Valorization of Peanut Skin as Agricultural Waste Using Various Extraction Methods: A Review
Abstract
:1. Introduction
2. Physicochemical Properties of Peanut Skin
3. Bioactive Compounds in Peanut Skin
3.1. Catechin
3.2. Resveratrol
3.3. Procyanidins
4. Antioxidant Activity of Peanut Skin Extract
5. Valorization of Peanut Skin by Conventional and Green Extraction
5.1. Soxhlet Extraction on Peanut Skin Valorization
5.2. MAE on Peanut Skin Valorization
5.3. UAE on Peanut Skin Valorization
5.4. ScCO2 Extraction on Peanut Skin Valorization
6. Future Perspective of Peanut Skin Valorization
7. Summary of Various Extraction Methods to Valorize the Peanut Skin
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AA | antioxidant activity |
| AH | antioxidant |
| CER | constant extraction rate |
| CHD | coronary heart disease |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl-hydrate |
| FER | falling extraction rate |
| GAE | gallic acid equivalent |
| IL | ionic liquid |
| MAE | microwave-assisted extraction |
| NADES | natural deep eutectic solvent |
| ORAC | oxygen radical absorbance capacity |
| R | radical |
| ROS | reactive oxygen species |
| ScCO2 | supercritical carbon dioxide |
| SWE | subcritical water extraction |
| TAA | total antioxidant activity |
| TE | Trolox equivalent |
| TFC | total flavonoid compounds |
| TPC | total phenolic compounds |
| UV | ultraviolet |
References
- FAO. Production of Peanut; FAO: Rome, Italy, 2022. [Google Scholar]
- Putra, N.R.; Rizkiyah, D.N.; Qomariyah, L.; Aziz, A.H.A.; Veza, I.; Yunus, M.A.C. Experimental and modeling for catechin and epicatechin recovery from peanut skin using subcritical ethanol. J. Food Process. Eng. 2023, 46, e14275. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Yian, L.N.; Ramli, W.D.; Yunus, M.A.C. Optimization of supercritical carbon dioxide and co-solvent ethanol extraction of wasted peanut skin using response surface methodology. MATEC Web Conf. 2018, 156, 2005. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Machmudah, S.; Jumakir, J.; Waluyo, W.; Che Yunus, M.A. Procyanidin and proanthocyanidin extraction from Arachis hypogaea skins by using supercritical carbon dioxide: Optimization and modeling. J. Food Process. Preserv. 2021, 45, e15689. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Rizkiyah, D.N.; Jusoh, W.M.S.W.; Idham, Z.; Putra, N.R.; Che Yunus, M.A. Investigation of Phenolic, Flavonoid and Antioxidant Recovery and Solubility from Roselle Using Supercritical Carbon Dioxide: Experimental and Modelling. J. Food Process. Preserv. 2022, 46, e16670. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Aziz, A.H.A.; Idham, Z.; Qomariyah, L.; Yunus, M.A.C. Extraction rate of Valuable Compounds from Peanut Skin Waste by Ethanol-Assisted Supercritical Carbon Dioxide: Modelling and Optimization. Malays. J. Fundam. Appl. Sci. 2022, 18, 157–170. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Veza, I.; Jumakir, J.; Waluyo, W.; Suparwoto, S.; Qomariyah, L.; Yunus, M.A.C. Solubilization and Extraction of Valuable Compounds from Peanut skin in Subcritical Water. J. Food Process. Preserv. 2022, 46, e17005. [Google Scholar] [CrossRef]
- Bharthare, P.; Shrivastava, P.; Singh, P.; Ttiwari, A. Peanut shell as renewable energy source and their utility in production of ethanol. Int. J. Adv. Res 2014, 2, 1–12. [Google Scholar]
- Jin, S.; Gao, M.; Kong, W.; Yang, B.; Kuang, H.; Yang, B.; Fu, Y.; Cheng, Y.; Li, H. Enhanced and sustainable pretreatment for bioconversion and extraction of resveratrol from peanut skin using ultrasound-assisted surfactant aqueous system with microbial consortia immobilized on cellulose. 3 Biotech 2020, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Kalender, Y.; Kaya, S.; Durak, D.; Uzun, F.G.; Demir, F. Protective effects of catechin and quercetin on antioxidant status, lipid peroxidation and testis-histoarchitecture induced by chlorpyrifos in male rats. Environ. Toxicol. Pharmacol. 2012, 33, 141–148. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, S.; Magnusdottir, S. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J. Agric. Food Chem. 2002, 50, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.M.; Resurreccion, A.V. Resveratrol in peanuts. Crit. Rev. Food Sci. Nutr. 2014, 54, 734–770. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-T.; Li, X.; Xie, M.-L.; Huang, Z.; Huang, Y.-X.; Wu, G.-X.; Peng, Z.-R.; Sun, Y.-N.; Ming, Q.-L.; Liu, Y.-X. Resveratrol: Review on its discovery, anti-leukemia effects and pharmacokinetics. Chem.-Biol. Interact. 2019, 306, 29–38. [Google Scholar] [CrossRef]
