Mechanism and Performance Analysis of Nanoparticle-Polymer Fluid for Enhanced Oil Recovery: A Review
Abstract
:1. Introduction
2. Polymer Flooding Mechanism and Influencing Factors
2.1. Common Polymers in Oil Displacement
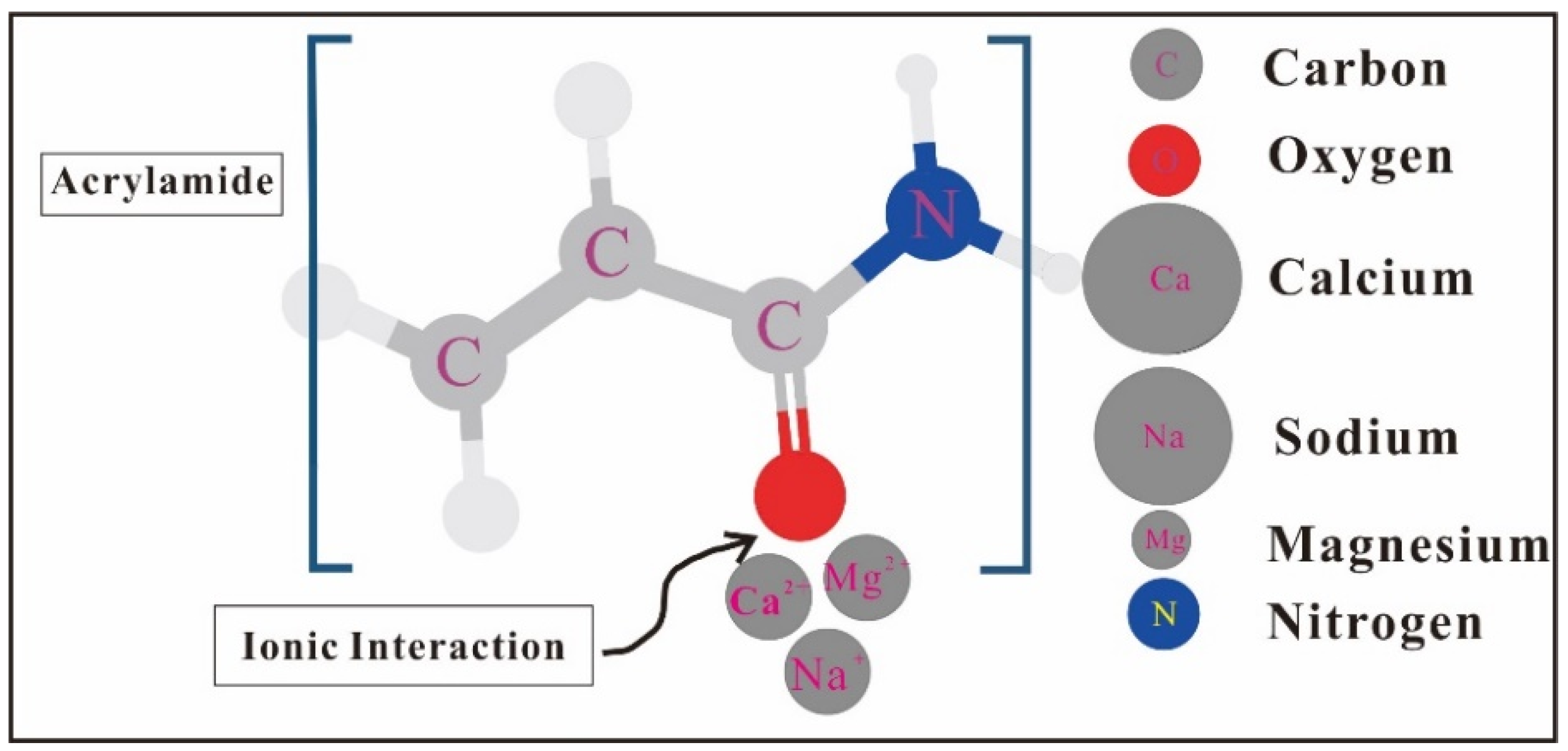
2.2. Influence of Salinity
2.3. Influence of Temperature
2.4. PH
2.5. Self-Factors of Polymer
3. Nanoparticles Commonly Used in Oil Displacement
3.1. Nanoparticles of Metal Oxide
3.2. Non-Metallic Oxide Nanoparticles
4. Nanoparticle Reinforced Polymer
4.1. Quantum Size Effect and Chemical Bonding between Nanoparticles and Polymers
4.2. Improvement of Polymer Properties by Nanoparticles
4.2.1. Improvement of Viscosity
4.2.2. Stability of Shear Property
4.2.3. Thermal Stability and Salt Tolerance
4.3. Advantages of Nano Polymer Fluids
5. EOR Mechanism of Nanoparticle-Polymer Fluid
5.1. IFT
5.2. Wettability
5.3. Stability Analysis
6. Current Challenges
7. Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, J.A.; Kolo, K.; Manshad, A.K.; Stephen, K.D. Potential application of low-salinity polymeric-nanofluid in carbonate oil reservoirs: IFT reduction, wettability alteration, rheology and emulsification characteristics. J. Mol. Liq. 2019, 284, 735–747. [Google Scholar] [CrossRef]
- Bahraminejad, H.; Manshad, A.K.; Riazi, M.; Ali, J.A.; Sajadi, S.; Keshavarz, A. CuO/TiO2/PAM as a Novel Introduced Hybrid Agent for Water-Oil Interfacial Tension and Wettability Optimization in Chemical Enhanced Oil Recovery. Energy Fuels 2019, 33, 10547–10560. [Google Scholar] [CrossRef]
- Asif, M.; Muneer, T. Energy supply, its demand and security issues for developed and emerging economies. Renew. Sustain. Energy Rev. 2007, 11, 1388–1413. [Google Scholar] [CrossRef]
- Izadi, N.; Koochi, M.M.; Amrollahi, A.; Pourkhalil, M. Investigation of functionalized polyelectrolyte polymer-coated Fe3O4 nanoparticles stabilized in high salinity brine at high temperatures as an EOR agent. J. Pet. Sci. Eng. 2019, 178, 1079–1091. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.; Agi, A.; Oseh, J.; Usman, J. Synergistic application of aluminium oxide nanoparticles and oilfield polyacrylamide for enhanced oil recovery. J. Pet. Sci. Eng. 2019, 182, 106345. [Google Scholar] [CrossRef]
- Saha, R.; Uppaluri, R.V.S.; Tiwari, P. Silica Nanoparticle Assisted Polymer Flooding of Heavy Crude Oil: Emulsification, Rheology, and Wettability Alteration Characteristics. Ind. Eng. Chem. Res. 2018, 57, 6364–6376. [Google Scholar] [CrossRef]
- Esmaeilnezhad, E.; Van, S.L.; Chon, B.H.; Choi, H.J.; Schaffie, M.; Gholizadeh, M.; Ranjbar, M. An experimental study on enhanced oil recovery utilizing nanoparticle ferrofluid through the application of a magnetic field. J. Ind. Eng. Chem. 2018, 58, 319–327. [Google Scholar] [CrossRef]
- Negin, C.; Saeedi, A.; Xie, Q. Most common surfactants employed in chemical enhanced oil recovery. Petroleum 2017, 3, 197–211. [Google Scholar] [CrossRef]
- Adasani, A.A.; Bai, B. Analysis of EOR projects and updated screening criteria. J. Pet. Sci. Eng. 2011, 79, 10–24. [Google Scholar] [CrossRef]
- Mariyate, J.; Bera, A. Recent progresses of microemulsions-based nanofluids as a potential tool for enhanced oil recovery. Fuel 2021, 306, 121640. [Google Scholar] [CrossRef]
- Jeirani, Z.; Jan, B.M.; Ali, B.S.; Noor, I.M.; See, C.H.; Saphanuchart, W. Formulation, optimization and application of triglyceride microemulsion in enhanced oil recovery. Ind. Crops Prod. 2013, 43, 6–14. [Google Scholar] [CrossRef]
- Thomas, S. Enhanced Oil Recovery—An Overview. Oil Gas Sci. Technol.-Rev. De L’ifp 2007, 63, 9–19. [Google Scholar] [CrossRef]
- Esfandyari, H.; Shadizadeh, S.R.; Esmaeilzadeh, F.; Davarpanah, A. Implications of anionic and natural surfactants to measure wettability alteration in EOR processes. Fuel 2020, 278, 118392. [Google Scholar] [CrossRef]
- Seetharaman, G.R.; Jadhav, R.M.; Sangwai, J.S. Effect of monovalent and divalent alkali [NaOH and Ca(OH)2] on the interfacial tension of pure hydrocarbon-water systems relevant for enhanced oil recovery. J. Pet. Sci. Eng. 2021, 197, 107892. [Google Scholar] [CrossRef]
- Tackie-Otoo, B.N.; Mohammed, A.A.A. Experimental investigation of the behaviour of a novel amino acid-based surfactant relevant to EOR application. J. Mol. Liq. 2020, 316, 113848. [Google Scholar] [CrossRef]
- Mei, Z.; Kang, X.; He, C.; Zhang, J. Polymer flood injection conformance behavior of heterogeneous heavy-oil reservoirs. Sci. Technol. Eng. 2018, 18, 61–66. [Google Scholar]
- Xing, E.; Tao, X.; Jin, L.; Ma, J.; Qv, Z. Determination and influence factors of resistance coefficient and residual resistance coefficient. Liaoning Chem. Ind. 2016, 45, 284–287. [Google Scholar]
- Sun, G. Insight into interactions between polymer and crude oil with dual polarization interferometry. Oilfield Chem. 2017, 34, 290–295. [Google Scholar]
