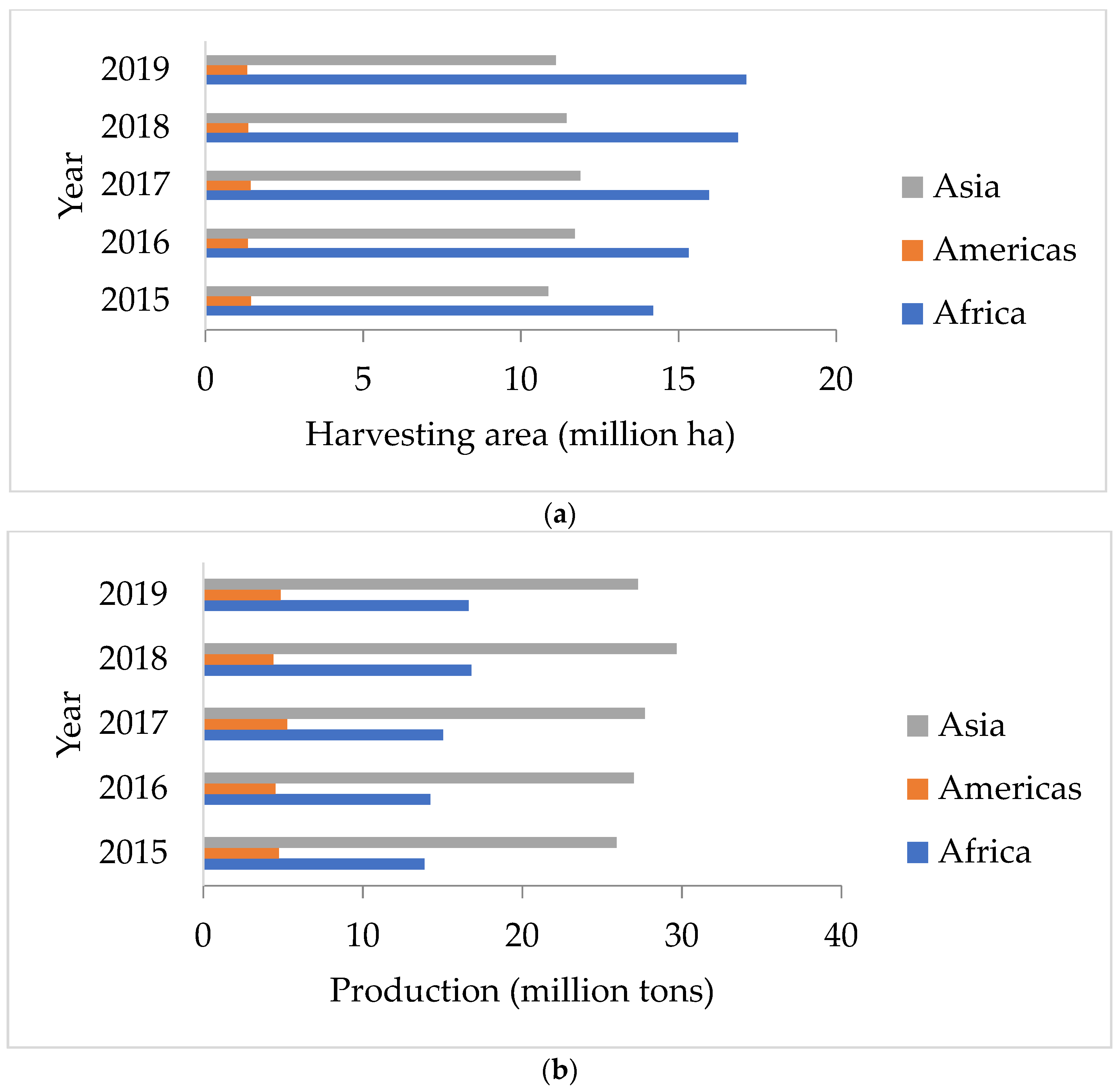Abstract
Peanuts (Arachis hypogea) can be made into various products, from oil to butter to roasted snack peanuts and candies, all from the kernels. However, the skin is usually thrown away, used as cheap animal feed, or as one of the ingredients in plant fertilizer due to its little value on the market. For the past ten years, studies have been conducted to determine the full extent of the skin’s bioactive substance repertoire and its powerful antioxidant potential. Alternatively, researchers reported that peanut skin could be used and be profitable in a less-intensive extraction technique. Therefore, this review explores the conventional and green extraction of peanut oil, peanut production, peanut physicochemical characteristics, antioxidant activity, and the prospects of valorization of peanut skin. The significance of the valorization of peanut skin is that it contains high antioxidant capacity, catechin, epicatechin resveratrol, and procyanidins, which are also advantageous. It could be exploited in sustainable extraction, notably in the pharmaceutical industries.
1. Introduction
Peanuts are vital crops for the food sector in the world. Peanuts are extensively cultivated in tropical and subtropical locations, and the crop has the ability to commercially advantage both food producers and industrial manufacturers. The average annual worldwide peanut output is 46 million tons, according to studies. As shown in Figure 1b, the Asian regions have the largest harvesting areas and yields compared with America and Africa [1].
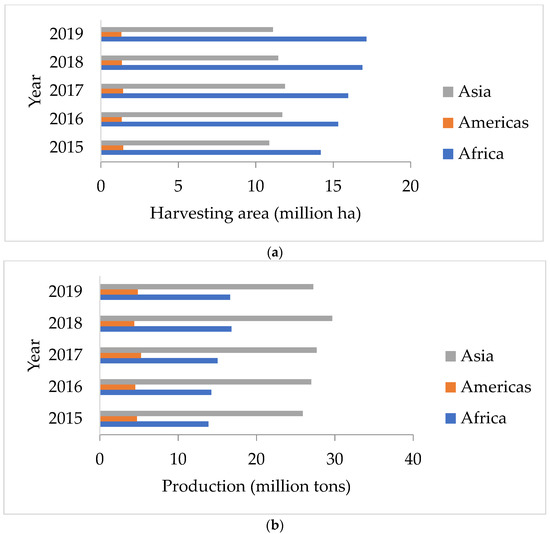
Figure 1.
(a) Harvest area and (b) production of peanuts in Asia, Americas, and Africa [1].
The majority of peanuts are processed to manufacture butter, oil, roasted peanuts, sweets, and desserts. During peanut manufacturing, substantial amounts of potentially polluting peanut skin byproducts are produced. Nevertheless, peanut skin is employed as animal feed and fertilizer after its reutilization. Significant quantities of peanut powder, shells, hulls, and vines are also classified as agricultural waste. Due to the fact that the great majority of studies concentrate on oil and kernel production, peanut byproducts such as peanut skin get little study [2].
Moreover, peanut skins are beneficial as they are rich in antioxidants, catechin, epicatechin, resveratrol, and anthocyanidins [3,4]. They may reduce the rate of free-radical-induced oxidation processes [5]. Antioxidants are able to neutralize and build stable compounds by donating additional hydrogen electrons to free radicals [6]. Previous research demonstrated that long-term consumption of peanut skin extract rich in plant polyphenols protects against cancer, cardiovascular disease, diabetes, osteoporosis, and neurological disorders [7,8]. Peanut skin also contains cellulose (40.5%), lignin (26.4%), and hemicellulose (14.7%) [9].
Catechin is the bioactive substance in the peanut skin, which accounts for 17 mg of catechin per g sample [10]. Catechin is commonly found in onions, chocolate, wine, grape skin, and tea [11,12,13,14]. It is a flavanol with five attached hydroxyl groups, making it a polar flavanol. It also has been identified as an effective antioxidant. Regarding antioxidant activity, catechin ranks second among the other antioxidants after quercetin [7]. Moreover, catechin is well-known for its many health benefits, which include anti-inflammatory, anti-HIV, antidepressant, and anti-hypertension properties. Therefore, it must be transformed from a zero-value material into a high-value product.
Resveratrol, a stilbene molecule that belongs to the polyphenol family, is often isolated from a wide variety of natural plants, especially from peanut skin. Due to its numerous positive characteristics, resveratrol is extensively used in the culinary and pharmaceutical industries. It is sensitive to structural deterioration and may involve chemical transformations during food preparation [15]. Several studies have consequently focused further on the different elements of resveratrol, such as its anti-aging, antioxidant, and anti-cancer properties [16].
Previously, the bioactive compounds of peanut skin were extracted by traditional techniques such as maceration and Soxhlet. However, the “green extraction” of plant materials has become a challenge for specific industrial experts. This method could provide a higher yield and quality extract with short extraction time, whilst being safe for human health. This is because the extract is free from toxic residues and organic solvents [17,18]. Thus, significant advancements in green extraction, such as microwave-assisted extraction (MAE) and supercritical carbon dioxide (ScCO2) extraction, have been introduced [19,20,21,22,23,24,25,26,27]. The extraction method has been crucial to ensuring the end product’s excellent function. Even if the traditional approach provided a larger yield, the long-term repercussions should be addressed, especially in terms of the environment and our health.
The fact that peanut skin includes high levels of antioxidants, catechin, epicatechin, and resveratrol, is also helpful and might be utilized in sustainable extraction processes, especially in the pharmaceutical sectors. Therefore, this article will provide an overview of a recent extraction to valorize the waste peanut skin into high-value products. Some of the optimization methods are briefly described to valorize peanut skin. The published research findings on peanut skin’s physicochemical qualities, antioxidant activity, and phenolic content were examined in greater depth.
