Regio- and Stereoselective Switchable Synthesis of (E)- and (Z)-N-Carbonylvinylated Pyrazoles
Abstract
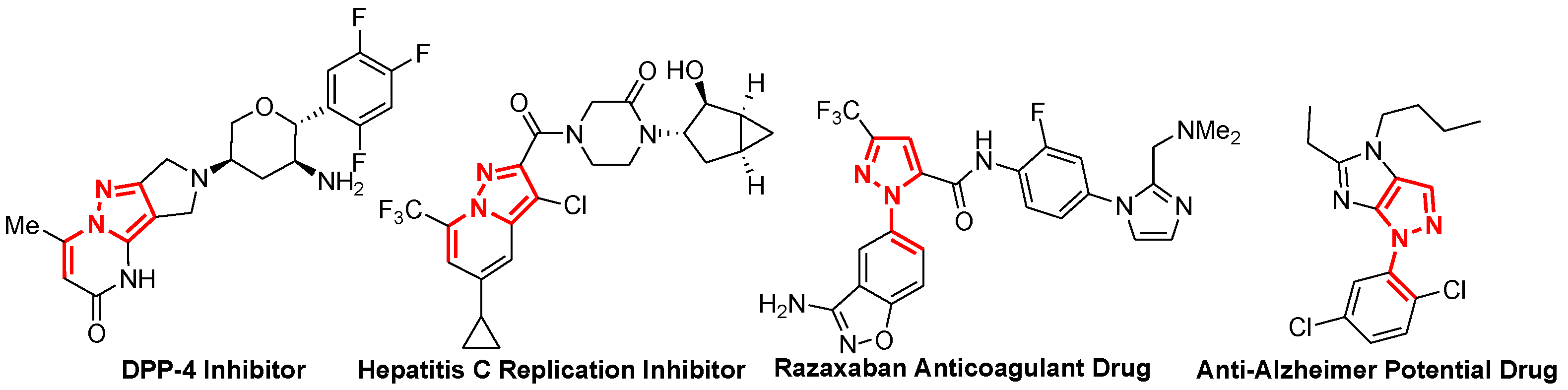
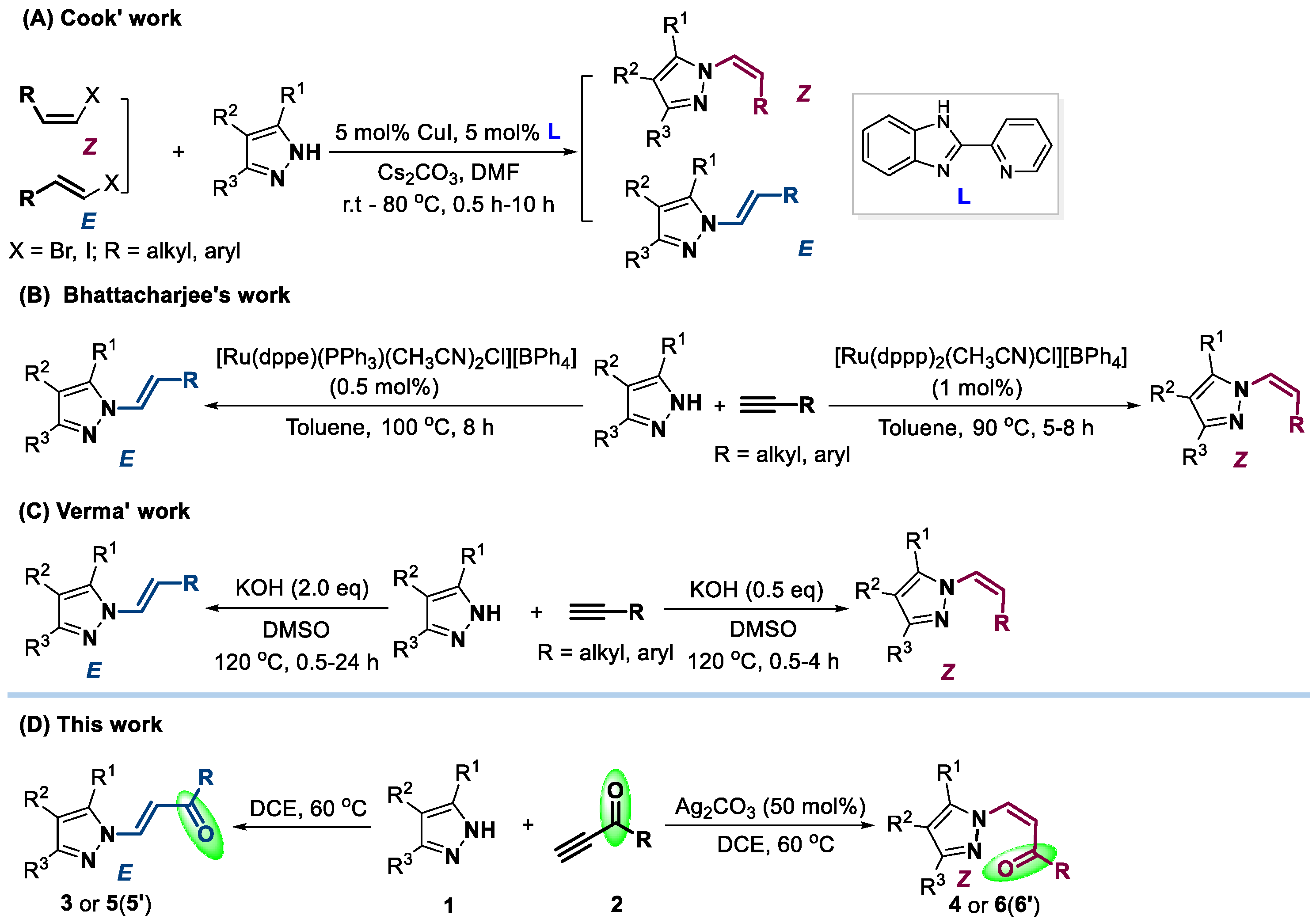
:1. Introduction
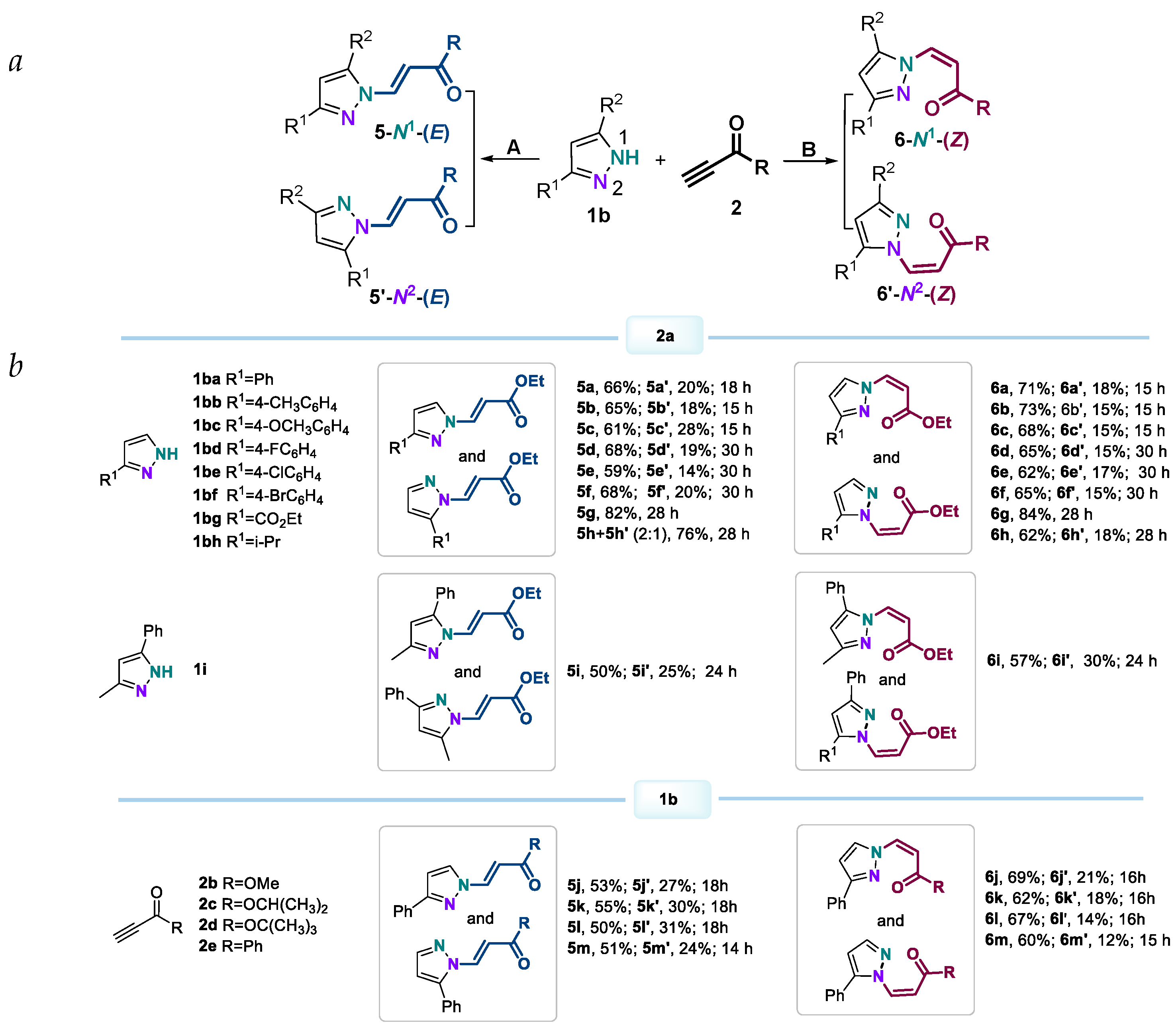
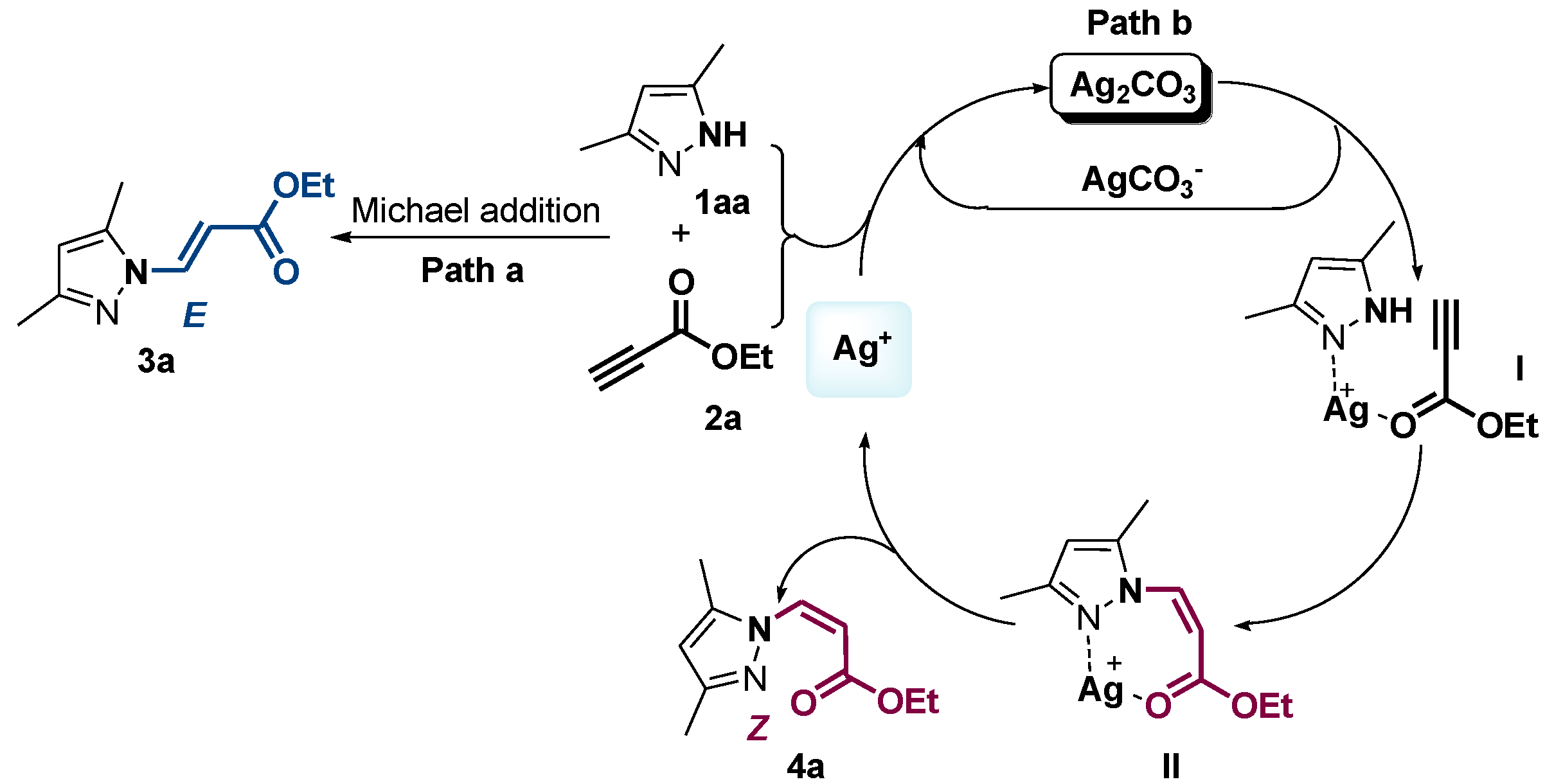
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Typical Procedure for the Synthesis of 3a
3.3. Typical Procedure for the Synthesis of 4a
3.4. Typical Procedure for the Synthesis of 5a and 5a’
3.5. Typical Procedure for the Synthesis of 6a and 6a’
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hu, C.H.; Valente, M.W.N.; Halpern, O.S.; Jusuf, S.; Khan, J.A.; Locke, G.A.; Duke, G.J.; Liu, X.; Duclos, F.J.; Wexler, R.R.; et al. Small molecule and macrocyclic pyrazole derived inhibitors of myeloperoxidase (MPO). Bioorg. Med. Chem. Lett. 2021, 42, 128010. [Google Scholar] [CrossRef]
- Oulous, A.; Daoudi, N.E.; Harit, T.; Cherfi, M.; Bnouham, M.; Malek, F. New pyrazole-tetrazole hybrid compounds as potent α-amylase and non-enzymatic glycation inhibitors. Bioorg. Med. Chem. Lett. 2022, 69, 128785. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zhou, M.-Z.; Ye, T.; Wang, P.-P.; Lu, R.; Wang, Y.-L.; Liu, C.-X.; Xiao, W.; Li, J.-Y.; Meng, Z.-B.; et al. Discovery of a Series of 5-Amide-1H-pyrazole-3-carboxyl Derivatives as Potent P2Y14R Antagonists with Anti-Inflammatory Characters. J. Med. Chem. 2022, 65, 15967–15990. [Google Scholar] [CrossRef]