- Abidin, A.Z.; Puspasari, T.; Nugroho, W.A. Polymers for enhanced oil recovery technology. Procedia Chem. 2012, 4, 11–16. [Google Scholar] [CrossRef]
- Li, H.; Zeng, J.; Chen, X.; Gao, L.; Zhao, Z.; Wen, J. Preparation of Polymer Encapsulated Nano Particle Plugging Agent and Its Plugging Performance. J. Liaoning Petrochem. Univ. 2023, 43, 8–12. [Google Scholar]
- Ma, X.; Lv, Y.; Liu, P.; Hao, Y.; Xia, N. Switch-on Fluorescence Analysis of Protease Activity with the Assistance of a Nickel Ion-Nitrilotriacetic Acid-Conjugated Magnetic Nanoparticle. Molecules 2023, 28, 3426. [Google Scholar] [CrossRef]
- Morozovska, A.N.; Eliseev, E.N.; Hertel, R.; Fomichov, Y.M.; Tulaidan, V.; Reshetnyak, V.Y.; Evans, D.R. Electric field control of three-dimensional vortex states in core-shell ferroelectric nanoparticles. Acta Mater. 2020, 200, 256–273. [Google Scholar] [CrossRef]
- Singh, Y.; Singh, N.K.; Sharma, A.; Singla, A.; Singh, D.; Rahim, E.A. Effect of ZnO nanoparticles concentration as additives to the epoxidized Euphorbia Lathyris oil and their tribological characterization. Fuel 2021, 285, 119148. [Google Scholar] [CrossRef]
- Foroozesh, J.; Kumar, S. Nanoparticles behaviors in porous media: Application to enhanced oil recovery. J. Mol. Liq. 2020, 316, 113876. [Google Scholar] [CrossRef]
- He, Y.; An, S.; Wang, J.; Zhao, L.; Wang, Y. Research progress of enhanced oil recovery by nanoparticles. Petrochem. Technol. 2022, 51, 1508–1516. [Google Scholar]
- Alnarabiji, M.S.; Yahya, N.; Nadeem, S.; Adil, M.; Baig, M.K.; Ghanem, O.B.; Azizi, K.; Ahmed, S.; Maulianda, B.; Klemeš, J.J.; et al. Nanofluid enhanced oil recovery using induced ZnO nanocrystals by electromagnetic energy: Viscosity increment. Fuel 2018, 233, 632–643. [Google Scholar] [CrossRef]
- Lu, T.; Li, Z.; Zhou, Y.; Zhang, C. Enhanced oil recovery of low-permeability cores by SiO2 nanofluid. Energy Fuels 2017, 31, 5612–5621. [Google Scholar] [CrossRef]
- Qu, M.; Hou, J.; Liang, T.; Xiao, L.; Yang, J.; Raj, I.; Shao, Y. Preparation and Interfacial Properties of Ultralow Concentrations of Amphiphilic Molybdenum Disulfide Nanosheets. Ind. Eng. Chem. Res. 2020, 59, 9066–9075. [Google Scholar] [CrossRef]
- Rezvani, H.; Panahpoori, D.; Riazi, M.; Parsaei, R.; Tabaei, M.; Cortés, F.B. A novel foam formulation by Al2O3/SiO2 nanoparticles for EOR applications: A mechanistic study. J. Mol. Liq. 2020, 304, 112730. [Google Scholar] [CrossRef]
- Esmaeilzadeh, P.; Hosseinpour, N.; Bahramian, A.; Fakhroueian, Z.; Arya, S. Effect of ZrO2 nanoparticles on the interfacial behavior of surfactant solutions at air-water and n-heptane-water interfaces. Fluid Phase Equilibria 2014, 361, 289–295. [Google Scholar] [CrossRef]
- Hill, D.; Barron, A.R.; Alexander, S. Controlling the wettability of plastic by thermally embedding coated aluminium oxide nanoparticles into the surface. J. Colloid Interface Sci. 2020, 567, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Hou, J.; Qu, M.; Zhao, M.; Raj, I. High-viscosity alpha-starch nanogel particles to enhance oil recovery. RSC Adv. 2020, 10, 8275–8285. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Shah, S.; Ahmed, R.; Ucan, S. Effects of nanoparticles and temperature on heavy oil viscosity. J. Pet. Sci. Eng. 2018, 167, 819–828. [Google Scholar] [CrossRef]
- Zhang, H.; Ramakrishnan, T.S.; Nikolov, A.; Wasan, D. Enhanced Oil Recovery Driven by Nanofilm Structural Disjoining Pressure: Flooding Experiments and Microvisualization. Energy Fuels 2016, 30, 2771–2779. [Google Scholar] [CrossRef]
- Cao, X.; Ji, Y.; Zhu, Y.; Zhao, F. Research advance and technology outlook of polymer flooding. Reserv. Eval. Dev. 2020, 10, 8–16. [Google Scholar]
- Zhao, Y.; Zhao, L.; Chang, G.; Chen, H.; Hao, L.; Zhao, N.; Zhao, C.; Geng, C.; Yang, W.; Li, Z. Fabrication of surfactant-biopolymer combined system with dual viscosity reduction and mobility controllability for heavy oil reservoirs. J. Mol. Liq. 2022, 368, 120777. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, J.; Wu, J. Application of polymer flooding technology in Daqing Oilfield. Acta Pet. Sin. 2005, 26, 74–78. [Google Scholar]
- Zhu, W.; Li, H.; Chen, Z.; Song, Z. Pore-scale experiments reveal distinct flow field of polymer flooding with viscoelasticity loss by high salinity. Colloids Surf. A 2023, 668, 131473. [Google Scholar] [CrossRef]
- Hazarika, K.; Gogoi, S.B.; Kumar, A. Polymer flooding and its effects on enhanced oil recovery special reference to Upper Assam Basin. Pet. Res. 2023, 8, 54–62. [Google Scholar] [CrossRef]
- Jadhawar, P.; Saeed, M. Low salinity water and polymer flooding in sandstone reservoirs: Upscaling from nano-to macro-scale using the maximum energy barrier. J. Pet. Sci. Eng. 2023, 220, 111247. [Google Scholar] [CrossRef]