- Mandana, B.; Russly, A.; Farah, S.; Noranizan, M.; Zaidul, I.; Ali, G. Antioxidant activity of winter melon (Benincasa hispida) seeds using conventional Soxhlet extraction technique. Int. Food Res. J. 2012, 19, 229–234. [Google Scholar]
- Salgın, U.; Salgın, S.; Ekici, D.D.; UludaĿ, G. Oil recovery in rosehip seeds from food plant waste products using supercritical CO2 extraction. J. Supercrit. Fluids 2016, 118, 194–202. [Google Scholar] [CrossRef]
- Sodeifian, G.; Saadati Ardestani, N.; Sajadian, S.A.; Ghorbandoost, S. Application of supercritical carbon dioxide to extract essential oil from Cleome coluteoides Boiss: Experimental, response surface and grey wolf optimization methodology. J. Supercrit. Fluids 2016, 114, 55–63. [Google Scholar] [CrossRef]
- Milala, J.; Grzelak-Błaszczyk, K.; Sójka, M.; Kosmala, M.; Dobrzyńska-Inger, A.; Rój, E. Changes of bioactive components in berry seed oils during supercritical CO2 extraction. J. Food Process. Preserv. 2018, 42, e13368. [Google Scholar] [CrossRef]
- De Melo, M.; Şen, A.; Silvestre, A.J.; Pereira, H.; Silva, C.M. Experimental and modeling study of supercritical CO2 extraction of Quercus cerris cork: Influence of ethanol and particle size on extraction kinetics and selectivity to friedelin. Sep. Purif. Technol. 2017, 187, 34–45. [Google Scholar] [CrossRef]
- Pour Hosseini, S.R.; Tavakoli, O.; Sarrafzadeh, M.H. Experimental optimization of SC-CO2 extraction of carotenoids from Dunaliella salina. J. Supercrit. Fluids 2017, 121, 89–95. [Google Scholar] [CrossRef]
- Chan, Y.H.; Yusup, S.; Quitain, A.T.; Chai, Y.H.; Uemura, Y.; Loh, S.K. Extraction of palm kernel shell derived pyrolysis oil by supercritical carbon dioxide: Evaluation and modeling of phenol solubility. Biomass Bioenergy 2018, 116, 106–112. [Google Scholar] [CrossRef]
- Taheri, S.; Brodie, G.; Gupta, D. Fluidisation of lentil seeds during microwave drying and disinfection could prevent detrimental impacts on their chemical and biochemical characteristics. LWT 2020, 129, 109534. [Google Scholar] [CrossRef]
- Sarip, M.S.M.; Yamashita, Y.; Morad, N.A.; Yunus, M.A.C.; Aziz, M.K.A. Modeling and Optimization of the Hot Compressed Water Extraction of Palm Oil Using Artificial Neural Network. J. Chem. Eng. Jpn. 2016, 49, 614–621. [Google Scholar] [CrossRef]
- Hans, N.; Naik, S.N.; Malik, A. Platform Molecules from Algae by Using Supercritical CO2 and Subcritical Water Extraction. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; pp. 229–243. [Google Scholar]
- Aguiló-Aguayo, I.; Walton, J.; Viñas, I.; Tiwari, B.K. Ultrasound assisted extraction of polysaccharides from mushroom by-products. LWT 2017, 77, 92–99. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I.; Dai, J. Peanut skin procyanidins: Composition and antioxidant activities as affected by processing. J. Food Compos. Anal. 2006, 19, 364–371. [Google Scholar] [CrossRef]
- Nepote, V.; Grosso, N.R.; Guzman, C. Extraction of antioxidant components from peanut skins. Grasas Aceites 2002, 53, 391–395. [Google Scholar] [CrossRef]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Putra, N.R.; Yunus, M.A.C.; Ruslan, M.S.H.; Idham, Z.; Idrus, F.N. Comparison extraction of peanut skin between CO2 supercritical fluid extraction and soxhlet extraction in term of oil yield and catechin. Pertanika J. Sci. Technol. 2018, 26, 799–810. [Google Scholar]
- Li, Z.; Huang, D.; Tang, Z.; Deng, C. Microwave-assisted extraction followed by CE for determination of catechin and epicatechin in green tea. J. Sep. Sci. 2010, 33, 1079–1084. [Google Scholar] [CrossRef]
- Katalinić, V.; Milos, M.; Modun, D.; Musić, I.; Boban, M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem. 2004, 86, 593–600. [Google Scholar] [CrossRef]
- Park, H.S.; Choi, H.-K.; Lee, S.J.; Park, K.W.; Choi, S.-G.; Kim, K.H. Effect of mass transfer on the removal of caffeine from green tea by supercritical carbon dioxide. J. Supercrit. Fluids 2007, 42, 205–211. [Google Scholar] [CrossRef]
- Ghassempour, A.; Mollayi, S.; Farzaneh, M.; Sharifi-Tehrani, A.; Aboul-Enein, H.Y. Variation of Catechin, epicatechin and their enantiomers concentrations before and after wheat cultivar-Puccinia triticina infection. Food Chem. 2011, 125, 1287–1290. [Google Scholar] [CrossRef]
- Casazza, A.A.; Pettinato, M.; Perego, P. Polyphenols from apple skins: A study on microwave-assisted extraction optimization and exhausted solid characterization. Sep. Purif. Technol. 2020, 240, 116640. [Google Scholar] [CrossRef]
- Okiyama, D.C.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E. Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Rémésy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 73160, (-)-Catechin. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Catechin (accessed on 1 February 2022).
