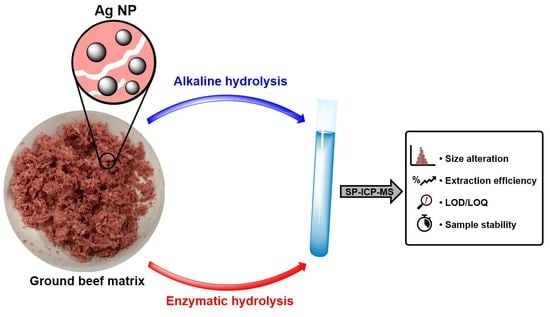
Analysis of Silver Nanoparticles in Ground Beef by Single Particle Inductively Coupled Plasma Mass Spectrometry (SP-ICP-MS)
Abstract
1. Introduction
2. Results and Discussion
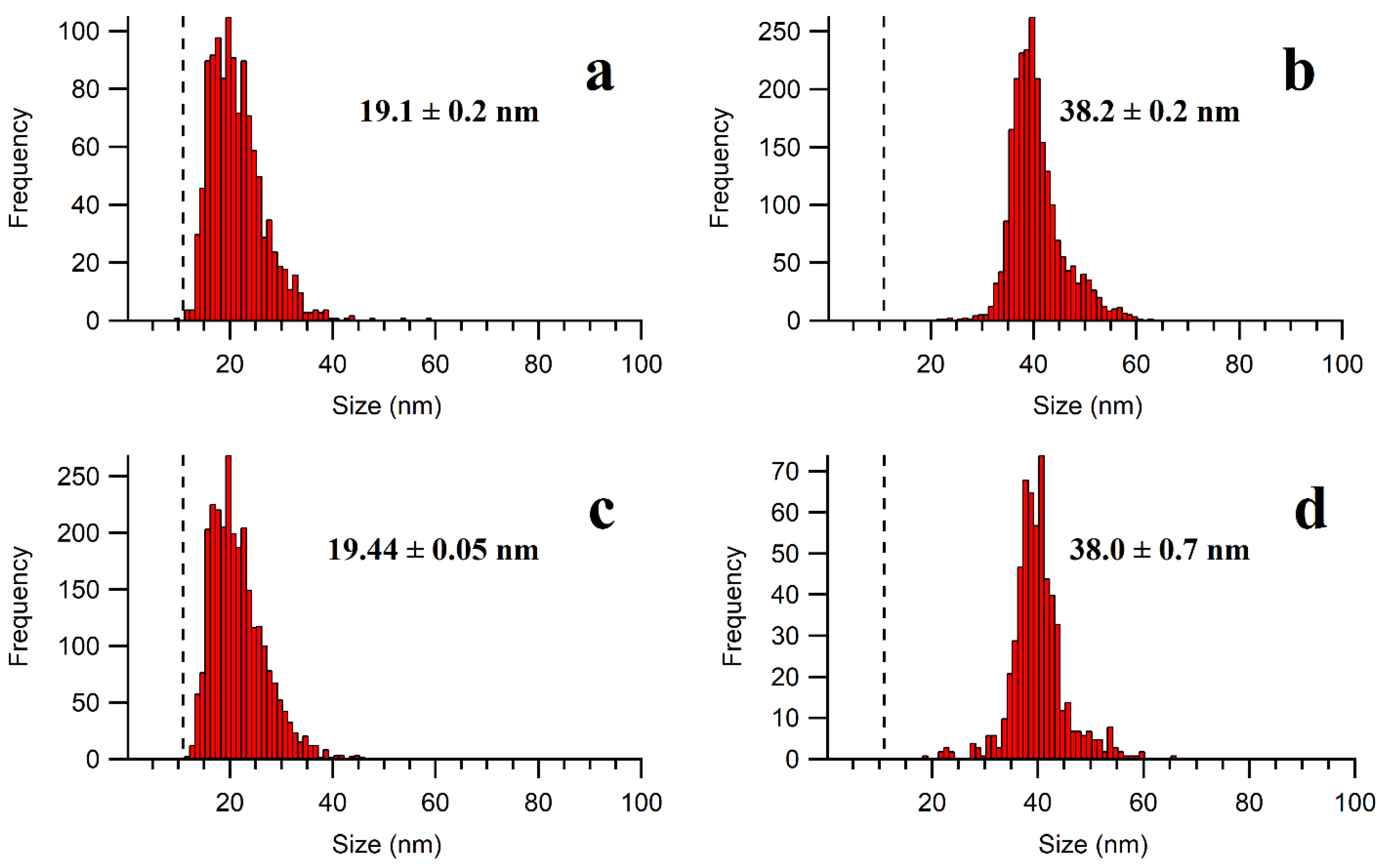
2.1. Optimization of NP Extraction by Enzymatic Hydrolysis
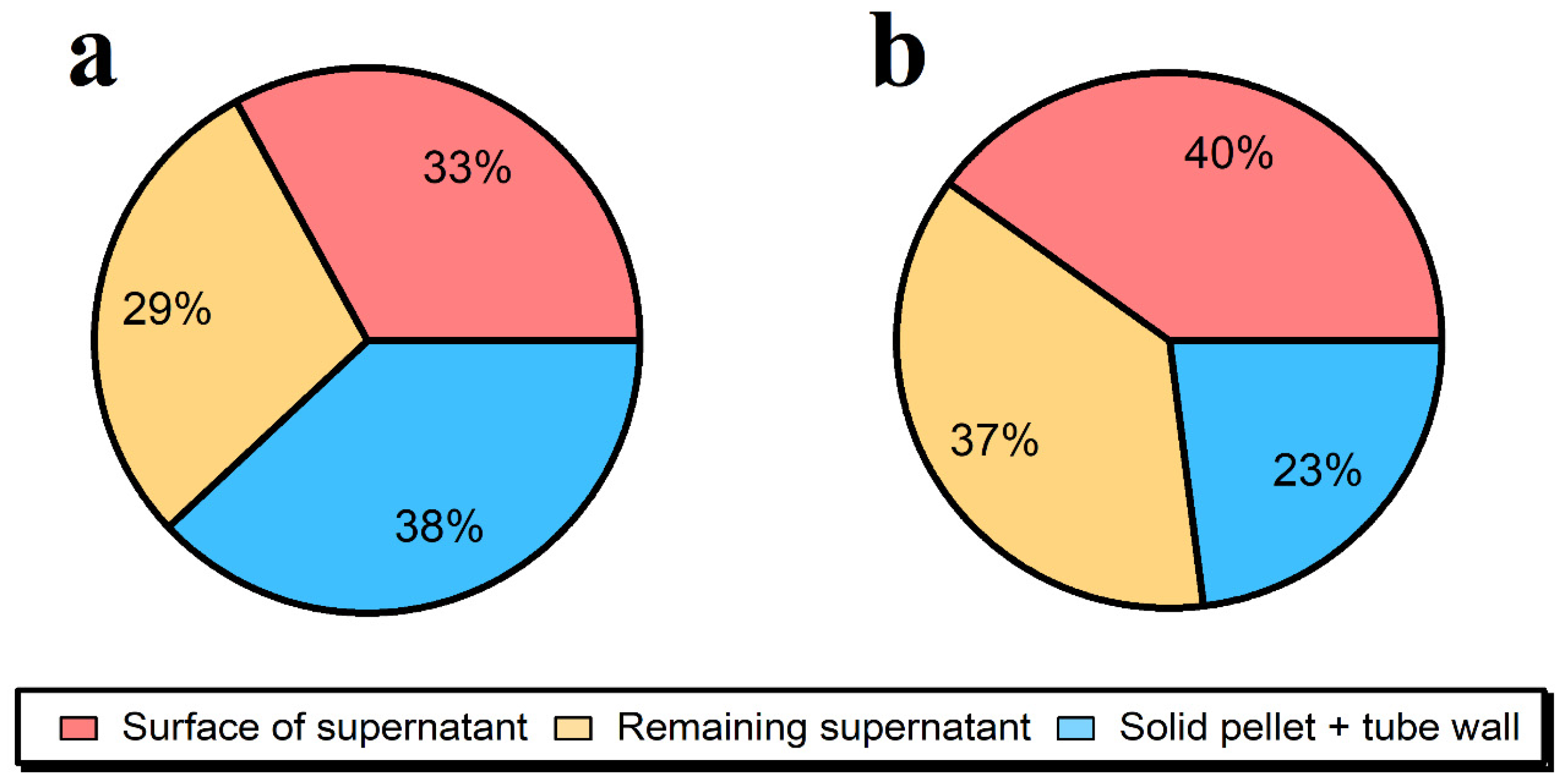
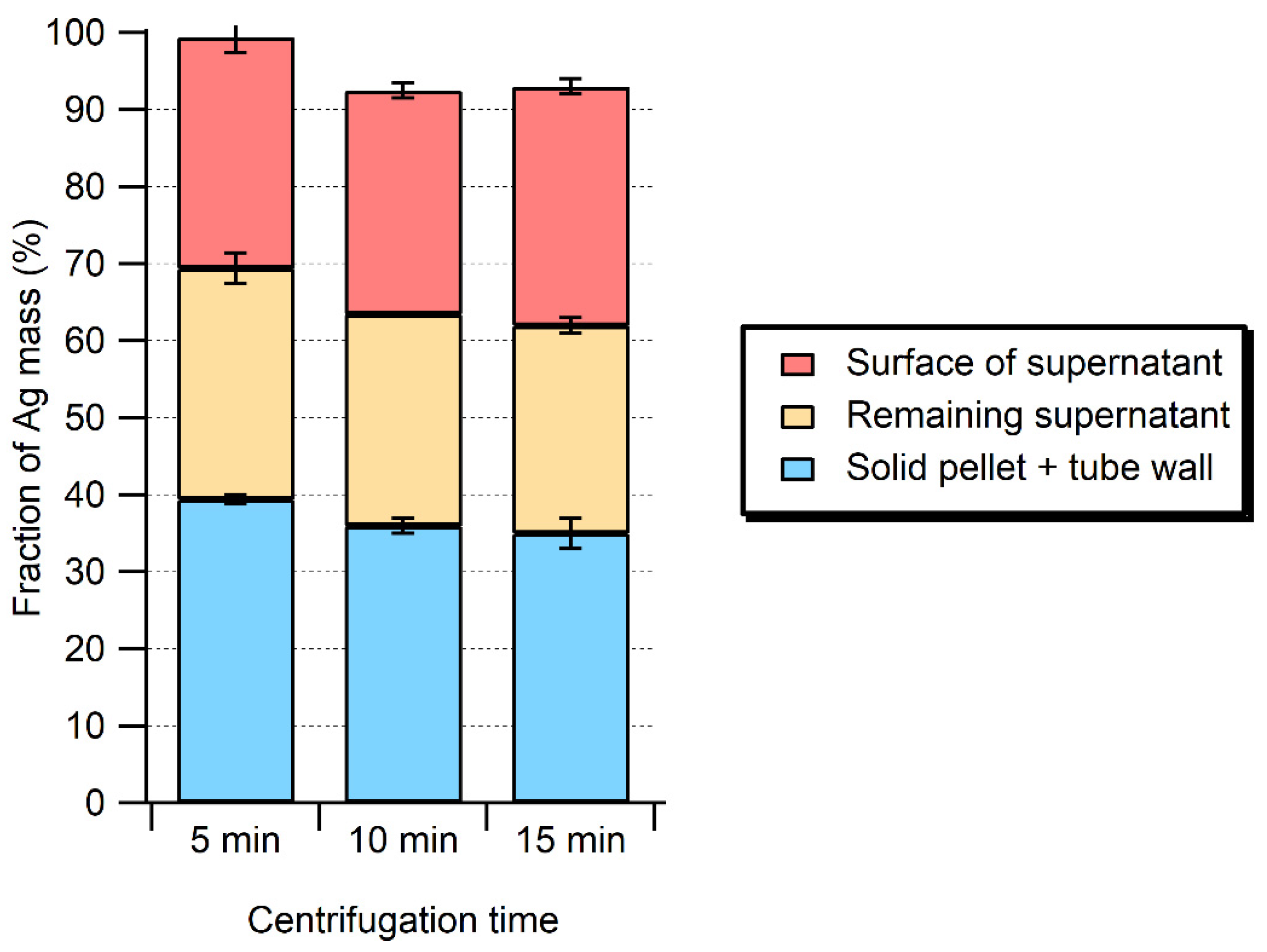
2.2. Fate of Ag NPs after the Enzymatic Extraction
2.3. Alkaline Hydrolysis of the Meat Matrix
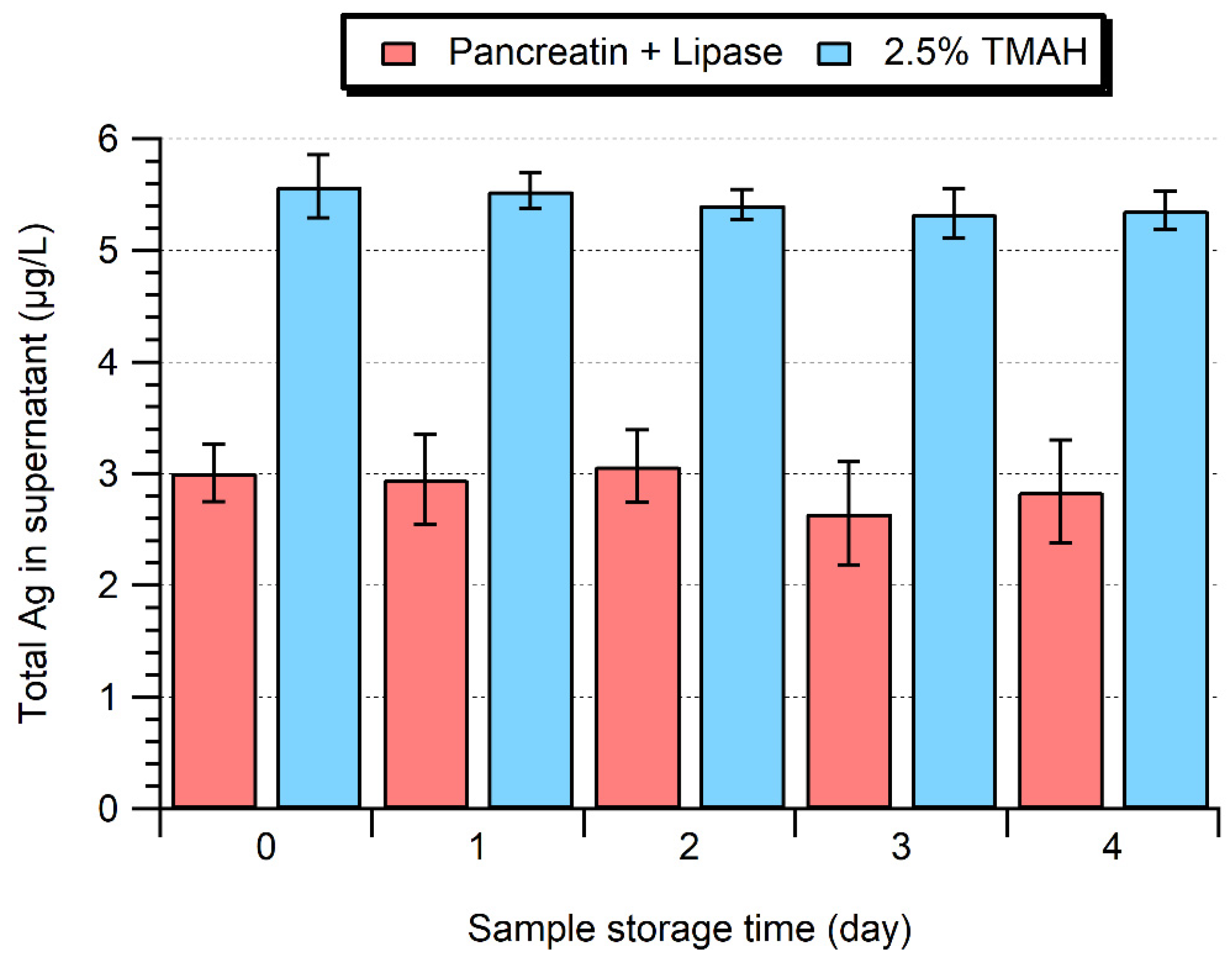
2.4. Stability of the Processed Samples over 4 Days
2.5. Evaluation of Analytical Performance
3. Materials and Methods
3.1. Chemicals and Lab Equipment
3.2. Preparation of a Meat Reference Material
3.3. Ultrasound-Assisted Enzymatic Hydrolysis for Ag NP Extraction
3.4. Ultrasound-Assisted Alkaline Hydrolysis for Ag NP Extraction
3.5. Stability of the Processed Samples
3.6. Mass Balances of Ag NPs
3.7. Total Ag Determination in Samples
3.8. ICP-MS and SP-ICP-MS Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; Abdalla, M.S.; Bechelany, M. Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations. Nanomaterials 2022, 12, 177. [Google Scholar] [CrossRef]
- Hofmann, T.; Lowry, G.V.; Ghoshal, S.; Tufenkji, N.; Brambilla, D.; Dutcher, J.R.; Gilbertson, L.M.; Giraldo, J.P.; Kinsella, J.M.; Landry, M.P.; et al. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food 2020, 1, 416–425. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef]
- He, X.; Hwang, H.M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef]