2. Physicochemical Properties of Peanut Skin
Peanut skin, also known as peanut coat, is a common byproduct of peanut factories, as most peanut butter manufacturers remove the seed’s skin (Figure 2). An astringent peanut peel will diminish the flavor of peanut butter. Despite this, peanut skin contains several bioactive components and antioxidants that promote and protect human health, such as phenolic acid, procyanidin, and catechin, to name a few. The skin of non-defatted peanuts contains 90–125 mg/gram of total phenolics, which include phenolic acids, flavonoids, and resveratrol [28].

Figure 2.
Peanut skin.
Soxhlet extraction is frequently used to extract peanut skin, as shown in Table 1. In each gram of dry skin, peanut skin has 140–150 mg of phenolic compounds [29]. The solvents of ethanol, methanol, and hexane were used to extract the phenolic compounds from peanut skin [28,30,31]. Prior studies also found that peanut skin contains 88% of the total antioxidant activity and 30% of the oil extract. Catechin, a bioactive compound, is also highly found in both green tea (17 mg/100 g) and peanut skin (16.1 mg/100 g) [32].

Table 1.
Antioxidant activity (AA) ranking of various bioactive compounds [33].
3. Bioactive Compounds in Peanut Skin
3.1. Catechin
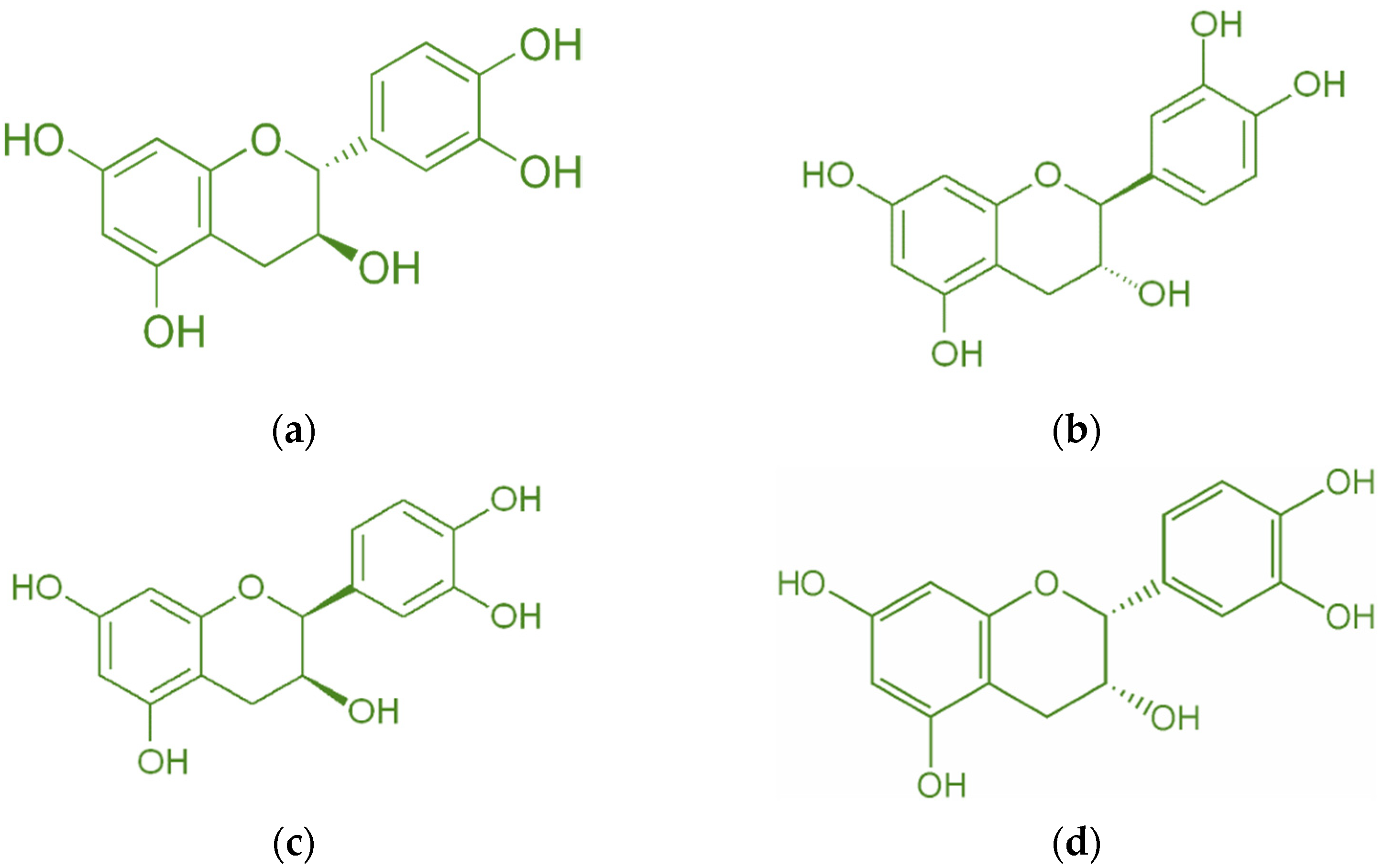
Catechin is a bioactive molecule found in peanut skin, but it can also be found in tea [34], grape skin [13], wheat [35], onion, apple skin [36], broccoli [12], cocoa [37], and red wine [38]. Catechin has a molecular weight of 290.26 g/mol and a melting point of 175 °C [39]. A high melting point makes it resistant to deterioration at high temperatures. Figure 3 depicts the catechin’s isomer. The most common isomer of catechin is (+)-catechin. The opposite stereoisomer is (−)-catechin, often called ent-catechin. Other names for the most common epicatechin isomer (2R,3R)-(−)-epicatechin are l-epicatechin, epicatechol, (−)-epicatechol, l-acacatechin, l-epicatechol, epi-catechin, 2,3-cis-epicatechin, or (−)-epicatechin. (+)-catechin is thought to exert pharmacological effects on the human body, such as cardioprotective, diuretic, and hypotensive effects [11]. Reactive oxygen species (ROS) and hydroxyl radicals have the potential to cause oxidative damage to bodily cells. Catechin, on the other hand, possesses antioxidant qualities that prevent this damage [34]. Catechin has been demonstrated to have superior antioxidant qualities and a greater capacity to scavenge free radicals than vitamin C, E, or -carotene carotene [40,41,42,43]. Moreover, catechins can transfer hydrogens through their hydroxyl groups.
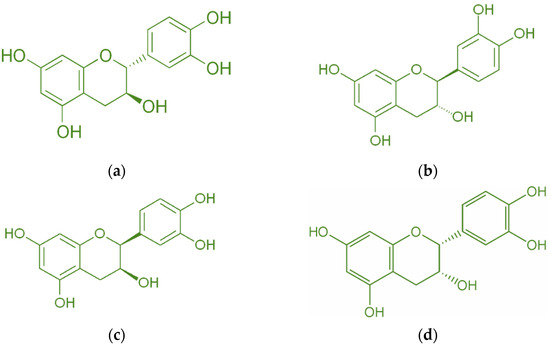
Figure 3.
Diastereoisomers of catechin (a) (+)-catechin, (b) (−)-catechin, (c) (+)-epicatechin, and (d) (−)-epicatechin.
Catechin belongs to the favan-3-ol family. Regular use can help prevent Parkinson’s, diabetes, cancer, and other diseases [44]. However, producing highly active catechin may be problematic because it is rapidly broken down when exposed to light due to UV radiation from the sun [2,45]. Furthermore, catechin reduces hemoglobin levels by delaying the oxidative process in hemoglobin cells [46]. Katalinić, et al. [33] used a bioassay to determine the antioxidant activity of many well-known antioxidants as shown in Table 1. Furthermore, Table 2 shows that various materials contain catechin as a bioactive compound [32]. The catechin content of peanut skin is comparable to that of green tea and higher than that of black tea [14]. Peanut skin contains a high concentration of catechin, prompting the development of peanut skin as a catechin resource to replace other catechin resources such as green and black tea.

Table 2.
Catechin recovery from various materials.