- Vuong, Q.V.; Golding, J.B.; Nguyen, M.; Roach, P.D. Extraction and isolation of catechins from tea. J. Sep. Sci. 2010, 33, 3415–3428. [Google Scholar] [CrossRef]
- Wan, X.; Li, D.; Zhang, Z. Antioxidant Properties and Mechanisms of Tea Polyphenols. In Tea and Tea Products: Chemistry and Health-Promoting Properties; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ho, C.-T.; Lin, J.-K.; Shahidi, F. Tea and Tea Products: Chemistry and Health-Promoting Properties; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Nakao, M.; Takio, S.; Ono, K. Alkyl peroxyl radical-scavenging activity of catechins. Phytochemistry 1998, 49, 2379–2382. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables–the millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar] [CrossRef]
- Gogoi, P.; Hazarika, S.; Dutta, N.N.; Rao, P.G. Kinetics and mechanism on laccase catalyzed synthesis of poly (allylamine)–catechin conjugate. Chem. Eng. J. 2010, 163, 86–92. [Google Scholar] [CrossRef]
- Lu, N.; Chen, P.; Yang, Q.; Peng, Y.-Y. Anti-and pro-oxidant effects of (+)-catechin on hemoglobin-induced protein oxidative damage. Toxicol. Vitr. 2011, 25, 833–838. [Google Scholar] [CrossRef]
- Ruslan, M.S.H.; Mohd Azizi, C.; Idham, Z.; Morad, N.A.; Ali, A. Parametric evaluation for extraction of catechin from Areca catechu Linn seeds using supercritical CO2 extraction. J. Teknol. 2015, 74, 87–92. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar]
- Abdel Raoof, G.F.; Mohamed, K.Y. Chapter 10—Natural Products for the Management of Diabetes. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 323–374. [Google Scholar]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Romero-Pérez, A.I.; Ibern-Gómez, M.; Lamuela-Raventós, R.M.; de la Torre-Boronat, M.C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 1999, 47, 1533–1536. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Hong, J.-Y.; Chun, H.S.; Lee, S.K.; Min, H.-Y. Ultrasonication-assisted extraction of resveratrol from grapes. J. Food Eng. 2006, 77, 725–730. [Google Scholar] [CrossRef]
- Liu, F.-C.; Tsai, H.-I.; Yu, H.-P. Organ-protective effects of red wine extract, resveratrol, in oxidative stress-mediated reperfusion injury. Oxidative Med. Cell. Longev. 2015, 2015, 568634. [Google Scholar] [CrossRef]
- Wang, Y.; Catana, F.; Yang, Y.; Roderick, R.; van Breemen, R.B. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J. Agric. Food Chem. 2002, 50, 431–435. [Google Scholar] [CrossRef]
- Salvador, I.; Massarioli, A.P.; Silva, A.P.; Malaguetta, H.; Melo, P.S.; Alencar, S.M. Can we conserve trans-resveratrol content and antioxidant activity during industrial production of chocolate? J. Sci. Food Agric. 2019, 99, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Siemann, E.; Creasy, L. Concentration of the phytoalexin resveratrol in wine. Am. J. Enol. Vitic. 1992, 43, 49–52. [Google Scholar] [CrossRef]
- Renaud, S.d.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Nemzer, B.; Stalmach, A.; Ali, S.; Combet, E. Polyphenolic and hydroxycinnamate contents of whole coffee fruits from China, India, and Mexico. J. Agric. Food Chem. 2013, 61, 5298–5309. [Google Scholar] [CrossRef]
- Leadbeater, N.; Ondruschka, B. Ionic liquids and their heating behaviour during microwave irradiation–a state of the art report and challenge to assessment. Letter and reply. Green Chem. 2003, 5, 677–678. [Google Scholar] [CrossRef]
