Graphene Oxide/Nitrocellulose Non-Covalent Hybrid as Solid Phase for Oligo-DNA Extraction from Complex Medium
Abstract
1. Introduction
2. Results and Discussion
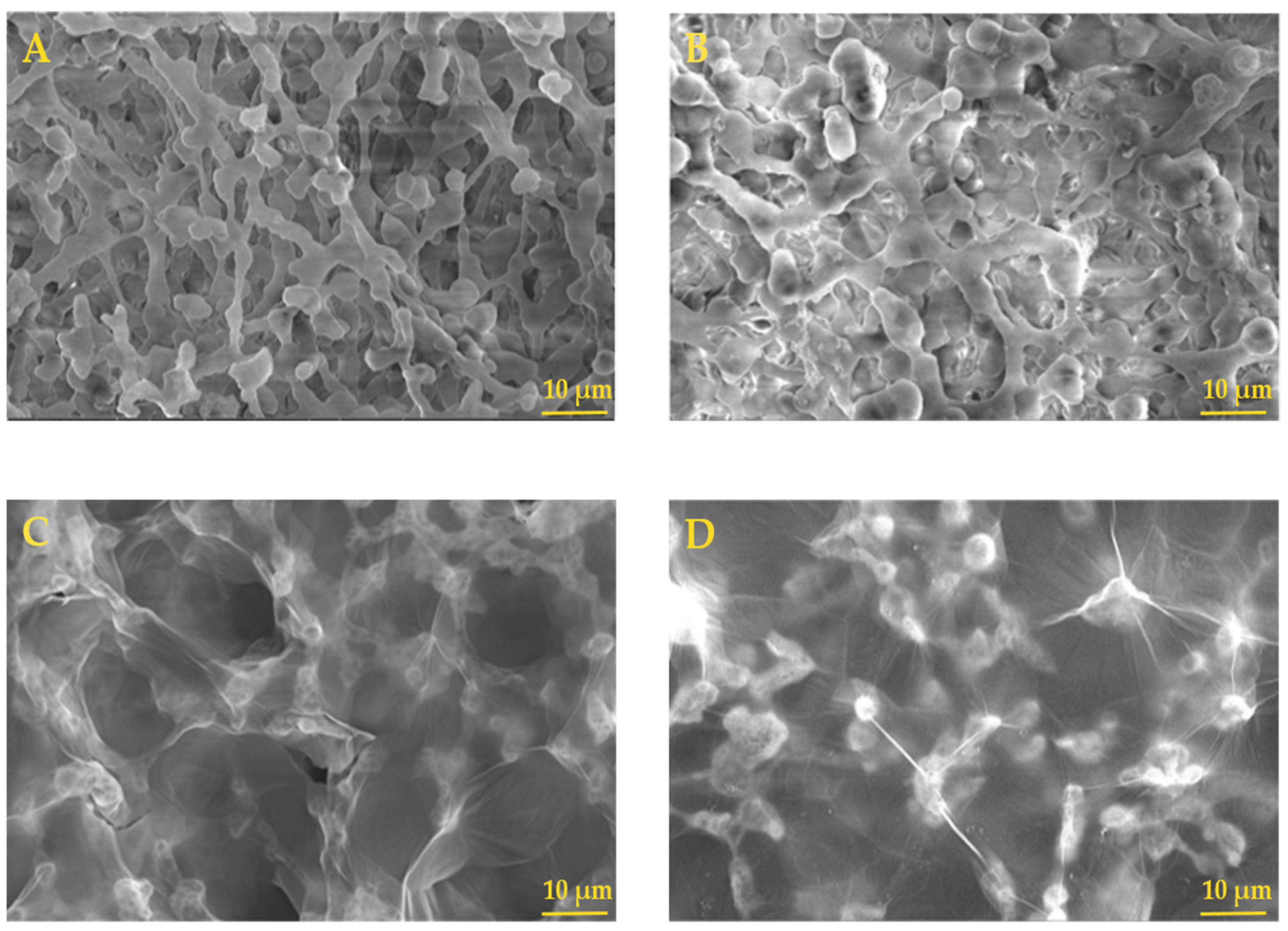
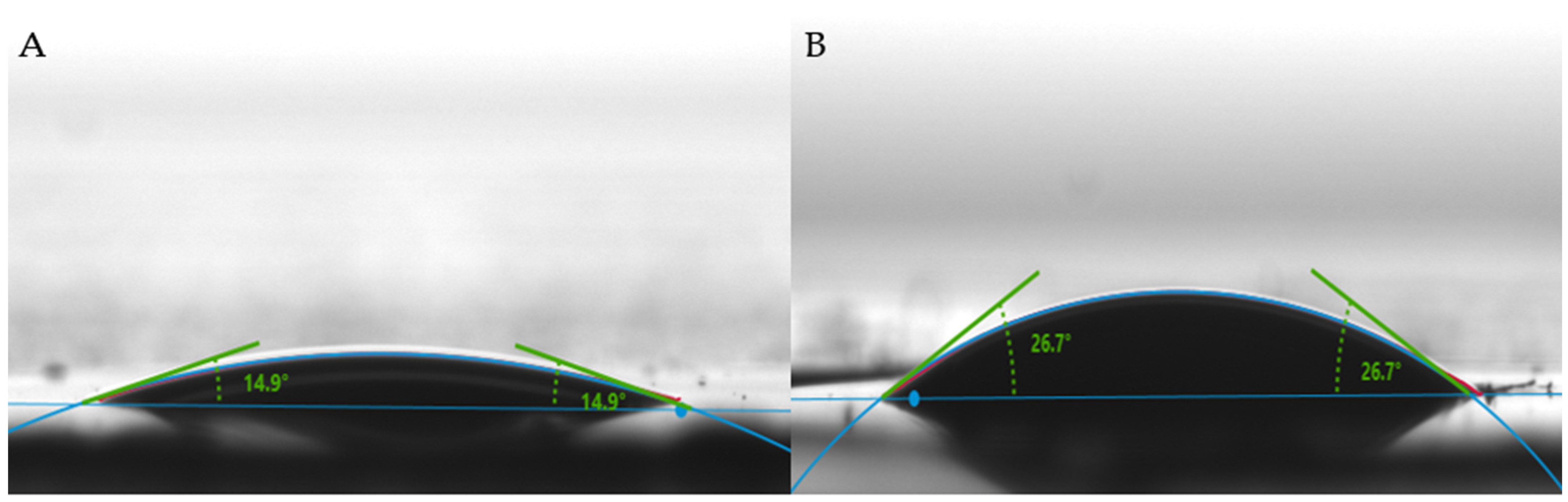
2.1. Characterization of NC–GO Hybrid Membrane Array
2.1.1. FTIR Spectra
2.1.2. Morphological Characterization
2.1.3. Wettability Properties
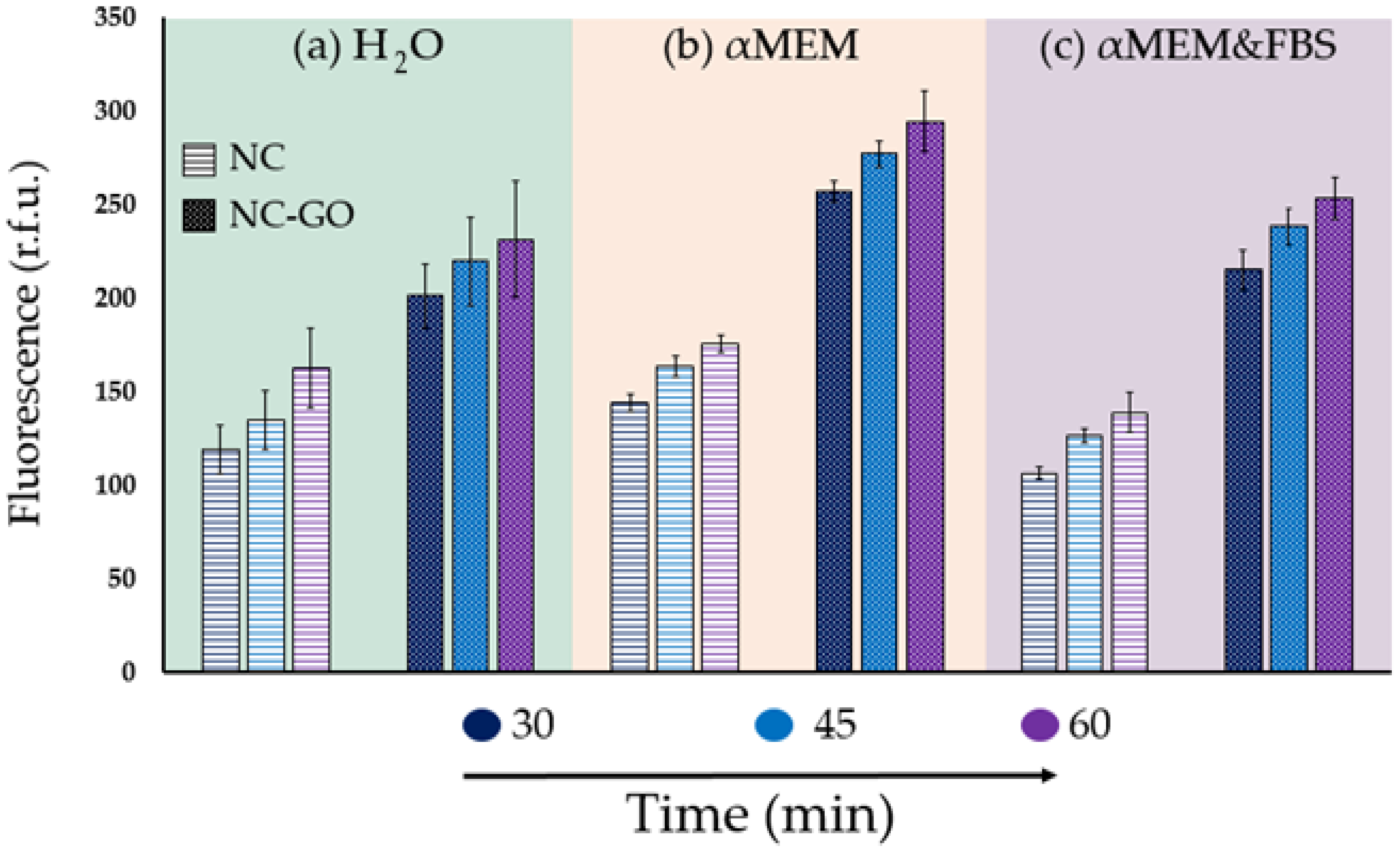
2.2. Assay Conditions Influence FAM–ssDNA Fluorescence: Detection and Extraction of Oligo DNA Using NC–GO Hybrid Membranes in Complex Media
3. Materials and Methods
3.1. Reagents
3.2. NC–GO Hybrid Membrane Preparation
3.3. Preparation and Desorption Procedure of FAM–ssDNA from NC and NC–GO Hybrid Membranes
3.4. Spectroflourimeter Assay
3.5. Membrane Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kazlauskas, D.; Varsani, A.; Koonin, E.V.; Krupovic, M. Multiple origins of prokaryotic and eukaryotic single-stranded DNA viruses from bacterial and archaeal plasmids. Nat. Commun. 2019, 10, 3425. [Google Scholar] [CrossRef] [PubMed]
- Krupovic, M.; Forterre, P. Single-stranded DNA viruses employ a variety of mechanisms for integration into host genomes. Ann. N. Y. Acad. Sci. 2015, 1341, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, G.; Zhao, Y.; Dai, X.; Liu, W.; Qu, F.; Huang, Y. Selection and Identification of an ssDNA Aptamer for Fibroblast Activation Protein. Molecules 2023, 28, 1682. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-P.; Chen, Y.-C.; Chen, W.-Y. Improve sample preparation process for miRNA isolation from the culture cells by using silica fiber membrane. Sci. Rep. 2020, 10, 21132. [Google Scholar] [CrossRef]
- Petrou, L.; Ladame, S. On-chip miRNA extraction platforms: Recent technological advances and implications for next generation point-of-care nucleic acid tests. Lab Chip 2022, 22, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Dairawan, M.; Shetty, P.J. The evolution of DNA extraction methods. Am. J. Biomed. Sci. Res. 2020, 8, 39–45. [Google Scholar]