- Wang, Z.W.; Zhang, Y.; Wang, H.; Wang, X.W.; Yu, Z.Z.; Zhao, L.W. Synthesis and biological evaluation of 4-(pyridine-4-oxy)-3-(tetrahydro-2H-pyran-4-yl)-pyrazole derivatives as novel, potent of ALK5 receptor inhibitors. Bioorg. Med. Chem. Lett. 2022, 61, 128552. [Google Scholar] [CrossRef]
- Witten, M.R.; Wu, L.; Lai, C.-T.; Kapilashrami, K.; Pusey, M.; Gallagher, K.; Chen, Y.; Yao, W. Inhibition of ALK2 with bicyclic pyridyllactams. Bioorg. Med. Chem. Lett. 2021, 55, 128452. [Google Scholar] [CrossRef]
- McBride, C.; Trzoss, L.; Povero, D.; Lazic, M.; Ambrus-Aikelin, G.; Santini, A.; Pranadinata, R.; Bain, G.; Stansfield, R.; Stafford, J.A.; et al. Overcoming Preclinical Safety Obstacles to Discover (S)-N-((1,2,3,5,6,7-Hexahydro-s-indacen-4-yl) carbamoyl)-6- (methylamino)-6,7-dihydro-5H-pyrazolo [5,1-b][1,3]oxazine-3-sulfonamide (GDC-2394): A Potent and Selective NLRP3 Inhibitor. J. Med. Chem. 2022, 65, 14721–14739. [Google Scholar] [CrossRef]
- Ragab, M.A.; Eldehna, W.M.; Nocentini, A.; Bonardi, A.; Okda, H.E.; Elgendy, B.; Ibrahim, T.S.; Abd-Alhaseeb, M.M.; Gratteri, P.; Supuran, C.T.; et al. 4-(5-Amino-pyrazol-1-yl)benzenesulfonamide derivatives as novel multitarget anti-inflammatory agents endowed with inhibitory activity against COX-2, 5-LOX and carbonic anhydrase: Design, synthesis, and biological assessments. Eur. J. Med. Chem. 2023, 250, 115180. [Google Scholar] [CrossRef]
- Short, K.M.; Estiarte, M.A.; Pham, S.M.; Williams, D.C.; Igoudin, L.; Dash, S.; Sandoval, N.; Datta, A.; Pozzi, N.; Di Cera, E.; et al. Discovery of novel N-acylpyrazoles as potent and selective thrombin inhibitors. Eur. J. Med. Chem. 2023, 246, 114855. [Google Scholar] [CrossRef]