3.2. Resveratrol
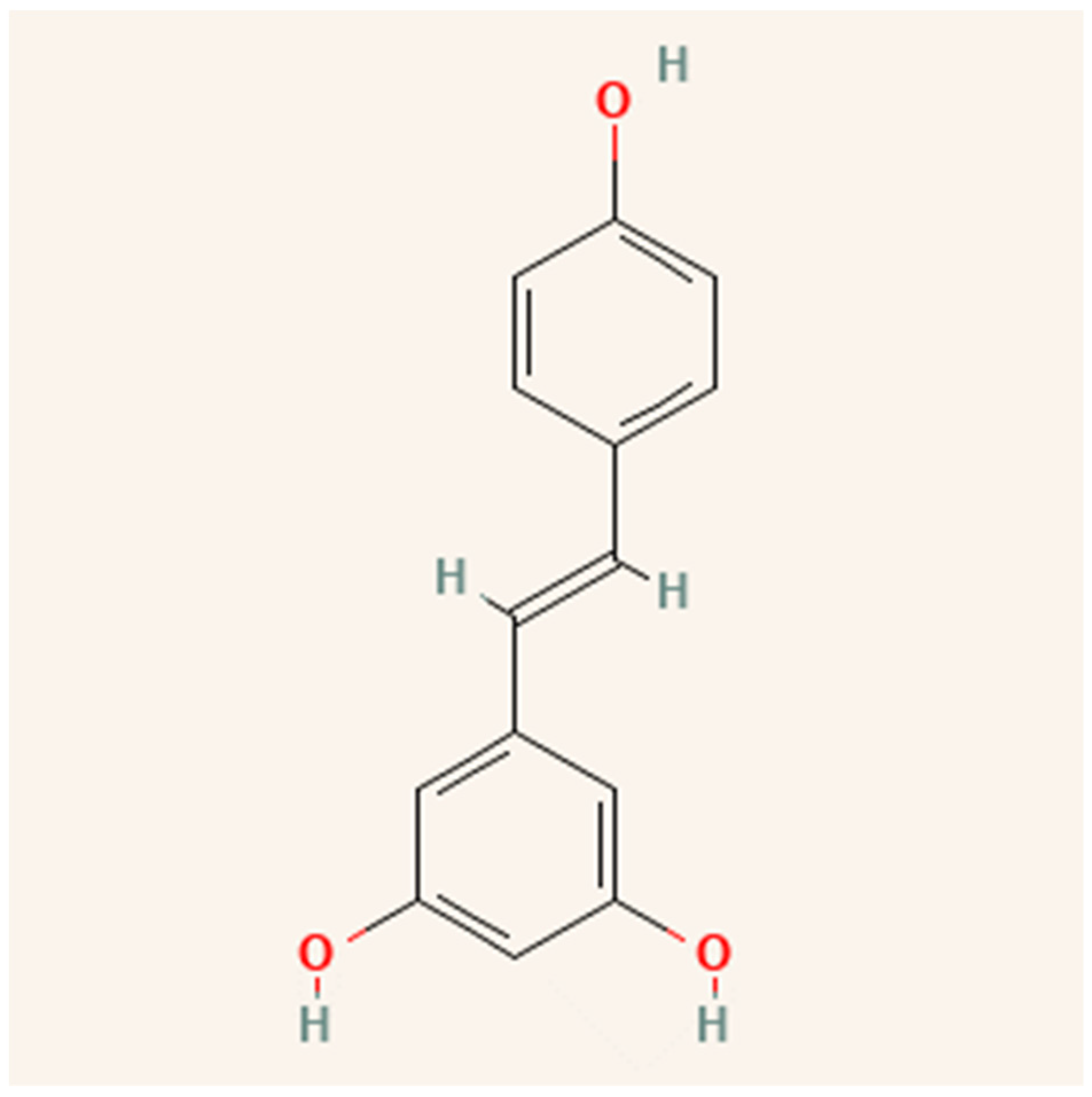
Resveratrol (3,4′,5-trihydroxystilbene) is a stilbene, a type of polyphenolic compound. Certain plants produce resveratrol and other stilbenes in response to stress, injury, fungal infection, or ultraviolet (UV) radiation [49]. As shown in Figure 4, resveratrol is a fat-soluble molecule with trans and cis molecular configurations [50]. Both cis- and trans-resveratrol occur as glucose-bound glucosides [51]. Romero-Pérez, et al. [52] discovered that resveratrol-3-O-glucoside, also known as piceid, is one of the most important resveratrol derivatives. Resveratrol can be found in grapes [53], wine [54], grape juice [55], peanuts [15], cocoa [56], and berries [57].
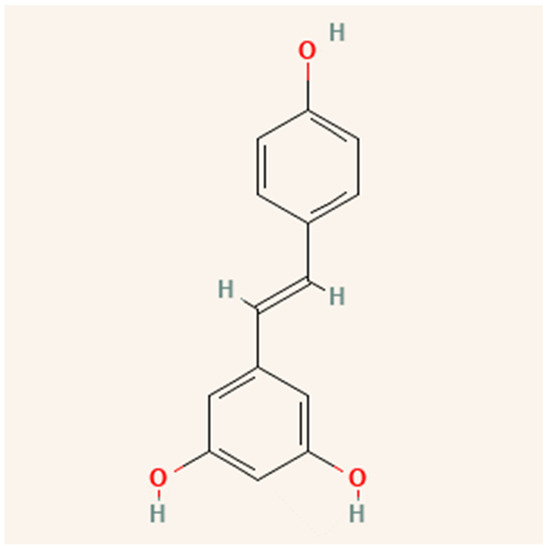
Figure 4.
Structure of resveratrol.
Researchers worldwide have investigated resveratrol’s health consequences since the early 1990s, when the presence of resveratrol in red wine was proven [58]. It was hypothesized, in particular, that moderate red wine consumption containing resveratrol could help explain why the French have a relatively low incidence of coronary heart disease (CHD) despite eating foods high in saturated fat, a phenomenon known as the “French Paradox” (cardiovascular disease) [59]. Since then, scientific interest has grown in resveratrol’s ability to prevent cancer, delay the onset of cardiovascular and neurological diseases, improve glycemic control in type 2 diabetes, and lengthen lifespan in experimental animals.
Resveratrol has been extracted from peanut skin using ethanol with the maceration method [29]. On the other hand, this technique has limitations including long extraction time and excessive solvent usage. Therefore, resveratrol was recently extracted from peanut skin using modern techniques such as MAE [30]. Modern extraction techniques and environmentally friendly solvents have been developed to extract resveratrol efficiently and effectively [10]. Ionic liquids (ILs) combined with MAE [60] are categorized as green solvents and are also used to extract resveratrol. This method has a high dissolution rate and absorbs more microwave radiation than organic solvents [61].
3.3. Procyanidins
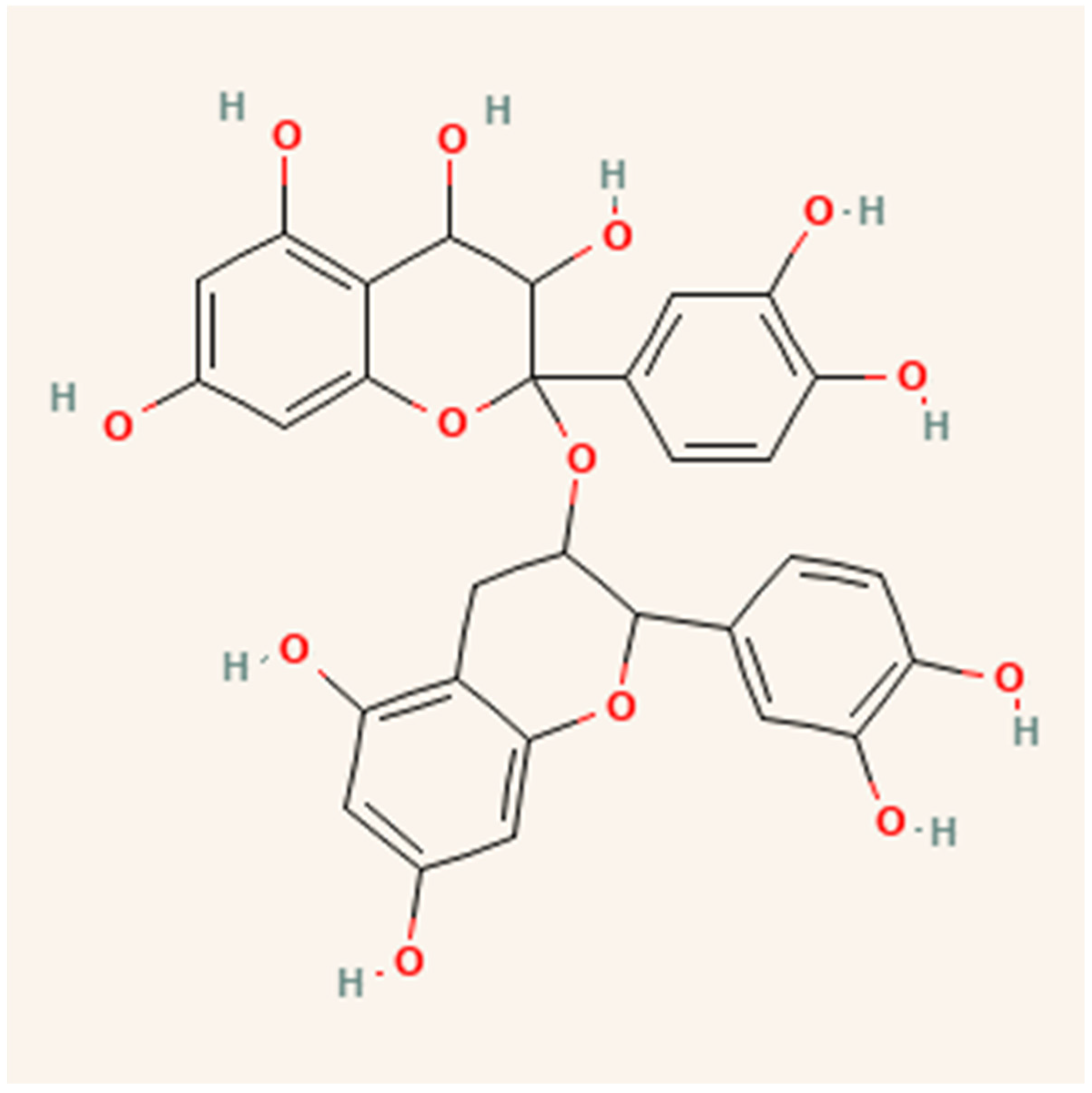
Many agro-industrial wastes from the food processing sectors, including cocoa, berries, grapes, apples, blueberries, plums, tea leaves, coffee, cinnamon, peanuts, and leguminous plants, include procyanidins and their monomers [62]. Depending on the origin and kind of the plant material, the procyanidin concentration may vary [60]. These molecules contain flavan-3-ol monomers as basic units in their structure, which are composed exclusively of (+) catechin and (–) epicatechin. (Epi)catechin monomers may be biosynthetic precursors of procyanidins [63] as shown in Figure 5.