- Valencia-Hernandez, L.J.; Wong-Paz, J.E.; Ascacio-Valdés, J.A.; Chávez-González, M.L.; Contreras-Esquivel, J.C.; Aguilar, C.N. Procyanidins: From agro-industrial waste to food as bioactive molecules. Foods 2021, 10, 3152. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Phenolic compounds of pomegranate byproducts (outer skin, mesocarp, divider membrane) and their antioxidant activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef]
- Sarnoski, P.J.; Johnson, J.V.; Reed, K.A.; Tanko, J.M.; O’Keefe, S.F. Separation and characterisation of proanthocyanidins in Virginia type peanut skins by LC–MSn. Food Chem. 2012, 131, 927–939. [Google Scholar] [CrossRef]
- Kaur, R.; Shekhar, S.; Prasad, K. Secondary Metabolites of Fruits and Vegetables with Antioxidant Potential. In Secondary Metabolites—Trends and Reviews; IntechOpen: London, UK, 2022. [Google Scholar]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Arsad, N.H.; Yunus, M.A.C.; Zaini, M.A.A.; Rahman, Z.A.; Idham, Z. Effect of Operating Conditions of Supercritical Carbon Dioxide on Piper Betle Leave Oil Yield and Antioxidant Activity. Int. J. Appl. Chem. 2016, 12, 741–751. [Google Scholar]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Yunus, M.A.C.; Machmudah, S.; Idham, Z.b.; Hazwan Ruslan, M.S. Effect of particle size on yield extract and antioxidant activity of peanut skin using modified supercritical carbon dioxide and soxhlet extraction. J. Food Process. Preserv. 2018, 42, e13689. [Google Scholar] [CrossRef]
- Shah, S.; Gani, A.; Ahmad, M.; Shah, A.; Gani, A.; Masoodi, F. In vitro antioxidant and antiproliferative activity of microwave-extracted green tea and black tea (Camellia sinensis): A comparative study. NutraFoods 2015, 14, 207–215. [Google Scholar] [CrossRef]
- Putra, N.R.; Wibobo, A.G.; Machmudah, S.; Winardi, S. Recovery of valuable compounds from palm-pressed fiber by using supercritical CO2 assisted by ethanol: Modeling and optimization. Sep. Sci. Technol. 2020, 55, 3126–3139. [Google Scholar] [CrossRef]
- Ozcan, T.; Sahin, S.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L. Assessment of antioxidant capacity by method comparison and amino acid characterisation in buffalo milk kefir. Int. J. Dairy Technol. 2019, 72, 65–73. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Zengin, G.; Bahadori, S.; Dinparast, L.; Movahhedin, N. Phenolic composition and functional properties of wild mint (Mentha longifolia var. calliantha (Stapf) Briq.). Int. J. Food Prop. 2018, 21, 183–193. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Jiménez, M.; Juarez, N.; Jiménez-Fernández, V.; Monribot-Villanueva, J.; Guerrero-Analco, J. Phenolic Compounds and Antioxidant Activity of Wild Grape (Vitis Tiliifolia). Ital. J. Food Sci. 2017, 30, 128–143. [Google Scholar]
- Hasmida, M.; Liza, M.; Nur Syukriah, A.; Harisun, Y.; Mohd Azizi, C.; Fadzilah Adibah, A. Total phenolic content and antioxidant activity of quercus infectoria galls using supercritical CO2 extraction technique and its comparison with soxhlet extraction. Pertanika J. Sci. Technol. 2015, 23, 287–295. [Google Scholar]
