives (Part 1); Ott, E., Spurlin, H.M., Grafflin, M.W., Eds.; Interscience: New York, NY, USA, 1954; pp. 347–392. [Google Scholar]
- Warwicker, J.O.; Jeffries, R.; Colbran, R.L.; Robinson, R.N. A review of the literature on the effect of caustic soda and other swelling agents on the fine structure of cotton. In Pamphlet 93 Shirley Inst.; Cotton, Silk and Man-made Fibres Research Association: Manchester, UK, 1966; p. 247. [Google Scholar]
- Chanzy, H.; Noe, P.; Paillet, M.; Smith, P. Swelling and Dissolution of Cellulose in Amineoxide/Water System. J. Appl. Polym. Sci. 1983, 37, 239–259. [Google Scholar]
- Cuissinat, C.; Navard, P. Swelling and Dissolution of Cellulose Part 1: Free Floating Cotton and Wood Fibres in N-Methylmorpholine-N-Oxide–Water Mixtures. Macromol. Symp. 2006, 244, 1–18. [Google Scholar] [CrossRef]
- Neale, S.M. 30—The Swelling of Cellulose, and its Affinity Relations With Aqueous Solutions. Part I—Experiments on the Behaviour of Cotton Cellulose and Regenerated Cellulose in Sodium Hydroxide Solution, and their Theoretical Interpretation. J. Text. Inst. Trans. 1929, 20, T373–T400. [Google Scholar] [CrossRef]
- Lindström, T.; Carlsson, G. The Effect of Carboxyl Groups and Their Ionic Form during Drying on the Hornification of Cellulose Fibers [PH, Paper Properties, Tensile Strength, Swelling]. Sven. Papperstidning 1983, 85, r146–r151. [Google Scholar]
- Scallan, A.M. The Effect of Acidic Groups on the Swelling of Pulps: A Review. Tappi J. 1983, 66, 73–75. [Google Scholar]
- Katz, S.; Liebergott, N.; Scallan, A.M. A Mechanism for the Alkali Strengthening of Mechanical Pulps. TAPPI J. Tech. Assoc. Pulp Pap. Ind. 1981, 64, 97–100. [Google Scholar]
- Katz, S.; Scallan, A.M. Ozone and Caustic Soda Treatments of Mechanical Pulp. Tappi J. 1983, 66, 85–87. [Google Scholar]
- Zhang, S.; Wang, W.; Li, F.; Yu, J. Swelling and dissolution of cellulose in NaOH aqueous solvent systems. Cell. Chem. Technol. 2013, 47, 671–679. [Google Scholar]
- Ehrhardt, A.; Groner, S.; Bechtold, T. Swelling Behavior of Cellulosic Fibers—Part I: Changes in Physical Properties. Fibres Text. East. Eur. 2007, 15, 46–48. [Google Scholar]
- Mahmud-Ali, A.; Bechtold, T. Aqueous Thiocyanate–Urea Solution as a Powerful Non-Alkaline Swelling Agent for Cellulose Fibres. Carbohydr. Polym. 2015, 116, 124–130. [Google Scholar] [CrossRef]
- Forest Products Laboratory (U.S.). Forest Products Laboratory Urea-Plasticized Wood (Uralloy); U.S. Dept. Laboratory, Forest Service, Forest Products Laboratory, Madison, Wisconsin in Cooperation with the University of Wisconsin Forest Products: Madison, WI, USA, 1943. [Google Scholar]
- Brown, W.F. Vulcanized Fibre—An Old Material with a New Relevancy. In Proceedings of the Electrical Insulation Conference and Electrical Manufacturing and Coil Winding Conference, Cincinnati, OH, USA, 28 October 1999. [Google Scholar]
- Halonen, H. Structural Changes during Cellulose Composite Processing. Ph.D. Thesis, KTH, Stockholm, Sweden, 2012. [Google Scholar]
- Künne, B.; Dumke, D. Vulcanized Fiber as a High–Strength Construction Material for Highly Loaded Construction Units. In Proceedings of the International Paper Physics Conference & 8th International Paper and Coating Chemistry, Stockholm, Sweden, 10–14 June 2012. [Google Scholar]
- Piltonen, P.; Hildebrandt, N.C.; Westerlind, B.; Valkama, J.-P.; Tervahartiala, T.; Illikainen, M. Green and Efficient Method for Preparing All-Cellulose Composites with NaOH/Urea Solvent. Compos. Sci. Technol. 2016, 135, 153–158. [Google Scholar] [CrossRef]
- Baghaei, B.; Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Arimoto, N.; Nishino, T.; Peijs, T. All-Cellulose Composites by Surface Selective Dissolution of Aligned Ligno-Cellulosic Fibres. Compos. Sci. Technol. 2008, 68, 2201–2207. [Google Scholar] [CrossRef]
- Nishino, T.; Arimoto, N. All-Cellulose Composite Prepared by Selective Dissolving of Fiber Surface. Biomacromolecules 2007, 8, 2712–2716. [Google Scholar] [CrossRef]
- Sjöström, E. Wood Chemistry, Fundamentals and Applications, 2nd ed.; Press, A., Ed.; Elsevier: San Diego, CA, USA, 1993; ISBN 9780080925899. [Google Scholar]
- Eklund, D.; Lindström, T. Paper Chemistry: An Introduction, 1st ed.; DT Paper Science Publications: Grankulla, Finland, 1991. [Google Scholar]
- Wohlert, M.; Benselfelt, T.; Wågberg, L.; Furó, I.; Berglund, L.A.; Wohlert, J. Cellulose and the Role of Hydrogen Bonds: Not in Charge of Everything. Cellulose 2022, 29, 1–23. [Google Scholar] [CrossRef]
- Bodvik, R.; Dedinaite, A.; Karlson, L.; Bergström, M.; Bäverbäck, P.; Pedersen, J.S.; Edwards, K.; Karlsson, G.; Varga, I.; Claesson, P.M. Aggregation and Network Formation of Aqueous Methylcellulose and Hydroxypropylmethylcellulose Solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 354, 162–171. [Google Scholar] [CrossRef]
- Zhang, L.; Ruan, D.; Gao, S. Dissolution and Regeneration of Cellulose in NaOH/Thiourea Aqueous Solution. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1521–1529. [Google Scholar] [CrossRef]
- Isogai, A.; Atalla, R.H. Dissolution of Cellulose in Aqueous NaOH Solutions. Cellulose 1998, 5, 309–319. [Google Scholar] [CrossRef]
- Cao, N.-J.; Xu, Q.; Chen, C.-S.; Gong, C.S.; Chen, L.F. Cellulose Hydrolysis Using Zinc Chloride as a Solvent and Catalyst. Appl. Biochem. Biotechnol. 1994, 45–46, 521–530. [Google Scholar] [CrossRef]
- Åstrand, P.-O.; Karlström, G.; Engdahl, A.; Nelander, B. Novel Model for Calculating the Intermolecular Part of the Infrared Spectrum for Molecular Complexes. J. Chem. Phys. 1995, 102, 3534–3554. [Google Scholar] [CrossRef]
- Shinoda, K. “Iceberg” Formation and Solubility. J. Phys. Chem. 1977, 81, 1300–1302. [Google Scholar] [CrossRef]