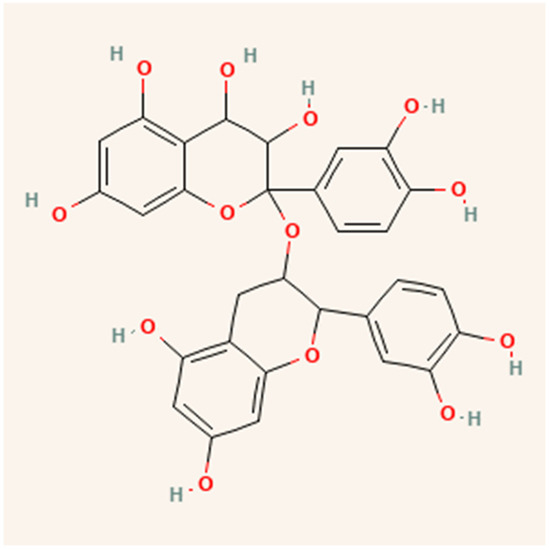
Figure 5.
Structure of procyanidins.
Sarnoski, et al. [64] found that there are several forms of proanthocyanidins in peanut skins, but A-type proanthocyanidins predominate. Peanut skins are mostly composed of dimeric, trimeric, and tetrameric proanthocyanidins species. Compared to single solvent extractions, the multistep extraction approach is an excellent method for concentrating procyanidins from peanut skins. The optimal parameters for ScCO2 extraction were 20.39 MPa, 333.23 K, and 0.17 mL/min for procyanidins and proanthocyanidins containing 2325.23 and 409.95 g/g, respectively [4].
4. Antioxidant Activity of Peanut Skin Extract
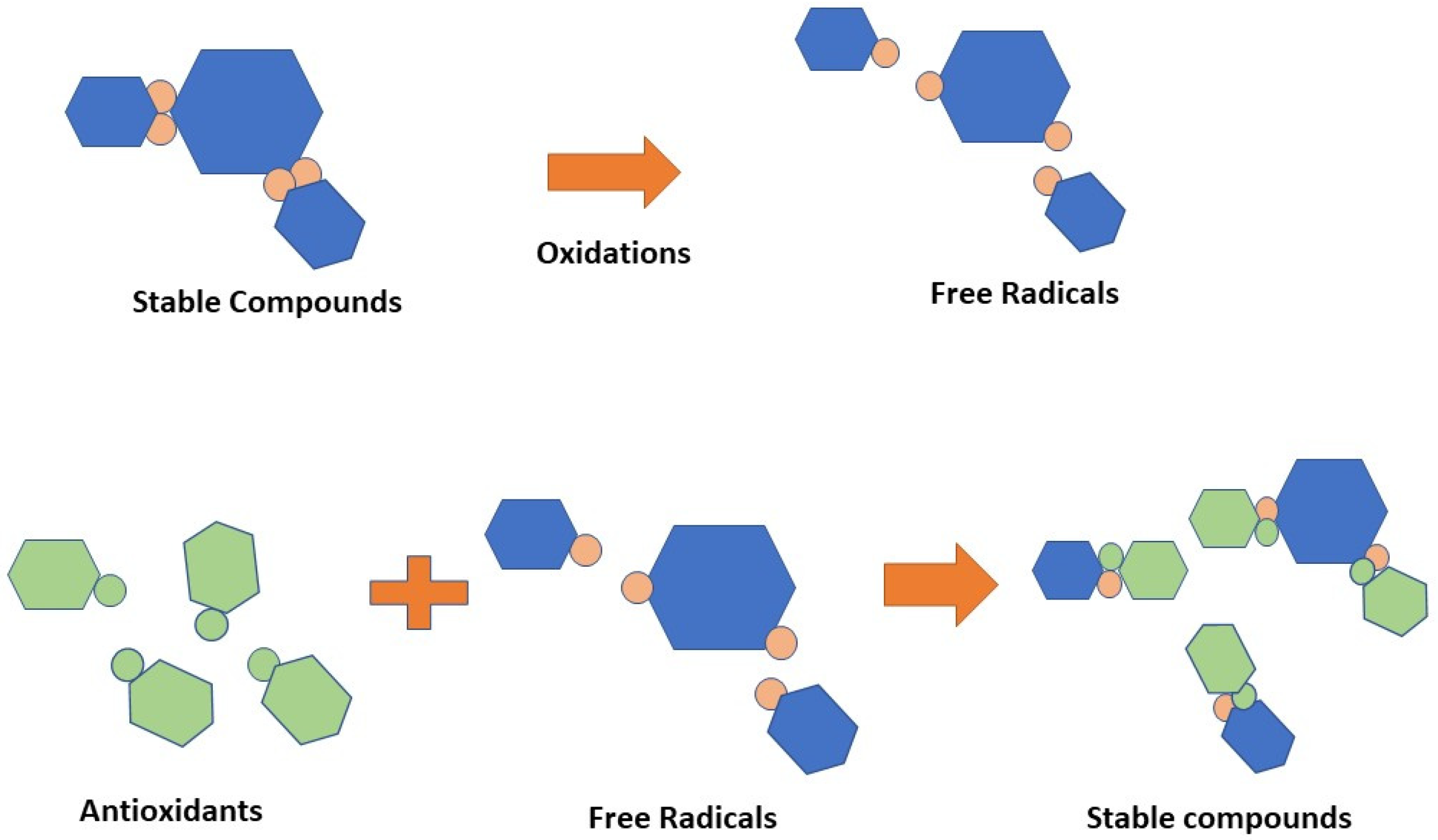
The body requires antioxidants to interact with free radicals and interrupt the chain reaction that leads to sickness [65]. Antioxidants are chemical classes that can hinder oxidation cycles, preventing or delaying the oxidative destruction of biomolecules. The antioxidant mechanism is depicted in Figure 6. Vitamin C, vitamin E, xanthophylls, carotenes, flavonoids, lignans, and stilbenes were the most common antioxidant-active compounds in the peanut skin [6,66,67,68,69,70].
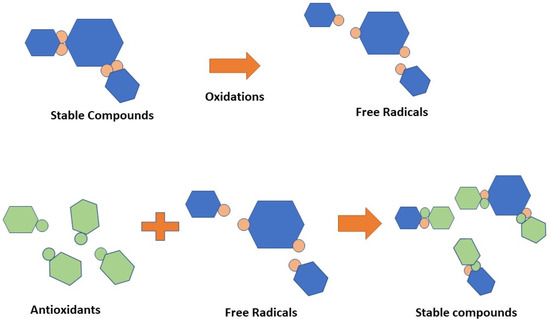
Figure 6.
Scheme of antioxidants.
The free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) is commonly used to test compounds’ ability to function as free radical scavengers and assess the antioxidant activity of antioxidant components [71]. The DPPH approach, which applies to the overall antioxidant capacity of the sample rather than any specific antioxidant component, can be employed for solid or liquid samples [72]. A measure of total antioxidant capacity will help us understand the antioxidant compound’s functional properties [73]. As indicated in Table 3, several researchers have successfully used the DPPH method to assess antioxidant activity. Table 4 shows that peanut skin offers higher antioxidant activity compared with several sources of antioxidant compounds. As a result, peanut skin can be used to replace renewable or sustainable sources in health and wellness goods.

Table 3.
Antioxidant activity from another material.

Table 4.
Previous studies on the valorization of peanut skin.
5. Valorization of Peanut Skin by Conventional and Green Extraction
Extraction is commonly defined as separating phytochemicals from plant components [84]. Plant materials differ in phenolic chemicals, flavonoid compounds, and tannins [85,86,87]. Different extraction conditions are required to obtain maximum extract yield recovery and excellent extract quality [3,4,7,78,88,89]. An extract’s number of bioactive compounds is critical for assessing its quality [90]. Many factors influence the efficiency and quality of bioactive chemical extraction, including the type of extraction solvent, solvent concentration, extraction temperature, extraction pH, and extraction duration [91]. There are two extraction processes based on overall separation: conventional and green. Soxhlet extraction is categorized as a conventional extraction method and is commonly applied to extract plants and herbs [92]. It uses a toxic solvent including methanol, n-hexane, and other toxic solvents. ScCO2, MAE, and UAE are categorized as green extraction methods due to possessing non-toxic solvents, shorter extraction times, low energy consumption, and providing higher quality extracts [90]. The previous studies of peanut skin extraction are shown in Table 4.
5.1. Soxhlet Extraction on Peanut Skin Valorization
In 1879, Von Soxhlet invented a novel extraction process, which became the most widely used leaching technology for an extended period. It is always a critical metric of success against which novel leaching strategies are measured. The benefits and drawbacks of this extraction process have been exploited to generate various changes to alleviate or reduce the extraction time while enhancing the quantity and quality of extract. This technique requires fewer minor procedures, can extract more sample mass, and appears devoid of matrix effects [93,94,95].
Ju and Howard [96] optimized the Soxhlet extraction conditions for maximum peanut skin recovery. Ethanol proportions (0 to 96% v/v), particle sizes (0 to 10 mm and non-crushed skin), and solid–liquid proposition (20 to 60 mL/g) were used in the extraction. The results showed that 70% ethanol, non-crushed peanut skin, and a solvent/solid ratio of 20 mL/g were the best conditions, yielding a maximum yield of 0.118 g/g. The solid–liquid solvent and ethanol concentration ratio were significant factors in increasing yield recovery.
Methanol, ethanol, and water were used as solvents, with concentrations ranging from 0 to 90%, temperatures ranging from 30 to 60 °C, and extraction times ranging from 10 to 30 min. The responses were TPC, ORAC level, and resveratrol content. TPC was highest in ethanol extracts, followed by methanol and water. The maximum TPC predicted was 118 mg/g. The highest ORAC activity was found in methanol extracts, which had 2149 mol of TE/g, followed by ethanol and water [81]. Nepote [96] also reported that ethanol is the best solvent for extracting peanut skin via Soxhlet extraction. This is because ethanol can extract both polar and nonpolar compounds. As a result, all bioactive compounds found in peanut skin can be extracted effectively [31].