- Bodoira, R.; Rossi, Y.; Montenegro, M.; Maestri, D.; Velez, A. Extraction of antioxidant polyphenolic compounds from peanut skin using water-ethanol at high pressure and temperature conditions. J. Supercrit. Fluids 2017, 128, 57–65. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Machmudah, S.; Yunus, M.A.C. Solubility of catechin and epicatechin from Arachis Hypogea skins wastes by using supercritical carbon dioxide-ethanol and its optimization. J. Food Meas. Charact. 2021, 15, 2031–2038. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I. Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem. 2005, 90, 199–206. [Google Scholar] [CrossRef]
- Ying, L. Study on extraction of peanut-skin red pigment with ultrasonic assisted. Cereals Oils 2009, 10, 12. [Google Scholar]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S.F. Optimizing the extraction of phenolic antioxidants from peanut skins using response surface methodology. J. Agric. Food Chem. 2009, 57, 3064–3072. [Google Scholar] [CrossRef]
- Wu, J.-G.; Chen, K.; Zhang, X.-Y. Extraction of peanut skin total polyphenols by microwave combined-assisted ethanol method. China Food Addit. 2010, 5, 103–106. [Google Scholar]
- Braga, G.C.; Melo, P.S.; Bergamaschi, K.B.; Tiveron, A.P.; Massarioli, A.P.; Alencar, S.M.d. Extraction yield, antioxidant activity andphenolics from grape, mango and peanut agro-industrial by-products. Ciência Rural 2016, 46, 1498–1504. [Google Scholar] [CrossRef]
- Chirinos, R.; Rogez, H.; Campos, D.; Pedreschi, R.; Larondelle, Y. Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers. Sep. Purif. Technol. 2007, 55, 217–225. [Google Scholar]
- Salih, A.M.; Al-Qurainy, F.; Nadeem, M.; Tarroum, M.; Khan, S.; Shaikhaldein, H.O.; Al-Hashimi, A.; Alfagham, A.; Alkahtani, J. Optimization method for phenolic compounds extraction from medicinal plant (Juniperus procera) and phytochemicals screening. Molecules 2021, 26, 7454. [Google Scholar] [CrossRef] [PubMed]
- Galgano, F.; Tolve, R.; Scarpa, T.; Caruso, M.C.; Lucini, L.; Senizza, B.; Condelli, N. Extraction kinetics of total polyphenols, flavonoids, and condensed tannins of lentil seed coat: Comparison of solvent and extraction methods. Foods 2021, 10, 1810. [Google Scholar] [CrossRef]
- Bashmil, Y.M.; Ali, A.; Bk, A.; Dunshea, F.R.; Suleria, H.A. Screening and characterization of phenolic compounds from australian grown bananas and their antioxidant capacity. Antioxidants 2021, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Abdul Aziz, A.H.; Putra, N.R.; Kong, H.; Che Yunus, M.A. Supercritical carbon dioxide extraction of sinensetin, isosinensetin, and rosmarinic acid from Orthosiphon stamineus leaves: Optimization and modeling. Arab. J. Sci. Eng. 2020, 45, 7467–7476. [Google Scholar] [CrossRef]
- Mohd-Nasir, H.; Putra, N.R.; Chuo, S.C.; Daud, N.M.; Hartati, H.; Bakeri, N.; Ruslan, M.S.; Mohd-Setapar, S.H.; Ahmad, A.; Md Salleh, L. Optimization of the supercritical carbon dioxide extraction of Quercus infectoria galls extracts and its bioactivities. J. Food Process. Preserv. 2021, 45, e15156. [Google Scholar] [CrossRef]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Norodin, N.S.M.; Sarkawi, N.S.; Hamzah, M.H.S.; Nasir, H.M.; Zaidel, D.N.A.; Yunus, M.A.C.; Salleh, L.M. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Gómez-Prieto, M.S.; del Castillo, M.L.R.; Flores, G.; Santa-María, G.; Blanch, G.P. Application of Chrastil’s model to the extraction in SC-CO2 of β-carotene and lutein in Mentha spicata L. J. Supercrit. Fluids 2007, 43, 32–36. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Faizal, A.N.M.; Che Yunus, M.A. Methods and Potential in Valorization of Banana Peels Waste by Various Extraction Processes: In Review. Sustainability 2022, 14, 10571. [Google Scholar] [CrossRef]