- Tan, C.; Yang, E.; Fang, Y.; Song, S.; Yang, C. Study on migration law of micro remaining oil and displacement effect of polymer flooding. China Energy Environ. Prot. 2022, 44, 173–176. [Google Scholar]
- Wei, B.; Romero-Zerón, L.; Rodrigue, D. Oil displacement mechanisms of viscoelastic polymers in enhanced oil recovery (EOR): A review. J. Pet. Explor. Prod. Technol. 2014, 4, 113–121. [Google Scholar] [CrossRef]
- Xue, W. Polymer flooding enhanced oil recovery technology and application. Chem. Eng. Equip. 2022, 307, 59–60. [Google Scholar]
- Chang, H.L. Polymer Flooding Technology—Yesterday, Today, and Tomorrow. J. Pet. Technol. 1978, 30, 1113–1128. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, L.; Davletshin, A.; Li, Z.; You, J.; Tan, S. Application of Polysaccharide Biopolymer in Petroleum Recovery. Polymers 2020, 12, 1860. [Google Scholar] [CrossRef]
- Gamini, A.; Mandel, M. Physicochemical Properties of Aqueous Xanthan Solutions: Static Light Scattering. Biopolymers 1994, 34, 783–797. [Google Scholar] [CrossRef]
- Rellegadla, S.; Bairwa, H.K.; Kumari, M.R.; Prajapat, G.; Nimesh, S.; Pareek, N.; Jain, S.; Agrawal, A. An Effective Approach for Enhanced Oil Recovery Using Nickel Nanoparticles Assisted Polymer Flooding. Energy Fuels 2018, 32, 11212–11221. [Google Scholar] [CrossRef]
- Adimule, V.; Kerur, S.S.; Chinnam, S.; Yallur, B.C.; Nandi, S.S. Guar gum and its nanocomposites as prospective materials for miscellaneous applications: A short review. Eng. Top. Catal. 2022, 02, 1–14. [Google Scholar]
- Guan, B.; Wang, Y.; He, Z. Development of CJ2-3 Type Recoverable Low Molecular Mass Guar Gum Fracturing Fluid. Oilfield Chem. 2006, 23, 27–31. [Google Scholar]
- Combariza, M.Y.; Martínez-Ramírez, A.P.; Blanco-Tirado, C. Perspectives in nanocellulose for crude oil recovery: A minireview. Energy Fuels 2021, 35, 15381–15397. [Google Scholar] [CrossRef]
- Zhu, J.; Xie, S.; Yang, Z.; Li, X.; Chen, J.; Zhang, X.; Zheng, N. A review of recent advances and prospects on nanocellulose properties and its applications in oil and gas production. J. Nat. Gas Sci. Eng. 2021, 96, 104253. [Google Scholar] [CrossRef]
- Zhang, H.; Li, T.; Chen, M. Reuse with Equivalent Performance of Retired Bumper Plastic of Retired Passenger Vehicle. Polym. Mater. Sci. Eng. 2016, 32, 177–181. [Google Scholar]
- Wang, D. Development and use of HPAM. Petrochem. Ind. Appl. 2010, 29, 8–15+26. [Google Scholar]
- Olajire, A.A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Afolabi, R.O. Effect of surfactant and hydrophobe content on the rheology of poly (acrylamide-co-N-dodecylacrylamide) for Potential enhanced oil recovery application. Am. J. Polym. Sci. 2015, 5, 41–46. [Google Scholar]
- Afolabi, R.O.; Oluyemi, G.F.; Officer, S.; Ugwu, J.O. Hydrophobically associating polymers for enhanced oil recovery—Part A: A review on the effects of some key reservoir conditions. J. Pet. Sci. Eng. 2019, 180, 681–698. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Liu, G. Review on polymer flooding technology. IOP Conf. Ser. Earth Environ. Sci. 2021, 675, 012199. [Google Scholar] [CrossRef]
- Pu, W.; Du, D.; Liu, R.; Gu, J.; Li, K.; Zhang, Y.; Liu, P. Synthesis and characterization of hyperbranched associative polyacrylamide. Rsc Adv. 2016, 6, 39522–39529. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymers for enhanced oil recovery: A paradigm for structure-property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Firozjaii, A.M.; Saghafi, H.R. Review on chemical enhanced oil recovery using polymer flooding: Fundamentals, experimental and numerical simulation. Petroleum 2020, 6, 115–122. [Google Scholar] [CrossRef]
- Pu, W.; Shen, C.; Wei, B.; Yang, Y. A comprehensive review of polysaccharide biopolymers for enhanced oil recovery (EOR) from flask to field. J. Ind. Eng. Chem. 2018, 61, 1–11. [Google Scholar] [CrossRef]
- Drumeanu, A.C. Some considerations concerning four-ball machine testing of the polyacrylamide solutions. IOP Conf. Ser. Mater. Sci. Eng. 2017, 174, 012040. [Google Scholar] [CrossRef]
- He, J.; Wang, X.; Wu, J. Influencing Factors on the Viscosity of Hydrophobically Associating Polymers. Oilfield Chem. 2011, 28, 259–262. [Google Scholar]
- Abdullahi, M.B.; Rajaei, K.; Junin, R.; Bayat, A.E. Appraising the impact of metal-oxide nanoparticles on rheological properties of HPAM in different electrolyte solutions for enhanced oil recovery. J. Pet. Sci. Eng. 2019, 172, 1057–1068. [Google Scholar] [CrossRef]
- Wang, H.; Dai, L.; Lu, K.; Wang, Z.; Li, Y.; Yang, J.; Bai, Y. Influence factors of apparent viscosity and molecular chain failuremechanism of polyacrylamide solution used in drilling and production. J. China Univ. Pet. (Ed. Nat. Sci.) 2022, 46, 115–125. [Google Scholar]
- Zhao, R.; Ma, W.; Xu, X.; Li, C.; Ma, G.; Zhang, Q. Research progress of chemical absorbents for carbon dioxide capture. Fine Chem. 2023, 40, 1–9. [Google Scholar]