5.2. MAE on Peanut Skin Valorization
The frequency range of microwaves is 300 MHz to 300 GHz [97]. In contrast to the traditional way, thermal energy is wasted to the environment. The heating in MAE is selective and localized. This new heating method might greatly shorten extraction time [98]. Microwave heating is dependent on polar solvent contact, which is determined by ionic conduction and dipole rotation [92,97,99]. Ionic conduction refers to the electrophoretic transport of ions in a variable electromagnetic current. The resistance of the solution to ion movement generates friction, which warms the liquid. Rotation of the dipoles realigns them with a rapidly changing electromagnetic current [100]. In one to two minutes, the radiation may hydrolyze the ether bonds of cellulose; thus, the component of plant cell walls becomes soluble into the solvents. High temperature promotes cellulose dehydration and decreases its mechanical strength in the cell wall enabling the solvent to more effectively promote the solubilization of compounds into the solvent [101].
Solvent selection in MAE for the valorization of peanut skin is influenced by the target analyte’s solubility, the solvent’s penetration, interaction with the sample matrix, and the dielectric constant [102]. Ethanol, methanol, and acetone were used to extract phenolic components from peanut skin, yielding a higher yield of polyphenols than ethanol extraction, despite the latter extract having superior antioxidant capabilities [103].
Ballard, Mallikarjunan, Zhou and O’Keefe [30] investigated the effects of microwave power (10% to 90%), irradiation time (30 to 150 s), and sample mass (1.5 to 3.5 g) on TPC and ORAC levels of peanut skin extracts. The optimized conditions of 90% power, 30 s of irradiation time, and 1.5 g of skins yielded a maximum TPC of 143.6 mg/g and an ORAC level of 2789 mol/g. Higher microwave power with shorter irradiation time was the best combination for obtaining high TPC and ORAC levels. Peanut skin pores will be broken by high microwave power. As a result, ethanol will easily penetrate the pores of peanut skin as a solvent. Therefore, the extract will be more soluble in the solvent and have less mass transfer resistance. Higher microwave power also increases solvent diffusivity [104]. The disadvantage of irradiation duration could not be ignored because exposing the compounds to microwave irradiation for an extended time decomposes the monomeric catechin and reduces efficiency [105].
Bai, et al. [106] discovered that microwave extraction had a higher extraction rate in total flavonoid recovery from peanut skin. The optimal extraction conditions were a microwave power of 690 W, an extraction time of 40 s, an ethanol concentration of 55%, and a material–liquid ratio of 1:20. The maximum total flavonoid yield was 3.18%. The simple microwave extraction method can quickly extract total flavonoids from peanut skin. The extraction rate will increase as the microwave power increases. Localized heating occurs in the sample because of microwave power. It causes MAE to degrade the plant matrix, allowing the extract to diffuse and dissolve in the solvent.
Meanwhile, increasing the power enhances the extraction yield and allows for a shorter irradiation time [107]. However, high microwave power may result in low extraction yields due to the destruction of thermally sensitive compounds. Increasing microwave power improves extraction yield until it becomes minor or declines [108]. A good solvent-to-solid ratio ensures homogeneous and efficient heating. Meanwhile, excessive solvent results in poor microwave heating because the solvent absorbs microwave radiation, necessitating more power. Since active compounds are concentrated in specific areas, a low solvent-to-solid ratio creates mass transfer barriers, limiting compound transport out of the cell matrix [109].
5.3. UAE on Peanut Skin Valorization
UAE is less costly, quicker, and more versatile than earlier techniques since it can employ solvents with varying polarity. Despite its benefits, this method has difficulty integrating numerous instruments and automation [110,111]. Gharibzahedi, et al. [112] discovered that ultrasonic frequency improves extraction yields more efficiently than the previous extraction method. Additionally, UAE extracts were less cytotoxic than solvent and hydrothermal extraction techniques. Irradiation also led to matrix disintegration, but ultrasonic waves promoted matrix hydration [113].
Jin, Gao, Kong, Yang, Kuang, Yang, Fu, Cheng and Li [10] developed an improved and sustainable technique for the extraction of resveratrol from peanut skin using microbial consortiums immobilized on cellulose and an ultrasound-assisted surfactant aqueous system pretreatment. The best immobilized microbial consortium on cellulose was formed by Aspergillus oryzae and Aspergillus niger yeast. Other ideal conditions included 3% Triton X-114, a liquid–solid ratio of 25:1, an ultrasonic power of 200 W, a culture temperature of 30 °C, and a culture period of 36 h. The study found that resveratrol concentration reached 96.58 g/g under these conditions.
The suggested pretreatment approach employing microbial consortiums immobilized on cellulose with ultrasound-assisted surfactant aqueous solution for the extraction and bioconversion of target chemicals from plant materials proved to be efficient, rapid, environmentally friendly, and inexpensive. As a result, the method described in this article could be a viable and efficient way of extracting resveratrol from peanut skin. It also could be widely used to produce targeted compounds from plant waste residue. Syahdi, et al. [114] discovered that many conditions were optimized for resveratrol, including solvent types with 70% ethanol and natural deep eutectic solvent (NADES). The results showed that a 1:20 solid-to-liquid NADES ratio and a 15 min extraction time provided more resveratrol (0.049 mg/g) than 70% ethanol (0.011 mg/g).
5.4. ScCO2 Extraction on Peanut Skin Valorization
ScCO2 is applied when the fluid condition exceeds the critical temperature of 31.1 °C and a pressure of 7.1 MPa. ScCO2 is advantageous due to its high diffusivity and broad density range. It depends on the solubility of the solvent. ScCO2 has a density, viscosity, and diffusivity that are intermediate between a gas and a liquid. The critical temperature is the highest temperature at which a gas can transform into a liquid phase when the pressure is increased. Meanwhile, the critical pressure is the highest pressure at which a liquid can transform into a gas when its temperature increases.
ScCO2 extraction of peanut skin has previously been studied by Putra, Rizkiyah, Zaini, Machmudah and Yunus [78]. Initially, they discovered that the best conditions were 0.17 mL/min, 21.86 MPa, and 332.23 K, with 3399.84 g/g of epicatechin and 752.03 g/g of catechin. The solubility of epicatechin and catechin was between 6.02 E-5 g/L and 1.21 E-5 g/L. Furthermore, catechin concentration increased with increasing temperature. The high temperature increases the diffusivity of the solvent, allowing it to penetrate the particle pore of the peanut skin. As a result, the catechin dissolves easily in the solvent. High temperatures also reduce the viscosity of carbon dioxide. The viscosity of a lighter solvent increases mass transfer and decreases resistance. Therefore, the higher catechin and epicatechin yield will be increased. A high-pressure environment was also suitable for extracting catechin and epicatechin. This is because the high pressure will increase the density of carbon dioxide, thus, enhancing carbon dioxide’s solvation power.
Moreover, ScCO2 could extract high procyanidins and proanthocyanidins as promising compounds from peanut skin [4]. The best conditions were 0.17 mL/min of ethanol, 20.39 MPa, and 333.23 K, with the outcomes of 409.95 g/g of proanthocyanidins and 325.23 g/g of procyanidins. With an average absolute relative deviation of 3.01%, the Chrastil model had the best connection to flavonoid solubility. Pressure and temperature increase the extraction efficiency of ScCO2 to recover both procyanidins and proanthocyanidins. As a modifier, ethanol improves the recovery of procyanidins and proanthocyanidins. This is because ethanol increases the polarity of carbon dioxide; thus, combining ethanol and ScCO2 may extract polar compounds. ScCO2’s only limitation is that it only works with nonpolar compounds and oil extracts. Thus, adding ethanol can overcome this limitation in extracting high polar compounds. Putra, et al. [115] also discovered higher TPC recovery from peanut skin when there were high pressure and high temperature conditions. The optimal conditions were 29.43 MPa, 319.16 K, 0.09 mL/min, and 290.12 mg/L TPC. The results showed that a high temperature condition improves phenolic content solubility while a low temperature environment improves flavonoid solubility.
6. Future Perspective of Peanut Skin Valorization
Attention has switched from emission reduction to a more pragmatic approach in waste management [92,99]. Waste products are still viable commodities, which are essential for sustainable growth. The processing, use, and disposal of agro-industrial wastes have made them a worldwide issue. As a consequence, population growth has led to the depletion of natural resources. In the biorefinery concept, “circular economy” has emerged lately. These models aim to use and manage waste from certain industrial processes as sustainable raw materials, with an emphasis on the economic and environmental issues.
Since peanut skin is generated in enormous quantities, it must be processed immediately to prevent putrefaction. Processing waste into goods with added value has the potential to reduce waste and become a feasible solution for the world’s population. It has high antioxidative, antibacterial, and antiviral capabilities that are extremely helpful and might be used in future nutraceutical and pharmaceutical sectors. In the past, extraction techniques, such as maceration and Soxhlet, were often utilized to obtain peanut skin. The “green extraction” of plant material has, however, overtaken the classic extraction method. Green extraction has been encouraged in order to improve production at a lower price. In the absence of an organic solvent, this method may also avoid the production of hazardous residues. As a consequence, significant advances have been achieved in green extraction methods, such as MAE and ScCO2.