- dos Santos, C.A.; Ingle, A.P.; Rai, M. The emerging role of metallic nanoparticles in food. Appl. Microbiol. Biotechnol. 2020, 104, 2373–2383. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag. Shelf 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Johnston, L.J.; Gonzalez-Rojano, N.; Wilkinson, K.J.; Xing, B. Key challenges for evaluation of the safety of engineered nanomaterials. NanoImpact 2020, 18, 100219. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Fernandez, M.J.F.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; Husøy, T.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar]
- Laycock, A.; Clark, N.J.; Clough, R.; Smith, R.; Handy, R.D. Determination of metallic nanoparticles in biological samples by single particle ICP-MS: A systematic review from sample collection to analysis. Environ. Sci. Nano 2022, 9, 420–453. [Google Scholar] [CrossRef] [PubMed]
- Pompeu de Moraes, D.; Gonzalez-Morales, S.; Toledano-Serrabona, J.; Sanchez-Garces, M.A.; Bettmer, J.; Montes-Bayon, M.; Corte-Rodriguez, M. Tracking soluble and nanoparticulated titanium released in vivo from metal dental implant debris using (single-particle)-ICP-MS. J. Trace Elem. Med. Biol. 2023, 77, 127143. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Wang, Y.J.; Hou, W.C. Bioaccumulation and depuration of TiO(2) nanoparticles by zebrafish through dietary exposure: Size- and number concentration-resolved analysis using single-particle ICP-MS. J. Hazard. Mater. 2022, 426, 127801. [Google Scholar] [CrossRef] [PubMed]