- Yang, H.; Zhang, Y.; Liu, W.; Lin, M. Influence of Salinity on the Property of Cross-Linked Polyacrylamide Microspheres. J. Petrochem. Univ. 2014, 27, 46–50. [Google Scholar]
- Dong, Z.; Song, J.; Lin, M.; Li, M. Influence of salt concentration on shear thickening properties of LPS. Oil Drill. Prod. Technol. 2009, 31, 81–84. [Google Scholar]
- Zhao, X.; Wang, Z.; Qiu, G.; Xin, Y.; Ni, J. Study on Influence Factors of the Initial Viscosity of HPAM Solution. Chem. Eng. Oil Gas 2009, 38, 231–234. [Google Scholar]
- Wang, Q.; Qi, H.; Zhang, X.; Li, D. Temperature Effect on Polyacrylamide Solution Spectrum Measurement. Res. Explor. Lab. 2018, 37, 18–20. [Google Scholar]
- Zhang, X.; Han, M.; Fuseni, A.; Alsofi, A.M. An approach to evaluate polyacrylamide-type polymers’ long-term stability under high temperature and high salinity environment. J. Pet. Sci. Eng. 2019, 180, 518–525. [Google Scholar] [CrossRef]
- Maghzi, A.; Kharrat, R.; Mohebbi, A.; Ghazanfari, M.H. The impact of silica nanoparticles on the performance of polymer solution in presence of salts in polymer flooding for heavy oil recovery. Fuel 2014, 123, 123–132. [Google Scholar] [CrossRef]
- Seright, R.S.; Campbell, A.R.; Mozley, P.S.; Han, P. Stability of Partially Hydrolyzed Polyacrylamides at Elevated Temperatures in the Absence of Divalent Cations. SPE J. 2009, 15, 341–348. [Google Scholar] [CrossRef]
- Wang, X. Temperature on ultra high molecular weight salt resistant polypropylene effect of amide viscosity and shear rate. Chem. Eng. Equip. 2021, 291, 26–28. [Google Scholar]
- Liu, P.; Mu, Z.; Wang, C.; Wang, Y. Experimental Study of Rheological Properties and Oil Displacement Efficiency in Oilfields for a Synthetic Hydrophobically Modified Polymer. Sci. Rep. 2017, 7, 8791. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Y. Study on the intrinsic viscosity changing with salts of AM-MADQUAT-NaAMPS copolymer. Appl. Chem. Ind. 2008, 198, 924–926. [Google Scholar]
- Luo, H.; Ma, Y. Study on the Effect of ASP Composite Flooding Alkalion the Viscosity of Polymer. Yunnan Chem. Technol. 2019, 46, 27–29. [Google Scholar]
- Urbissinova, T.S.; Trivedi, J.J.; Kuru, E. Effect of Elasticity During Viscoelastic Polymer Flooding: A Possible Mechanism of Increasing the Sweep Efficiency. J. Can. Pet. Technol. 2010, 49, 49–56. [Google Scholar] [CrossRef]
- Li, K.; Sun, W.; Li, F.; Qu, Y.; Yang, Y. Novel Method for Characterizing Single-Phase Polymer Flooding. SPE J. 2014, 19, 695–702. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, J.; Zhang, L.; Liu, Y.; Guo, B.; Yuan, Z.; Yang, H. Adsorption Law of Polymer in Injection Well of Bohai SZ 36-1 Oilfield. Oilfield Chem. 2019, 36, 672–676. [Google Scholar]
- Shi, L.; Zhu, S.; Ye, Z.; Xu, X.; Zhao, W. Adsorption and retention of polymers with different associative energy in porous media. Appl. Chem. Ind. 2019, 330, 1786–1790. [Google Scholar]
- Mageswari, A.; Srinivasan, R.; Subramanian, P.; Ramesh, N.; Gothandam, K.M. Nanomaterials: Classification, Biological Synthesis and Characterization. Nanosci. Food Agric. 3 2016, 23, 31–71. [Google Scholar]
- Ogolo, N.; Olafuyi, O.; Onyekonwu, M.O. Enhanced Oil Recovery Using Nanoparticles. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 8–11 April 2012. [Google Scholar]
- Karimi, A.; Fakhroueian, Z.; Bahramian, A.; Khiabani, N.P.; Darabad, J.B.; Azin, R.; Arya, S. Wettability Alteration in Carbonates using Zirconium Oxide Nanofluids: EOR Implications. Energy Fuels 2012, 26, 1028–1036. [Google Scholar] [CrossRef]
- Pereira, M.L.; Maia, K.C.B.; Silva, W.C.; Leite, A.C.; Francisco, A.D.S.; Vasconcelos, T.; Nascimento, R.; Grasseschi, D. Fe3O4 nanoparticles as surfactant carriers for enhanced oil recovery and scale prevention. ACS Appl. Nano Mater. 2020, 3, 5762–5772. [Google Scholar] [CrossRef]
- Zargar, G.; Arabpour, T.; Manshad, A.K.; Ali, J.A.; Sajadi, S.M.; Keshavarz, A.; Mohammadi, A.H. Experimental investigation of the effect of green TiO2/Quartz nanocomposite on interfacial tension reduction, wettability alteration, and oil recovery improvement. Fuel 2020, 263, 116599. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, T.; Wu, H.; You, Z.; Shang, D.; Hou, J. Enhanced Oil Recovery Using Oleic Acid-Modified Titania Nanofluids: Underlying Mechanisms and Oil-Displacement Performance. Energy Fuels 2020, 34, 5813–5822. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, Q.; Cheng, H. The Present Research Situation of Surface Modification of White Carbon. China Non-Met. Miner. Ind. 2008, 26, 12–15. [Google Scholar]
- Moghaddam, R.N.; Bahramian, A.; Fakhroueian, Z.; Karimi, A.; Arya, S. Comparative Study of Using Nanoparticles for Enhanced Oil Recovery: Wettability Alteration of Carbonate Rocks. Energy Fuels 2015, 29, 2111–2119. [Google Scholar] [CrossRef]
- Chen, X.; Ke, J.; Zhu, S.; Zhao, B.; Yang, W. Research on Properties of Polyprogylene/Nano-SiO2 Composite. J. Wuhan Univ. Technol. 2003, 25, 8–11. [Google Scholar]
