Application of Metal-Based Nanomaterials in In Vitro Diagnosis of Tumor Markers: Summary and Prospect
Abstract
1. Introduction
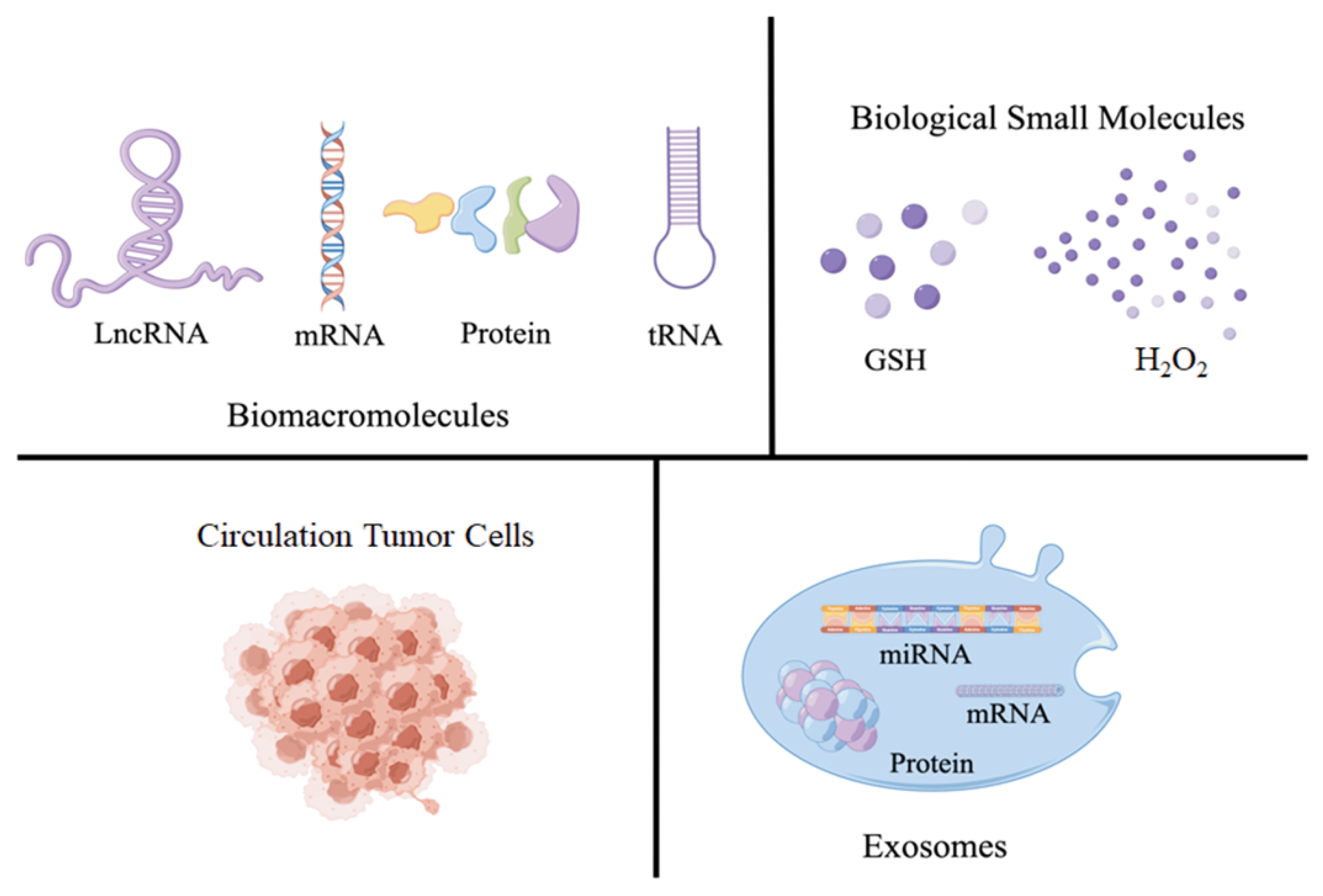
2. Introduction for Tumor Markers
3. The Catalytic Properties of MNPs for Detecting Tumor Markers
3.1. Construction of Electrocatalytic-Activity-Based Electrochemical Biosensor
3.2. Chemical Catalysis-Based Tumor Marker Determination
3.3. Methods-Based Photocatalytic Properties for Tumor Marker Detection
4. The Optical Properties of MNPs for Detecting Tumor Markers
4.1. Construction of MNPs’ Fluorescence Biosensor
4.2. Build a Surface-Enhanced Raman Sensor
| Materials | Tumor Marker | Linearity Range | Limit of Detection | Ref. |
|---|---|---|---|---|
| Small gold nanorods (Au NRs) | Exosomes of breast cancer cells | 106~108 particles/mL | 2 × 106 particles/mL | [94] |
| Gold nanostar@4-mercaptobenzoic acid@nanoshell structures (AuNS@4-MBA@Au) | Exosomes of liver cancer patients | 40~4.0 × 107 particles/μL | 27 particles/μL | [95] |
| Magnetic bead MB@SiO2@Au@aptamer | Exosomes of breast, colorectal, and prostate cancer | - | 32, 73, and 203 particles/μL | [96] |
| Fe3O4@TiO2 nanoparticles | PD-L1 exosome | 5 × 103~2 × 105 particles/mL | 1 particles/μL | [97] |
| Fe3O4@Ag-DNA-Au@Ag@DTNB | miRNA | 3 aM~100 pM | 1.8 aM | [98] |
| Plasmonic head-flocked gold nanopillars@LNA detection probe | miRNA | 1 aM~100 nM | 1 aM | [99] |
| Functionalized gold nanoparticles (Au NPs) | muc-4 | 10 ng/mL~100 μg/mL | 33 ng/mL | [100] |
| Silver/chitosan nanoparticles (Ag@CS NPs) | Platelet-derived growth factor BB | 10 pg/mL~5.0 ng/mL | 3.2 pg/mL | [101] |
| Fe3O4 nanoring (R-Fe3O4) | Interleukin-6 | 0.1~1000 pg/mL | 0.028 pg/mL | [102] |
| 4-MBA-encoded Au NPs (AuNP-MBA) | MCF-7 | 5~500 cells/mL | 5 cells/mL | [103] |
| Triangular silver nanoprisms (Ag NPR) | HeLa cell | 1–100 cells/mL | 1 cell/mL | [104] |
| Poly(ethyleneimine) (PEI)-stabilized superparamagnetic iron oxide nanoparticles (SPION-PEI) | HeLa cell | 1–25 cells/mL | 1 cell/mL | [105] |
4.3. Determination of Tumor Markers by Surface Plasmon Resonance Characteristics of MNPs
5. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, M.; Chen, W. Epidemiology of lung cancer in China. Thorac. Cancer 2019, 10, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-H.; Shen, L.; Li, J.; Zhou, Z.-W.; Liang, H.; Zhang, X.-T.; Tang, L.; Xin, Y.; Jin, J.; Zhang, Y.-J.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun. 2019, 39, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-H.; Zhang, X.-T.; Li, Y.-F.; Tang, L.; Qu, X.-J.; Ying, J.-E.; Zhang, J.; Sun, L.-Y.; Lin, R.-B.; Qiu, H.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021, 41, 747–795. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Cuo, Z.; Zhu, P.; Chen, J.; Huang, Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med. 2019, 8, 1958–1975. [Google Scholar] [CrossRef]
- Gezici, S.; Sekeroglu, N. Current Perspectives in the Application of Medicinal Plants Against Cancer: Novel Therapeutic Agents. Anti Cancer Agents Med. Chem. 2019, 19, 101–111. [Google Scholar] [CrossRef]
- Mitra, T.; Bhattacharya, R. Phytochemicals modulate cancer aggressiveness: A review depicting the anticancer efficacy of dietary polyphenols and their combinations. J. Cell. Physiol. 2020, 235, 7696–7708. [Google Scholar] [CrossRef]
- Wu, M.; Meng, Q.; Chen, Y.; Zhang, L.; Li, M.; Cai, X.; Li, Y.; Yu, P.; Zhang, L.; Shi, J. Large Pore-Sized Hollow Mesoporous Organosilica for Redox-Responsive Gene Delivery and Synergistic Cancer Chemotherapy. Adv. Mater. 2016, 28, 1963–1969. [Google Scholar] [CrossRef]
- Jia, L.; Shen, J.; Li, Z.; Zhang, D.; Zhang, Q.; Liu, G.; Zheng, D.; Tian, X. In vitro and in vivo evaluation of paclitaxel-loaded mesoporous silica nanoparticles with three pore sizes. Int. J. Pharm. 2013, 445, 12–19. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef]
- Song, Y.; Li, D.; Lu, Y.; Jiang, K.; Yang, Y.; Xu, Y.; Dong, L.; Yan, X.; Ling, D.; Yang, X.; et al. Ferrimagnetic mPEG-b-PHEP copolymer micelles loaded with iron oxide nanocubes and emodin for enhanced magnetic hyperthermia-chemotherapy. Natl. Sci. Rev. 2020, 7, 723–736. [Google Scholar] [CrossRef]
- Cheng, K.; Peng, S.; Xu, C.; Sun, S. Porous Hollow Fe3O4 Nanoparticles for Targeted Delivery and Controlled Release of Cisplatin. J. Am. Chem. Soc. 2009, 131, 10637–10644. [Google Scholar] [CrossRef]