- Loeschner, K.; Navratilova, J.; Kobler, C.; Molhave, K.; Wagner, S.; von der Kammer, F.; Larsen, E.H. Detection and characterization of silver nanoparticles in chicken meat by asymmetric flow field flow fractionation with detection by conventional or single particle ICP-MS. Anal. Bioanal. Chem. 2013, 405, 8185–8195. [Google Scholar] [CrossRef]
- Gruszka, J.; Malejko, J.; Bajguz, A.; Godlewska-Zylkiewicz, B. Method development for speciation analysis of silver nanoparticles and silver ions in green algae and surface waters at environmentally relevant concentrations using single particle ICP-MS. J. Anal. Atom. Spectrom 2022, 37, 1208–1222. [Google Scholar] [CrossRef]
- Taboada-Lopez, M.V.; Herbello-Hermelo, P.; Dominguez-Gonzalez, R.; Bermejo-Barrera, P.; Moreda-Pineiro, A. Enzymatic hydrolysis as a sample pre-treatment for titanium dioxide nanoparticles assessment in surimi (crab sticks) by single particle ICP-MS. Talanta 2019, 195, 23–32. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Y.; Tou, F.Y.; Niu, Z.S.; Guo, X.P.; Liu, C.; Yan, J.; Wu, J.Y.; Xu, M.; Hou, L.J.; et al. Extraction and quantification of metal-containing nanoparticles in marine shellfish based on single particle inductively coupled plasma-mass spectrometry technique. J. Hazard. Mater. 2022, 424 Pt A, 127383. [Google Scholar] [CrossRef]
- Lopez-Mayan, J.J.; Del-Angel-Monroy, S.; Pena-Vazquez, E.; Barciela-Alonso, M.C.; Bermejo-Barrera, P.; Moreda-Pineiro, A. Titanium dioxide nanoparticles assessment in seaweeds by single particle inductively coupled plasma—Mass spectrometry. Talanta 2022, 236, 122856. [Google Scholar] [CrossRef]