- Ellermann, M.; Valot, G.; Cancho Grande, Y.; Hassfeld, J.; Kinzel, T.; Koebberling, J.; Beyer, K.; Roehrig, S.; Sperzel, M.; Stampfuss, J.; et al. Piperidinylpyrazolopyrimidinones and Their Use. WO2016/071216 A1, 30 October 2016. [Google Scholar]
- Miller, J.F.; Chong, P.Y.; Shotwell, J.B.; Catalano, J.G.; Tai, V.W.-F.; Fang, J.; Banka, A.L.; Roberts, C.D.; Youngman, M.; Zhang, H.; et al. Hepatitis C Replication Inhibitors That Target the Viral NS4B Protein. J. Med. Chem. 2014, 57, 2107–2120. [Google Scholar] [CrossRef]
- Quan, M.L.; Lam, P.Y.S.; Han, Q.; Pinto, D.J.P.; He, M.Y.; Li, R.H.; Ellis, C.D.; Clark, C.G.; Teleha, C.A.; Sun, J.H.; et al. Discovery of 1-(3′-Aminobenzisoxazol-5′-yl)-3-trifluoro-methyl-N-[2-fluoro-4-[(2′-dimethylaminomethyl) imidazol-1-yl] phenyl]-1H-pyrazole-5-carboxyamide Hydrochloride (Razaxaban), a Highly Potent, Selective, and Orally Bioavailable Factor Xa Inhibitor. J. Med. Chem. 2005, 48, 1729–1744. [Google Scholar] [CrossRef]
- Toque, H.A.F.; Priviero, F.; Teixeira, C.E.; Perissutti, E.; Fiorino, F.; Severino, B.; Frecentese, F.; Lorenzetti, R.; Baracat, J.S.; Santagada, V.; et al. Synthesis and Pharmacological Evaluations of Sildenafil Analogues for Treatment of Erectile Dysfunction. J. Med. Chem. 2008, 51, 2807–2815. [Google Scholar] [CrossRef]
- Iddon, B.; Tønder, J.E.; Hosseini, M.; Begtrup, M. The N-vinyl group as a protection group of the preparation of 3(5)-substituted pyrazoles via bromine–lithium exchange. Tetrahedron 2007, 63, 56–61. [Google Scholar] [CrossRef]