- He, H.; Li, R.; Chen, Y.; Pan, P.; Tong, W.; Dong, X.; Chen, Y.; Yu, D. Integrated DNA and RNA extraction using magnetic beads from viral pathogens causing acute respiratory infections. Sci. Rep. 2017, 7, 45199. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kariper, İ.A. Graphene and graphene oxide based aerogels: Synthesis, characteristics and supercapacitor applications. J. Energy Storage 2020, 27, 101038. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Kapitanova, O.O.; Eremina, E.A.; Goodilin, E.A. Preparation, chemical features, structure and applications of membrane materials based on graphene oxide. Mendeleev Commun. 2021, 31, 137–148. [Google Scholar] [CrossRef]
- Wu, X.; Mu, F.; Wang, Y.; Zhao, H. Graphene and Graphene-Based Nanomaterials for DNA Detection: A Review. Molecules 2018, 23, 2050. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xu, Z.; Fang, H.; Lei, X. Unexpected sequence adsorption features of polynucleotide ssDNA on graphene oxide. Phys. Chem. Chem. Phys. 2020, 22, 11740–11746. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Wang, Y.; Du, Z.; Mao, X.; Sun, D.; Liu, J.; Zhou, Y.; Xu, X. Studies on binding of single-stranded DNA with reduced graphene oxide–silver nanocomposites. IET Nanobiotechnol. 2020, 14, 308–313. [Google Scholar] [CrossRef]
- Chiticaru, E.A.; Pilan, L.; Damian, C.-M.; Vasile, E.; Burns, J.S.; Ioniţă, M. Influence of Graphene Oxide Concentration when Fabricating an Electrochemical Biosensor for DNA Detection. Biosensors 2019, 9, 113. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Pan, G. Detection of a Double-Stranded MGMT Gene Using Electrochemically Reduced Graphene Oxide (ErGO) Electrodes Decorated with AuNPs and Peptide Nucleic Acids (PNA). Biosensors 2022, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, G.; Yang, A.; Wu, J.; Yan, F.; Ju, H. A DNA-functionalized graphene field-effect transistor for quantitation of vascular endothelial growth factor. Sens. Actuators B Chem. 2020, 351, 130964. [Google Scholar] [CrossRef]