- Laughton, S.; Laycock, A.; Bland, G.; von der Kammer, F.; Hofmann, T.; Casman, E.A.; Lowry, G.V. Methanol-based extraction protocol for insoluble and moderately water-soluble nanoparticles in plants to enable characterization by single particle ICP-MS. Anal. Bioanal. Chem. 2021, 413, 299–314. [Google Scholar] [CrossRef]
- Vidmar, J.; Buerki-Thurnherr, T.; Loeschner, K. Comparison of the suitability of alkaline or enzymatic sample pre-treatment for characterization of silver nanoparticles in human tissue by single particle ICP-MS. J. Anal. At. Spectrom. 2018, 33, 752–761. [Google Scholar] [CrossRef]
- Clark, N.J.; Clough, R.; Boyle, D.; Handy, R.D. Development of a suitable detection method for silver nanoparticles in fish tissue using single particle ICP-MS. Environ. Sci. Nano 2019, 6, 3388–3400. [Google Scholar] [CrossRef]
- Sung, H.K.; Jo, E.; Kim, E.; Yoo, S.-K.; Lee, J.-W.; Kim, P.-J.; Kim, Y.; Eom, I.-C. Analysis of gold and silver nanoparticles internalized by zebrafish (Danio rerio) using single particle-inductively coupled plasma-mass spectrometry. Chemosphere 2018, 209, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Jreije, I.; Hadioui, M.; Wilkinson, K.J. Sample preparation for the analysis of nanoparticles in natural waters by single particle ICP-MS. Talanta 2022, 238, 123060. [Google Scholar] [CrossRef] [PubMed]
- Fréchette-Viens, L.; Hadioui, M.; Wilkinson, K.J. Practical limitations of single particle ICP-MS in the determination of nanoparticle size distributions and dissolution: Case of rare earth oxides. Talanta 2017, 163, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Bajorath, J.; Hinrichs, W.; Saenger, W. The enzymatic activity of proteinase K is controlled by calcium. Eur. J. Biochem. 1988, 176, 441–447. [Google Scholar] [CrossRef]