- Kihlman, M.; Medronho, B.F.; Romano, A.L.; Germgard, U.; Lindman, B. Cellulose Dissolution in an Alkali Based Solvent: Influence of Additives and Pretreatments. J. Braz. Chem. Soc. 2013, 24, 295–303. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L. Solubility of Cellulose in NaOH/Urea Aqueous Solution. Polym. J. 2000, 32, 866–870. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Rapid Dissolution of Cellulose in LiOH/Urea and NaOH/Urea Aqueous Solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef]
- Kronberg, B. The Hydrophobic Effect. Curr. Opin. Colloid Interface Sci. 2016, 22, 14–22. [Google Scholar] [CrossRef]
- Biermann, O.; Hädicke, E.; Koltzenburg, S.; Müller-Plathe, F. Hydrophilicity and Lipophilicity of Cellulose Crystal Surfaces. Angew. Chemie Int. Ed. 2001, 40, 3822–3825. [Google Scholar] [CrossRef]
- Yamane, C.; Aoyagi, T.; Ago, M.; Sato, K.; Okajima, K.; Takahashi, T. Two Different Surface Properties of Regenerated Cellulose Due to Structural Anisotropy. Polym. J. 2006, 38, 819–826. [Google Scholar] [CrossRef]
- Miyamoto, H.; Umemura, M.; Aoyagi, T.; Yamane, C.; Ueda, K.; Takahashi, K. Structural Reorganization of Molecular Sheets Derived from Cellulose II by Molecular Dynamics Simulations. Carbohydr. Res. 2009, 344, 1085–1094. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Haigler, C.H.; White, A.R.; Brown, R.M.; Cooper, K.M. Alteration of in Vivo Cellulose Ribbon Assembly by Carboxymethylcellulose and Other Cellulose Derivatives. J. Cell Biol. 1982, 94, 64–69. [Google Scholar] [CrossRef]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef]
- Lindman, B.; Medronho, B.; Alves, L.; Norgren, M.; Nordenskiöld, L. Hydrophobic Interactions Control the Self-Assembly of DNA and Cellulose. Q. Rev. Biophys. 2021, 54, e3. [Google Scholar] [CrossRef]
- Lindman, B.; Medronho, B.; Alves, L.; Costa, C.; Edlund, H.; Norgren, M. The Relevance of Structural Features of Cellulose and Its Interactions to Dissolution, Regeneration, Gelation and Plasticization Phenomena. Phys. Chem. Chem. Phys. 2017, 19, 23704–23718. [Google Scholar] [CrossRef]
- Despa, F.; Berry, R.S. Hydrophobe-Water Interactions: Methane as a Model. Biophys. J. 2008, 95, 4241–4245. [Google Scholar] [CrossRef]
- Stephenson, B.C.; Goldsipe, A.; Beers, K.J.; Blankschtein, D. Quantifying the Hydrophobic Effect. 1. A Computer Simulation−Molecular-Thermodynamic Model for the Self-Assembly of Hydrophobic and Amphiphilic Solutes in Aqueous Solution. J. Phys. Chem. B 2007, 111, 1025–1044. [Google Scholar] [CrossRef]
- Stock, P.; Monroe, J.I.; Utzig, T.; Smith, D.J.; Shell, M.S.; Valtiner, M. Unraveling Hydrophobic Interactions at the Molecular Scale Using Force Spectroscopy and Molecular Dynamics Simulations. ACS Nano 2017, 11, 2586–2597. [Google Scholar] [CrossRef]
- Elder, R.M.; Pfaendtner, J.; Jayaraman, A. Effect of Hydrophobic and Hydrophilic Surfaces on the Stability of Double-Stranded DNA. Biomacromolecules 2015, 16, 1862–1869. [Google Scholar] [CrossRef]
- Mancera, R.L. Computer Simulation of the Effect of Salt on the Hydrophobic Effect. J. Chem. Soc. Faraday Trans. 1998, 94, 3549–3559. [Google Scholar] [CrossRef]
- Bergenstråhle, M.; Wohlert, J.; Himmel, M.E.; Brady, J.W. Simulation Studies of the Insolubility of Cellulose. Carbohydr. Res. 2010, 345, 2060–2066. [Google Scholar] [CrossRef]
- Nishiyama, Y. Molecular Interactions in Nanocellulose Assembly. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170047. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Bioni, T.A.; Dignani, M.T. Understanding Cellulose Dissolution in Ionic Liquid-Dimethyl Sulfoxide Binary Mixtures: Quantification of the Relative Importance of Hydrogen Bonding and Hydrophobic Interactions. J. Mol. Liq. 2021, 322, 114848. [Google Scholar] [CrossRef]
- Medronho, B.; Duarte, H.; Alves, L.; Antunes, F.; Romano, A.; Lindman, B. Probing Cellulose Amphiphilicity. Nord. Pulp Pap. Res. J. 2015, 30, 58–66. [Google Scholar] [CrossRef]
- Alves, L.C.H. Cellulose Solutions: Dissolution, Regeneration, Solution Structure and Molecular Interactions. Ph.D. Thesis, University of Coimbra, Coimbra, Portugal, 2015. [Google Scholar]
- Costa, C.; Medronho, B.; Eivazi, A.; Svanedal, I.; Lindman, B.; Edlund, H.; Norgren, M. Lignin Enhances Cellulose Dissolution in Cold Alkali. Carbohydr. Polym. 2021, 274, 118661. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Medronho, B.F.; Antunes, F.E.; Romano, A.; Miguel, M.G.; Lindman, B. On the Role of Hydrophobic Interactions in Cellulose Dissolution and Regeneration: Colloidal Aggregates and Molecular Solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 483, 257–263. [Google Scholar] [CrossRef]
- Abe, M.; Fukaya, Y.; Ohno, H. Fast and Facile Dissolution of Cellulose with Tetrabutylphosphonium Hydroxide Containing 40 Wt% Water. Chem. Commun. 2012, 48, 1808. [Google Scholar] [CrossRef]
- Abe, M.; Kuroda, K.; Ohno, H. Maintenance-Free Cellulose Solvents Based on Onium Hydroxides. ACS Sustain. Chem. Eng. 2015, 3, 1771–1776. [Google Scholar] [CrossRef]
- Ema, T.; Komiyama, T.; Sunami, S.; Sakai, T. Synergistic Effect of Quaternary Ammonium Hydroxide and Crown Ether on the Rapid and Clear Dissolution of Cellulose at Room Temperature. RSC Adv. 2014, 4, 2523–2525. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Filipe, A.; Antunes, F.E.; Lindman, B.; Topgaard, D.; Davidovich, I.; Talmon, Y. New Insights on the Role of Urea on the Dissolution and Thermally-Induced Gelation of Cellulose in Aqueous Alkali. Gels 2018, 4, 87. [Google Scholar] [CrossRef]
- Pereira, A.; Duarte, H.; Nosrati, P.; Gubitosi, M.; Gentile, L.; Romano, A.; Medronho, B.; Olsson, U. Cellulose Gelation in NaOH Solutions Is Due to Cellulose Crystallization. Cellulose 2018, 25, 3205–3210. [Google Scholar] [CrossRef]