- Nitu, F.R.; Burns, J.S.; Ionită, M. Oligonucleotide Detection and Optical Measurement with Graphene Oxide in the Presence of Bovine Serum Albumin Enabled by Use of Surfactants and Salts. Coatings 2020, 10, 420. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, L.; Yue, P.; Shi, L.; Wang, M.; Zhou, L.; Zhao, H.; Kong, W. Development of a graphene oxide nanosheet and double-stranded DNA structure based fluorescent “signal off” aptasensor for ochratoxin A detection in malt. Food Chem. X 2022, 14, 100308. [Google Scholar] [CrossRef]
- Qu, H.; Fan, C.; Chen, M.; Zhang, X.; Yan, Q.; Wang, Y.; Zhang, S.; Gong, Z.; Shi, L.; Li, X.; et al. Recent advances of fluorescent biosensors based on cyclic signal amplification technology in biomedical detection. J. Nanobiotechnol. 2021, 19, 403. [Google Scholar] [CrossRef]
- Zheng, P.; Wu, N. Fluorescence and Sensing Applications of Graphene Oxide and Graphene Quantum Dots: A Review. Chem. Asian J. 2017, 12, 2343–2353. [Google Scholar] [CrossRef]
- Wang, M.; Chu, Y.; Qiang, L.; Han, Y.; Zhang, Y.; Han, L. Rapid, amplification-free and high-throughput SARS-CoV-2 RNA detection via a reduced-graphene-oxide based fluorescence assay. Sens. Diagn. 2022, 1, 262–269. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Shen, A.; Zhou, X.; Hu, J. Highly selective and sensitive method for cysteine detection based on fluorescence resonance energy transfer between FAM-tagged ssDNA and graphene oxide. Talanta 2012, 93, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Ishihara, S.; Kurita, R. A Multi-Fluorescent DNA/Graphene Oxide Conjugate Sensor for Signature-Based Protein Discrimination. Sensors 2017, 17, 2194. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Milhem, R.M.; Panicker, N.G.; Rizvi, T.A.; Mustafa, F. Electrical characterization of DNA supported on nitrocellulose membranes. Sci. Rep. 2016, 6, 29089. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, X.; Liao, Y.; Xing, D. Sensitive monitoring of RNA transcription levels using a graphene oxide fluorescence switch. Chin. Sci. Bull. 2012, 58, 2634–2639. [Google Scholar] [CrossRef]