- Gunkel, F.A.; Gassen, H.G. Proteinase K from Tritirachium album Limber. Eur. J. Biochem. 1989, 179, 185–194. [Google Scholar] [CrossRef]
- Gimenez-Ingalaturre, A.C.; Rubio, E.; Chueca, P.; Laborda, F.; Goñi, P. Contribution to optimization and standardization of antibacterial assays with silver nanoparticles: The culture medium and their aggregation. J. Microbiol. Methods 2022, 203, 106618. [Google Scholar] [CrossRef]
- Peters, R.J.B.; Rivera, Z.H.; van Bemmel, G.; Marvin, H.J.P.; Weigel, S.; Bouwmeester, H. Development and validation of single particle ICP-MS for sizing and quantitative determination of nano-silver in chicken meat. Anal. Bioanal. Chem. 2014, 406, 3875–3885. [Google Scholar] [CrossRef]
- Taboada-López, M.V.; Alonso-Seijo, N.; Herbello-Hermelo, P.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Determination and characterization of silver nanoparticles in bivalve molluscs by ultrasound assisted enzymatic hydrolysis and sp-ICP-MS. Microchem. J. 2019, 148, 652–660. [Google Scholar] [CrossRef]
- Chetwynd, A.J.; Lynch, I. The rise of the nanomaterial metabolite corona, and emergence of the complete corona. Environ. Sci. Nano 2020, 7, 1041–1060. [Google Scholar] [CrossRef]
- Steinmetz, L.; Geers, C.; Balog, S.; Bonmarin, M.; Rodriguez-Lorenzo, L.; Taladriz-Blanco, P.; Rothen-Rutishauser, B.; Petri-Fink, A. A comparative study of silver nanoparticle dissolution under physiological conditions. Nanoscale Adv. 2020, 2, 5760–5768. [Google Scholar] [CrossRef] [PubMed]