- Pons, T.; Bouccara, S.; Loriette, V.; Lequeux, N.; Pezet, S.; Fragola, A. In Vivo Imaging of Single Tumor Cells in Fast-Flowing Bloodstream Using Near-Infrared Quantum Dots and Time-Gated Imaging. ACS Nano 2019, 13, 3125–3131. [Google Scholar] [CrossRef]
- Qi, G.-B.; Gao, Y.-J.; Wang, L.; Wang, H. Self-Assembled Peptide-Based Nanomaterials for Biomedical Imaging and Therapy. Adv. Mater. 2018, 30, e1703444. [Google Scholar] [CrossRef]
- Shen, K.; Huang, Y.; Li, Q.; Chen, M.; Wu, L. Self-Assembled Polysaccharide-Diphenylalanine/Au Nanospheres for Photothermal Therapy and Photoacoustic Imaging. ACS Omega 2019, 4, 18118–18125. [Google Scholar] [CrossRef]
- Yokoi, A.; Matsuzaki, J.; Yamamoto, Y.; Yoneoka, Y.; Takahashi, K.; Shimizu, H.; Uehara, T.; Ishikawa, M.; Ikeda, S.-I.; Sonoda, T.; et al. Integrated extracellular microRNA profiling for ovarian cancer screening. Nat. Commun. 2018, 9, 4319. [Google Scholar] [CrossRef]
- Yang, H.; Liao, C.; Zhang, Z.; Zhan, P.; Chen, Y.-R. Wheel drive-based DNA sensing system for highly specific and rapid one-step detection of MiRNAs at the attomolar level. Talanta 2023, 257, 124371. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, C.; Lai, Y. A serum miRNAs signature for early diagnosis of bladder cancer. Ann. Med. 2023, 55, 736–745. [Google Scholar] [CrossRef]
- Fischer, S.; Rothermundt, C.; Stalder, O.; Terbuch, A.; Hermanns, T.; Zihler, D.; Muller, B.; Fankhauser, C.D.; Hirschi-Blickenstorfer, A.; Seifert, B.; et al. The Value of Tumour Markers in the Detection of Relapse-Lessons Learned from the Swiss Austrian German Testicular Cancer Cohort Study. Eur. Urol. Open Sci. 2023, 50, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, P.; Wu, R.; Lu, K.; Zhou, H. Identifying the Best Marker Combination in CEA, CA125, CY211, NSE, and SCC for Lung Cancer Screening by Combining ROC Curve and Logistic Regression Analyses: Is It Feasible? Dis. Markers 2018, 2018, 2082840. [Google Scholar] [CrossRef]
- Li, M.; Yao, B.; Jing, C.; Chen, H.; Zhang, Y.; Zhou, N. Engineering a G-quadruplex-based logic gate platform for sensitive assay of dual biomarkers of ovarian cancer. Anal. Chim. Acta 2022, 1198, 339559. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, M.; Jin, G.; Jiang, Y.; Luan, Y. Cu-MOF chemodynamic nanoplatform via modulating glutathione and H2O2 in tumor microenvironment for amplified cancer therapy. J. Colloid Interface Sci. 2021, 587, 358–366. [Google Scholar] [CrossRef]
- Hussain, M.; Nisar, A.; Qian, L.; Karim, S.; Khan, M.; Liu, Y.; Sun, H.; Ahmad, M. Ni and Co synergy in bimetallic nanowires for the electrochemical detection of hydrogen peroxide. Nanotechnology 2021, 32, 205501. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Cheng, D.; Li, P.; Xu, Z.; Zhu, X.; Zhang, Y.; Li, H.; Liu, X.; Liu, M.; Yao, S. Au/Metal-Organic Framework Nanocapsules for Electrochemical Determination of Glutathione. ACS Appl. Nano Mater. 2021, 4, 4853–4862. [Google Scholar] [CrossRef]
- Sharma, S.; Zhuang, R.; Long, M.; Pavlovic, M.; Kang, Y.; Ilyas, A.; Asghar, W. Circulating tumor cell isolation, culture, and downstream molecular analysis. Biotechnol. Adv. 2018, 36, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Yang, H.C.; Rhee, W.J. Simultaneous multiplexed detection of exosomal microRNAs and surface proteins for prostate cancer diagnosis. Biosens. Bioelectron. 2019, 146, 111749. [Google Scholar] [CrossRef]
- Zhai, Q.; Cheng, W. Soft and stretchable electrochemical biosensors. Mater. Today Nano 2019, 7, 100041. [Google Scholar] [CrossRef]
- Prakash, S.; Chakrabarty, T.; Singh, A.K.; Shahi, V.K. Polymer thin films embedded with metal nanoparticles for electrochemical biosensors applications. Biosens. Bioelectron. 2013, 41, 43–53. [Google Scholar] [CrossRef]
- Savk, A.; Aydin, H.; Cellat, K.; Sen, F. A novel high performance non-enzymatic electrochemical glucose biosensor based on activated carbon-supported Pt-Ni nanocomposite. J. Mol. Liq. 2020, 300, 112355. [Google Scholar] [CrossRef]
- Zabihollahpoor, A.; Rahimnejad, M.; Najafpour, G.; Moghadanmia, A.A. Gold nanoparticle prepared by electrochemical deposition for electrochemical determination of gabapentin as an antiepileptic drug. J. Electroanal. Chem. 2019, 835, 281–286. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, J.; Feng, Z.; Wang, F.; Xie, S.; Bu, S. Immunoassay for tumor markers in human serum based on Si nanoparticles and SiC@Ag SERS-active substrate. Analyst 2016, 141, 2534–2541. [Google Scholar] [CrossRef]
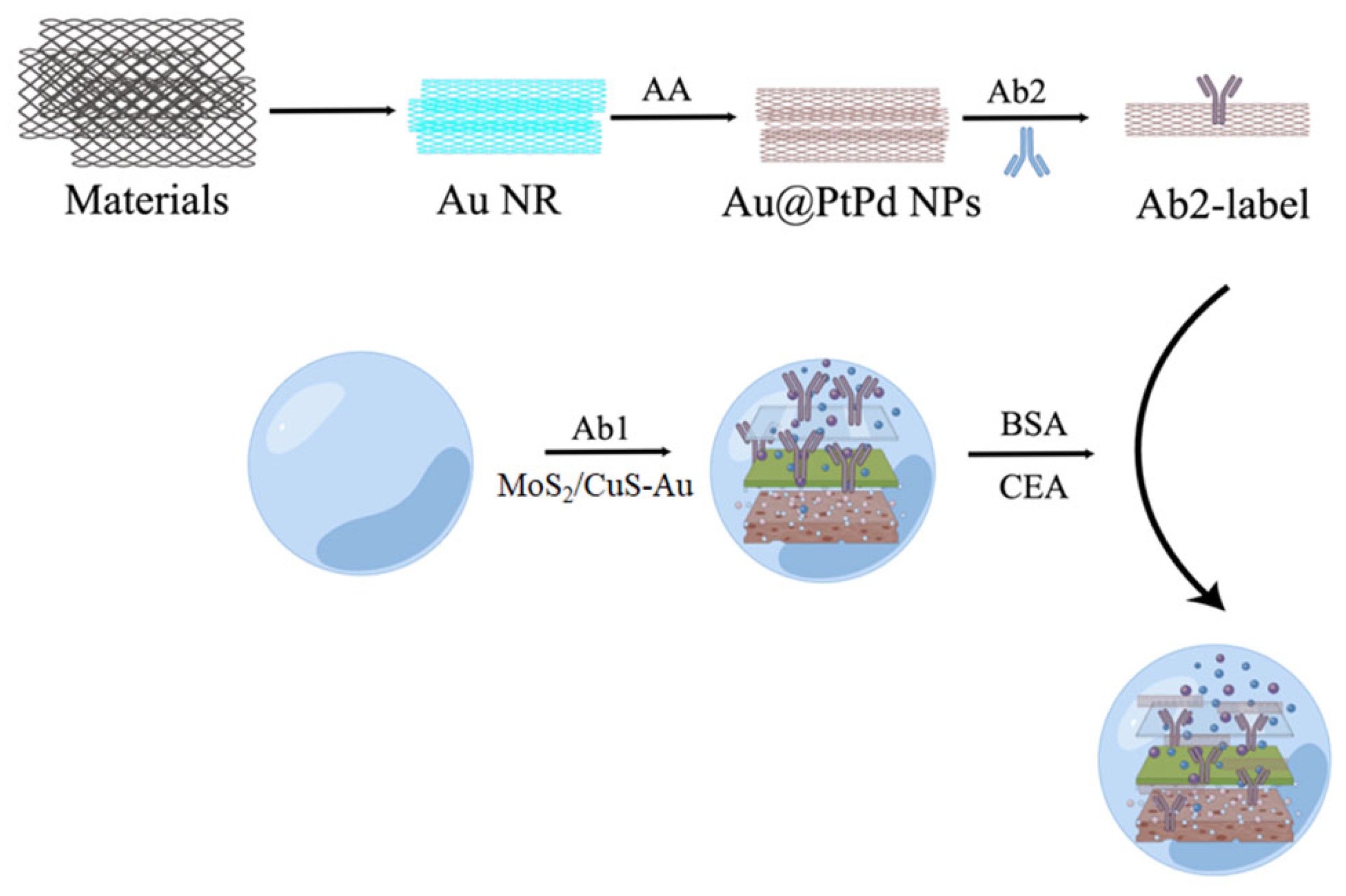