- Bhanuchandra, M.; Kuram, M.R.; Sahoo, A.K. Silver(I)- Catalyzed Reaction between Pyrazole and Propargyl Acetates: Stereoselective Synthesis of the Scorpionate Ligands (E)-Allyl-gem-dipyrazoles (ADPs). J. Org. Chem. 2013, 78, 11824–11834. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, X.; Wan, J.-P. Recent advances in transition metal-free annulation toward heterocycle diversity based on the C–N bond cleavage of enaminone platform. Org. Biomol. Chem. 2022, 20, 2356–2369. [Google Scholar] [CrossRef]
- Kurma, S.H.; Sridhar, B.; Bhimapaka, C.R. Direct Access for the Regio- and Stereoselective Synthesis of N-Alkenylpyrazoles and Chromenopyrazoles. J. Org. Chem. 2021, 86, 2271–2282. [Google Scholar] [CrossRef]
- Chen, S.J.; Li, J.H.; He, Z.Q.; Chen, G.S.; Zhuang, Y.Y.; Chen, C.P.; Liu, Y.L. N-Trifluoropropylation of Azoles through N-Vinylation and Sequential Hydrogenation. J. Org. Chem. 2022, 87, 15703–15712. [Google Scholar] [CrossRef]
- Liao, Q.; Zhang, L.Y.; Li, S.T.; Xi, C.J. Domino N-H/C-H Bond Activation: Copper-Catalyzed Synthesis of Nitrogen-Bridgehead Heterocycles Using Azoles and 1,4-Dihalo-1,3-dienes. Org. Lett. 2011, 13, 228–231. [Google Scholar] [CrossRef]
- Kaddouri, H.; Vicente, V.; Ouali, A.; Ouazzani, F.; Taillefer, M. Copper-Catalyzed Arylation of Nucleophiles by Using Butadienyl-phosphines as Ligands: Mechanistic Insight. Angew. Chem. Int. Ed. 2009, 48, 333–336. [Google Scholar] [CrossRef]