- Baccaro, M.; Undas, A.K.; de Vriendt, J.; van den Berg, J.H.J.; Peters, R.J.B.; van den Brink, N.W. Ageing, dissolution and biogenic formation of nanoparticles: How do these factors affect the uptake kinetics of silver nanoparticles in earthworms? Environ. Sci. Nano 2018, 5, 1107–1116. [Google Scholar] [CrossRef]
- Campagnolo, L.; Massimiani, M.; Vecchione, L.; Piccirilli, D.; Toschi, N.; Magrini, A.; Bonanno, E.; Scimeca, M.; Castagnozzi, L.; Buonanno, G.; et al. Silver nanoparticles inhaled during pregnancy reach and affect the placenta and the foetus. Nanotoxicology 2017, 11, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.P.; Coleman, J.G.; Bednar, A.J.; Kennedy, A.J.; Ranville, J.F.; Higgins, C.P. Extraction and Analysis of Silver and Gold Nanoparticles from Biological Tissues Using Single Particle Inductively Coupled Plasma Mass Spectrometry. Environ. Sci. Technol. 2013, 47, 14315–14323. [Google Scholar] [CrossRef]
- Ishizaka, T.; Nagano, K.; Tasaki, I.; Tao, H.; Gao, J.-Q.; Harada, K.; Hirata, K.; Saito, S.; Tsujino, H.; Higashisaka, K.; et al. Optimization and Evaluation of Pretreatment Method for sp-ICP-MS to Reveal the Distribution of Silver Nanoparticles in the Body. Nanoscale Res. Lett. 2019, 14, 180. [Google Scholar] [CrossRef]
- Laborda, F.; Gimenez-Ingalaturre, A.C.; Bolea, E.; Castillo, J.R. About detectability and limits of detection in single particle inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2020, 169, 105883. [Google Scholar] [CrossRef]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining Transport Efficiency for the Purpose of Counting and Sizing Nanoparticles via Single Particle Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef]