- Medronho, B.; Pereira, A.; Duarte, H.; Gentile, L.; Costa, A.; Romano, A.; Olsson, U. Probing Cellulose-Solvent Interactions with Self-Diffusion NMR: Onium Hydroxide Concentration and Co-Solvent Effects. Carbohydr. Polym. 2023, 303, 120440. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, P.; Hu, K.; Zhang, L.; Cheng, G. Dissolution of Cellulose in Aqueous NaOH/Urea Solution: Role of Urea. Cellulose 2014, 21, 1183–1192. [Google Scholar] [CrossRef]
- Swensson, B.; Larsson, A.; Hasani, M. Probing Interactions in Combined Hydroxide Base Solvents for Improving Dissolution of Cellulose. Polymers 2020, 12, 1310. [Google Scholar] [CrossRef]
- Isobe, N.; Noguchi, K.; Nishiyama, Y.; Kimura, S.; Wada, M.; Kuga, S. Role of Urea in Alkaline Dissolution of Cellulose. Cellulose 2013, 20, 97–103. [Google Scholar] [CrossRef]
- Wang, S.; Sun, P.; Liu, M.; Lu, A.; Zhang, L. Weak Interactions and Their Impact on Cellulose Dissolution in an Alkali/Urea Aqueous System. Phys. Chem. Chem. Phys. 2017, 19, 17909–17917. [Google Scholar] [CrossRef]
- Gustavsson, S.; Alves, L.; Lindman, B.; Topgaard, D. Polarization Transfer Solid-State NMR: A New Method for Studying Cellulose Dissolution. RSC Adv. 2014, 4, 31836–31839. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Antunes, F.E.; Topgaard, D.; Lindman, B. Dissolution State of Cellulose in Aqueous Systems. 1. Alkaline Solvents. Cellulose 2016, 23, 247–258. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Antunes, F.E.; Topgaard, D.; Lindman, B. Dissolution State of Cellulose in Aqueous Systems. 2. Acidic Solvents. Carbohydr. Polym. 2016, 151, 707–715. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Filipe, A.; Romano, A.; Rasteiro, M.G.; Lindman, B.; Topgaard, D.; Davidovich, I.; Talmon, Y. Revisiting the Dissolution of Cellulose in H3PO4(Aq) through Cryo-TEM, PTssNMR and DWS. Carbohydr. Polym. 2021, 252, 117122. [Google Scholar] [CrossRef]
- Medronho, B.; Duarte, H.; Alves, L.; Antunes, F.E.; Romano, A.; Valente, A.J.M. The Role of Cyclodextrin-Tetrabutylammonium Complexation on the Cellulose Dissolution. Carbohydr. Polym. 2016, 140, 136–143. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Antunes, F.E.; Fernandez-Garcia, M.P.; Ventura, J.; Araujo, J.P.; Romano, A.; Lindman, B. Unusual Extraction and Characterization of Nanocrystalline Cellulose from Cellulose Derivatives. J. Mol. Liq. 2015, 210, 106–112. [Google Scholar] [CrossRef]
- Malaspina, D.C.; Faraudo, J. Molecular Insight into the Wetting Behavior and Amphiphilic Character of Cellulose Nanocrystals. Adv. Colloid Interface Sci. 2019, 267, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Kimura, S.; Wada, M.; Kuga, S. Mechanism of Cellulose Gelation from Aqueous Alkali-Urea Solution. Carbohydr. Polym. 2012, 89, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
- Bialik, E.; Stenqvist, B.; Fang, Y.; Östlund, Å.; Furó, I.; Lindman, B.; Lund, M.; Bernin, D. Ionization of Cellobiose in Aqueous Alkali and the Mechanism of Cellulose Dissolution. J. Phys. Chem. Lett. 2016, 7, 5044–5048. [Google Scholar] [CrossRef]
- Gentile, L.; Olsson, U. Cellulose–Solvent Interactions from Self-Diffusion NMR. Cellulose 2016, 23, 2753–2758. [Google Scholar] [CrossRef]
- Behrens, M.A.; Holdaway, J.A.; Nosrati, P.; Olsson, U. On the Dissolution State of Cellulose in Aqueous Tetrabutylammonium Hydroxide Solutions. RSC Adv. 2016, 6, 30199–30204. [Google Scholar] [CrossRef]
- Gubitosi, M.; Duarte, H.; Gentile, L.; Olsson, U.; Medronho, B. On Cellulose Dissolution and Aggregation in Aqueous Tetrabutylammonium Hydroxide. Biomacromolecules 2016, 17, 2873–2881. [Google Scholar] [CrossRef]
- Medronho, B.; Duarte, H.; Magalhães, S.; Alves, L.; Valente, A.J.M.; Romano, A. From a New Cellulose Solvent to the Cyclodextrin Induced Formation of Hydrogels. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 532, 548–555. [Google Scholar] [CrossRef]
- Kostag, M.; Gericke, M.; Heinze, T.; El Seoud, O.A. Twenty-Five Years of Cellulose Chemistry: Innovations in the Dissolution of the Biopolymer and Its Transformation into Esters and Ethers. Cellulose 2019, 26, 139–184. [Google Scholar] [CrossRef]
- Idström, A.; Gentile, L.; Gubitosi, M.; Olsson, C.; Stenqvist, B.; Lund, M.; Bergquist, K.-E.; Olsson, U.; Köhnke, T.; Bialik, E. On the Dissolution of Cellulose in Tetrabutylammonium Acetate/Dimethyl Sulfoxide: A Frustrated Solvent. Cellulose 2017, 24, 3645–3657. [Google Scholar] [CrossRef]
- Casarano, R.; Pires, P.A.R.; Borin, A.C.; Seoud, O.A. El Novel Solvents for Cellulose: Use of Dibenzyldimethylammonium Fluoride/Dimethyl Sulfoxide (DMSO) as Solvent for the Etherification of the Biopolymer and Comparison with Tetra(1-Butyl)Ammonium Fluoride/DMSO. Ind. Crop. Prod. 2014, 54, 185–191. [Google Scholar] [CrossRef]
- Heinze, T.; Dicke, R.; Koschella, A.; Kull, A.H.; Klohr, E.-A.; Koch, W. Effective Preparation of Cellulose Derivatives in a New Simple Cellulose Solvent. Macromol. Chem. Phys. 2000, 201, 627–631. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Xin, P.-P.; Li, J.-X.; Shao, Y.-Y.; Huang, C.-B.; Pan, H. Room-Temperature Dissolution and Mechanistic Investigation of Cellulose in a Tetra-Butylammonium Acetate/Dimethyl Sulfoxide System. ACS Sustain. Chem. Eng. 2016, 4, 2286–2294. [Google Scholar] [CrossRef]
- Jiang, Z.; Miao, J.; Yu, Y.; Zhang, L. Effective Preparation of Bamboo Cellulose Fibers in Quaternary Ammonium/DMSO Solvent. BioResources 2016, 11, 4536–4549. [Google Scholar] [CrossRef]
- Kostag, M.; Liebert, T.; El Seoud, O.A.; Heinze, T. Efficient Cellulose Solvent: Quaternary Ammonium Chlorides. Macromol. Rapid Commun. 2013, 34, 1580–1584. [Google Scholar] [CrossRef]