- Guerin, T. The extraction of aged polycyclic aromatic hydrocarbon (PAH) residues from a clay soil using sonication and a Soxhlet procedure: A comparative study. J. Environ. Monit. 1999, 1, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Shiroma, C.; Rodriguez-Saona, L. Application of NIR and MIR spectroscopy in quality control of potato chips. J. Food Compos. Anal. 2009, 22, 596–605. [Google Scholar] [CrossRef]
- Wu, T.R.; Wang, H.L.; Jiang, S.W.; Liu, D.D.; Wei, F. Optimization of Extraction of Tannins from Banana Peel Using Response Surface Methodology. Appl. Mech. Mater. 2014, 678, 566–571. [Google Scholar]
- Ju, Z.; Howard, L.R. Subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin. J. Food Sci. 2005, 70, S270–S276. [Google Scholar] [CrossRef]
- Letellier, M.; Budzinski, H.; Charrier, L.; Capes, S.; Dorthe, A. Optimization by factorial design of focused microwave assisted extraction of polycyclic aromatic hydrocarbons from marine sediment. Fresenius J. Anal. Chem. 1999, 364, 228–237. [Google Scholar] [CrossRef]
- Huie, C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002, 373, 23–30. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Che Yunus, M.A.; Veza, I.; Harny, I.; Tirta, A. Waste to Wealth of Apple Pomace Valorization by Past and Current Extraction Processes: A Review. Sustainability 2023, 15, 830. [Google Scholar] [CrossRef]
- Zuloaga, O.; Etxebarria, N.; Fernández, L.A.; Madariaga, J.M. Optimisation and comparison of microwave-assisted extraction and Soxhlet extraction for the determination of polychlorinated biphenyls in soil samples using an experimental design approach. Talanta 1999, 50, 345–357. [Google Scholar] [CrossRef]
- Latha, C. Microwave-assisted extraction of embelin from Embelia ribes. Biotechnol. Lett. 2007, 29, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, M.J.; Bélanger, J.M.; Padilla, F.C.; Paré, J.J. Influence of solvent, matrix dielectric properties, and applied power on the liquid-phase microwave-assisted processes (MAP™) extraction of ginger (Zingiber officinale). Food Res. Int. 2003, 36, 499–504. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Gujar, J.; Wagh, S.; Gaikar, V. Experimental and modeling studies on microwave-assisted extraction of thymol from seeds of Trachyspermum ammi (TA). Sep. Purif. Technol. 2010, 70, 257–264. [Google Scholar] [CrossRef]
- Farzaneh, V.; Carvalho, I.S. Modelling of microwave assisted extraction (MAE) of anthocyanins (TMA). J. Appl. Res. Med. Aromat. Plants 2017, 6, 92–100. [Google Scholar] [CrossRef]
- Bai, L.-S.; Yang, Y.; Lv, D.-D. Microwave extraction of total flavonoids in peanut skins. J. Chin. Med. Mater. 2012, 35, 977–980. [Google Scholar]