To meet growing demand, however, the process’s efficiency, predictability, and reproducibility of product quality must be enhanced. As emphasized in this study, microwave, ultrasonic, and ScCO2 are among the successful and trustworthy innovative techniques explored for integrating the pectin extraction method, with varied degrees of performance. Even if these processes are quantitatively and qualitatively suitable for laboratory use, a lack of expertise hinders their usual industrial use. They cannot be used for scale-up, since continuous methods are preferable.
Some of these innovations are too costly for new and small producers; however, this may not be the case for bigger specialty chemical/ingredient businesses. However, careful optimization of the process parameters of the most recent techniques is necessary. Eventually, market participants will adopt one or more of these methods to make specialized catechin and resveratrol, most likely with microwave heating for rapid mass transfer. In addition to industrially produced peanut skin, several research institutions and labs have focused on identifying and exploiting peanut skin as a raw material for catechin synthesis. In addition, there is little study on the recovery of catechin/bioactive chemicals from peanut skin using environmentally friendly methods such as UAE and ScCO2. However, classic extraction techniques such as Soxhlet and MAE were commonly used. As a consequence, there is a technical gap in the use of environmentally friendly methods to extract this bioactive compound.
7. Summary of Various Extraction Methods to Valorize the Peanut Skin
Table 5 compares the benefits and drawbacks of various extraction techniques for valorizing peanut skin. The traditional Soxhlet process yields more but has lower quality than Soxhlet ScCO2. MAE outperforms Soxhlet extraction in terms of quality because it uses a shorter extraction time at a lower temperature. The UAE is also improving the Soxhlet extraction process, resulting in less energy consumption, a shorter extraction time, and a higher quality extract. However, ultrasonic waves have been shown to degrade certain phenolic acids and produce highly reactive hydroxyl radicals inside the gas, which are drawbacks of this method. Meanwhile, ScCO2 is a simple way to valorize the peanut skin and is categorized as green and sustainable. This is because CO2 extracts catechin, resveratrol, and antioxidant compounds from peanut skin, and it is inert and safe for health and wellness products, making it a green solvent. Furthermore, the extraction time is reduced compared to previous techniques such as Soxhlet, MAE, and UAE. Thus, energy consumption can be reduced. The only disadvantage of ScCO2 is that it is operated in high pressure conditions. Therefore, this will impact upon higher cost production and safety.

Table 5.
Summary of advantages and disadvantages of peanut extraction.
Author Contributions
Conceptualization, N.R.P.; methodology, A.H.A.A.; software, D.N.R.; validation, D.N.R.; writing—original draft preparation, N.R.P.; writing—review and editing, A.H.A.A.; J.J., W.W., S.S., I.I. and L.Q. visualization, N.R.P.; supervision, M.A.C.Y.; Funding: A.S.H.M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the Professional Development Research University grant (R.J130000.7113.05E53) from Universiti Teknologi Malaysia for supporting this work and the Faculty of Resilience, Rabdan Academy, Abu Dhabi, United Arab Emirates for the publication fee.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request from the authors.
Acknowledgments
The authors would like to acknowledge the Professional Development Research University grant (R.J130000.7113.05E53) from Universiti Teknologi Malaysia and the Faculty of Resilience, Rabdan Academy, Abu Dhabi, United Arab Emirates for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| AA | antioxidant activity |
| AH | antioxidant |
| CER | constant extraction rate |
| CHD | coronary heart disease |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl-hydrate |
| FER | falling extraction rate |
| GAE | gallic acid equivalent |
| IL | ionic liquid |
| MAE | microwave-assisted extraction |
| NADES | natural deep eutectic solvent |
| ORAC | oxygen radical absorbance capacity |
| R | radical |
| ROS | reactive oxygen species |
| ScCO2 | supercritical carbon dioxide |
| SWE | subcritical water extraction |
| TAA | total antioxidant activity |
| TE | Trolox equivalent |
| TFC | total flavonoid compounds |
| TPC | total phenolic compounds |
| UV | ultraviolet |
References
- FAO. Production of Peanut; FAO: Rome, Italy, 2022. [Google Scholar]
- Putra, N.R.; Rizkiyah, D.N.; Qomariyah, L.; Aziz, A.H.A.; Veza, I.; Yunus, M.A.C. Experimental and modeling for catechin and epicatechin recovery from peanut skin using subcritical ethanol. J. Food Process. Eng. 2023, 46, e14275. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Yian, L.N.; Ramli, W.D.; Yunus, M.A.C. Optimization of supercritical carbon dioxide and co-solvent ethanol extraction of wasted peanut skin using response surface methodology. MATEC Web Conf. 2018, 156, 2005. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Machmudah, S.; Jumakir, J.; Waluyo, W.; Che Yunus, M.A. Procyanidin and proanthocyanidin extraction from Arachis hypogaea skins by using supercritical carbon dioxide: Optimization and modeling. J. Food Process. Preserv. 2021, 45, e15689. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Rizkiyah, D.N.; Jusoh, W.M.S.W.; Idham, Z.; Putra, N.R.; Che Yunus, M.A. Investigation of Phenolic, Flavonoid and Antioxidant Recovery and Solubility from Roselle Using Supercritical Carbon Dioxide: Experimental and Modelling. J. Food Process. Preserv. 2022, 46, e16670. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Aziz, A.H.A.; Idham, Z.; Qomariyah, L.; Yunus, M.A.C. Extraction rate of Valuable Compounds from Peanut Skin Waste by Ethanol-Assisted Supercritical Carbon Dioxide: Modelling and Optimization. Malays. J. Fundam. Appl. Sci. 2022, 18, 157–170. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Veza, I.; Jumakir, J.; Waluyo, W.; Suparwoto, S.; Qomariyah, L.; Yunus, M.A.C. Solubilization and Extraction of Valuable Compounds from Peanut skin in Subcritical Water. J. Food Process. Preserv. 2022, 46, e17005. [Google Scholar] [CrossRef]
- Bharthare, P.; Shrivastava, P.; Singh, P.; Ttiwari, A. Peanut shell as renewable energy source and their utility in production of ethanol. Int. J. Adv. Res 2014, 2, 1–12. [Google Scholar]
- Jin, S.; Gao, M.; Kong, W.; Yang, B.; Kuang, H.; Yang, B.; Fu, Y.; Cheng, Y.; Li, H. Enhanced and sustainable pretreatment for bioconversion and extraction of resveratrol from peanut skin using ultrasound-assisted surfactant aqueous system with microbial consortia immobilized on cellulose. 3 Biotech 2020, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Kalender, Y.; Kaya, S.; Durak, D.; Uzun, F.G.; Demir, F. Protective effects of catechin and quercetin on antioxidant status, lipid peroxidation and testis-histoarchitecture induced by chlorpyrifos in male rats. Environ. Toxicol. Pharmacol. 2012, 33, 141–148. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, S.; Magnusdottir, S. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J. Agric. Food Chem. 2002, 50, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.M.; Resurreccion, A.V. Resveratrol in peanuts. Crit. Rev. Food Sci. Nutr. 2014, 54, 734–770. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-T.; Li, X.; Xie, M.-L.; Huang, Z.; Huang, Y.-X.; Wu, G.-X.; Peng, Z.-R.; Sun, Y.-N.; Ming, Q.-L.; Liu, Y.-X. Resveratrol: Review on its discovery, anti-leukemia effects and pharmacokinetics. Chem.-Biol. Interact. 2019, 306, 29–38. [Google Scholar] [CrossRef]
- Mandana, B.; Russly, A.; Farah, S.; Noranizan, M.; Zaidul, I.; Ali, G. Antioxidant activity of winter melon (Benincasa hispida) seeds using conventional Soxhlet extraction technique. Int. Food Res. J. 2012, 19, 229–234. [Google Scholar]
- Salgın, U.; Salgın, S.; Ekici, D.D.; UludaĿ, G. Oil recovery in rosehip seeds from food plant waste products using supercritical CO2 extraction. J. Supercrit. Fluids 2016, 118, 194–202. [Google Scholar] [CrossRef]
- Sodeifian, G.; Saadati Ardestani, N.; Sajadian, S.A.; Ghorbandoost, S. Application of supercritical carbon dioxide to extract essential oil from Cleome coluteoides Boiss: Experimental, response surface and grey wolf optimization methodology. J. Supercrit. Fluids 2016, 114, 55–63. [Google Scholar] [CrossRef]
- Milala, J.; Grzelak-Błaszczyk, K.; Sójka, M.; Kosmala, M.; Dobrzyńska-Inger, A.; Rój, E. Changes of bioactive components in berry seed oils during supercritical CO2 extraction. J. Food Process. Preserv. 2018, 42, e13368. [Google Scholar] [CrossRef]
- De Melo, M.; Şen, A.; Silvestre, A.J.; Pereira, H.; Silva, C.M. Experimental and modeling study of supercritical CO2 extraction of Quercus cerris cork: Influence of ethanol and particle size on extraction kinetics and selectivity to friedelin. Sep. Purif. Technol. 2017, 187, 34–45. [Google Scholar] [CrossRef]