- Jia, Y.; Li, Y.; Zhang, S.; Wang, P.; Liu, Q.; Dong, Y. Mulberry-like Au@PtPd porous nanorods composites as signal amplifiers for sensitive detection of CEA. Biosens. Bioelectron. 2020, 149, 111842. [Google Scholar] [CrossRef] [PubMed]
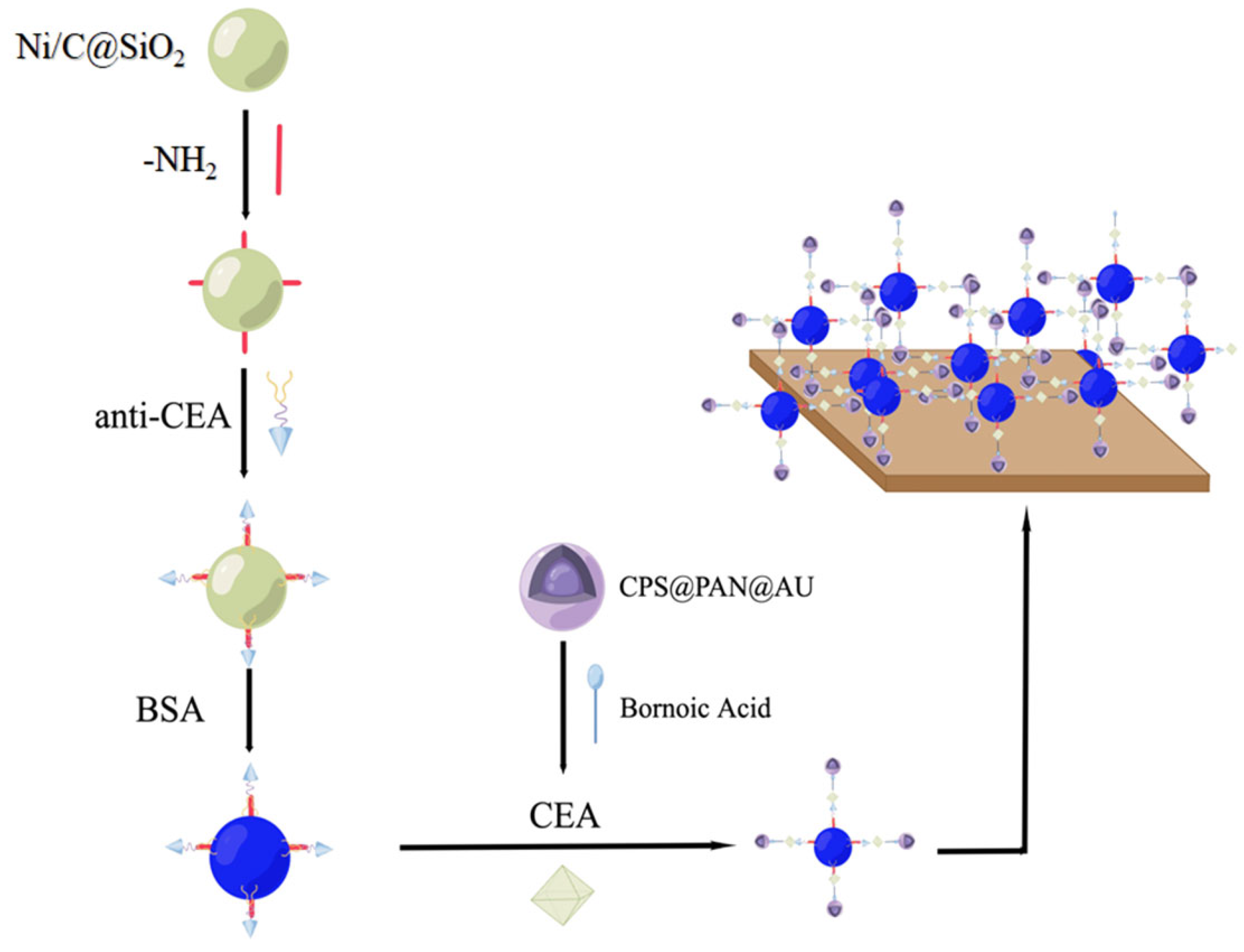
- Song, D.; Zheng, J.; Myung, N.V.; Xu, J.; Zhang, M. Sandwich-type electrochemical immunosensor for CEA detection using magnetic hollow Ni/C@SiO2 nanomatrix and boronic acid functionalized CPS@PANI@Au probe. Talanta 2021, 225, 122006. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, J.; Li, B.; Liu, J.; Xu, J.J.; Chen, H.Y. Dual-Mode SERS and Electrochemical Detection of miRNA Based on Popcorn-like Gold Nanofilms and Toehold-Mediated Strand Displacement Amplification Reaction. Anal. Chem. 2021, 93, 6120–6127. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Huang, K.-J.; Wu, X.; Ma, Y.-Y.; Song, D.-L.; Du, C.-Y.; Chang, S.-H. Ultrasensitive supersandwich-type biosensor for enzyme-free amplified microRNA detection based on N-doped graphene/Au nanoparticles and hemin/G-quadruplexes. J. Mater. Chem. B 2018, 6, 2134–2142. [Google Scholar] [CrossRef]
- Li, Y.; Yu, C.; Yang, B.; Liu, Z.; Xia, P.; Wang, Q. Target-catalyzed hairpin assembly and metal-organic frameworks mediated nonenzymatic co-reaction for multiple signal amplification detection of miR-122 in human serum. Biosens. Bioelectron. 2018, 102, 307–315. [Google Scholar] [CrossRef]
- Li, Y.; Tian, R.; Zheng, X.; Huang, R. Amplified electrochemical detection of nucleic acid hybridization via selective preconcentration of unmodified gold nanoparticles. Anal. Chim. Acta 2016, 934, 59–65. [Google Scholar] [CrossRef]
- Lu, Z.; Tang, H.; Wu, D.; Xia, Y.; Wu, M.; Yi, X.; Li, H.; Wang, J. Amplified voltammetric detection of miRNA from serum samples of glioma patients via combination of conducting magnetic microbeads and ferrocene-capped gold nanoparticle/streptavidin conjugates. Biosens. Bioelectron 2016, 86, 502–507. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Liu, L.; Hu, Y.; Xu, Z.; Liu, L.; Wu, N.; Li, N. Microstructure evolution of sandwich graphite oxide/interlayer-embedded Au nanoparticles induced from gamma-rays for carcinoembryonic antigen biosensor. Nanotechnology 2019, 30, 495501. [Google Scholar] [CrossRef]
- Guo, C.; Su, F.; Song, Y.; Hu, B.; Wang, M.; He, L.; Peng, D.; Zhang, Z. Aptamer-Templated Silver Nanoclusters Embedded in Zirconium Metal-Organic Framework for Bifunctional Electrochemical and SPR Aptasensors toward Carcinoembryonic Antigen. ACS Appl. Mater. Interfaces 2017, 9, 41188–41199. [Google Scholar] [CrossRef]
- Chen, P.; Hua, X.; Liu, J.; Liu, H.; Xia, F.; Tian, D.; Zhou, C. A dual amplification electrochemical immunosensor based on HRP-Au@Ag NPs for carcinoembryonic antigen detection. Anal. Biochem. 2019, 574, 23–30. [Google Scholar] [CrossRef]
- Wang, X.; Chu, C.; Shen, L.; Deng, W.; Yan, M.; Ge, S.; Yu, J.; Song, X. An ultrasensitive electrochemical immunosensor based on the catalytical activity of MoS2-Au composite using Ag nanospheres as labels. Sens. Actuators B Chem. 2015, 206, 30–36. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Zhang, Y.; Pang, X.; Cao, W.; Du, B.; Wei, Q. Sandwich-type electrochemical immunosensor for CEA detection based on Ag/MoS2@Fe3O4 and an analogous ELISA method with total internal reflection microscopy. Sens. Actuators B Chem. 2018, 266, 561–569. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, N.; Ma, Z. Platinum porous nanoparticles hybrid with metal ions as probes for simultaneous detection of multiplex cancer biomarkers. Biosens. Bioelectron. 2014, 53, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, N.; Yuan, J.; Ma, Z. Triple tumor markers assay based on carbon-gold nanocomposite. Biosens. Bioelectron. 2015, 70, 161–166. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Han, X.; Cui, Y.; Wang, W. Electrochemical DNA biosensor fabrication with hollow gold nanospheres modified electrode and its enhancement in DNA immobilization and hybridization. Biosens. Bioelectron. 2010, 25, 1640–1645. [Google Scholar] [CrossRef]
