Photocatalytic Degradation of Food and Juices Dyes via Photocatalytic Nanomaterials Synthesized through Green Synthetic Route: A Systematic Review
Abstract
:1. Introduction
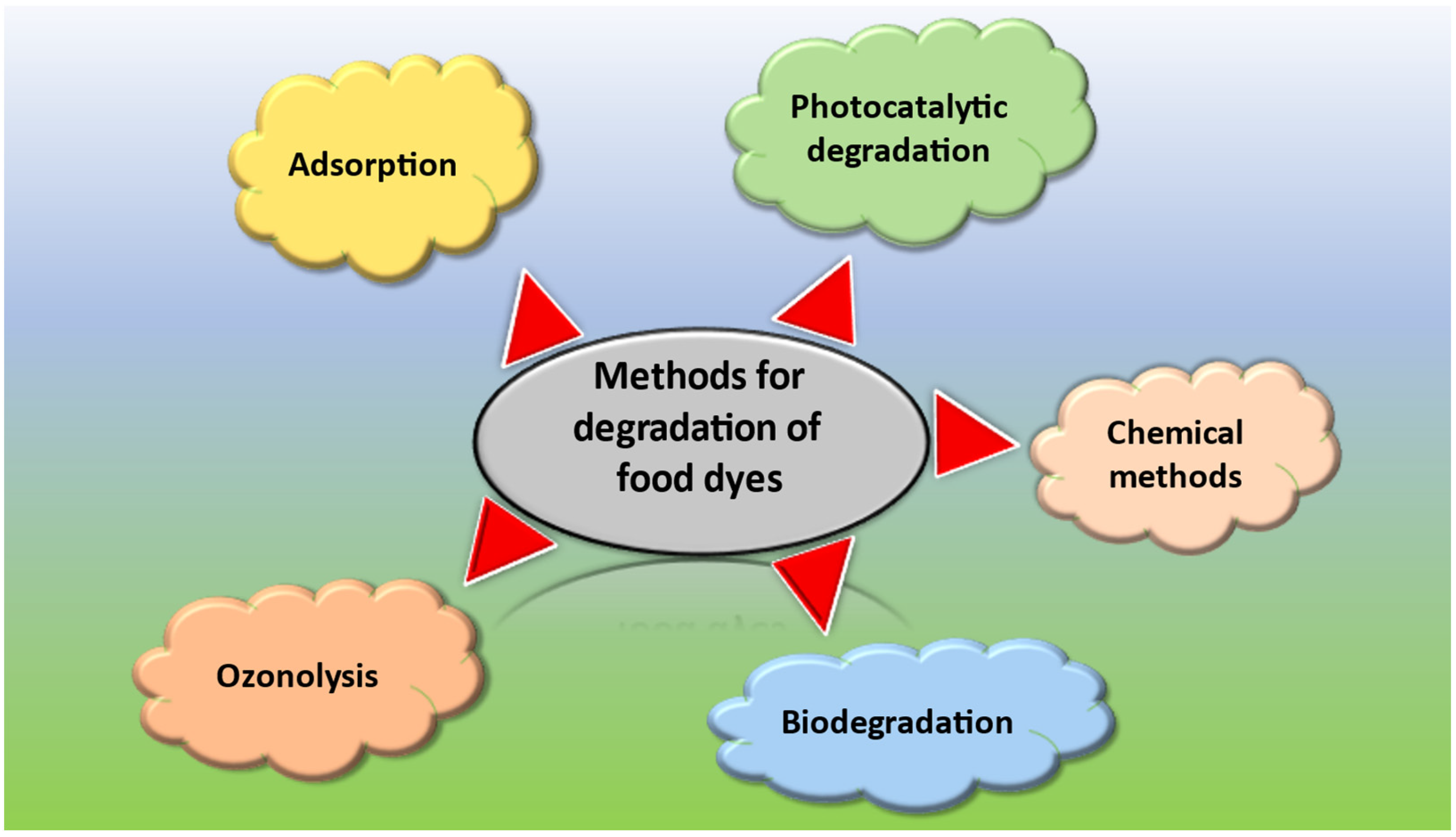
2. Methods Used for the Degradation of Food Dyes
- High efficiency: As the NPs possess a large surface-area-to-volume ratio, they offer many active sites within a small area and can thus increase the degradation efficiency even at a smaller concentration of the dye [34].
- Selectivity: Photocatalytic degradation using NPs is a selective process that targets specific compounds and leaves the other compounds in the food matrix unharmed. This is because the photocatalysts are activated by light on a specific wavelength which can be tailored to specific dyes [35].
- Environmental friendliness: The most important advantage of using photocatalysis over the other is the environmental friendliness of this method as it would most commonly result in the production of water and carbon dioxide as the end products which are not harmful to the environment, and we can thus reduce the toxicity of the dyes to a greater extent [36].