- Miao, J.; Sun, H.; Yu, Y.; Song, X.; Zhang, L. Quaternary Ammonium Acetate: An Efficient Ionic Liquid for the Dissolution and Regeneration of Cellulose. RSC Adv. 2014, 4, 36721. [Google Scholar] [CrossRef]
- Ren, F.; Wang, J.; Yu, J.; Zhong, C.; Xie, F.; Wang, S. Dissolution of Cellulose in Ionic Liquid–DMSO Mixtures: Roles of DMSO/IL Ratio and the Cation Alkyl Chain Length. ACS Omega 2021, 6, 27225–27232. [Google Scholar] [CrossRef]
- Rinaldi, R. Instantaneous Dissolution of Cellulose in Organic Electrolyte Solutions. Chem. Commun. 2011, 47, 511–513. [Google Scholar] [CrossRef]
- Sun, H.; Miao, J.; Yu, Y.; Zhang, L. Dissolution of Cellulose with a Novel Solvent and Formation of Regenerated Cellulose Fiber. Appl. Phys. A 2015, 119, 539–546. [Google Scholar] [CrossRef]
- Bioni, T.; Arêas, E.; Couto, L.; Favarin, G.; El Seoud, O. Dissolution of Cellulose in Mixtures of Ionic Liquid and Molecular Solvents: Relevance of Solvent-Solvent and Cellulose-Solvent Interactions. Nord. Pulp Pap. Res. J. 2015, 30, 105–111. [Google Scholar] [CrossRef]
- Andanson, J.-M.; Bordes, E.; Devémy, J.; Leroux, F.; Pádua, A.A.H.; Gomes, M.F.C. Understanding the Role of Co-Solvents in the Dissolution of Cellulose in Ionic Liquids. Green Chem. 2014, 16, 2528. [Google Scholar] [CrossRef]
- Lethesh, K.C.; Evjen, S.; Venkatraman, V.; Shah, S.N.; Fiksdahl, A. Highly Efficient Cellulose Dissolution by Alkaline Ionic Liquids. Carbohydr. Polym. 2020, 229, 115594. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Tang, J.; Lan, A.; Yang, Y.; Gibril, M.; Yu, M. Efficient Preparation of High Concentration Cellulose Solution with Complex DMSO/ILs Solvent. J. Polym. Res. 2016, 23, 32. [Google Scholar] [CrossRef]
- Wen, Y.-C.; Kuo, H.-C.; Jia, H.-W. Multinuclear NMR Spectroscopy for Differentiation of Molecular Configurations and Solvent Properties between Acetone and Dimethyl Sulfoxide. J. Mol. Struct. 2016, 1109, 154–160. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Ionic Liquids and Deep Eutectic Solvents in Natural Products Research: Mixtures of Solids as Extraction Solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Zhang, X.; You, T.-T.; Xu, F. Deep Eutectic Solvents (DESs) for Cellulose Dissolution: A Mini-Review. Cellulose 2019, 26, 205–213. [Google Scholar] [CrossRef]
- Costa, C.; Medronho, B.; Lindman, B.; Edlund, H.; Norgren, M. Cellulose as a Natural Emulsifier: From Nanocelluloses to Macromolecules. In Cellulose Science and Derivatives; IntechOpen: London, UK, 2021. [Google Scholar]
- Costa; Medronho; Filipe; Mira; Lindman; Edlund; Norgren Emulsion Formation and Stabilization by Biomolecules: The Leading Role of Cellulose. Polymers 2019, 11, 1570. [CrossRef]
- Costa, C.; Medronho, B.; Filipe, A.; Romano, A.; Lindman, B.; Edlund, H.; Norgren, M. On the Formation and Stability of Cellulose-Based Emulsions in Alkaline Systems: Effect of the Solvent Quality. Carbohydr. Polym. 2022, 286, 119257. [Google Scholar] [CrossRef]
- Costa, C.; Mira, I.; Benjamins, J.-W.; Lindman, B.; Edlund, H.; Norgren, M. Interfacial Activity and Emulsion Stabilization of Dissolved Cellulose. J. Mol. Liq. 2019, 292, 111325. [Google Scholar] [CrossRef]
- Costa, C.; Rosa, P.; Filipe, A.; Medronho, B.; Romano, A.; Liberman, L.; Talmon, Y.; Norgren, M. Cellulose-Stabilized Oil-in-Water Emulsions: Structural Features, Microrheology, and Stability. Carbohydr. Polym. 2021, 252, 117092. [Google Scholar] [CrossRef]
- Medronho, B.; Filipe, A.; Costa, C.; Romano, A.; Lindman, B.; Edlund, H.; Norgren, M. Microrheology of Novel Cellulose Stabilized Oil-in-Water Emulsions. J. Colloid Interface Sci. 2018, 531, 225–232. [Google Scholar] [CrossRef]
- Miyamoto, H.; Rein, D.M.; Ueda, K.; Yamane, C.; Cohen, Y. Molecular Dynamics Simulation of Cellulose-Coated Oil-in-Water Emulsions. Cellulose 2017, 24, 2699–2711. [Google Scholar] [CrossRef]
- Napso, S.; Rein, D.M.; Fu, Z.; Radulescu, A.; Cohen, Y. Structural Analysis of Cellulose-Coated Oil-in-Water Emulsions Fabricated from Molecular Solution. Langmuir 2018, 34, 8857–8865. [Google Scholar] [CrossRef]
- Rein, D.M.; Khalfin, R.; Cohen, Y. Cellulose as a Novel Amphiphilic Coating for Oil-in-Water and Water-in-Oil Dispersions. J. Colloid Interface Sci. 2012, 386, 456–463. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic Nanorods of Various Aspect Ratios for Oil in Water Pickering Emulsions. Soft Matter 2013, 9, 952–959. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering Emulsions Stabilized by Bacterial Cellulose Nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of Cellulose Nanocrystals Amphiphilic Properties to Stabilize Oil/Water Interface. Biomacromolecules 2012, 13, 267–275. [Google Scholar] [CrossRef]
- Lefroy, K.S.; Murray, B.S.; Ries, M.E.; Curwen, T.D. A Natural, Cellulose-Based Microgel for Water-in-Oil Emulsions. Food Hydrocoll. 2021, 113, 106408. [Google Scholar] [CrossRef]
- O’sullivan, A.C. Cellulose: The Structure Slowly Unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- From, M.; Larsson, P.T.; Andreasson, B.; Medronho, B.; Svanedal, I.; Edlund, H.; Norgren, M. Tuning the Properties of Regenerated Cellulose: Effects of Polarity and Water Solubility of the Coagulation Medium. Carbohydr. Polym. 2020, 236, 116068. [Google Scholar] [CrossRef]
- Yang, Q.; Fukuzumi, H.; Saito, T.; Isogai, A.; Zhang, L. Transparent Cellulose Films with High Gas Barrier Properties Fabricated from Aqueous Alkali/Urea Solutions. Biomacromolecules 2011, 12, 2766–2771. [Google Scholar] [CrossRef] [PubMed]
- Dahlström, C.; Eivazi, A.; Nejström, M.; Zhang, R.; Iftikhar, H.; Rojas, O.; Medronho, B.; Norgren, M. Effects of Film Microstructure on the Triboelectric Performance of Regenerated Cellulose. ACS Appl. Mater. Interfaces. unpublished.