- Nguyen, P.T.; Do, H.B.; Pham, D.K.; Nguyen, H.A.; Dao, O.P.; Nguyen, B.D. Evaluation on the EOR Potential Capacity of the Synthesized Composite Silica-Core/Polymer-Shell Nanoparticles Blended with Surfactant Systems for the HPHT Offshore Reservoir Conditions. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Pu, C. Factors analysis of polymer flooding effect. Chem. Eng. Des. Commun. 2019, 45, 24–25. [Google Scholar]
- Xu, H.; Sun, X.; Han, Y.; He, D.; Dong, W. PerformanceEvaluationand Microstructure Study of Ultra High Molecular Weight Polyme. Pet. Drill. Tech. 2013, 41, 114–118. [Google Scholar]
- Mou, S.; Shi, S.; Fang, K.; Wen, Q. Research Progress on the Application of Nanomaterial and Technology in Petroleum Exploration. Oilfield Chem. 2019, 36, 564–570. [Google Scholar]
- Santamaria, O.; Lopera, S.H.; Riazi, M.; Minale, M.; Cortés, F.B.; Franco, C.A. Phenomenological study of the micro- and macroscopic mechanisms during polymer flooding with SiO2 nanoparticles. J. Pet. Sci. Eng. 2021, 198, 108135. [Google Scholar] [CrossRef]
- Yousefvand, H.; Jafari, A. Enhanced Oil Recovery Using Polymer/nanosilica. Procedia Mater. Sci. 2015, 11, 565–570. [Google Scholar] [CrossRef]
- Sharma, T.; Iglauer, S.; Sangwai, J.S. Silica Nanofluids in an Oilfield Polymer Polyacrylamide: Interfacial Properties, Wettability Alteration, and Applications for Chemical Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2016, 55, 12387–12397. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Oseh, J.O.; Usman, J. Effect of aluminium oxide nanoparticles on oilfield polyacrylamide: Rheology, interfacial tension, wettability and oil displacement studies. J. Mol. Liq. 2019, 296, 111863. [Google Scholar] [CrossRef]
- Rezaei, A.; Abdi-Khangah, M.; Mohebbi, A.; Tatar, A.; Mohammadi, A.H. Using surface modified clay nanoparticles to improve rheological behavior of Hydrolized Polyacrylamid (HPAM) solution for enhanced oil recovery with polymer flooding. J. Mol. Liq. 2016, 222, 1148–1156. [Google Scholar] [CrossRef]
- Bera, A.; Shah, S.; Shah, M.; Agarwal, J.; Vij, R.K. Mechanistic study on silica nanoparticles-assisted guar gum polymer flooding for enhanced oil recovery in sandstone reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124833. [Google Scholar] [CrossRef]
- Ma, W. Research on Preparation and Mechanical Properties of PP/POE Blends and Nano-composites. South China Univ. Technol. 2011, 12, 36–48. [Google Scholar]
- Bhardwaj, P.; Singh, S.; Singh, V.; Aggarwal, S.; Mandal, U.K. Nanosize Polyacrylamide/SiO2 Composites by Inverse Microemulsion Polymerization. Int. J. Polym. Mater. Polym. Biomater. 2008, 57, 404–416. [Google Scholar] [CrossRef]
- Maurya, N.K.; Mandal, A. Studies on behavior of suspension of silica nanoparticle in aqueous polyacrylamide solution for application in enhanced oil recovery. Pet. Sci. Technol. 2016, 34, 429–436. [Google Scholar] [CrossRef]
- Hu, Z.; Haruna, M.; Gao, H.; Nourafkan, E.; Wen, D. Rheological Properties of Partially Hydrolyzed Polyacrylamide Seeded by Nanoparticles. Ind. Eng. Chem. Res. 2017, 56, 3456–3463. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Yekeen, N.; Augustine, A. Polymer Bulletin Hybrid suspension of polymer and nanoparticles for enhanced oil recovery. Polym. Bull. 2019, 76, 6193–6230. [Google Scholar] [CrossRef]
- Khoramian, R.; Ramazani, A.; Hekmatzadeh, M.; Kharrat, R.; Asadian, E. Graphene Oxide Nanosheets for Oil Recovery. ACS Appl. Nano Mater. 2019, 2, 5730–5742. [Google Scholar] [CrossRef]
- Wang, S.; Liu, N.; Su, J.; Li, L.; Long, F.; Zou, Z.; Jiang, X.; Gao, Y. Highly Stretchable and Self-Healable Supercapacitor with Reduced Graphene Oxide Based Fiber Springs. ACS Nano 2017, 11, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Druetta, P.; Picchioni, F. Polymer and nanoparticles flooding as a new method for Enhanced Oil Recovery. J. Pet. Sci. Eng. 2019, 177, 479–495. [Google Scholar] [CrossRef]
- Haruna, M.A.; Pervaiz, S.; Hu, Z.; Nourafkan, E.; Wen, D. Improved rheology and high-temperature stability of hydrolyzed polyacrylamide using graphene oxide nanosheet. J. Appl. Polym. Sci. 2019, 136, 47582. [Google Scholar] [CrossRef]
- Maghzi, A.; Mohebbi, A.; Kharrat, R.; Ghazanfari, M.H. An Experimental Investigation of Silica Nanoparticles Effect on the Rheological Behavior of Polyacrylamide Solution to Enhance Heavy Oil Recovery. Pet. Sci. Technol. 2013, 31, 500–508. [Google Scholar] [CrossRef]
- Khalilinezhad, S.S.; Cheraghian, G.; Roayaei, E.; Tabatabaee, H.; Karambeigi, M.S. Improving heavy oil recovery in the polymer flooding process by utilizing hydrophilic silica nanoparticles. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 45, 1779. [Google Scholar]