- Li, Q.; Tang, D.; Tang, J.; Su, B.; Chen, G.; Wei, M. Magneto-controlled electrochemical immunosensor for direct detection of squamous cell carcinoma antigen by using serum as supporting electrolyte. Biosens. Bioelectron. 2011, 27, 153–159. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, X.; Liu, S.; Zheng, L.; Bu, Y.; Deng, H.; Chen, R.; Peng, H.; Lin, X.; Chen, W. Colorimetric acid phosphatase sensor based on MoO3 nanozyme. Anal. Chim. Acta 2020, 1105, 162–168. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Su, D.; Zhang, Y.; Lu, H.; Yan, X.; Bai, J.; Gao, Y.; Lu, G. The DNA controllable peroxidase mimetic activity of MoS2 nanosheets for constructing a robust colorimetric biosensor. Nanoscale 2020, 12, 19420–19428. [Google Scholar] [CrossRef]
- Zheng, S.; Gu, H.; Yin, D.; Zhang, J.; Li, W.; Fu, Y. Biogenic synthesis of AuPd nanocluster as a peroxidase mimic and its application for colorimetric assay of acid phosphatase. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124444. [Google Scholar] [CrossRef]
- Ge, J.; Cai, R.; Chen, X.; Wu, Q.; Zhang, L.; Jiang, Y.; Cui, C.; Wan, S.; Tan, W. Facile approach to prepare HSA-templated MnO2 nanosheets as oxidase mimic for colorimetric detection of glutathione. Talanta 2019, 195, 40–45. [Google Scholar] [CrossRef]
- Zhan, Y.; Zeng, Y.; Li, L.; Guo, L.; Luo, F.; Qiu, B.; Huang, Y.; Lin, Z. Cu2+-Modified Boron Nitride Nanosheets-Supported Subnanometer Gold Nanoparticles: An Oxidase-Mimicking Nanoenzyme with Unexpected Oxidation Properties. Anal. Chem. 2019, 92, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, A.; Mao, X.; Chen, Q.; Huang, Y. Engineered Mn/Co oxides nanocomposites by cobalt doping of Mn-BTC—New oxidase mimetic for colorimetric sensing of acid phosphatase. Sens. Actuators B Chem. 2019, 299, 126928. [Google Scholar] [CrossRef]
- Jin, L.; Sun, Y.; Shi, L.; Li, C.; Shen, Y. PdPt bimetallic nanowires with efficient oxidase mimic activity for the colorimetric detection of acid phosphatase in acidic media. J. Mater. Chem. B 2019, 7, 4561–4567. [Google Scholar] [CrossRef]
- He, S.-B.; Yang, L.; Yang, Y.; Noreldeen, H.A.A.; Wu, G.-W.; Peng, H.-P.; Deng, H.-H.; Chen, W. Carboxylated chitosan enabled platinum nanozyme with improved stability and ascorbate oxidase-like activity for a fluorometric acid phosphatase sensor. Carbohydr. Polym. 2022, 298, 120120. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Q.; Weng, C.; Li, X.; Yan, X.; Yang, W.; Li, B.; Zhou, X. Label-Free Colorimetric Detection of Acid Phosphatase and Screening of Its Inhibitors Based on Biomimetic Oxidase Activity of MnO2 Nanosheets. ACS Biomater. Sci. Eng. 2020, 6, 3132–3138. [Google Scholar] [CrossRef]
- Hong, L.; Liu, A.-L.; Li, G.-W.; Chen, W.; Lin, X.-H. Chemiluminescent cholesterol sensor based on peroxidase-like activity of cupric oxide nanoparticles. Biosens. Bioelectron. 2013, 43, 1–5. [Google Scholar] [CrossRef]
- Jin, L.; Meng, Z.; Zhang, Y.; Cai, S.; Zhang, Z.; Li, C.; Shang, L.; Shen, Y. Ultrasmall Pt Nanoclusters as Robust Peroxidase Mimics for Colorimetric Detection of Glucose in Human Serum. ACS Appl. Mater. Interfaces 2017, 9, 10027–10033. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, Y.; Li, J.; Lu, M.; Jiang, Z.; Hu, X. Smart CuS Nanoparticles as Peroxidase Mimetics for the Design of Novel Label-Free Chemiluminescent Immunoassay. ACS Appl. Mater. Interfaces 2016, 8, 12031–12038. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, C.; Li, X.; Wu, K.; Gao, L.; Lyu, X.; Zhang, X.; Zhang, X.; Luo, X.; Liu, Q. Two-dimensional porphyrin-Co9S8 nanocomposites with synergistic peroxidase-like catalysis: Synthesis and application toward colorimetric biosensing of H2O2 and glutathione. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 248–258. [Google Scholar] [CrossRef]
- Huang, X.; Nan, Z. Porous 2D FeS2 nanosheets as a peroxidase mimic for rapid determination of H2O2. Talanta 2020, 216, 120995. [Google Scholar] [CrossRef]
- Li, X.; Qian, C.; Tian, Y.; Yao, N.; Duan, Y.; Huang, Z. Pt-Ru bimetallic nanoclusters with super peroxidase-like activity for ultra-sensitive lateral flow immunoassay. Chem. Eng. J. 2023, 457, 141324. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, W.; Li, X.; Wang, D.; Shuang, S.; Dong, C. Dendritic Mesoporous Silica Nanoparticle-Tuned High-Affinity MnO2 Nanozyme for Multisignal GSH Sensing and Target Cancer Cell Detection. ACS Sustain. Chem. Eng. 2022, 10, 5911–5921. [Google Scholar] [CrossRef]
- Chen, M.; Wu, L.; Ye, H.; Liang, N.; Zhao, L. Biocompatible BSA-AuNP@ZnCo2O4 nanosheets with oxidase-like activity: Colorimetric biosensing and antitumor activity. Microchem. J. 2022, 175, 107208. [Google Scholar] [CrossRef]