- Xiao, W.; Han, L.; Shi, B. Microwave-assisted extraction of flavonoids from Radix Astragali. Sep. Purif. Technol. 2008, 62, 614–618. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Mandal, V.; Mandal, S.C. Design and performance evaluation of a microwave based low carbon yielding extraction technique for naturally occurring bioactive triterpenoid: Oleanolic acid. Biochem. Eng. J. 2010, 50, 63–70. [Google Scholar] [CrossRef]
- Ishak, N.A.; Razak, N.A.A.; Dek, M.S.P.; Baharuddin, A.S. Production of High Tannin Content and Antioxidant Activity Extract from an Unripe Peel of Musa acuminata (Cavendish) Using Ultrasound-Assisted Extraction (UAE). BioResources 2020, 15, 1877–1893. [Google Scholar]
- De la Guardia, M.; Armenta, S. Origins of green analytical chemistry. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2011; Volume 57, pp. 1–23. [Google Scholar]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: Optimization, characterization and bioactivity. Carbohydr. Polym. 2019, 222, 114992. [Google Scholar] [CrossRef]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Syahdi, R.R.; Nadyana, R.; Putri, R.H.; Santi, R.; Mun’im, A. Application of green extraction methods to resveratrol extraction from peanut (Arachis Hypogaea L.) skin. Int. J. Appl. Pharm 2020, 12, 38–42. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Machmudah, S.; Shalleh, L.M.; Che Yunus, M.A. Recovery and solubility of flavonoid and phenolic contents from Arachis Hypogea in supercritical carbon dioxide assisted by ethanol as cosolvent. J. Food Process. Preserv. 2020, 44, e14768. [Google Scholar] [CrossRef]
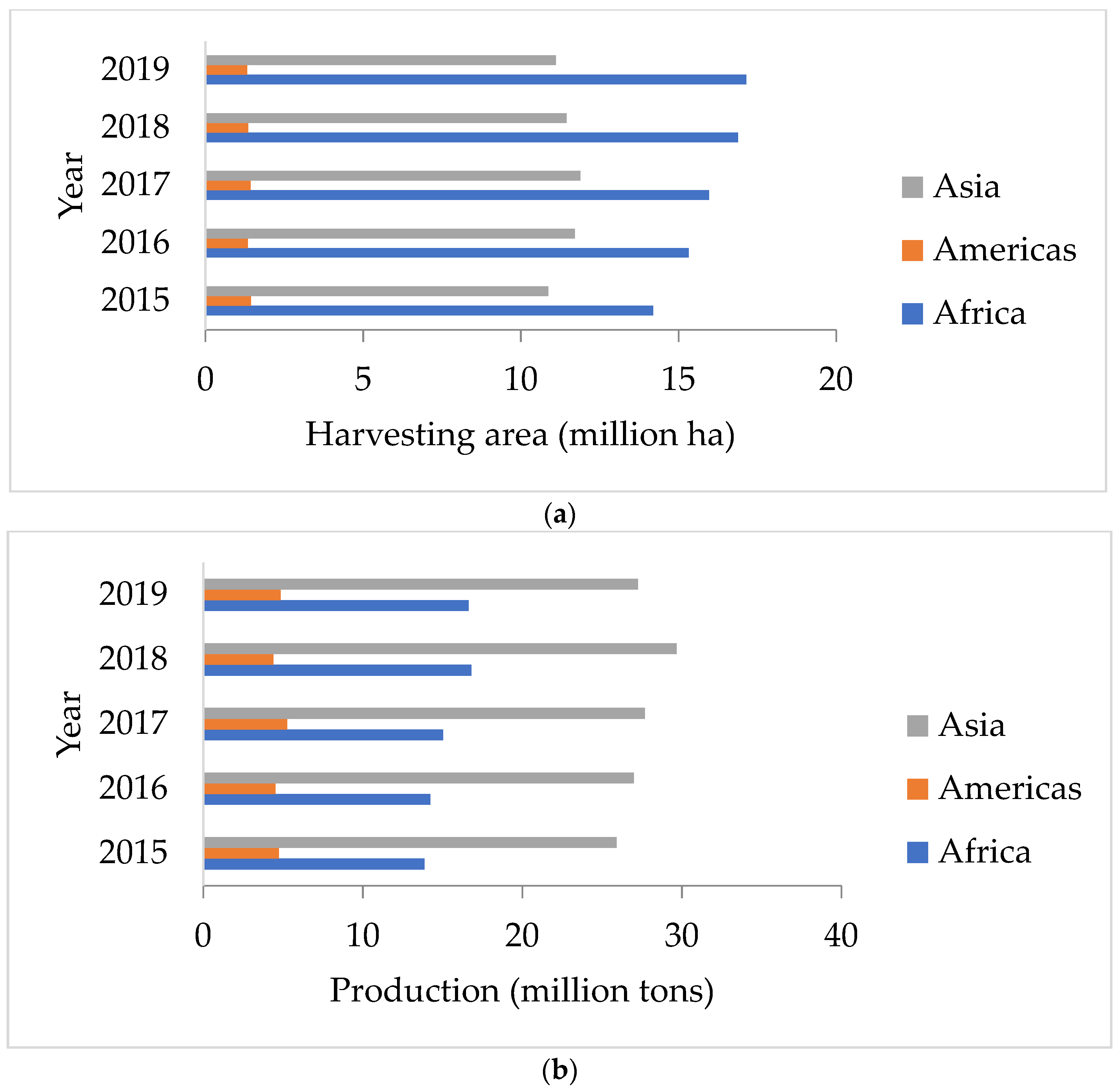




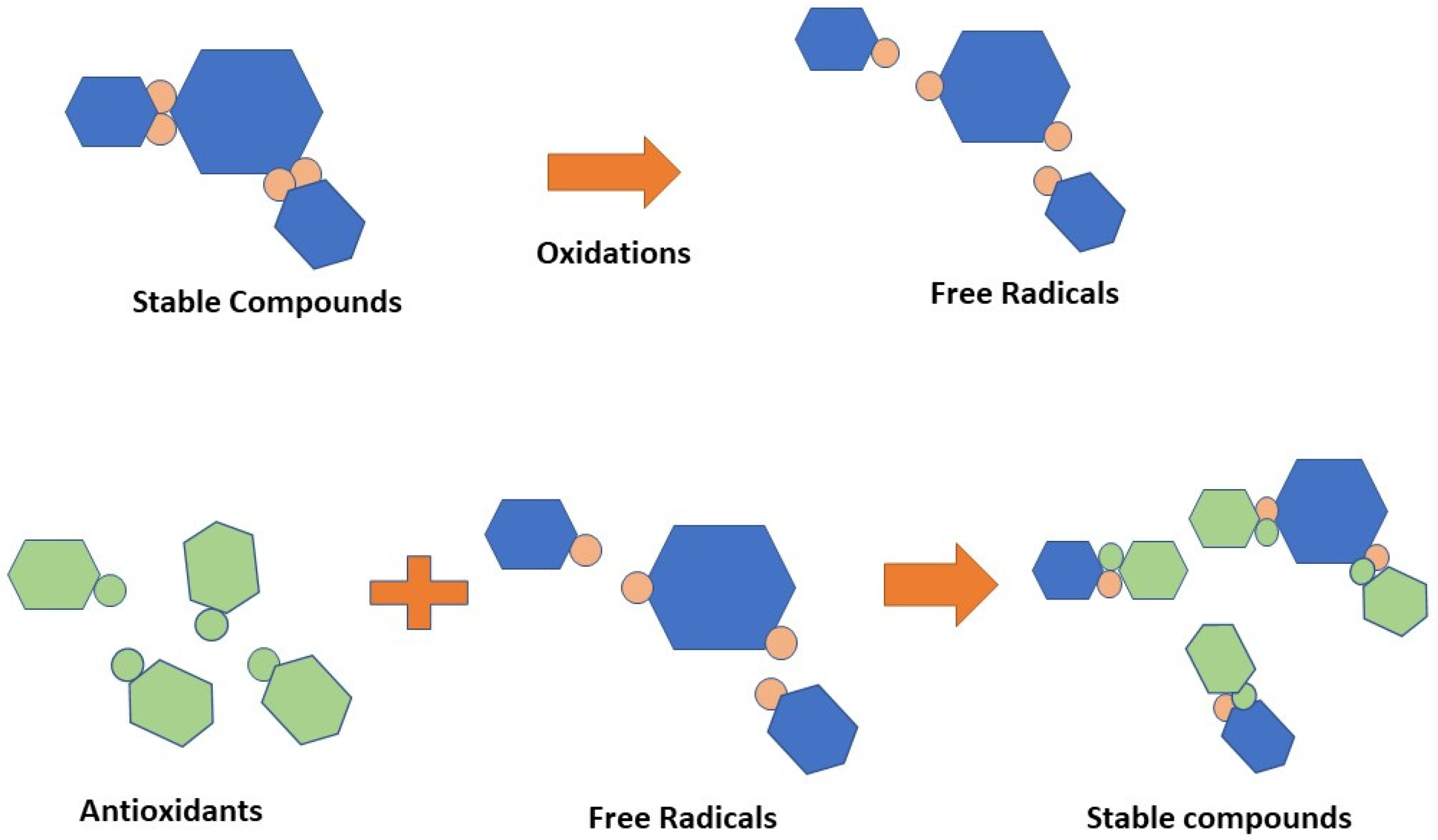
| Rank | Bioactive Compounds | AA (%) |
|---|---|---|
| 1 | Quercetin | 95 |
| 2 | Catechin | 94 |
| 3 | Trolox | 92 |
| 4 | Vitamin E | 91 |
| 5 | Butylated Hydroxytoluene (BHT) | 49 |
| Materials | Catechin (mg/g) | Source |
|---|---|---|
| Peanut skin | 16.1 | [28] |
| Areca catechu | 0.0716 | [47] |
| Green tea | 17.7 | [32] |
| Grape skin | 12 | [13] |
| Black tea | 5.6 | [14] |
| Spearmint | 0.14 | [48] |
| Materials | AA (%) | Reference |
|---|---|---|
| Peanut skin | 97.70 | [28] |
| Limnophila aromatica | 88.56 | [74] |
| Green tea | 86.05 | [69] |
| Grape skin | 91.39 | [75] |
| Black tea | 59.34 | [69] |
| Piper betle leaves | 92.34 | [67] |
| Quercus infectoria galls | 96.97 | [76] |
| Source | Results |
|---|---|
| Nepote, Grosso and Guzman [29] | The best Soxhlet extraction conditions were ethanol 70% and solvent/solid ratio of 1:20 g/mL. The highest yield obtained was 0.12 g/g. |
| Ballard, Mallikarjunan, Zhou and O’Keefe [30] | The optimum MAE variable was 90% of power, 30 s of irradiation time, and 1.5 g. The highest TPC and ORAC of skins were 143.6 mg/g and 2789 μmol/g, respectively. |
| Bodoira, et al. [77] | The maximum TPC was achieved using 60.5% ethanol at a temperature of 220 °C and a 7 g/min flow rate. |
| Putra, Yunus, Ruslan, Idham and Idrus [31] | Soxhlet extraction gave the highest yield (36.22%) using ethanol compared with ScCO2 extraction (15.47%) at 30 MPa, 70 °C. The extracts of ScCO2 extraction yielded the higher catechin (208.73 µg/g) compared with Soxhlet extraction (42.24 µg/g). |
| Putra, Rizkiyah, Zaini, Yunus, Machmudah, Idham and Hazwan Ruslan [68] | Mean particle size of 425 µm gave the highest yield extract and antioxidant activity by using ScCO2 extraction (15.53% extract, 93.43% antioxidant activity) and Soxhlet extraction (36.28%, 62.21% antioxidant activity). |