- Kang, W.; Cao, C.; Guo, S.; Tang, X.; Lashari, Z.A.; Gao, Y.; Zhang, X. Muhammad Waseem Iqbal, Hongbin Yang Mechanism of silica nanoparticles’ better-thickening effect on amphiphilic polymers in high salinity condition. J. Mol. Liq. 2019, 277, 254–260. [Google Scholar] [CrossRef]
- Zhu, D.; Wei, L.; Wang, B.; Feng, Y. Aqueous Hybrids of Silica Nanoparticles and Hydrophobically Associating Hydrolyzed Polyacrylamide Used for EOR in High-Temperature and High-Salinity Reservoirs. Energies 2014, 7, 3858–3871. [Google Scholar] [CrossRef]
- Cheraghian, G. Effect of nano titanium dioxide on heavy oil recovery during polymer flooding. Pet. Sci. Technol. 2016, 34, 633–641. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Y.; Jiang, L.; Xu, L.; Feng, W. Analysis of preparation and dispersion characterization of surface modified nano-silica particles. Exp. Technol. Manag. 2019, 36, 159–162. [Google Scholar]
- Cao, J.; Song, T.; Wang, X.; Zhu, Y.; Wang, S.; Zhao, M.; Miao, Y.; Zhang, J. Studies on the rheological properties of amphiphilic nanosilica and a partially hydrolyzed polyacrylamide hybrid for enhanced oil recovery. Chem. Eng. Sci. 2019, 206, 146–155. [Google Scholar] [CrossRef]
- Nguyen, B.D.; Ngo, T.K.; Bui, T.H.; Pham, D.K.; Dinh, X.L.; Nguyen, P.T. The impact of graphene oxide particles on viscosity stabilization for diluted polymer solutions using in enhanced oil recovery at HTHP offshore reservoirs. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 6, 015012. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.; Yu, H.; Niu, L.; Yu, L.; Ni, D.; Zhang, Z. Functional Janus-SiO2 Nanoparticles Prepared by a Novel “Cut the Gordian Knot” Method and Their Potential Application for Enhanced Oil Recovery. ACS Appl. Mater. Interfaces 2020, 12, 24201–24208. [Google Scholar] [CrossRef]
- Alvarez, N.; Anna, S.; Saigal, T.; Tilton, R.; Walker, L. Interfacial Dynamics and Rheology of Polymer-Grafted Nanoparticles at Air-Water and Xylene-Water Interfaces. Langmuir 2012, 28, 8052–8063. [Google Scholar] [CrossRef]
- Qi, L.; Song, C.; Wang, T.; Li, Q. Polymer-Coated Nanoparticles for Reversible Emulsification and Recovery of Heavy Oil. Langmuir 2018, 34, 6522–6528. [Google Scholar] [CrossRef]
- Bila, A.; Torsaeter, O. Experimental Investigation of Polymer-Coated Silica Nanoparticles for EOR under Harsh Reservoir Conditions of High Temperature and Salinity. Nanomaterials 2021, 11, 765. [Google Scholar] [CrossRef]
- Xu, L.; Liu, X.; Ding, H.; Zhang, H.; Liu, L.; Li, J.; Gong, H.; Dong, M. Temperature/salt tolerance and oil recovery of xanthan gum solution enhanced by surface-modified nanosilicas. Pet. Sci. 2023, 20, 577–589. [Google Scholar] [CrossRef]
- Keykhosravi, A.; Vanani, M.B.; Aghayari, C. TiO2 nanoparticle-induced Xanthan Gum Polymer for EOR: Assessing the underlying mechanisms in oil-wet carbonates. J. Pet. Sci. Eng. 2021, 204, 108756. [Google Scholar] [CrossRef]
- Li, Q.; Wei, B.; Lu, L.; Li, Y.; Wen, Y.; Pu, W.; Li, H. Chongyang Wang Investigation of physical properties and displacement mechanisms of surface-grafted nano-cellulose fluids for enhanced oil recovery. Fuel 2017, 207, 352–364. [Google Scholar] [CrossRef]
- Tiong, A.C.; Tan, I.; Foo, H.C.; Lam, M.K.; Mahmud, H.B.; Lee, K.T.; Show, P.K. Study on the synergism of cellulose nanocrystals and janus graphene oxide for enhanced oil recovery. Geoenergy Sci. Eng. 2023, 221, 111242. [Google Scholar] [CrossRef]
- Asl, F.O.; Zargar, G.; Manshad, A.K.; Arif, M.; Iglauer, S.; Keshavarz, A. Impact of PAM-ZnO nanocomposite on oil recovery. Fuel 2023, 332, 125941. [Google Scholar] [CrossRef]
- Haruna, M.A.; Gardy, J.; Yao, G.; Hu, Z.; Hondow, N.; Wen, D. Nanoparticle modified polyacrylamide for enhanced oil recovery at harsh conditions. Fuel 2020, 268, 117186. [Google Scholar] [CrossRef]
- Li, Q.; Yu, X.; Wang, L.; Qu, S.; Wu, W.; Ji, R.; Luo, Y.; Yang, H. Nano-silica hybrid polyacrylamide/polyethylenimine gel for enhanced oil recovery at harsh conditions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127898. [Google Scholar] [CrossRef]
- Zheng, C.; Cheng, Y.; Wei, Q.; Li, X.; Zhang, Z. Suspension of surface-modified nano-SiO2 in partially hydrolyzed aqueous solution of polyacrylamide for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2017, 524, 169–177. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Medeiros, F.S.; Diniz, B.R.; Viana, M.M.; Caliman, V.; Silva, G.G. Nanofluids based on hydrolyzed polyacrylamide and aminated graphene oxide for enhanced oil recovery in different reservoir conditions. Fuel 2022, 310, 122299. [Google Scholar] [CrossRef]
- Cao, J.; Xu, G.; Wang, X.; Wang, H.; Zhang, J.; Liu, C. Tug-of-war between hydrogen bond and hydrophobic interaction of bisfunctionalized graphene oxide/hydrolyzed polyacrylamide allows thickening and salt-resistance in enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129909. [Google Scholar] [CrossRef]