- Ding, C.; Wang, X.; Song, K.; Zhang, B.; Wang, J.; Zhao, Z.; Chang, H.; Wei, W. Visible light enabled colorimetric tumor marker detection using ternary GO-C3N4-AgBr heterojunction nanophotocatalyst. Sens. Actuators B Chem. 2018, 268, 376–382. [Google Scholar] [CrossRef]
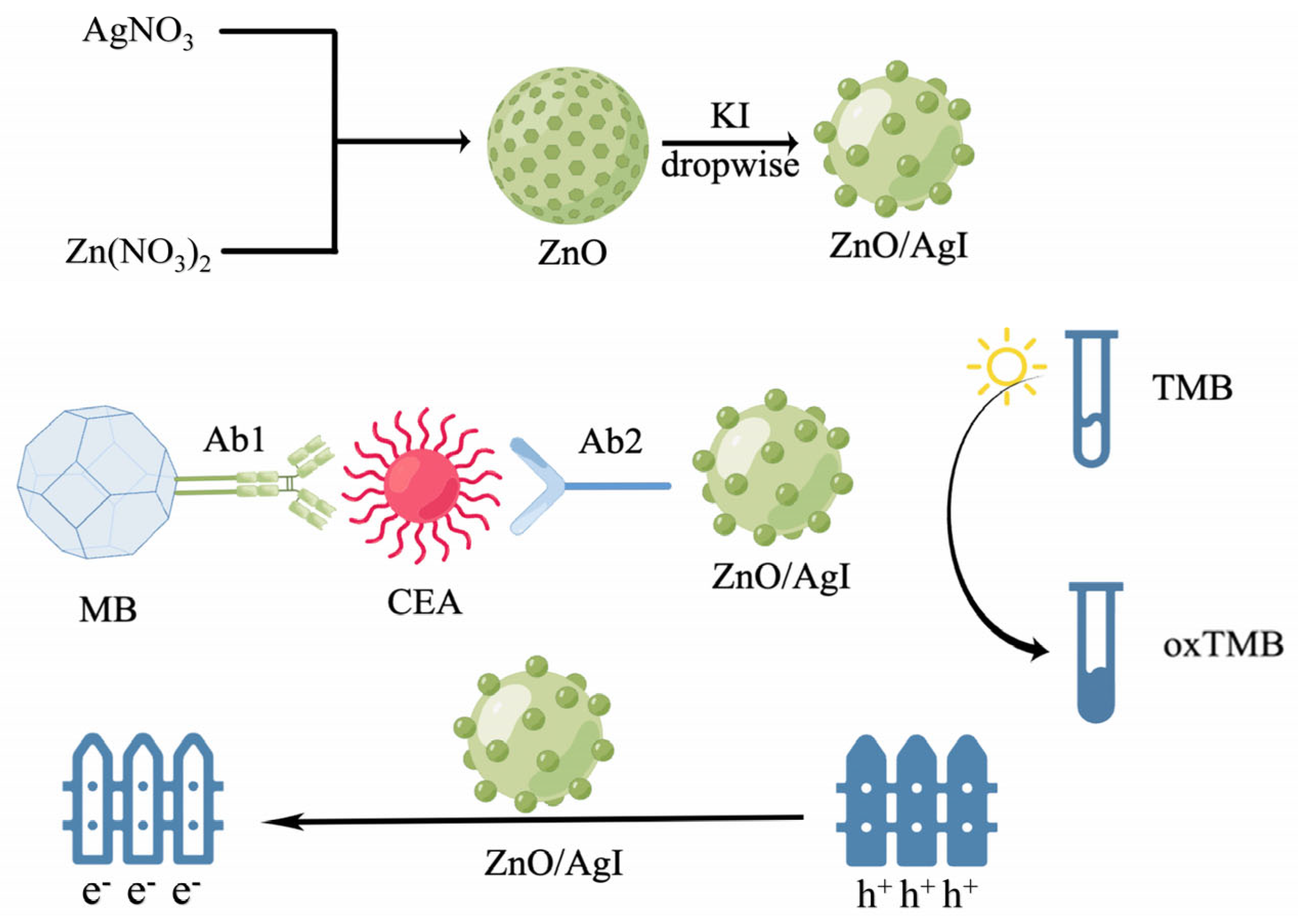
- Zhang, B.; Wang, X.; Cheng, Y. Photochromic immunoassay for tumor marker detection based on ZnO/AgI nanophotocatalyst. Mikrochim. Acta 2022, 189, 77. [Google Scholar] [CrossRef]
- Zhou, M.; Higaki, T.; Hu, G.; Sfeir, M.Y.; Chen, Y.; Jiang, D.-E.; Jin, R. Three-orders-of-magnitude variation of carrier lifetimes with crystal phase of gold nanoclusters. Science 2019, 364, 279–282. [Google Scholar] [CrossRef]
- Luo, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From Aggregation-Induced Emission of Au(I)-Thiolate Complexes to Ultrabright Au(0)@Au(I)-Thiolate Core-Shell Nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar] [CrossRef]
- Jung, J.; Kang, S.; Han, Y.-K. Ligand effects on the stability of thiol-stabilized gold nanoclusters: Au25(SR)18−, Au38(SR)24, and Au102(SR)44. Nanoscale 2012, 4, 4206–4210. [Google Scholar] [CrossRef]
- Jia, P.; Ding, C.; Sun, Z.; Song, L.; Zhang, D.; Yan, Z.; Zhang, Z.; Su, F.; Mostafa, A.A.; Huang, Y. DNA precisely regulated Au nanorods/Ag2S quantum dots satellite structure for ultrasensitive detection of prostate cancer biomarker. Sens. Actuators B Chem. 2021, 347, 130585. [Google Scholar] [CrossRef]
- Tian, F.; Cai, L.; Chang, J.; Li, S.; Liu, C.; Li, T.; Sun, J. Label-free isolation of rare tumor cells from untreated whole blood by interfacial viscoelastic microfluidics. Lab A Chip 2018, 18, 3436–3445. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; He, Y.; Huang, K.; Wang, X.; Zhou, R.; Liu, T.; Qu, R.; Zhou, J.; Peng, W.; et al. Homogeneous Visual and Fluorescence Detection of Circulating Tumor Cells in Clinical Samples via Selective Recognition Reaction and Enzyme-Free Amplification. ACS Nano 2021, 15, 11634–11643. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Y.; Ding, J.; Meng, S.; Li, C.; Yin, X. Design of a Biocompatible and Ratiometric Fluorescent probe for the Capture, Detection, Release, and Reculture of Rare Number CTCs. Anal. Chem. 2018, 90, 13290–13298. [Google Scholar] [CrossRef]
- Lian, W.; Tu, D.; Hu, P.; Song, X.; Gong, Z.; Chen, T.; Song, J.; Chen, Z.; Chen, X. Broadband excitable NIR-II luminescent nano-bioprobes based on CuInSe2 quantum dots for the detection of circulating tumor cells. Nano Today 2020, 35, 100943. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, C.; Cheng, S.; Xian, Y. Multivalent Aptamer Functionalized Ag2S Nanodots/Hybrid Cell Membrane-Coated Magnetic Nanobioprobe for the Ultrasensitive Isolation and Detection of Circulating Tumor Cells. Adv. Funct. Mater. 2020, 30, 1909781. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, H.; Yang, F.; Cheng, Y.; Dai, W.; Meng, X.; Dong, H.; Zhang, X. Uniform palladium nanosheets for fluorimetric detection of circulating tumor DNA. Anal. Chim. Acta 2020, 1139, 164–168. [Google Scholar] [CrossRef]
- Chen, X.; Yang, L.; Liang, S.; Dang, P.; Jin, D.; Cheng, Z.; Lin, J. Entropy-driven strand displacement reaction for ultrasensitive detection of circulating tumor DNA based on upconversion and Fe3O4 nanocrystals. Sci. China-Mater. 2021, 64, 2593–2600. [Google Scholar] [CrossRef]
- Wang, J.; Hua, G.; Li, L.; Li, D.; Wang, F.; Wu, J.; Ye, Z.; Zhou, X.; Ye, S.; Yang, J.; et al. Upconversion nanoparticle and gold nanocage satellite assemblies for sensitive ctDNA detection in serum. Analyst 2020, 145, 5553–5562. [Google Scholar] [CrossRef]
- Chen, H.; Luo, D.; Shang, B.; Cao, J.; Wei, J.; Chen, Q.; Chen, J. Immunoassay-type biosensor based on magnetic nanoparticle capture and the fluorescence signal formed by horseradish peroxidase catalysis for tumor-related exosome determination. Microchim. Acta 2020, 187, 282. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Luo, X.; Wan, Q.; Qiu, R.; Wang, S. An ultrasensitive homogeneous aptasensor for carcinoembryonic antigen based on upconversion fluorescence resonance energy transfer. Talanta 2019, 195, 33–39. [Google Scholar] [CrossRef]
- Wang, K.; Ding, Y.; Yang, W.; Wen, X.; Zhao, H.; Liu, Y.; Hong, X. Fluorescence-infrared absorption dual-mode nanoprobes based on carbon dots@SiO2 nanorods for ultrasensitive and reliable detection of carcinoembryonic antigen. Talanta 2021, 230, 122342. [Google Scholar] [CrossRef]