- Ouali, A.; Laurent, R.; Caminade, A.M.; Majoral, J.P.; Taillefer, M. Enhanced Catalytic Properties of Copper in O- and N-Arylation and Vinylation Reactions, Using Phosphorus Dendrimers as Ligands. J. Am. Chem. Soc. 2006, 128, 15990–15991. [Google Scholar] [CrossRef]
- Taillefer, M.; Ouali, A.; Renard, B.; Spindler, J.F. Mild Copper-Catalyzed Vinylation Reactionsof Azolesand Phenolswith Vinyl Bromides. Chem. Eur. J. 2006, 12, 5301–5313. [Google Scholar] [CrossRef]
- Davydov, D.V.; Sazonov, P.K.; Oprunenko, Y.F. Regioselectivity of the Chan-Lam coupling of ambident nitropyrazoles with trans-styrylboronic acid. Mendeleev Commun. 2020, 30, 185–187. [Google Scholar] [CrossRef]
- Xin, J.R.; He, Y.H.; Guan, Z. Metal-Free Aerobic Oxidative Direct C-H Amination of Electron-Deficient Alkenes via Photoredox Catalysis. Org. Chem. Front. 2018, 5, 1684–1688. [Google Scholar] [CrossRef]
- Tsuchimoto, T.; Aoki, K.; Wagatsuma, T.; Suzuki, Y. Lewis Acid Catalyzed Addition of Pyrazoles to Alkynes: Selective Synthesis of Double and Single Addition Products. Eur. J. Org. Chem. 2008, 2008, 4035–4040. [Google Scholar] [CrossRef]
- Das, U.K.; Bhattacharjee, M. A Moisture- and Air-Stable Cationic Ruthenium Complex as Catalyst for Highly Atom-Economical Stereo- and Regioselective Vinylation of Azoles. Chem. Eur. J. 2012, 18, 5180–5183. [Google Scholar] [CrossRef]
- Velena, W.A.; Berg, J.P.V.; Hansen, M.J.; Szymanski, W.; Driessen, A.J.M.; Feringa, B.L. Optical control of antibacterial activity. Nat. Chem. 2013, 5, 924–928. [Google Scholar] [CrossRef]