- Torul, H.; Yarali, E.; Eksin, E.; Ganguly, A.; Benson, J.; Tamer, U.; Papakonstantinou, P.; Erdem, A. Paper-Based Electrochemical Biosensors for Voltammetric Detection of miRNA Biomarkers Using Reduced Graphene Oxide or MoS2 Nanosheets Decorated with Gold Nanoparticle Electrodes. Biosensors 2021, 11, 236. [Google Scholar] [CrossRef]
- Erkocyigit, B.A.; Ozufuklar, O.; Yardim, A.; Celik, E.G.; Timur, S. Biomarker Detection in Early Diagnosis of Cancer: Recent Achievements in Point-of-Care Devices Based on Paper Microfluidics. Biosensors 2023, 13, 387. [Google Scholar] [CrossRef]
- Musico, Y.L.F.; Santos, C.M.; Dalida, M.L.P.; Rodrigues, D.F. Surface Modification of Membrane Filters Using Graphene and Graphene Oxide-Based Nanomaterials for Bacterial Inactivation and Removal. ACS Sustain. Chem. Eng. 2014, 2, 1559–1565. [Google Scholar] [CrossRef]
- Vlasceanu, G.M.; Șelaru, A.; Dinescu, S.; Balta, C.; Herman, H.; Gharbia, S.; Hermenean, A.; Ionita, M.; Costache, M. Comprehensive Appraisal of Graphene–Oxide Ratio in Porous Biopolymer Hybrids Targeting Bone-Tissue Regeneration. Nanomaterials 2020, 10, 1444. [Google Scholar] [CrossRef]
- Lujanienė, G.; Novikau, R.; Joel, E.F.; Karalevičiūtė, K.; Šemčuk, S.; Mažeika, K.; Talaikis, M.; Pakštas, V.; Tumėnas, S.; Mažeika, J.; et al. Preparation of Graphene Oxide-Maghemite-Chitosan Composites for the Adsorption of Europium Ions from Aqueous Solutions. Molecules 2022, 27, 8035. [Google Scholar] [CrossRef]
- Costa, M.N.; Veigas, B.; Jacob, J.M.; Santos, D.S.; Gomes, J.; Baptista, P.V.; Martins, R.; Inácio, J.; Fortunato, E. A low cost, safe, disposable, rapid and self-sustainable paper-based platform for diagnostic testing: Lab-on-paper. Nanotechnology 2014, 25, 094006. [Google Scholar] [CrossRef] [PubMed]
- Gismatulina, Y.A.; Budaeva, V.V.; Sakovich, G.V. Nitrocellulose Synthesis from Miscanthus Cellulose. Propellants Explos. Pyrotech. 2017, 43, 96–100. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.; Deng, L.; Zhao, X.; Yan, Q.; Cai, Y.; Lin, J.; Bai, Y.; Liu, S.; Zhang, Y. Use of nitrocellulose membranes as a scaffold in cell culture. Cytotechnology 2013, 65, 71–81. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, S.A.; Aladresi, A.A.M.; Alharbi, S.A.; Chinnathambi, A. Ovalbumin-mediated synthesis and simultaneous functionalization of graphene with increased protein stability. Green Chem. Lett. Rev. 2020, 13, 60–67. [Google Scholar] [CrossRef]
- Ji, P.; Zhang, W.; Ai, S.; Zhang, Y.; Liu, J.; Liu, J.; He, P.; Li, Y. Hybridization of graphene oxide into nanogels to acquire higher photothermal effects for therapeutic delivery. Nanotechnology 2019, 30, 115701. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, E.; Ghitman, J.; Biru, E.I.; Pircalabioru, G.G.; Vasile, E.; Iovu, H. Synthesis and Characterization of Electrospun Composite Scaffolds Based on Chitosan-Carboxylated Graphene Oxide with Potential Biomedical Applications. Materials 2021, 14, 2535. [Google Scholar] [CrossRef]
- Elder, R.M.; Jayaraman, A. Structure and thermodynamics of ssDNA oligomers near hydrophobic and hydrophilic surfaces. Soft Matter 2013, 9, 11521–11533. [Google Scholar] [CrossRef]
- El-Ghazali, S.; Khatri, M.; Kobayashi, S.; Kim, I.S. An overview of medical textile materials. Med. Text. Nat. Resour. 2022, 1, 3–42. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Xue, Q.; Chang, C.; Wang, R.; Liu, Y.; Xie, H. Highly efficient detection of ofloxacin in water by samarium oxide and β-cyclodextrin-modified laser-induced graphene electrode. Microchem. J. 2023, 186, 108353. [Google Scholar] [CrossRef]
- Wei, X.-Q.; Hao, L.-Y.; Shao, X.-R.; Zhang, Q.; Jia, X.-Q.; Zhang, Z.-R.; Lin, Y.-F.; Peng, Q. Insight into the Interaction of Graphene Oxide with Serum Proteins and the Impact of the Degree of Reduction and Concentration. ACS Appl. Mater. Interfaces 2015, 7, 13367–13374. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, C.; Chiu, L.-Y.; Abbasi, K.; Tolbert, B.S.; Sauve, G.; Yen, Y.; Liu, C.-C. Application of bioconjugation chemistry on biosensor fabrication for detection of TAR-DNA binding protein 43. Biosens. Bioelectron. 2018, 117, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.X.; Yeap, S.K.; Ho, W.Y. Transfection types, methods and strategies: A technical review. PeerJ 2021, 9, e11165. [Google Scholar] [CrossRef] [PubMed]