- Yamane, C. Structure Formation of Regenerated Cellulose from Its Solution and Resultant Features of High Wettability: A Review. Nord. Pulp Pap. Res. J. 2015, 30, 78–91. [Google Scholar] [CrossRef]
- EPNOE Research Roadmap 2040. Available online: https://www.epnoe.eu/discover/research-roadmap/ (accessed on 15 May 2023).
- Wang, J.; Wang, L.; Gardner, D.J.; Shaler, S.M.; Cai, Z. Towards a Cellulose-Based Society: Opportunities and Challenges. Cellulose 2021, 28, 4511–4543. [Google Scholar] [CrossRef]
- Su, Y.; Yang, B.; Liu, J.; Sun, B.; Cao, C.; Zou, X.; Lutes, R.; He, Z. Prospects for Replacement of Some Plastics in Packaging with Lignocellulose Materials: A Brief Review. BioResources 2018, 13, 4550–4576. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent Advances in Regenerated Cellulose Materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Yang, Q.; Saito, T.; Isogai, A. Facile Fabrication of Transparent Cellulose Films with High Water Repellency and Gas Barrier Properties. Cellulose 2012, 19, 1913–1921. [Google Scholar] [CrossRef]
- Wu, R.-L.; Wang, X.-L.; Li, F.; Li, H.-Z.; Wang, Y.-Z. Green Composite Films Prepared from Cellulose, Starch and Lignin in Room-Temperature Ionic Liquid. Bioresour. Technol. 2009, 100, 2569–2574. [Google Scholar] [CrossRef]
- Shih, C.-M.; Shieh, Y.-T.; Twu, Y.-K. Preparation and Characterization of Cellulose/Chitosan Blend Films. Carbohydr. Polym. 2009, 78, 169–174. [Google Scholar] [CrossRef]
- Sixta, H.; Michud, A.; Hauru, L.; Asaadi, S.; Ma, Y.; King, A.W.T.; Kilpeläinen, I.; Hummel, M. Ioncell-F: A High-Strength Regenerated Cellulose Fibre. Nord. Pulp Pap. Res. J. 2015, 30, 43–57. [Google Scholar] [CrossRef]
- Sixta, H.; Hummel, M.; Le Boulch, K.; Kilpeläinen, I.; King, A.W.T.; Helminen, J.; Hellsten, S. Process for Making Cellulose Fibre or Film. U.S. Patent 11,549,200 B2, 10 June 2023. [Google Scholar]
- Rånby, B.G.; Banderet, A.; Sillén, L.G. Aqueous Colloidal Solutions of Cellulose Micelles. Acta Chem. Scand. 1949, 3, 649–650. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chemie Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Benselfelt, T.; Kummer, N.; Nordenström, M.; Fall, A.B.; Nyström, G.; Wågberg, L. The Colloidal Properties of Nanocellulose. ChemSusChem 2023, 16, e202201955. [Google Scholar] [CrossRef]
- Solhi, L.; Guccini, V.; Heise, K.; Solala, I.; Niinivaara, E.; Xu, W.; Mihhels, K.; Kröger, M.; Meng, Z.; Wohlert, J.; et al. Understanding Nanocellulose–Water Interactions: Turning a Detriment into an Asset. Chem. Rev. 2023, 123, 1925–2015. [Google Scholar] [CrossRef]
- Tardy, B.L.; Mattos, B.D.; Otoni, C.G.; Beaumont, M.; Majoinen, J.; Kämäräinen, T.; Rojas, O.J. Deconstruction and Reassembly of Renewable Polymers and Biocolloids into Next Generation Structured Materials. Chem. Rev. 2021, 121, 14088–14188. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing Fibrillated Cellulose as a Sustainable Technological Material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, G.; Zhu, Y.; Cheng, W.; Cao, K.; Xu, G.; Zhao, D.; Yu, H. A Scalable Bacterial Cellulose Ionogel for Multisensory Electronic Skin. Research 2022, 2022, 9814767. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, C.; Yang, L.; Ao, X. Highly Stretchable, Self-Adhesive, Antidrying Ionic Conductive Organohydrogels for Strain Sensors. Molecules 2023, 28, 2817. [Google Scholar] [CrossRef]
- Yang, J.C.; Mun, J.; Kwon, S.Y.; Park, S.; Bao, Z.; Park, S. Electronic Skin: Recent Progress and Future Prospects for Skin-Attachable Devices for Health Monitoring, Robotics, and Prosthetics. Adv. Mater. 2019, 31, 1904765. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Araki, T.; Kurihira, N.; Kasuga, T.; Sekitani, T.; Nogi, M.; Koga, H. Skin-Adhesive, -Breathable, and -Compatible Nanopaper Electronics for Harmonious On-Skin Electrophysiological Monitoring. Adv. Mater. Interfaces 2023, 10, 2202263. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Qiao, H.; Li, M.; Wang, C.; Zhang, Y.; Zhou, H. Progress, Challenge, and Perspective of Fabricating Cellulose. Macromol. Rapid Commun. 2022, 43, 2200208. [Google Scholar] [CrossRef]