- Verma, A.K.; Patel, M.; Joshi, M.; Likhar, P.R.; Tiwari, R.K.; Parang, K. Base-Mediated Chemo- and Stereoselective Addition of 5- Aminoindole/Tryptamine and Histamines onto Alkynes. J. Org. Chem. 2014, 79, 172–186. [Google Scholar] [CrossRef]
- Kabir, M.S.; Lorenz, M.; Namjoshi, O.A.; Cook, J.M. First Application of an Efficient and Versatile Ligand for Copper-Catalyzed Cross-Coupling Reactions of Vinyl Halides with N-Heterocycles and Phenols. Org. Lett. 2010, 12, 464–467. [Google Scholar] [CrossRef]
- Das, U.K.; Mandal, S.; Anoop, A.; Bhattacharjee, M. Structure-Selectivity Relationship in Ruthenium Catalyzed Regio- and Stereoselective Addition of Alkynes to Pyrazoles: An Experimental and Theoretical Investigation. J. Org. Chem. 2014, 79, 9979–9991. [Google Scholar] [CrossRef]
- Garg, V.; Kumar, P.; Verma, A.K. Chemo-, Regio-, and Stereoselective N-Alkenylation of Pyrazoles/Benzpyrazoles Using Activated and Unactivated Alkynes. J. Org. Chem. 2017, 82, 10247–10262. [Google Scholar] [CrossRef]
- Yu, H.F.; Wang, W.J. Catalyst free cyclocondensation of β-ethylthio-β-indolyl-α, β-unsaturated ketones with hydrazines: Efficient synthesis of 3-pyrazolyl indoles. Synth. Commun. 2020, 50, 1133–1140. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Yu, H.F.; Liao, P.Q.; Wang, W.J. Green and Efficient Synthesis of 3-Pyrazolyl Indoles in Water. Chem. Res. Chin. Univ. 2020, 36, 847–852. [Google Scholar] [CrossRef]