- Zhao, J.; Wen, D. Pore-scale simulation of wettability and interfacial tension effects on flooding process for enhanced oil recovery. RSC Adv. 2017, 7, 41391–41398. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Ni, J. Study on chemical flooding relative permeability curves based on the extended capillary number theory. Oil Gas Geol. 2017, 38, 379–384. [Google Scholar]
- Manshad, A.K.; Olad, M.; Taghipour, S.A.; Nowrouzi, I.; Mohammadi, A.H. Effects of water soluble ions on interfacial tension (IFT) between oil and brine in smart and carbonated smart water injection process in oil reservoirs. J. Mol. Liq. 2016, 223, 987–993. [Google Scholar] [CrossRef]
- Corredor, L.M.; Husein, M.M.; Maini, B.B. A review of polymer nanohybrids for oil recovery. Adv. Colloid Interface Sci. 2019, 272, 102018. [Google Scholar] [CrossRef] [PubMed]
- Fanchi, J.R. Shared Earth Modeling: Methodologies for Integrated Reservoir Simulations; Gulf Professional Publishing: Oxford, UK, 2022. [Google Scholar]
- Cheraghian, G.; Hemmati, M.; Masihi, M.; Bazgir, S. An experimental investigation of the enhanced oil recovery and improved performance of drilling fluids using titanium dioxide and fumed silica nanoparticles. J. Nanostruct. Chem. 2013, 3, 78. [Google Scholar] [CrossRef]
- Bayat, A.E.; Junin, R.; Samsuri, A.; Piroozian, A.; Hokmabadi, M. Impact of Metal Oxide Nanoparticles on Enhanced Oil Recovery from Limestone Media at Several Temperatures. Energy Fuels 2014, 28, 6255–6266. [Google Scholar] [CrossRef]
- Saleh, N.; Sarbu, T.; Sirk, K.; Lowry, G.V.; Matyjaszewski, K.; Tilton, R.D. Oil-in-water emulsions stabilized by highly charged polyelectrolyte-grafted silica nanoparticles. Langmuir 2005, 21, 9873–9878. [Google Scholar] [CrossRef] [PubMed]
- Craig, F.C. The Reservoir Engineering Aspects of Waterflooding (Monograph Volume 3–Henry, L. Doherty Series); Society of Petroleum Engineers: Wilkes-Barre, PA, USA, 2015. [Google Scholar]
- Sedaghat, M.H.; Ghazanfari, M.H.; Masihi, M.; Rashtchian, D. Experimental Investigation of Microscopic/Macroscopic Efficiency of Polymer Flooding in Fractured Heavy Oil Five-Spot Systems. J. Energy Resour. Technol. 2013, 135, 032901. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, W.; Wang, D.; Zeng, Y. The Effect of Elasticity on Displacement Efficiency in the Lab and Results of High Concentration Polymer Flooding in the Field. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008. [Google Scholar]
- Joonaki, E.; Ghanaatian, S. The Application of Nanofluids for Enhanced Oil Recovery: Effects on Interfacial Tension and Coreflooding Process. Pet. Sci. Technol. 2014, 32, 2599–2607. [Google Scholar] [CrossRef]
- Maurya, N.K.; Kushwaha, P.; Mandal, A. Studies on interfacial and rheological properties of water soluble polymer grafted nanoparticle for application in enhanced oil recovery. J. Taiwan Inst. Chem. Eng. 2017, 70, 319–330. [Google Scholar] [CrossRef]
- Li, W.; Zhou, X.; Liu, B.; Lv, F. Study on the dispersion of nano particles in cryoprotectant. Cryog. Supercond. 2013, 41, 13–16. [Google Scholar]
- Fedele, L.; Colla, L.; Bobbo, S.; Barison, S. Experimental stability analysis of different water-based nanofluids. Nanoscale Res. Lett. 2011, 6, 300. [Google Scholar] [CrossRef] [PubMed]
- Ehtesabi, H.; Ahadian, M.M.; Taghikhani, V.; Ghazanfari, M.H. Enhanced heavy oil recovery in sandstone cores using TiO2 nanofluids. Energy Fuels 2013, 28, 423–430. [Google Scholar] [CrossRef]
- Krishnans, S.J.; Nagarajan, P.K. Influence of stability and particle shape effects for an entropy generation based optimized selection of magnesia nanofluid for convective heat flow applications. Appl. Surf. Sci. 2019, 489, 560–575. [Google Scholar]
- Rao, Y.Q. Nanofluids: Stability, phase diagram, rheology and applications. Particuology 2010, 8, 549–555. [Google Scholar] [CrossRef]
- Fikri, M.A.; Faizal, W.M.; Adli, H.K.; Bo, Z.; Jiang, X.X.; Ramadhan, A.I. Investigation on stability of TiO2-SiO2 nanofluids with ratio (70:30) in W/EG mixture (60:40). IOP Conf. Ser. Mater. Sci. Eng. 2021, 1062, 012020. [Google Scholar] [CrossRef]
- Ghadimi, A.; Saidur, R.; Metselaar, H.S.C. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Cacua, K.; Ordoñez, F.; Zapata, C.; Herrera, B.; Pabón, E.; Buitrago-Sierra, R. Surfactant concentration and pH effects on the zeta potential values of alumina nanofluids to inspect stability. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123960. [Google Scholar] [CrossRef]
- Ismay, M.J.; Doroodchi, E.; Moghtaderi, B. Effects of colloidal properties on sensible heat transfer in water-based titania nanofluids. Chem. Eng. Res. Des. 2013, 91, 426–436. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Magazù, S.; Calandra, P. Amphiphiles Self-Assembly: Basic Concepts and Future Perspectives of Supramolecular Approaches. Adv. Condens. Matter Phys. 2015, 2015, 151683. [Google Scholar] [CrossRef]