| Extraction | Meat Sample (Spiked with Ag NP) | Recovery of Ag Mass (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Extraction Medium a | Sonication Time (min) | Centrifugation | Supernatant Transfer b | Ag NP Size (nm) | Lyophilization | Mass (g) | Replicates | ||
| Speed (g) | Time (min) | ||||||||
| Ultrapure water only | 15 | 50 | 15 | Yes | 40 | No | 0.5 | 3 | 0.9 ± 0.8 |
| Pancreatin + Lipase | 15 | 50 | 15 | Yes | 40 | No | 0.5 | 3 | 38 ± 3 |
| 15 | 50 | 15 | Yes | 40 | Yes | 0.1 | 3 | 39 ± 5 | |
| 0 c | 500 | 3 | No | 40 | Yes | 0.1 | 2 | 15 ± 7 | |
| 15 | 500 | 3 | No | 40 | Yes | 0.1 | 3 | 63 ± 8 | |
| 15 | 500 | 3 | No | 40 | Yes | 0.025 | 3 | 67 ± 12 | |
| 15 | 500 | 3 | No | 40 | Yes | 0.01 | 3 | 62 ± 7 | |
| 15 | 500 | 2 | No | 40 | Yes | 0.02 | 3 | 63 ± 3 | |
| 15 | 500 | 3 | No | 40 | Yes | 0.05 | 2 | 42 ± 2 | |
| 15 | 500 | 3 | No | 20 | Yes | 0.02 | 3 | 62 ± 7 | |
| Pancreatin + Lipase + 1% SDS | 15 | 500 | 2 | No | 40 | Yes | 0.02 | 3 | 39 ± 6 |
| Proteinase K + Lipase + 1% SDS | 15 | 500 | 2 | No | 40 | Yes | 0.02 | 3 | 43 ± 2 |
| Extraction | Meat Sample | Recovery of Ag Mass (%) | ||||
|---|---|---|---|---|---|---|
| Extraction Medium | Sonication Time (min) | Centrifugation | Mass (g) | Replicates | ||
| Speed (g) | Time (min) | |||||
| 10% TMAH | 15 | 500 | 2 | 0.02 | 3 | 92 ± 6 |
| 10% TMAH, ionic Ag spike | 15 | 500 | 2 | 0.02 | 3 | 89 ± 5 |
| 2.5% TMAH | 15 | 500 | 2 | 0.02 | 3 | 96.1 ± 0.3 |
| 2.5% TMAH, ionic Ag spike | 15 | 500 | 2 | 0.02 | 3 | 114 ± 2 |
| Particle Size LoDsize, LoQsize (nm) | Particle Number Concentration LoDnumber b, LoQnumber | Dissolved Metal Concentration LoDdiss, LoQdiss | ||||