- Zhao, X.B.; Jiang, S.A.; Wang, N.; Yu, H.F. Green and complementary regioselective synthesis of 3-(1-substituted pyrazol-3(or5)-yl) indoles from β-ethyltho-β-indolyl-α, β-unsaturated ketones in water. Synth. Commun. 2020, 50, 3404–3412. [Google Scholar] [CrossRef]
- Yu, H.F.; Liao, P.Q.; Mei, Z.M. Regioselective Synthesis of Isomeric 3-(1-substituted Pyrazol-3(5)-yl) Indoles from β-Ethylthio- β-Indolyl-α, β-unsaturated Ketones. Synthesis 2020, 52, 2111–2120. [Google Scholar]
- Zhang, X.; Qiu, D.X.; Qiu, W.T.; Wang, H.R.; Zhao, Z.W.; Yu, H.F.; Che, G.B. Ag2CO3 Catalyzed Aza-Michael Addition of Pyrazoles to α, β-unsaturated Carbonyl Compounds: A New Access to N-Alkylated Pyrazole Derivatives. Tetrahedron 2023, 134, 133305. [Google Scholar] [CrossRef]
- Bai, X.F.; Xu, Z.; Xia, C.G.; Zheng, Z.J.; Xu, L.W. Aromatic-Amide-Derived Nonbiaryl Atropisomer as Highly Efficient Ligand for Asymmetric Silver-Catalyzed [3 + 2] Cycloaddition. ACS Catal. 2015, 5, 6016–6020. [Google Scholar] [CrossRef]
- Li, J.Y.; Kim, H.Y.; Oh, K. Enantiodivergent Brucine Diol-Catalyzed 1, 3-Dipolar Cycloaddition of Azomethine Ylides with α, β-Unsaturated Ketones. Adv. Synth. Catal. 2016, 358, 984–993. [Google Scholar] [CrossRef]
- Gao, Y.L.; Hu, Z.Y.; Dong, J.H.; Liu, J.; Xu, X.X. Chemoselective Double Annulation of Two Different Isocyanides: Rapid Access to Trifluoromethylated Indole-Fused Heterocycles. Org. Lett. 2017, 19, 5292–5295. [Google Scholar] [CrossRef]
- CCDC 2248238 (3l), CCDC 2248246 (4o), CCDC 2248247 (5c), CCDC 2248248 (6c) and CCDC 2248252 (5k’). The Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/datarequest/cif (accessed on 13 March 2023).