- Pour Hosseini, S.R.; Tavakoli, O.; Sarrafzadeh, M.H. Experimental optimization of SC-CO2 extraction of carotenoids from Dunaliella salina. J. Supercrit. Fluids 2017, 121, 89–95. [Google Scholar] [CrossRef]
- Chan, Y.H.; Yusup, S.; Quitain, A.T.; Chai, Y.H.; Uemura, Y.; Loh, S.K. Extraction of palm kernel shell derived pyrolysis oil by supercritical carbon dioxide: Evaluation and modeling of phenol solubility. Biomass Bioenergy 2018, 116, 106–112. [Google Scholar] [CrossRef]
- Taheri, S.; Brodie, G.; Gupta, D. Fluidisation of lentil seeds during microwave drying and disinfection could prevent detrimental impacts on their chemical and biochemical characteristics. LWT 2020, 129, 109534. [Google Scholar] [CrossRef]
- Sarip, M.S.M.; Yamashita, Y.; Morad, N.A.; Yunus, M.A.C.; Aziz, M.K.A. Modeling and Optimization of the Hot Compressed Water Extraction of Palm Oil Using Artificial Neural Network. J. Chem. Eng. Jpn. 2016, 49, 614–621. [Google Scholar] [CrossRef]
- Hans, N.; Naik, S.N.; Malik, A. Platform Molecules from Algae by Using Supercritical CO2 and Subcritical Water Extraction. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; pp. 229–243. [Google Scholar]
- Aguiló-Aguayo, I.; Walton, J.; Viñas, I.; Tiwari, B.K. Ultrasound assisted extraction of polysaccharides from mushroom by-products. LWT 2017, 77, 92–99. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I.; Dai, J. Peanut skin procyanidins: Composition and antioxidant activities as affected by processing. J. Food Compos. Anal. 2006, 19, 364–371. [Google Scholar] [CrossRef]
- Nepote, V.; Grosso, N.R.; Guzman, C. Extraction of antioxidant components from peanut skins. Grasas Aceites 2002, 53, 391–395. [Google Scholar] [CrossRef]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Putra, N.R.; Yunus, M.A.C.; Ruslan, M.S.H.; Idham, Z.; Idrus, F.N. Comparison extraction of peanut skin between CO2 supercritical fluid extraction and soxhlet extraction in term of oil yield and catechin. Pertanika J. Sci. Technol. 2018, 26, 799–810. [Google Scholar]
- Li, Z.; Huang, D.; Tang, Z.; Deng, C. Microwave-assisted extraction followed by CE for determination of catechin and epicatechin in green tea. J. Sep. Sci. 2010, 33, 1079–1084. [Google Scholar] [CrossRef]
- Katalinić, V.; Milos, M.; Modun, D.; Musić, I.; Boban, M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem. 2004, 86, 593–600. [Google Scholar] [CrossRef]
- Park, H.S.; Choi, H.-K.; Lee, S.J.; Park, K.W.; Choi, S.-G.; Kim, K.H. Effect of mass transfer on the removal of caffeine from green tea by supercritical carbon dioxide. J. Supercrit. Fluids 2007, 42, 205–211. [Google Scholar] [CrossRef]
- Ghassempour, A.; Mollayi, S.; Farzaneh, M.; Sharifi-Tehrani, A.; Aboul-Enein, H.Y. Variation of Catechin, epicatechin and their enantiomers concentrations before and after wheat cultivar-Puccinia triticina infection. Food Chem. 2011, 125, 1287–1290. [Google Scholar] [CrossRef]
- Casazza, A.A.; Pettinato, M.; Perego, P. Polyphenols from apple skins: A study on microwave-assisted extraction optimization and exhausted solid characterization. Sep. Purif. Technol. 2020, 240, 116640. [Google Scholar] [CrossRef]
- Okiyama, D.C.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E. Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Rémésy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 73160, (-)-Catechin. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Catechin (accessed on 1 February 2022).
- Vuong, Q.V.; Golding, J.B.; Nguyen, M.; Roach, P.D. Extraction and isolation of catechins from tea. J. Sep. Sci. 2010, 33, 3415–3428. [Google Scholar] [CrossRef]
- Wan, X.; Li, D.; Zhang, Z. Antioxidant Properties and Mechanisms of Tea Polyphenols. In Tea and Tea Products: Chemistry and Health-Promoting Properties; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ho, C.-T.; Lin, J.-K.; Shahidi, F. Tea and Tea Products: Chemistry and Health-Promoting Properties; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Nakao, M.; Takio, S.; Ono, K. Alkyl peroxyl radical-scavenging activity of catechins. Phytochemistry 1998, 49, 2379–2382. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables–the millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar] [CrossRef]
- Gogoi, P.; Hazarika, S.; Dutta, N.N.; Rao, P.G. Kinetics and mechanism on laccase catalyzed synthesis of poly (allylamine)–catechin conjugate. Chem. Eng. J. 2010, 163, 86–92. [Google Scholar] [CrossRef]
- Lu, N.; Chen, P.; Yang, Q.; Peng, Y.-Y. Anti-and pro-oxidant effects of (+)-catechin on hemoglobin-induced protein oxidative damage. Toxicol. Vitr. 2011, 25, 833–838. [Google Scholar] [CrossRef]
- Ruslan, M.S.H.; Mohd Azizi, C.; Idham, Z.; Morad, N.A.; Ali, A. Parametric evaluation for extraction of catechin from Areca catechu Linn seeds using supercritical CO2 extraction. J. Teknol. 2015, 74, 87–92. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar]
- Abdel Raoof, G.F.; Mohamed, K.Y. Chapter 10—Natural Products for the Management of Diabetes. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 323–374. [Google Scholar]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Romero-Pérez, A.I.; Ibern-Gómez, M.; Lamuela-Raventós, R.M.; de la Torre-Boronat, M.C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 1999, 47, 1533–1536. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Hong, J.-Y.; Chun, H.S.; Lee, S.K.; Min, H.-Y. Ultrasonication-assisted extraction of resveratrol from grapes. J. Food Eng. 2006, 77, 725–730. [Google Scholar] [CrossRef]
- Liu, F.-C.; Tsai, H.-I.; Yu, H.-P. Organ-protective effects of red wine extract, resveratrol, in oxidative stress-mediated reperfusion injury. Oxidative Med. Cell. Longev. 2015, 2015, 568634. [Google Scholar] [CrossRef]
- Wang, Y.; Catana, F.; Yang, Y.; Roderick, R.; van Breemen, R.B. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J. Agric. Food Chem. 2002, 50, 431–435. [Google Scholar] [CrossRef]
- Salvador, I.; Massarioli, A.P.; Silva, A.P.; Malaguetta, H.; Melo, P.S.; Alencar, S.M. Can we conserve trans-resveratrol content and antioxidant activity during industrial production of chocolate? J. Sci. Food Agric. 2019, 99, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Siemann, E.; Creasy, L. Concentration of the phytoalexin resveratrol in wine. Am. J. Enol. Vitic. 1992, 43, 49–52. [Google Scholar] [CrossRef]
- Renaud, S.d.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Nemzer, B.; Stalmach, A.; Ali, S.; Combet, E. Polyphenolic and hydroxycinnamate contents of whole coffee fruits from China, India, and Mexico. J. Agric. Food Chem. 2013, 61, 5298–5309. [Google Scholar] [CrossRef]
- Leadbeater, N.; Ondruschka, B. Ionic liquids and their heating behaviour during microwave irradiation–a state of the art report and challenge to assessment. Letter and reply. Green Chem. 2003, 5, 677–678. [Google Scholar] [CrossRef]
- Valencia-Hernandez, L.J.; Wong-Paz, J.E.; Ascacio-Valdés, J.A.; Chávez-González, M.L.; Contreras-Esquivel, J.C.; Aguilar, C.N. Procyanidins: From agro-industrial waste to food as bioactive molecules. Foods 2021, 10, 3152. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Phenolic compounds of pomegranate byproducts (outer skin, mesocarp, divider membrane) and their antioxidant activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef]
- Sarnoski, P.J.; Johnson, J.V.; Reed, K.A.; Tanko, J.M.; O’Keefe, S.F. Separation and characterisation of proanthocyanidins in Virginia type peanut skins by LC–MSn. Food Chem. 2012, 131, 927–939. [Google Scholar] [CrossRef]
- Kaur, R.; Shekhar, S.; Prasad, K. Secondary Metabolites of Fruits and Vegetables with Antioxidant Potential. In Secondary Metabolites—Trends and Reviews; IntechOpen: London, UK, 2022. [Google Scholar]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Arsad, N.H.; Yunus, M.A.C.; Zaini, M.A.A.; Rahman, Z.A.; Idham, Z. Effect of Operating Conditions of Supercritical Carbon Dioxide on Piper Betle Leave Oil Yield and Antioxidant Activity. Int. J. Appl. Chem. 2016, 12, 741–751. [Google Scholar]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Yunus, M.A.C.; Machmudah, S.; Idham, Z.b.; Hazwan Ruslan, M.S. Effect of particle size on yield extract and antioxidant activity of peanut skin using modified supercritical carbon dioxide and soxhlet extraction. J. Food Process. Preserv. 2018, 42, e13689. [Google Scholar] [CrossRef]
- Shah, S.; Gani, A.; Ahmad, M.; Shah, A.; Gani, A.; Masoodi, F. In vitro antioxidant and antiproliferative activity of microwave-extracted green tea and black tea (Camellia sinensis): A comparative study. NutraFoods 2015, 14, 207–215. [Google Scholar] [CrossRef]
- Putra, N.R.; Wibobo, A.G.; Machmudah, S.; Winardi, S. Recovery of valuable compounds from palm-pressed fiber by using supercritical CO2 assisted by ethanol: Modeling and optimization. Sep. Sci. Technol. 2020, 55, 3126–3139. [Google Scholar] [CrossRef]