|---|---|---|---|---|---|---|
| Extraction Medium a | Extract/Water (×102 mL−1) | Meat c (×106 g−1) | Extract/Water (ng L−1) | Meat c (ng g−1) | ||
| Pancreatin + Lipase | LoD | 10.9 ± 1.0 | 6.0 ± 2.3 | 4.8 ± 1.9 | 0.2 ± 0.2 | 2.0 ± 1.9 |
| LoQ | 13.7 ± 1.2 | 10.9 ± 4.5 | 8.6 ± 3.4 | 0.9 ± 0.9 | 7.0 ± 6.8 | |
| TMAH | LoD | 10.5 ± 1.7 | 11.5 ± 3.0 | 5.7 ± 1.5 | 0.43 ± 0.01 | 2.1 ± 0.1 |
| LoQ | 13.2 ± 2.2 | 21.2 ± 5.4 | 10.6 ± 2.7 | 1.52 ± 0.05 | 7.6 ± 0.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalifoux, A.; Hadioui, M.; Amiri, N.; Wilkinson, K.J. Analysis of Silver Nanoparticles in Ground Beef by Single Particle Inductively Coupled Plasma Mass Spectrometry (SP-ICP-MS). Molecules 2023, 28, 4442. https://doi.org/10.3390/molecules28114442
Chalifoux A, Hadioui M, Amiri N, Wilkinson KJ. Analysis of Silver Nanoparticles in Ground Beef by Single Particle Inductively Coupled Plasma Mass Spectrometry (SP-ICP-MS). Molecules. 2023; 28(11):4442. https://doi.org/10.3390/molecules28114442
Chicago/Turabian StyleChalifoux, Alexandre, Madjid Hadioui, Nesrine Amiri, and Kevin J. Wilkinson. 2023. "Analysis of Silver Nanoparticles in Ground Beef by Single Particle Inductively Coupled Plasma Mass Spectrometry (SP-ICP-MS)" Molecules 28, no. 11: 4442. https://doi.org/10.3390/molecules28114442
APA StyleChalifoux, A., Hadioui, M., Amiri, N., & Wilkinson, K. J. (2023). Analysis of Silver Nanoparticles in Ground Beef by Single Particle Inductively Coupled Plasma Mass Spectrometry (SP-ICP-MS). Molecules, 28(11), 4442. https://doi.org/10.3390/molecules28114442