| Entry | Cat. (x mol%) | Solvent | Temp. (°C) | Time (h) | Yield (%) b | |
|---|---|---|---|---|---|---|
| 3a | 4a | |||||
| 1 | none | DCE | 25 | 24 | 60 | 30 |
| 2 | none | DCE | 40 | 12 | 75 | 19 |
| 3 | none | DCE | 50 | 10 | 84 | 12 |
| 4 | none | DCE | 60 | 8 | 90 | 9 |
| 5 | none | DCE | 70 | 6 | 89 | 9 |
| 6 | none | toluene | 60 | 9 | 85 | 9 |
| 7 | none | 1,4-dioxane | 60 | 12 | 82 | 11 |
| 8 | Ag2CO3 (10) | DCE | 60 | 24 | 38 | 51 |
| 9 | Ag2CO3 (10) | DCE | 20 | 72 | 17 | 29 |
| 10 | Ag2CO3 (20) | DCE | 60 | 16 | 25 | 62 |
| 11 | Ag2CO3 (30) | DCE | 60 | 11 | 23 | 69 |
| 12 | Ag2CO3 (40) | DCE | 60 | 7 | 16 | 75 |
| 13 | Ag2CO3 (50) | DCE | 60 | 5 | 9 | 81 |
| 14 | Ag2CO3 (60) | DCE | 60 | 4 | 8 | 76 |
| 15 | Ag2CO3 (70) | DCE | 60 | 3 | 6 | 73 |
| 16 | Ag2CO3 (50) | DCE | 70 | 4 | 12 | 77 |
| 17 | Ag2CO3 (50) | DCE | 50 | 13 | 18 | 68 |
| 18 | AgOAc (50) | DCE | 60 | 17 | 21 | 61 |
| 19 | AgNO3 (50) | DCE | 60 | 15 | 14 | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, Z.; Yu, H.; Che, G. Regio- and Stereoselective Switchable Synthesis of (E)- and (Z)-N-Carbonylvinylated Pyrazoles. Molecules 2023, 28, 4347. https://doi.org/10.3390/molecules28114347
Zhang X, Zhang Z, Yu H, Che G. Regio- and Stereoselective Switchable Synthesis of (E)- and (Z)-N-Carbonylvinylated Pyrazoles. Molecules. 2023; 28(11):4347. https://doi.org/10.3390/molecules28114347
Chicago/Turabian StyleZhang, Xue, Zheyu Zhang, Haifeng Yu, and Guangbo Che. 2023. "Regio- and Stereoselective Switchable Synthesis of (E)- and (Z)-N-Carbonylvinylated Pyrazoles" Molecules 28, no. 11: 4347. https://doi.org/10.3390/molecules28114347
APA StyleZhang, X., Zhang, Z., Yu, H., & Che, G. (2023). Regio- and Stereoselective Switchable Synthesis of (E)- and (Z)-N-Carbonylvinylated Pyrazoles. Molecules, 28(11), 4347. https://doi.org/10.3390/molecules28114347