- Erbaş, A.; Marko, J.F. How do DNA-bound proteins leave their binding sites? The role of facilitated dissociation. Curr. Opin. Chem. Biol. 2019, 53, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Cholko, T.; Kaushik, S.; Chang, C.-E.A. Dynamics and molecular interactions of single-stranded DNA in nucleic acid biosensors with varied surface properties. Phys. Chem. Chem. Phys. 2019, 21, 16367–16380. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Kempaiah, R.; Huang, P.-J.J.; Maheshwari, V.; Liu, J. Adsorption and Desorption of DNA on Graphene Oxide Studied by Fluorescently Labeled Oligonucleotides. Langmuir 2011, 27, 2731–2738. [Google Scholar] [CrossRef]
- Park, J.S.; Goo, N.-I.; Kim, D.-E. Mechanism of DNA Adsorption and Desorption on Graphene Oxide. Langmuir 2014, 30, 12587–12595. [Google Scholar] [CrossRef]
- Liu, M.; Ding, X.; Wang, X.; Li, J.; Yang, H.; Yin, Y. Extraction of DNA from complex biological sample matrices using guanidinium ionic liquid modified magnetic nanocomposites. RSC Adv. 2019, 9, 23119–23128. [Google Scholar] [CrossRef]
- Zhao, X.H.; Kong, R.M.; Zhang, X.B.; Meng, H.M.; Liu, W.N.; Tan, W.; Shen, G.L.; Yu, R.Q. Graphene-DNAzyme based biosensor for amplified fluorescence “turn-on” detection of Pb2+ with a high selectivity. Anal. Chem. 2011, 83, 5062–5066. [Google Scholar] [CrossRef]
- He, Y.; Jiao, B.; Tang, H. Interaction of single-stranded DNA with graphene oxide: Fluorescence study and its application for S1 nuclease detection. RSC Adv. 2014, 4, 18294–18300. [Google Scholar] [CrossRef]
- Huang, P.-J.J.; Liu, J. Separation of Short Single- and Double-Stranded DNA Based on Their Adsorption Kinetics Difference on Graphene Oxide. Nanomaterials 2013, 3, 221–228. [Google Scholar] [CrossRef]