- Li, G.; Zeng, J.; Liu, H.; Ding, P.; Liang, J.; Nie, X.; Zhou, Z. A fluorometric aptamer nanoprobe for alpha-fetoprotein by exploiting the FRET between 5-carboxyfluorescein and palladium nanoparticles. Microchim. Acta 2019, 186, 314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ji, F.; Zhang, T.; Wang, F.; Li, Y.; Yu, Z.; Jin, X.; Ruan, B. An fluorescent aptasensor for sensitive detection of tumor marker based on the FRET of a sandwich structured QDs-AFP-AuNPs. Talanta 2019, 197, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Hu, Y.; Zhang, X.-J.; Yang, X.-T.; Tang, Y.-Y. Colorimetric and fluorometric dual-channel detection of alpha-fetoprotein based on the use ofZnS-CdTe hierarchical porous nanospheres. Microchim. Acta 2019, 186, 124. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xu, H.; Zhou, B.; Xu, S.; Shen, B.; Dong, B.; Yin, Z.; Xu, S.; Sun, L.; Lv, J.; et al. Double Stopband Bilayer Photonic Crystal Based Upconversion Fluorescence PSA Sensor. Sens. Actuators B Chem. 2021, 326, 128816. [Google Scholar] [CrossRef]
- Rong, Z.; Bai, Z.; Li, J.; Tang, H.; Shen, T.; Wang, Q.; Wang, C.; Xiao, R.; Wang, S. Dual-color magnetic-quantum dot nanobeads as versatile fluorescent probes in test strip for simultaneous point-of-care detection of free and complexed prostate-specific antigen. Biosens. Bioelectron. 2019, 145, 111719. [Google Scholar] [CrossRef]
- Li, F.; Li, G.; Cao, S.; Liu, B.; Ren, X.; Kang, N.; Qiu, F. Target-triggered entropy-driven amplification system-templated silver nanoclusters for multiplexed microRNA analysis. Biosens. Bioelectron. 2021, 172, 112757. [Google Scholar] [CrossRef]
- Plou, J.; Garcia, I.; Charconnet, M.; Astobiza, I.; Garcia-Astrain, C.; Matricardi, C.; Mihi, A.; Carracedo, A.; Liz-Marzan, L.M. Multiplex SERS Detection of Metabolic Alterations in Tumor Extracellular Media. Adv. Funct. Mater. 2020, 30, 1910335. [Google Scholar] [CrossRef]
- Wei, W.; Yu, D.; Du, Y.; Ding, Y.; Huang, Q. One-step fabrication of Au-Ag alloys and its application for catalysts and SERS sensors. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2022, 267, 120476. [Google Scholar] [CrossRef]
- Xiem Thi, V.; Long Toan, T.; Hai Van, P.; Mai Thi Tuyet, N. Tunable Plasmonic Properties of Bimetallic Au-Cu Nanorods for SERS-Based Sensing Application. J. Electron. Mater. 2022, 51, 1857–1865. [Google Scholar]
- Korolkov, I.V.; Shumskaya, A.; Kozlovskiy, A.L.; Kaliyekperov, M.E.; Lissovskaya, L.I.; Zdorovets, M.V. Magnetic-plasmonic Ni nanotubes covered with gold for improvement of SERS analysis. J. Alloys Compd. 2022, 901, 163661. [Google Scholar] [CrossRef]
- Cong, S.; Yuan, Y.; Chen, Z.; Hou, J.; Yang, M.; Su, Y.; Zhang, Y.; Li, L.; Li, Q.; Geng, F.; et al. Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies. Nat. Commun. 2015, 6, 7800. [Google Scholar] [CrossRef]
- Wilson, R.E., Jr.; O’Connor, R.; Gallops, C.E.; Kwizera, E.A.; Noroozi, B.; Morshed, B.I.; Wang, Y.; Huang, X. Immunomagnetic Capture and Multiplexed Surface Marker Detection of Circulating Tumor Cells with Magnetic Multicolor Surface-Enhanced Raman Scattering Nanotags. ACS Appl. Mater. Interfaces 2020, 12, 47220–47232. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Y.; Liu, Z.; Zhang, W.; Yi, K.; Zhang, X.; Zhang, X.; Jiang, C.; Yang, S.; Wang, F.; et al. Beehive-Inspired Macroporous SERS Probe for Cancer Detection through Capturing and Analyzing Exosomes in Plasma. ACS Appl. Mater. Interfaces 2020, 12, 5136–5146. [Google Scholar] [CrossRef]
- Kwizera, E.A.; O’Connor, R.; Vinduska, V.; Williams, M.; Butch, E.R.; Snyder, S.E.; Chen, X.; Huang, X. Molecular Detection and Analysis of Exosomes Using Surface-Enhanced Raman Scattering Gold Nanorods and a Miniaturized Device. Theranostics 2018, 8, 2722–2738. [Google Scholar] [CrossRef]
- Tian, Y.-F.; Ning, C.-F.; He, F.; Yin, B.-C.; Ye, B.-C. Highly sensitive detection of exosomes by SERS using gold nanostar@ Raman reporter@ nanoshell structures modified with a bivalent cholesterollabeled DNA anchor. Analyst 2018, 143, 4915–4922. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, S.; Wang, Y.; Li, N.; Li, L.; Lu, J.; Wang, Z.; Chen, B.; Cui, Y. Screening and multiple detection of cancer exosomes using an SERS-based method. Nanoscale 2018, 10, 9053–9062. [Google Scholar] [CrossRef]
- Pang, Y.; Shi, J.; Yang, X.; Wang, C.; Sun, Z.; Xiao, R. Personalized detection of circling exosomal PD-L1 based on Fe3O4@TiO2 isolation and SERS immunoassay. Biosens. Bioelectron. 2020, 148, 111800. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, C.; Lu, L.; Wang, C.; Sun, Z.; Xiao, R. Dual-SERS biosensor for one-step detection of microRNAs in exosome and residual plasma of blood samples for diagnosing pancreatic cancer. Biosens. Bioelectron. 2019, 130, 204–213. [Google Scholar] [CrossRef]