| Putra, et al. [78] | Higher pressure and lower temperature conditions increase the peanut skin oil recovery using ScCO2 extraction. |
| Yu, et al. [79] | Peanut skin was extracted using Soxhlet extraction that contains a highest TPC (125 mg/g and TAA (3.39 mMTE/mM). |
| Ying [80] | The optimum UAE conditions were ethanol of 60%, solid–liquid ratio of 1:8, temperature of 50 °C, and extraction time of 20 min. The maximum yield obtained was 33.25%. |
| Ballard, et al. [81] | In the extraction of peanut skin using MAE, ethanol gave the highest TPC recovery of 118 mg/g and yield of 30.8% at temperature of 30.9 °C and extraction time of 12 min. However, methanol offered the highest ORAC activity of 2149 μmol/g. |
| Wu, et al. [82] | The optimum MAE parameters were the solid–solvent ratio of 1:25, ethanol of 75%, extraction time of 2 min, and microwave power of 540 W, with the responses of TPC being 183.25 mg/g. |
| Braga, et al. [83] | The highest content of total flavonoids was obtained from peanut skin, with 2.44 mg/g compared to grape (1.76 mg/g) and mango (1.70 mg/g). |
| Methods | Benefits | Drawbacks |
| Soxhlet | Less energy consumption Less quantity solvent Higher yield | Toxic solvent High temperature Long extraction time Low quality of extract |
| MAE | Shorter extraction time with higher yield extract instead of Soxhlet extraction Less solvent consumption Better quality of extracts compared to Soxhlet | High temperature |
| UAE | Low energy consumption Fewer extraction times Higher extraction yields No high pressure or temperature High purity of extract | Unsuitable for phenolic recovery |
| ScCO2 | Suitable for nonpolar compounds extract Shorter extraction time Safe solvent for health and wellness products Higher quality of extract | High pressure extraction High cost of operation Low extraction efficiency for polar compounds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putra, N.R.; Rizkiyah, D.N.; Che Yunus, M.A.; Abdul Aziz, A.H.; Md Yasir, A.S.H.; Irianto, I.; Jumakir, J.; Waluyo, W.; Suparwoto, S.; Qomariyah, L. Valorization of Peanut Skin as Agricultural Waste Using Various Extraction Methods: A Review. Molecules 2023, 28, 4325. https://doi.org/10.3390/molecules28114325
Putra NR, Rizkiyah DN, Che Yunus MA, Abdul Aziz AH, Md Yasir ASH, Irianto I, Jumakir J, Waluyo W, Suparwoto S, Qomariyah L. Valorization of Peanut Skin as Agricultural Waste Using Various Extraction Methods: A Review. Molecules. 2023; 28(11):4325. https://doi.org/10.3390/molecules28114325
Chicago/Turabian StylePutra, Nicky Rahmana, Dwila Nur Rizkiyah, Mohd Azizi Che Yunus, Ahmad Hazim Abdul Aziz, Ahmad Shah Hizam Md Yasir, Irianto Irianto, Jumakir Jumakir, Waluyo Waluyo, Suparwoto Suparwoto, and Lailatul Qomariyah. 2023. "Valorization of Peanut Skin as Agricultural Waste Using Various Extraction Methods: A Review" Molecules 28, no. 11: 4325. https://doi.org/10.3390/molecules28114325
APA StylePutra, N. R., Rizkiyah, D. N., Che Yunus, M. A., Abdul Aziz, A. H., Md Yasir, A. S. H., Irianto, I., Jumakir, J., Waluyo, W., Suparwoto, S., & Qomariyah, L. (2023). Valorization of Peanut Skin as Agricultural Waste Using Various Extraction Methods: A Review. Molecules, 28(11), 4325. https://doi.org/10.3390/molecules28114325