- Wen, Y.; Niu, Z.; Ma, Y.; Ma, J.; Chen, L. Graphene oxide-based microspheres for the dispersive solid-phase extraction of non-steroidal estrogens from water samples. J. Chromatogr. A 2014, 1368, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Xu, Y.; Lu, Y.; Xing, W. Reduced Graphene Oxide-Based Solid-Phase Extraction for the Enrichment and Detection of microRNA. Anal. Chem. 2017, 89, 10137–10140. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, Z.; Zhang, X.; Liu, J. Mechanisms of DNA Sensing on Graphene Oxide. Anal. Chem. 2013, 85, 7987–7993. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, Y.; Olsen, T.R.; Sun, N.; Zhang, W.; Pei, R.; Lin, Q. A graphene aptasensor for biomarker detection in human serum. Electrochimica. Acta 2018, 290, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.; Mohapatra, P.; Mishra, P.P. Direct observation of adsorption and desorption of ds-DNA on graphene oxide and graphene oxide-gold nanoparticle hybrid material: A kineto-mechanistic investigation. Appl. Surf. Sci. 2022, 577, 151696. [Google Scholar] [CrossRef]
- Jayagopal, A.; Halfpenny, K.C.; Perez, J.W.; Wright, D.W. Wright, Hairpin DNA-Functionalized Gold Colloids for the Imaging ofmRNA in Live Cells. Am. Chem. Soc. 2010, 132, 9789–9796. [Google Scholar] [CrossRef]
- Nitu, F.R.; Savu, L.; Muraru, S.; Stoian, I.; Ionită, M. Label-Free Homogeneous microRNA Detection in Cell Culture Medium Based on Graphene Oxide and Specific Fluorescence Quenching. Nanomaterials 2021, 11, 368. [Google Scholar] [CrossRef]
- Li, F.-F. Comprehensive Review of Recent Research Advances on Flame-Retardant Coatings for Building Materials: Chemical Ingredients, Micromorphology, and Processing Techniques. Molecules 2023, 28, 1842. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.; Mason, J.O.; Lathe, R. Hybridization Parameters Revisited: Solutions Containing SDS. Biotechniques 2002, 33, 54–58. [Google Scholar] [CrossRef]
- Zhang, F.; Li, S.; Zhang, Q.; Liu, J.; Zeng, S.; Liu, M.; Sun, D. Adsorption of different types of surfactants on graphene oxide. J. Mol. Liq. 2019, 276, 338–346. [Google Scholar] [CrossRef]
- Available online: https://en.mgitech.cn/Uploads/Temp/file/20200526/5ecc87d664e02.pdf (accessed on 16 May 2023).