- Lee, J.U.; Kim, W.H.; Lee, H.S.; Park, K.H.; Sim, S.J. Quantitative and Specific Detection of Exosomal miRNAs for Accurate Diagnosis of Breast Cancer Using a Surface-Enhanced Raman Scattering Sensor Based on Plasmonic Head-Flocked Gold Nanopillars. Small 2019, 15, 1804968. [Google Scholar] [CrossRef]
- Wang, G.; Lipert, R.J.; Jain, M.; Kaur, S.; Chakraboty, S.; Torres, M.P.; Batra, S.K.; Brand, R.E.; Porter, M.D. Detection of the Potential Pancreatic Cancer Marker MUC4 in Serum Using Surface-Enhanced Raman Scattering. Anal. Chem. 2011, 83, 2554–2561. [Google Scholar] [CrossRef]
- He, J.; Li, G.; Hu, Y. Aptamer Recognition Induced Target-Bridged Strategy for Proteins Detection Based on Magnetic Chitosan and Silver/Chitosan Nanoparticles Using Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2015, 87, 11039–11047. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Xu, D.; Shang, Y.; Li, Y.; Gu, Y.; Yang, G.; Qu, L. Highly sensitive SERS detection of IL-6 in serum by Au@Fe3O4 nanoring-based sandwich immunoassay. Sens. Actuators B Chem. 2023, 375, 132897. [Google Scholar] [CrossRef]
- Wu, X.; Luo, L.; Yang, S.; Ma, X.; Li, Y.; Dong, C.; Tian, Y.; Zhang, L.; Shen, Z.; Wu, A. Improved SERS Nanoparticles for Direct Detection of Circulating Tumor Cells in the Blood. ACS Appl. Mater. Interfaces 2015, 7, 9965–9971. [Google Scholar] [CrossRef]
- Ruan, H.; Wu, X.; Yang, C.; Li, Z.; Xia, Y.; Xue, T.; Shen, Z.; Wu, A. A Supersensitive CTC Analysis System Based on Triangular Silver Nanoprisms and SPION with Function of Capture, Enrichment, Detection, and Release. ACS Biomater. Sci. Eng. 2018, 4, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Wang, S.; Ou, G.; Li, Y.; Ruan, H.; Li, Z.; Ma, Y.; Zou, R.; Qiu, J.; Shen, Z.; et al. Detection of circulating tumor cells based on improved SERS-active magnetic nanoparticles. Anal. Methods 2019, 11, 2918–2928. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.-P.; Yong, K.-T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef]
- Szymanska, B.; Lukaszewski, Z.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. A biosensor for determination of the circulating biomarker CA125/MUC16 by Surface Plasmon Resonance Imaging. Talanta 2020, 206, 120187. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, L.; Yang, X.; Liu, X.; Nie, W.; Zheng, Y.; Cheng, Q.; Wang, K. Direct quantification of cancerous exosomes via surface plasmon resonance with dual gold nanoparticle-assisted signal amplification. Biosens. Bioelectron. 2019, 135, 129–136. [Google Scholar] [CrossRef]
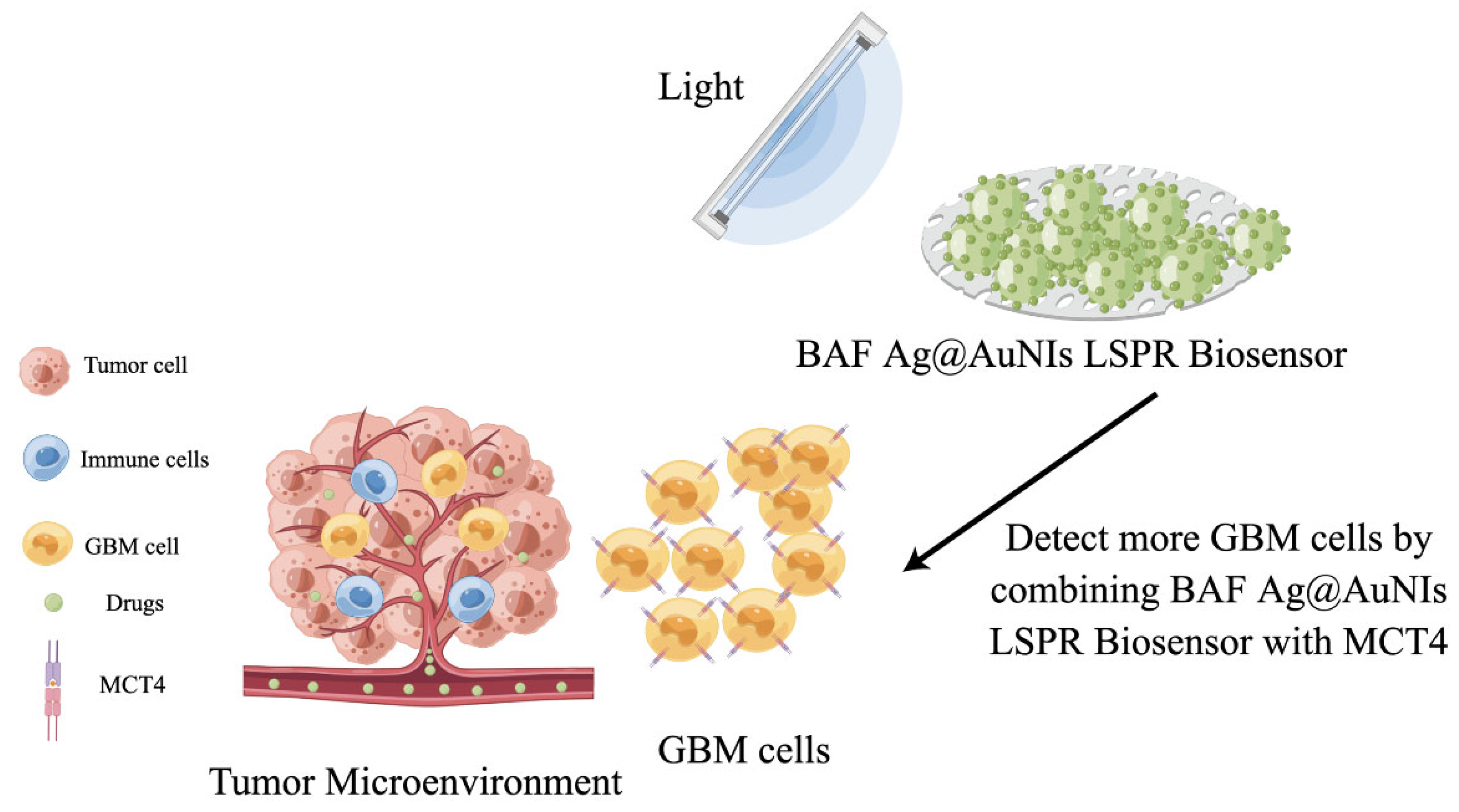
- Liu, L.; Thakur, A.; Li, W.K.; Qiu, G.; Yang, T.; He, B.; Lee, Y.; Wu, C.-M.L. Site specific biotinylated antibody functionalized Ag@AuNIs LSPR biosensor for the ultrasensitive detection of exosomal MCT4, a glioblastoma progression biomarker. Chem. Eng. J. 2022, 446, 137383. [Google Scholar] [CrossRef]


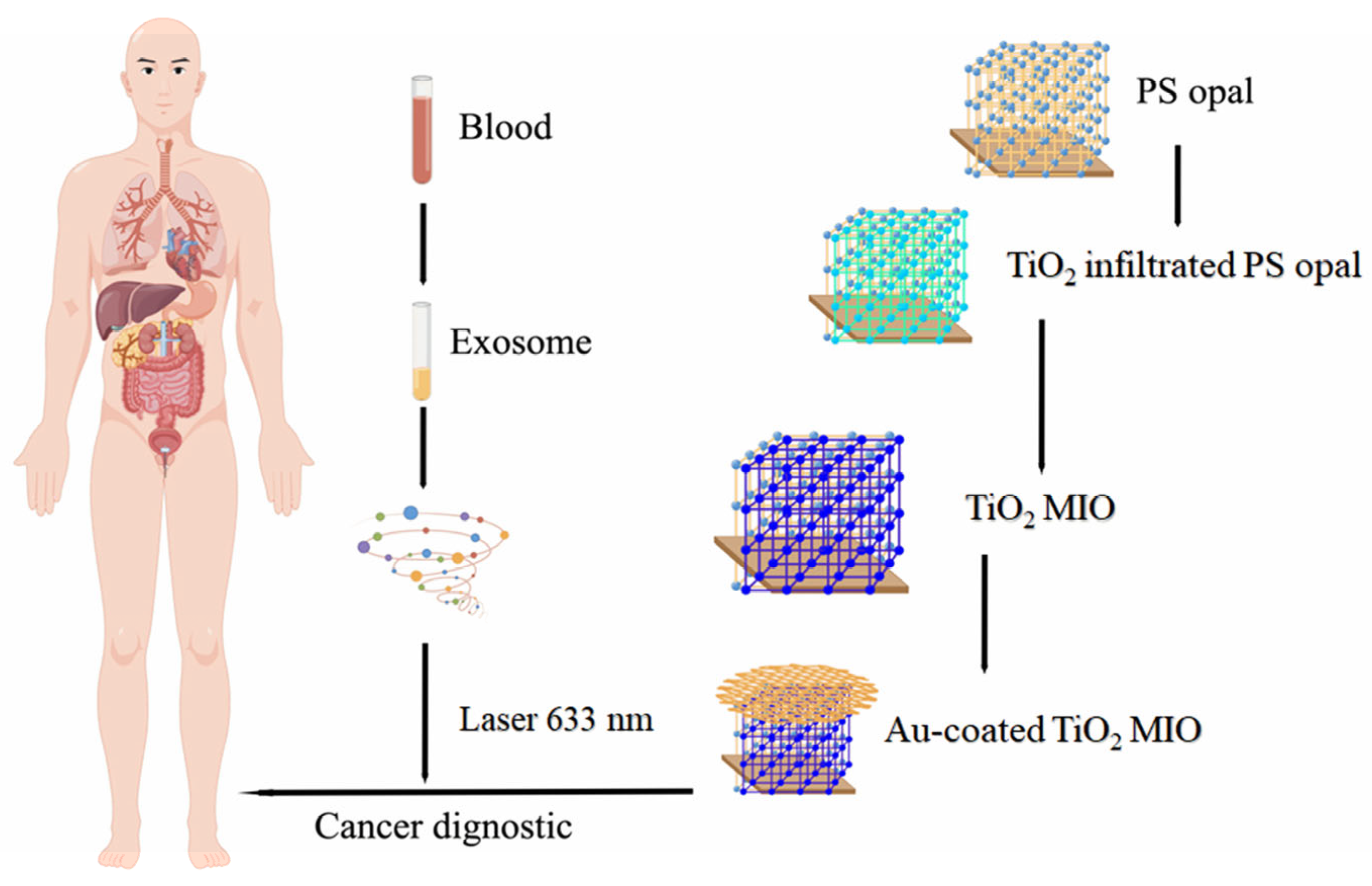
| Materials | Tumor Marker | Linearity Range | Limit of Detection | Ref. |
|---|---|---|---|---|
| N-doped graphene/Au nanoparticles (NG-AuNPs) | miRNA | 10 fM~1 nM | 0.17 fM | [34] |
| Multifunctional iron-based metal–organic frameworks (PdNPs@Fe-MOFs) | miRNA | 0.01 fM~10 pM | 0.003 fM | [35] |
| Unmodified gold nanoparticles (AuNPs) | miRNA | 0.05~0.9 pM | 16 fM | [36] |
| Gold nanoparticle (AuNP)-coated magnetic microbeads (AuNP-MMBs) | miRNA | 5 fM~100 fM | 0.14 fM | [37] |
| Graphite oxide–gold (GO-Au) nanocomposites | CEA | 1~40 ng/mL | 15.8 ng/mL | [38] |
| Silver nanoclusters (AgNCs@Apt@UiO-66) | CEA | 0.01~10 ng/mL | 0.3 ng/mL | [39] |
| Au@Ag nanoparticles (Au@Ag NPs) | CEA | 0.0001~100 ng/mL | 0.05 pg/mL | [40] |
| MoS2–Au composite–Ag NPs | CEA | 1 pg/mL~50 ng/mL | 0.27 pg/mL | [41] |
| Ag/MoS2@Fe3O4 | CEA | 0.0001~20 ng/mL | 0.03 pg/mL | [42] |
| Platinum porous nanoparticles (Pt PNPs) | CEA, AFP | 0.05 ng/mL~200 ng/mL | 0.002, 0.05 ng/mL | [43] |
| Carbon–gold nanocomposite (CGN) | CEA, PSA, AFP | 0.01~100 ng/mL | 2.7, 4.8 and 3.1 pg/mL | [44] |
| Hollow gold nanospheres (HGN) | DNA | 1~10 nM | 1 pM | [45] |
| Magnetic mesoporous nanogold/thionine/NiCo2O4 | SCCA | 2.5 pg/mL~15 ng/mL | 1.0 pg/mL | [46] |
| Materials | Tumor Marker | Linearity Range | Limit of Detection | Ref. |