- Ozcan, T.; Sahin, S.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L. Assessment of antioxidant capacity by method comparison and amino acid characterisation in buffalo milk kefir. Int. J. Dairy Technol. 2019, 72, 65–73. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Zengin, G.; Bahadori, S.; Dinparast, L.; Movahhedin, N. Phenolic composition and functional properties of wild mint (Mentha longifolia var. calliantha (Stapf) Briq.). Int. J. Food Prop. 2018, 21, 183–193. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Jiménez, M.; Juarez, N.; Jiménez-Fernández, V.; Monribot-Villanueva, J.; Guerrero-Analco, J. Phenolic Compounds and Antioxidant Activity of Wild Grape (Vitis Tiliifolia). Ital. J. Food Sci. 2017, 30, 128–143. [Google Scholar]
- Hasmida, M.; Liza, M.; Nur Syukriah, A.; Harisun, Y.; Mohd Azizi, C.; Fadzilah Adibah, A. Total phenolic content and antioxidant activity of quercus infectoria galls using supercritical CO2 extraction technique and its comparison with soxhlet extraction. Pertanika J. Sci. Technol. 2015, 23, 287–295. [Google Scholar]
- Bodoira, R.; Rossi, Y.; Montenegro, M.; Maestri, D.; Velez, A. Extraction of antioxidant polyphenolic compounds from peanut skin using water-ethanol at high pressure and temperature conditions. J. Supercrit. Fluids 2017, 128, 57–65. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Machmudah, S.; Yunus, M.A.C. Solubility of catechin and epicatechin from Arachis Hypogea skins wastes by using supercritical carbon dioxide-ethanol and its optimization. J. Food Meas. Charact. 2021, 15, 2031–2038. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I. Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem. 2005, 90, 199–206. [Google Scholar] [CrossRef]
- Ying, L. Study on extraction of peanut-skin red pigment with ultrasonic assisted. Cereals Oils 2009, 10, 12. [Google Scholar]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S.F. Optimizing the extraction of phenolic antioxidants from peanut skins using response surface methodology. J. Agric. Food Chem. 2009, 57, 3064–3072. [Google Scholar] [CrossRef]
- Wu, J.-G.; Chen, K.; Zhang, X.-Y. Extraction of peanut skin total polyphenols by microwave combined-assisted ethanol method. China Food Addit. 2010, 5, 103–106. [Google Scholar]
- Braga, G.C.; Melo, P.S.; Bergamaschi, K.B.; Tiveron, A.P.; Massarioli, A.P.; Alencar, S.M.d. Extraction yield, antioxidant activity andphenolics from grape, mango and peanut agro-industrial by-products. Ciência Rural 2016, 46, 1498–1504. [Google Scholar] [CrossRef]
- Chirinos, R.; Rogez, H.; Campos, D.; Pedreschi, R.; Larondelle, Y. Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers. Sep. Purif. Technol. 2007, 55, 217–225. [Google Scholar]
- Salih, A.M.; Al-Qurainy, F.; Nadeem, M.; Tarroum, M.; Khan, S.; Shaikhaldein, H.O.; Al-Hashimi, A.; Alfagham, A.; Alkahtani, J. Optimization method for phenolic compounds extraction from medicinal plant (Juniperus procera) and phytochemicals screening. Molecules 2021, 26, 7454. [Google Scholar] [CrossRef] [PubMed]
- Galgano, F.; Tolve, R.; Scarpa, T.; Caruso, M.C.; Lucini, L.; Senizza, B.; Condelli, N. Extraction kinetics of total polyphenols, flavonoids, and condensed tannins of lentil seed coat: Comparison of solvent and extraction methods. Foods 2021, 10, 1810. [Google Scholar] [CrossRef]
- Bashmil, Y.M.; Ali, A.; Bk, A.; Dunshea, F.R.; Suleria, H.A. Screening and characterization of phenolic compounds from australian grown bananas and their antioxidant capacity. Antioxidants 2021, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Abdul Aziz, A.H.; Putra, N.R.; Kong, H.; Che Yunus, M.A. Supercritical carbon dioxide extraction of sinensetin, isosinensetin, and rosmarinic acid from Orthosiphon stamineus leaves: Optimization and modeling. Arab. J. Sci. Eng. 2020, 45, 7467–7476. [Google Scholar] [CrossRef]
- Mohd-Nasir, H.; Putra, N.R.; Chuo, S.C.; Daud, N.M.; Hartati, H.; Bakeri, N.; Ruslan, M.S.; Mohd-Setapar, S.H.; Ahmad, A.; Md Salleh, L. Optimization of the supercritical carbon dioxide extraction of Quercus infectoria galls extracts and its bioactivities. J. Food Process. Preserv. 2021, 45, e15156. [Google Scholar] [CrossRef]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Norodin, N.S.M.; Sarkawi, N.S.; Hamzah, M.H.S.; Nasir, H.M.; Zaidel, D.N.A.; Yunus, M.A.C.; Salleh, L.M. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Gómez-Prieto, M.S.; del Castillo, M.L.R.; Flores, G.; Santa-María, G.; Blanch, G.P. Application of Chrastil’s model to the extraction in SC-CO2 of β-carotene and lutein in Mentha spicata L. J. Supercrit. Fluids 2007, 43, 32–36. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Faizal, A.N.M.; Che Yunus, M.A. Methods and Potential in Valorization of Banana Peels Waste by Various Extraction Processes: In Review. Sustainability 2022, 14, 10571. [Google Scholar] [CrossRef]
- Guerin, T. The extraction of aged polycyclic aromatic hydrocarbon (PAH) residues from a clay soil using sonication and a Soxhlet procedure: A comparative study. J. Environ. Monit. 1999, 1, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Shiroma, C.; Rodriguez-Saona, L. Application of NIR and MIR spectroscopy in quality control of potato chips. J. Food Compos. Anal. 2009, 22, 596–605. [Google Scholar] [CrossRef]
- Wu, T.R.; Wang, H.L.; Jiang, S.W.; Liu, D.D.; Wei, F. Optimization of Extraction of Tannins from Banana Peel Using Response Surface Methodology. Appl. Mech. Mater. 2014, 678, 566–571. [Google Scholar]
- Ju, Z.; Howard, L.R. Subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin. J. Food Sci. 2005, 70, S270–S276. [Google Scholar] [CrossRef]
- Letellier, M.; Budzinski, H.; Charrier, L.; Capes, S.; Dorthe, A. Optimization by factorial design of focused microwave assisted extraction of polycyclic aromatic hydrocarbons from marine sediment. Fresenius J. Anal. Chem. 1999, 364, 228–237. [Google Scholar] [CrossRef]
- Huie, C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002, 373, 23–30. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Che Yunus, M.A.; Veza, I.; Harny, I.; Tirta, A. Waste to Wealth of Apple Pomace Valorization by Past and Current Extraction Processes: A Review. Sustainability 2023, 15, 830. [Google Scholar] [CrossRef]
- Zuloaga, O.; Etxebarria, N.; Fernández, L.A.; Madariaga, J.M. Optimisation and comparison of microwave-assisted extraction and Soxhlet extraction for the determination of polychlorinated biphenyls in soil samples using an experimental design approach. Talanta 1999, 50, 345–357. [Google Scholar] [CrossRef]
- Latha, C. Microwave-assisted extraction of embelin from Embelia ribes. Biotechnol. Lett. 2007, 29, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, M.J.; Bélanger, J.M.; Padilla, F.C.; Paré, J.J. Influence of solvent, matrix dielectric properties, and applied power on the liquid-phase microwave-assisted processes (MAP™) extraction of ginger (Zingiber officinale). Food Res. Int. 2003, 36, 499–504. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Gujar, J.; Wagh, S.; Gaikar, V. Experimental and modeling studies on microwave-assisted extraction of thymol from seeds of Trachyspermum ammi (TA). Sep. Purif. Technol. 2010, 70, 257–264. [Google Scholar] [CrossRef]
- Farzaneh, V.; Carvalho, I.S. Modelling of microwave assisted extraction (MAE) of anthocyanins (TMA). J. Appl. Res. Med. Aromat. Plants 2017, 6, 92–100. [Google Scholar] [CrossRef]
- Bai, L.-S.; Yang, Y.; Lv, D.-D. Microwave extraction of total flavonoids in peanut skins. J. Chin. Med. Mater. 2012, 35, 977–980. [Google Scholar]
- Xiao, W.; Han, L.; Shi, B. Microwave-assisted extraction of flavonoids from Radix Astragali. Sep. Purif. Technol. 2008, 62, 614–618. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Mandal, V.; Mandal, S.C. Design and performance evaluation of a microwave based low carbon yielding extraction technique for naturally occurring bioactive triterpenoid: Oleanolic acid. Biochem. Eng. J. 2010, 50, 63–70. [Google Scholar] [CrossRef]
- Ishak, N.A.; Razak, N.A.A.; Dek, M.S.P.; Baharuddin, A.S. Production of High Tannin Content and Antioxidant Activity Extract from an Unripe Peel of Musa acuminata (Cavendish) Using Ultrasound-Assisted Extraction (UAE). BioResources 2020, 15, 1877–1893. [Google Scholar]
- De la Guardia, M.; Armenta, S. Origins of green analytical chemistry. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2011; Volume 57, pp. 1–23. [Google Scholar]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: Optimization, characterization and bioactivity. Carbohydr. Polym. 2019, 222, 114992. [Google Scholar] [CrossRef]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Syahdi, R.R.; Nadyana, R.; Putri, R.H.; Santi, R.; Mun’im, A. Application of green extraction methods to resveratrol extraction from peanut (Arachis Hypogaea L.) skin. Int. J. Appl. Pharm 2020, 12, 38–42. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Machmudah, S.; Shalleh, L.M.; Che Yunus, M.A. Recovery and solubility of flavonoid and phenolic contents from Arachis Hypogea in supercritical carbon dioxide assisted by ethanol as cosolvent. J. Food Process. Preserv. 2020, 44, e14768. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).