- Nishino, T.; Matsuda, I.; Hirao, K. All-Cellulose Composite. Macromolecules 2004, 37, 7683–7687. [Google Scholar] [CrossRef]
- Gindl, W.; Keckes, J. All-Cellulose Nanocomposite. Polymer 2005, 46, 10221–10225. [Google Scholar] [CrossRef]
- Pullawan, T.; Wilkinson, A.N.; Zhang, L.N.; Eichhorn, S.J. Deformation Micromechanics of All-Cellulose Nanocomposites: Comparing Matrix and Reinforcing Components. Carbohydr. Polym. 2014, 100, 31–39. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, C.-N.; Saito, T.; Isogai, A. Cellulose–Clay Layered Nanocomposite Films Fabricated from Aqueous Cellulose/LiOH/Urea Solution. Carbohydr. Polym. 2014, 100, 179–184. [Google Scholar] [CrossRef]
- Han, D.; Yan, L.; Chen, W.; Li, W.; Bangal, P.R. Cellulose/Graphite Oxide Composite Films with Improved Mechanical Properties over a Wide Range of Temperature. Carbohydr. Polym. 2011, 83, 966–972. [Google Scholar] [CrossRef]
- Morgado, D.L.; Frollini, E.; Castellan, A.; Rosa, D.S.; Coma, V. Biobased Films Prepared from NaOH/Thiourea Aqueous Solution of Chitosan and Linter Cellulose. Cellulose 2011, 18, 699–712. [Google Scholar] [CrossRef]
- Almeida, E.V.R.; Frollini, E.; Castellan, A.; Coma, V. Chitosan, Sisal Cellulose, and Biocomposite Chitosan/Sisal Cellulose Films Prepared from Thiourea/NaOH Aqueous Solution. Carbohydr. Polym. 2010, 80, 655–664. [Google Scholar] [CrossRef]
- Yang, J.; Dahlström, C.; Edlund, H.; Lindman, B.; Norgren, M. PH-Responsive Cellulose–Chitosan Nanocomposite Films with Slow Release of Chitosan. Cellulose 2019, 26, 3763–3776. [Google Scholar] [CrossRef]
- Yang, J.; Duan, J.; Zhang, L.; Lindman, B.; Edlund, H.; Norgren, M. Spherical Nanocomposite Particles Prepared from Mixed Cellulose–Chitosan Solutions. Cellulose 2016, 23, 3105–3115. [Google Scholar] [CrossRef]
- Qi, H.; Liu, J.; Gao, S.; Mäder, E. Multifunctional Films Composed of Carbon Nanotubes and Cellulose Regenerated from Alkaline–Urea Solution. J. Mater. Chem. A 2013, 1, 2161–2168. [Google Scholar] [CrossRef]
- Qi, H.; Mäder, E.; Liu, J. Unique Water Sensors Based on Carbon Nanotube–Cellulose Composites. Sensors Actuators B Chem. 2013, 185, 225–230. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, L.; Cai, J.; Cheng, G.; Zhang, H.; Li, J.; Wang, X. A Facile Construction of Supramolecular Complex from Polyaniline and Cellulose in Aqueous System. Macromolecules 2011, 44, 4565–4568. [Google Scholar] [CrossRef]
- Shi, X.; Lu, A.; Cai, J.; Zhang, L.; Zhang, H.; Li, J.; Wang, X. Rheological Behaviors and Miscibility of Mixture Solution of Polyaniline and Cellulose Dissolved in an Aqueous System. Biomacromolecules 2012, 13, 2370–2378. [Google Scholar] [CrossRef]
- Shi, X.; Hu, Y.; Fu, F.; Zhou, J.; Wang, Y.; Chen, L.; Zhang, H.; Li, J.; Wang, X.; Zhang, L. Construction of PANI–Cellulose Composite Fibers with Good Antistatic Properties. J. Mater. Chem. A 2014, 2, 7669–7673. [Google Scholar] [CrossRef]
- Qi, H.; Sui, X.; Yuan, J.; Wei, Y.; Zhang, L. Electrospinning of Cellulose-Based Fibers From NaOH/Urea Aqueous System. Macromol. Mater. Eng. 2010, 295, 695–700. [Google Scholar] [CrossRef]
- Ye, D.; Lei, X.; Li, T.; Cheng, Q.; Chang, C.; Hu, L.; Zhang, L. Ultrahigh Tough, Super Clear, and Highly Anisotropic Nanofiber-Structured Regenerated Cellulose Films. ACS Nano 2019, 13, 4843–4853. [Google Scholar] [CrossRef]
- Eivazihollagh, A.; Bäckström, J.; Dahlström, C.; Carlsson, F.; Ibrahem, I.; Lindman, B.; Edlund, H.; Norgren, M. One-Pot Synthesis of Cellulose-Templated Copper Nanoparticles with Antibacterial Properties. Mater. Lett. 2017, 187, 170–172. [Google Scholar] [CrossRef]
- IEA Net Zero by 2050. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 15 May 2023).
- Chen, C.; Hu, L. Nanocellulose toward Advanced Energy Storage Devices: Structure and Electrochemistry. Acc. Chem. Res. 2018, 51, 3154–3165. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Beguin, F.; Frackowiak, E. Supercapacitors: Materials, Systems, and Applications; Wiley VCH: Weinheim, Germany, 2013. [Google Scholar]