|---|---|---|---|---|
| Co/Mn oxide nanocomposite | ACP | 0.02~1.0 U/L | 8.2 mU/L | [52] |
| PdPt bimetallic alloy nanowires (Pd/Pt NWs) | ACP | 0.17~2.67 U/L | 0.06 U/L | [53] |
| Carboxylated chitosan modified Pt nanoclusters (CC-Pt NCs) | ACP | 0.25~18 U/L | 1.31 × 10−3 U/L | [54] |
| MnO2 nanosheets | ACP | 0.075~0.45 mU/mL | 0.046 mU/mL | [55] |
| Molybdenum oxide nanoparticles (MoO3 NPs) | ACP | 0.09~7.3 U/L | 0.011 U/L | [47] |
| CuO nanoparticles | cholesterol | 0.625~12.5 μM | 0.17 μM | [56] |
| Ultrasmall Pt nanoclusters (Pt NCs) | glucose | 0~200 µM | 0.28 µM | [57] |
| CuS nanoparticles (CuS NPs) | AFP | 0.1–60 ng/mL | 0.07 ng/mL | [58] |
| Two-dimensional Co9S8 nanocomposites (H2TCPP-Co9S8 nanocomposites) | H2O2 | 10~200 μM | 8.19 μM | [59] |
| Porous 2D FeS2 nanosheets | H2O2 | 0.02~4.00 μM | 7.60 nM | [60] |
| Pt–Ru bimetallic nanoclusters (Pt–Ru NCs) | H2O2 | 0.1~5 μg/mL | 0.08 μg/mL | [61] |
| Dendritic mesoporous silica nanoparticle-MnO2 | GSH | 2~250 μM | 0.654 μM | [62] |
| BSA-AuNP@ZnCo2O4 nanosheets | GSH | 0.25~17.50 U/L | 0.137 U/L | [63] |
| Materials | Tumor Marker | Linearity Range | Limit of Detection | Ref. |
|---|---|---|---|---|
| CuInSe2@ZnS nanoprobes | MCF-7 cells | 10~5000 cell/well | 12 cell/well | [73] |
| Functionalized Ag2S nanodot | MCF-7 cells | 6–10 cell/mL | - | [74] |
| Pd nanosheets | ctDNA | 1~100 nM | 0.63 nM | [75] |
| Fe3O4 nanoparticles | ctDNA | 100 amol/L~1 nmol/L | 1.6 amol/L | [76] |
| Gold nanocages (Au NCs) | ctDNA | 5 pmol/L~1000 pmol/L | 6.30 pmol/L | [77] |
| Fe3O4 magnetic nanoparticle | Hepatic carcinoma-specific exosomes | 576 (±15)~5.76 × 107 (±5.1 × 105) particles/mL | 200 (±9) particles/mL | [78] |
| Ln-upconversion nanoparticles (UCNPs) | CEA | 0.03~6 ng/mL | 10.7 pg/mL | [79] |
| Carbon dots@SiO2 nanorods | CEA | 1 fg/mL~10 ng/mL | 794.6 ag/mL | [80] |
| Palladium nanoparticles (PdNPs) | AFP | 5.0~150.0 ng/mL | 1.38 ng/mL | [81] |
| Anti-AFP antibody functional gold nanoparticles (Au NPs) | AFP | 0.50~45 ng/mL | 400 pg/mL | [82] |
| ZnS nanospheres modified with CdTe quantum dots | AFP | 0.04~64 ng·mL | 10 pg/mL | [83] |
| NaYF4:Yb3+, Er3+@NaYF4:Yb3+ UCNPs | PSA | 0.1~10 ng/mL | 0.01 ng/mL | [84] |
| Fe3O4 magnetic-quantum dot nanobeads | PSA | 0.01~100 ng mL | 0.061 ng/mL | [85] |
| Entropy-driven amplification system-templated silver nanoclusters (Ag NCs) | miRNA | 0~50 nM | 8.7 pM | [86] |
| Technology | Basic Principle | Advantage | Disadvantage |
|---|---|---|---|
| Electrochemical sensors | Converts an interaction signal between a biometric element and a recognition target into a detectable electrical signal | High selectivity; mass production and integration; rapid and low cost; simple and suitable for complicated situation | Lack of specificity for the captured cancer cells; lack of ability to detect intracellular protein markers |
| Fluorescence | Change of fluorescence spectrum and fluorescence intensity | High selectivity and stability; simplicity and rapidity; good accuracy; biocompatible | Spectral overlap; photobleaching; nonspecific binding labeling |
| SERS | Difference in Raman scattering spectra of different molecule | High selectivity and sensitivity; noninvasive and nondestructive | Expensive and complicated equipment; batch-to-batch reproductivity of SERS substrate |
| SPR | Refractive index changes occurring from the capture of a molecule on the plasmonic surface | Real-time; free-label; high accuracy; suitable for different biofluids | Limited detection; interference from complex samples |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, S.; Lin, N. Application of Metal-Based Nanomaterials in In Vitro Diagnosis of Tumor Markers: Summary and Prospect. Molecules 2023, 28, 4370. https://doi.org/10.3390/molecules28114370
Yang X, Zhang S, Lin N. Application of Metal-Based Nanomaterials in In Vitro Diagnosis of Tumor Markers: Summary and Prospect. Molecules. 2023; 28(11):4370. https://doi.org/10.3390/molecules28114370
Chicago/Turabian StyleYang, Xiaobo, Shaodian Zhang, and Nong Lin. 2023. "Application of Metal-Based Nanomaterials in In Vitro Diagnosis of Tumor Markers: Summary and Prospect" Molecules 28, no. 11: 4370. https://doi.org/10.3390/molecules28114370
APA StyleYang, X., Zhang, S., & Lin, N. (2023). Application of Metal-Based Nanomaterials in In Vitro Diagnosis of Tumor Markers: Summary and Prospect. Molecules, 28(11), 4370. https://doi.org/10.3390/molecules28114370