| Nanoparticle and Concentration (wt%) | Polymer and Concentration (ppm) | Porous Media | IFT (mN/m) | Contact Angle (°) | Oil Recovery (%OOIP) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| P | With NPs | P | With NPs | P | NPF | ||||
| SiO2 0.5 | HPAM 800 | Sandstone | - | - | - | - | 26.32 | 35.0 | [96] |
| SiO2 0.1–0.5 | PAM 1000 | Glass micromodel | - | - | - | - | 50.6 | 61.8 | [72] |
| SiO2 0.1–0.5 | PAM 2000 | Glass micromodel | - | - | - | - | 55.0 | 65.0 | [72] |
| SiO2 0.1–0.5 | PAM 3000 | Glass micromodel | - | - | - | - | 58.0 | 68.0 | [72] |
| SiO2 1.0 | PAM 1000 | Sandstone | 18.03 | 10.22 | - | - | 58.1 | 62.2 | [97] |
| SiO2 0.1 | Xanthan gum 5000 | Sandstone | 17.8 | 10.75 | 72.7 | 22.8 | 14.5 | 16.3 | [6] |
| SiO2 0.3 | Xanthan gum 5000 | Sandstone | 17.8 | 8.67 | 66.5 | - | - | - | [6] |
| SiO2 0.5 | xanthan gum 5000 | Sandstone | 17.8 | 8.54 | 50.4 | 18.84 | 14.5 | 20.8 | [6] |
| SiO2 0.1 | HPAM 2000 | Sandstone | 21.8 | 11.5 | 100.3 | 78.6 | 54.7 | 60.81 | [98] |
| Al2O3 0.1 | HPAM 2000 | Sandstone | 21.8 | 9.3 | 100.3 | 60.6 | 54.7 | 65.3 | [98] |
| Surface Modified Clay | HPAM 2000 | carbonate | - | - | - | - | 25.0 | 34.0 | [99] |
| SiO2 0.2 | Guar gum 4000 | Sandstone | - | - | - | 115.0 | 23.02 | 35.97 | [100] |
| Nanoparticle | Polymer | Preparation Method | Contact Angle (°) | IFT (mN/m) | Rock | Recovery Factor OOIP (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Water | NPF | Water | NPF | ||||||
| Bare silica nanoparticles | PDMAEMA homopolymer | Surface grafted | - | - | 39.5 | 19.0 | - | - | [119] |
| SiO2 | DMAEMA polymer | Coated | 75.9 | 62.2 | 27.0 | 14.0 | Sandstone | 9.9 | [120] |
| Fe3O4 | Anionic polymer | Coated | 160.0 | 114.0 | 11.23 | 7.92 | Carbonate rock | 16.2–17.1 | [4] |
| SiO2/Al2O3 | Methacrylate | Coated | 143.3 | 48.75 | 10.3 | 6.5 | Sandstone | 6.0 | [121] |
| Surface modified SiO2 (AANP) | Xanthan gum | Radical polymerization method | - | - | 31.5 | 25.1 | - | 18.5 | [122] |
| TiO2 | Xanthan gum | Homogeneous miscible | 148.0 | 28.0 | 24.5 | 10.8 | Natural rock | 25.0 | [123] |
| ZnO/SiO2 | Xanthan gum | Coated | 132.6 | 34.1 | 19.68 | 9.45 | Carbonate rock | 19.3 | [1] |
| SiO2 | Guar gum | Homogeneous miscible | 115.0 | 73.0 | - | - | Sandstone | 44.28 | [100] |
| Nano | Cellulose | Surface grafted | 101.8 | 56.8 | - | - | Carbonate rock | 6.0 | [124] |
| Janus GO | Cellulose nanocrystals | Radical polymerization method | 166.0 | 37.0 | 20.2 | 14.4 | Edwards White | 22.96 | [125] |
| CuO/TiO2 | PAM | - | 151.0 | 14.7 | 28.0 | 15.0 | Carbonate rock | - | [2] |
| ZnO | PAM | Homogeneous miscible | 145.86 | 83.6 | 29.18 | 5.52 | Carbonate rock | 26.34 | [126] |
| Aminated SiO2 (M-SiO2) | PAM | Radical polymerization method | - | - | - | - | - | 17.5 | [127] |
| Surface modified SiO2 (C-SiO2) | PAM/PEI | Radical polymerization method | - | - | - | - | - | - | [128] |
| Al2O3 | HPAM | Radical polymerization method | 111.8 | 25.1 | - | - | Sandstone | 37.6 | [5] |
| SiO2 | HPAM | Radical polymerization method | 111.8 | 31.0 | - | - | Sandstone | 33.0 | [5] |
| aminated SiO2 | HPAM | Homogeneous miscible | - | - | 24.0 | 14.0 | - | 17.3 | [116] |
| Dispersible nano-SiO2 | HPAM | Homogeneous miscible | 141.8 | - | - | - | Quartz sand | 10.54 | [129] |
| Aminated GO | HPAM | Radical polymerization method | - | - | - | - | - | - | [130] |
| Bisfunctionalized GO | HPAM | Radical polymerization method | - | - | - | - | - | 21.5 | [131] |
| Particle Wettability | Contact Angle Range (°) | Characteristics of Surface Unsaturated Bonds |
|---|---|---|
| Strong hydrophilic particles | 0 | Metal bond, ionic bond |
| Weakly hydrophilic particles | 0–40 | Surface ionic bond or covalent bond |
| Hydrophobic particle | 40–90 | Molecular bonds are dominant, and local regions are strong bonds |
| Strongly hydrophobic particles | 90 | Intermolecular bonding force |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, W.; Li, J.; Han, R.; Lu, C. Mechanism and Performance Analysis of Nanoparticle-Polymer Fluid for Enhanced Oil Recovery: A Review. Molecules 2023, 28, 4331. https://doi.org/10.3390/molecules28114331
Sun Y, Zhang W, Li J, Han R, Lu C. Mechanism and Performance Analysis of Nanoparticle-Polymer Fluid for Enhanced Oil Recovery: A Review. Molecules. 2023; 28(11):4331. https://doi.org/10.3390/molecules28114331
Chicago/Turabian StyleSun, Yuanxiu, Weijie Zhang, Jie Li, Ruifang Han, and Chenghui Lu. 2023. "Mechanism and Performance Analysis of Nanoparticle-Polymer Fluid for Enhanced Oil Recovery: A Review" Molecules 28, no. 11: 4331. https://doi.org/10.3390/molecules28114331
APA StyleSun, Y., Zhang, W., Li, J., Han, R., & Lu, C. (2023). Mechanism and Performance Analysis of Nanoparticle-Polymer Fluid for Enhanced Oil Recovery: A Review. Molecules, 28(11), 4331. https://doi.org/10.3390/molecules28114331