- Kötz, R.; Carlen, M. Principles and Applications of Electrochemical Capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Shaker, M.; Ghazvini, A.A.S.; Cao, W.; Riahifar, R.; Ge, Q. Biomass-Derived Porous Carbons as Supercapacitor Electrodes—A Review. New Carbon Mater. 2021, 36, 546–572. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Andres, B.; Forsberg, S.; Dahlström, C.; Blomquist, N.; Olin, H. Enhanced Electrical and Mechanical Properties of Nanographite Electrodes for Supercapacitors by Addition of Nanofibrillated Cellulose. Phys. status solidi 2014, 251, 2581–2586. [Google Scholar] [CrossRef]
- Hajian, A.; Lindström, S.B.; Pettersson, T.; Hamedi, M.M.; Wågberg, L. Understanding the Dispersive Action of Nanocellulose for Carbon Nanomaterials. Nano Lett. 2017, 17, 1439–1447. [Google Scholar] [CrossRef]
- Han, J.; Wang, S.; Zhu, S.; Huang, C.; Yue, Y.; Mei, C.; Xu, X.; Xia, C. Electrospun Core–Shell Nanofibrous Membranes with Nanocellulose-Stabilized Carbon Nanotubes for Use as High-Performance Flexible Supercapacitor Electrodes with Enhanced Water Resistance, Thermal Stability, and Mechanical Toughness. ACS Appl. Mater. Interfaces 2019, 11, 44624–44635. [Google Scholar] [CrossRef]
- Andres, B.; Dahlström, C.; Blomquist, N.; Norgren, M.; Olin, H. Cellulose Binders for Electric Double-Layer Capacitor Electrodes: The Influence of Cellulose Quality on Electrical Properties. Mater. Des. 2018, 141, 342–349. [Google Scholar] [CrossRef]
- Teng, G.; Lin, S.; Xu, D.; Heng, Y.; Hu, D. Renewable Cellulose Separator with Good Thermal Stability Prepared via Phase Inversion for High-Performance Supercapacitors. J. Mater. Sci. Mater. Electron. 2020, 31, 7916–7926. [Google Scholar] [CrossRef]
- Blomquist, N.; Koppolu, R.; Dahlström, C.; Toivakka, M.; Olin, H. Influence of Substrate in Roll-to-Roll Coated Nanographite Electrodes for Metal-Free Supercapacitors. Sci. Rep. 2020, 10, 5282. [Google Scholar] [CrossRef]
- Gui, Z.; Zhu, H.; Gillette, E.; Han, X.; Rubloff, G.W.; Hu, L.; Lee, S.B. Natural Cellulose Fiber as Substrate for Supercapacitor. ACS Nano 2013, 7, 6037–6046. [Google Scholar] [CrossRef]
- Dahlström, C.; López Durán, V.; Keene, S.T.; Salleo, A.; Norgren, M.; Wågberg, L. Ion Conductivity through TEMPO-Mediated Oxidated and Periodate Oxidated Cellulose Membranes. Carbohydr. Polym. 2020, 233, 115829. [Google Scholar] [CrossRef]
- Schlemmer, W.; Selinger, J.; Hobisch, M.A.; Spirk, S. Polysaccharides for Sustainable Energy Storage—A Review. Carbohydr. Polym. 2021, 265, 118063. [Google Scholar] [CrossRef]
- Lestriez, B.; Bahri, S.; Sandu, I.; Roue, L.; Guyomard, D. On the Binding Mechanism of CMC in Si Negative Electrodes for Li-Ion Batteries. Electrochem. commun. 2007, 9, 2801–2806. [Google Scholar] [CrossRef]
- Bridel, J.-S.; Azaïs, T.; Morcrette, M.; Tarascon, J.-M.; Larcher, D. Key Parameters Governing the Reversibility of Si/Carbon/CMC Electrodes for Li-Ion Batteries. Chem. Mater. 2010, 22, 1229–1241. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Xuan, Y.; Zhou, L.; Wang, D.; Li, Z.; Lin, H.; Tretiak, S.; Wang, H.; Wang, L.; et al. Multifunctional Cellulose Nanocrystals as a High-Efficient Polysulfide Stopper for Practical Li–S Batteries. ACS Appl. Mater. Interfaces 2020, 12, 17592–17601. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Zhan, H. Advanced Separators for Lithium-Ion Batteries. IOP Conf. Ser. Earth Environ. Sci. 2022, 1011, 012009. [Google Scholar] [CrossRef]
- Leijonmarck, S.; Cornell, A.; Lindbergh, G.; Wågberg, L. Single-Paper Flexible Li-Ion Battery Cells through a Paper-Making Process Based on Nano-Fibrillated Cellulose. J. Mater. Chem. A 2013, 1, 4671. [Google Scholar] [CrossRef]
- Gonçalves, R.; Lizundia, E.; Silva, M.M.; Costa, C.M.; Lanceros-Méndez, S. Mesoporous Cellulose Nanocrystal Membranes as Battery Separators for Environmentally Safer Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 3749–3761. [Google Scholar] [CrossRef]
- Chinnam, P.R.; Zhang, H.; Wunder, S.L. Blends of Pegylated Polyoctahedralsilsesquioxanes (POSS-PEG) and Methyl Cellulose as Solid Polymer Electrolytes for Lithium Batteries. Electrochim. Acta 2015, 170, 191–201. [Google Scholar] [CrossRef]