- Renart, J.; Behrens, M.M.; Fernandez-Renart, M.; Martinez, J.L. Immunoblotting Techniques, Immunoassay; Diamandis, E., Christopoulos, T., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 537–554. [Google Scholar]
- Available online: https://www.thermofisher.com/ro/en/home/references/ambion-tech-support/rna-tools-and-calculators/dna-and-rna-molecular-weights-and-conversions.html (accessed on 16 May 2023).


| Fluorescent Signal before Adding FAM–ssDNA Sample | Fluorescent Signal after Adding FAM–ssDNA Sample | Fluorescent Signal of the Desorption Solution after Incubation of NC–GO Hybrid Membrane without FAM–ssDNA | |||||
|---|---|---|---|---|---|---|---|
| H2O | αMEM | αMEM + FBS | H2O | αMEM | αMEM + FBS | αMEM | αMEM + FBS |
| Average fluorescent signal (r.f.u.) | |||||||
| 68.2 ± 8.1 | 1685.6 ± 209.6 | 2016.6 ± 231.7 | 3796.8 ± 486.5 | 5493.0 ± 785.4 | 5504.2 ± 764.3 | 74.7 ± 8.5 | 73.9 ± 10.3 |
| Incubation Media | Mass (pg) and Standard Deviation of Oligo ssDNA Desorbed from NC Membrane at Different Adsorption Times | Mass (pg) and Standard Deviation of Oligo ssDNA Desorbed from NC–GO Hybrid Membrane at Different Adsorption Times | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | 30 min | 45 min | 60 min | 30 min | 45 min | 60 min | ||||||
| U/M | pg | s.d. | pg | s.d. | pg | s.d. | pg | s.d. | pg | s.d. | pg | s.d. |
| H2O | 139.1 | ±15.4 | 157.5 | ±18.2 | 189.4 | ±24.7 | 234.8 | ±20.1 | 256.4 | ±27.6 | 269.9 | ±36.3 |
| αMEM | 168.2 | ±5.4 | 191.2 | ±6.3 | 205.0 | ±5.0 | 299.8 | ±6.1 | 323.4 | ±8.0 | 343.6 | ±19.1 |
| αMEM + FBS | 124.3 | ±3.9 | 147.5 | ±4.8 | 162.1 | ±12.6 | 251.1 | ±12.3 | 278.2 | ±11.2 | 296.2 | ±12.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toader, G.A.; Nitu, F.R.; Ionita, M. Graphene Oxide/Nitrocellulose Non-Covalent Hybrid as Solid Phase for Oligo-DNA Extraction from Complex Medium. Molecules 2023, 28, 4599. https://doi.org/10.3390/molecules28124599
Toader GA, Nitu FR, Ionita M. Graphene Oxide/Nitrocellulose Non-Covalent Hybrid as Solid Phase for Oligo-DNA Extraction from Complex Medium. Molecules. 2023; 28(12):4599. https://doi.org/10.3390/molecules28124599
Chicago/Turabian StyleToader, Georgian A., Florentin R. Nitu, and Mariana Ionita. 2023. "Graphene Oxide/Nitrocellulose Non-Covalent Hybrid as Solid Phase for Oligo-DNA Extraction from Complex Medium" Molecules 28, no. 12: 4599. https://doi.org/10.3390/molecules28124599
APA StyleToader, G. A., Nitu, F. R., & Ionita, M. (2023). Graphene Oxide/Nitrocellulose Non-Covalent Hybrid as Solid Phase for Oligo-DNA Extraction from Complex Medium. Molecules, 28(12), 4599. https://doi.org/10.3390/molecules28124599