- Paracha, R.N.; Ray, S.; Easteal, A.J. Grafting of LiAMPS on Ethyl Cellulose: A Route to the Fabrication of Superior Quality Polyelectrolyte Gels for Rechargeable Lithium Ion Batteries. J. Mater. Sci. 2012, 47, 3698–3705. [Google Scholar] [CrossRef]
- Al Haj, Y.; Mousavihashemi, S.; Robertson, D.; Borghei, M.; Pääkkönen, T.; Rojas, O.J.; Kontturi, E.; Kallio, T.; Vapaavuori, J. Biowaste-Derived Electrode and Electrolyte Materials for Flexible Supercapacitors. Chem. Eng. J. 2022, 435, 135058. [Google Scholar] [CrossRef]
- Calkins, C.R. Calkins, Studies of Dielectric Properties of Chemical Pulps. Tappi J. 1950, 33, 278–285. [Google Scholar]
- Hollertz, R.; Wågberg, L.; Pitois, C. Effect of Composition and Morphology on the Dielectric Response of Cellulose-Based Electrical Insulation. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2339–2348. [Google Scholar] [CrossRef]
- Fan, F.-R.; Tian, Z.-Q.; Lin Wang, Z. Flexible Triboelectric Generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Wang, Z.L. Triboelectric Nanogenerators as New Energy Technology and Self-Powered Sensors—Principles, Problems and Perspectives. Faraday Discuss. 2014, 176, 447–458. [Google Scholar] [CrossRef]
- Mondal, R.; Hasan, M.A.M.; Zhang, R.; Olin, H.; Yang, Y. Nanogenerators-Based Self-Powered Sensors. Adv. Mater. Technol. 2022, 7, 2200282. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, H.; Li, Q.; Zhang, R.; Gao, S.; Wang, F.; Li, G.; Chen, B.; Yu, H.; Liu, S.; et al. Multi-Dimensional, Transparent and Foldable Cellulose-Based Triboelectric Nanogenerator for Touching Password Recognition. Nano Energy 2022, 98, 107307. [Google Scholar] [CrossRef]
- Zhang, R.; Hummelgård, M.; Örtegren, J.; Olsen, M.; Andersson, H.; Yang, Y.; Zheng, H.; Olin, H. The Triboelectricity of the Human Body. Nano Energy 2021, 86, 106041. [Google Scholar] [CrossRef]
- Zhang, R.; Olin, H. Material Choices for Triboelectric Nanogenerators: A Critical Review. EcoMat 2020, 2, e12062. [Google Scholar] [CrossRef]
- Zhang, R.; Hummelgård, M.; Örtegren, J.; Andersson, H.; Olsen, M.; Chen, W.; Wang, P.; Dahlström, C.; Eivazi, A.; Norgren, M. Energy Harvesting Using Wastepaper-Based Triboelectric Nanogenerators. Adv. Eng. Mater. 2023, 2300107. [Google Scholar] [CrossRef]
- Zhang, R.; Dahlström, C.; Zou, H.; Jonzon, J.; Hummelgård, M.; Örtegren, J.; Blomquist, N.; Yang, Y.; Andersson, H.; Olsen, M.; et al. Cellulose-Based Fully Green Triboelectric Nanogenerators with Output Power Density of 300 W m−2. Adv. Mater. 2020, 32, 2002824. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal Crystallinity Index Revisited by the Simulation of X-Ray Diffraction Patterns of Cotton Cellulose Iβ and Cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Isobe, N.; Nishiyama, Y.; Kimura, S.; Wada, M.; Kuga, S. Origin of Hydrophilicity of Cellulose Hydrogel from Aqueous LiOH/Urea Solvent Coagulated with Alkyl Alcohols. Cellulose 2014, 21, 1043–1050. [Google Scholar] [CrossRef]
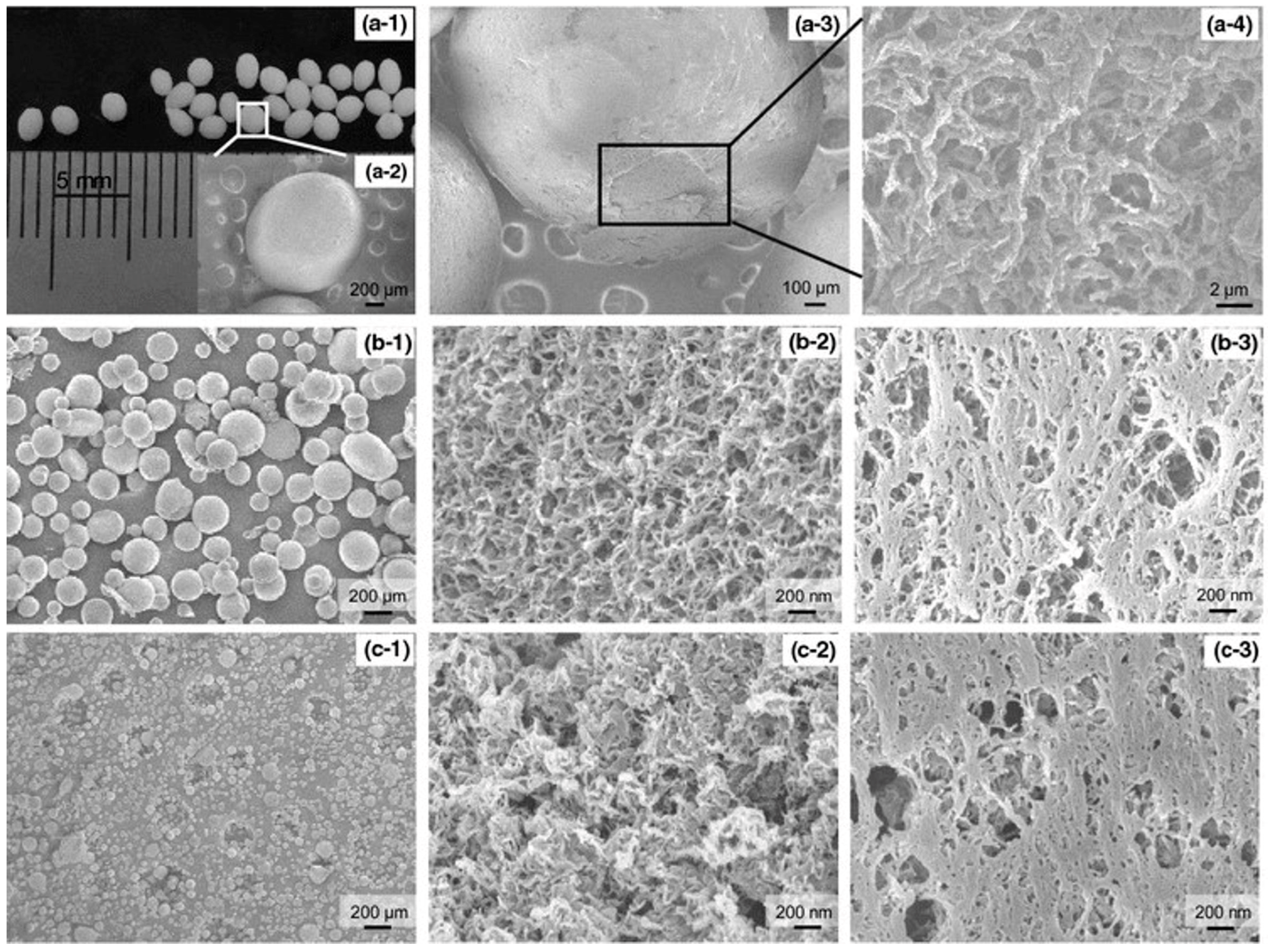

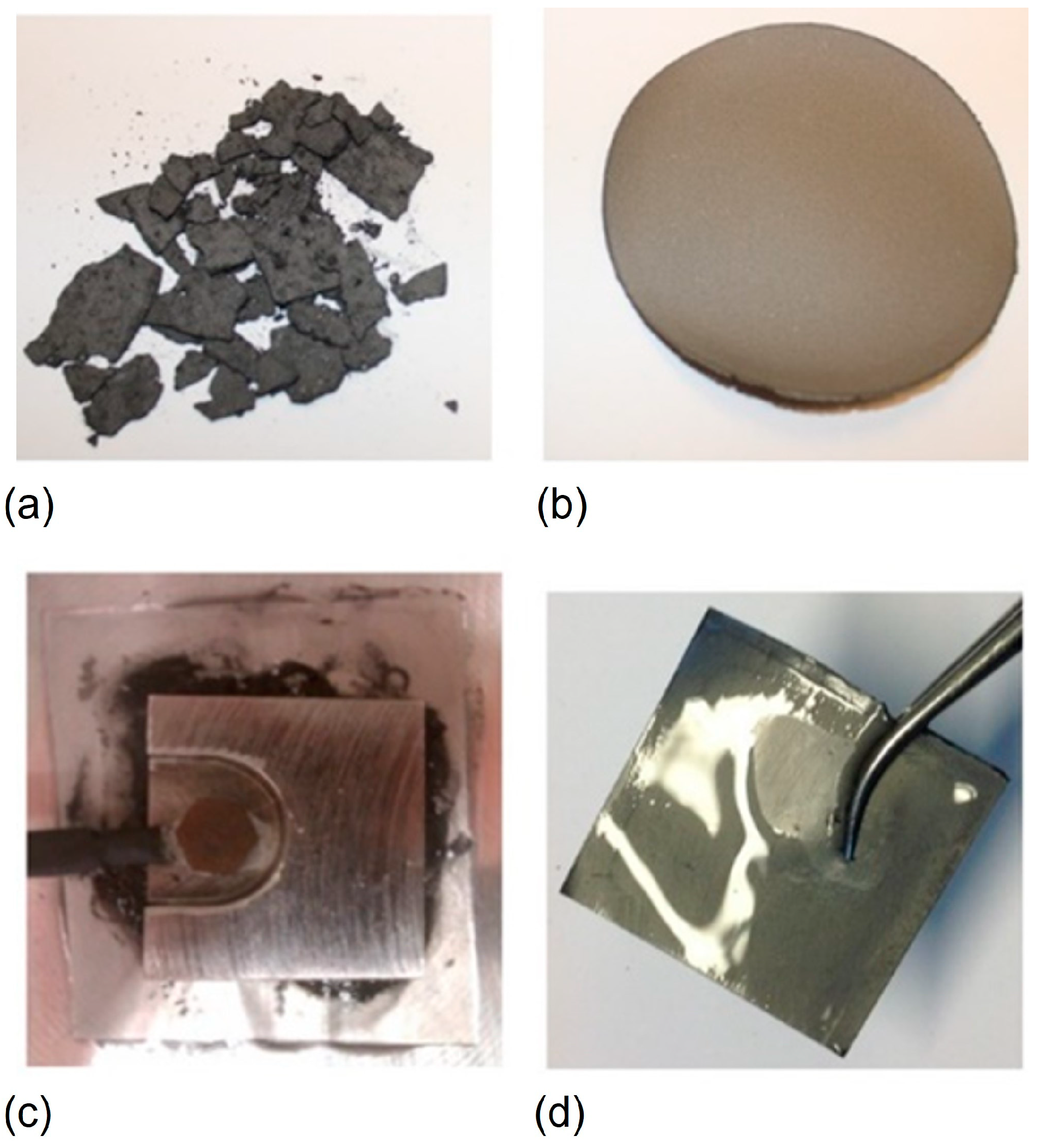


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norgren, M.; Costa, C.; Alves, L.; Eivazi, A.; Dahlström, C.; Svanedal, I.; Edlund, H.; Medronho, B. Perspectives on the Lindman Hypothesis and Cellulose Interactions. Molecules 2023, 28, 4216. https://doi.org/10.3390/molecules28104216
Norgren M, Costa C, Alves L, Eivazi A, Dahlström C, Svanedal I, Edlund H, Medronho B. Perspectives on the Lindman Hypothesis and Cellulose Interactions. Molecules. 2023; 28(10):4216. https://doi.org/10.3390/molecules28104216
Chicago/Turabian StyleNorgren, Magnus, Carolina Costa, Luís Alves, Alireza Eivazi, Christina Dahlström, Ida Svanedal, Håkan Edlund, and Bruno Medronho. 2023. "Perspectives on the Lindman Hypothesis and Cellulose Interactions" Molecules 28, no. 10: 4216. https://doi.org/10.3390/molecules28104216
APA StyleNorgren, M., Costa, C., Alves, L., Eivazi, A., Dahlström, C., Svanedal, I., Edlund, H., & Medronho, B. (2023). Perspectives on the Lindman Hypothesis and Cellulose Interactions. Molecules, 28(10), 4216. https://doi.org/10.3390/molecules28104216