- Versatility: photocatalytic degradation can be used to degrade many toxic organic compounds, including those that are resistant to other methods; therefore, its properties are highly versatile [37].
- Cost effectiveness: This method is relatively low cost as compared to the other as it does not involve the use of any expensive material or compound. All that is required is NPs, such as ZnO or TiO2, and exposure to UV light that can be obtained by exposure to the sunlight [38].
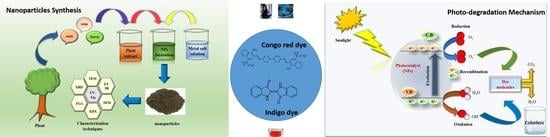
2.1. Various Approaches for Synthesis of Photocatalytic NPs
2.2. Green Synthesis and Characterization of Photocatalytic NPs
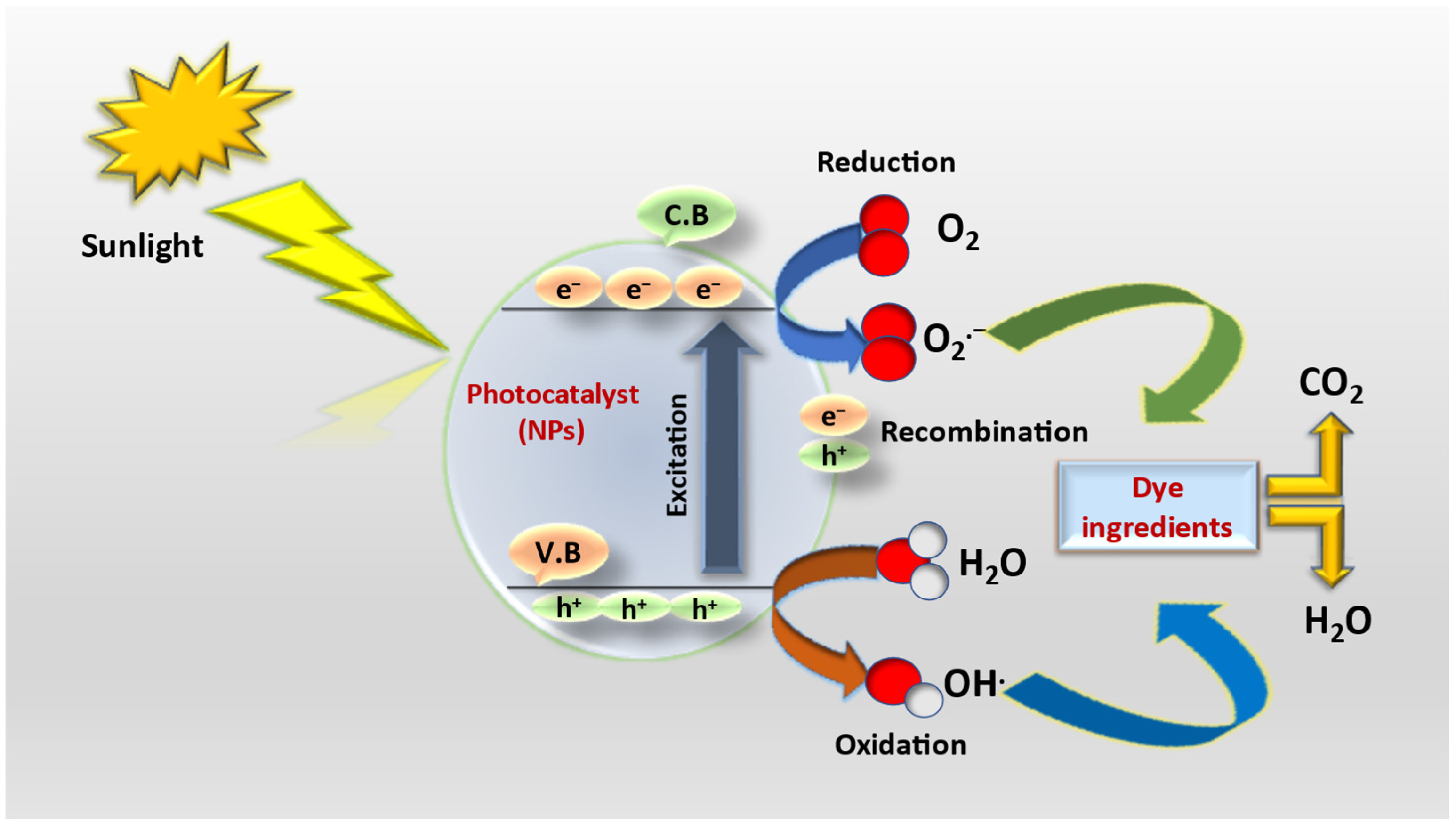
3. Mechanism of Dye Degradation by Metal and Metal Oxide NPs
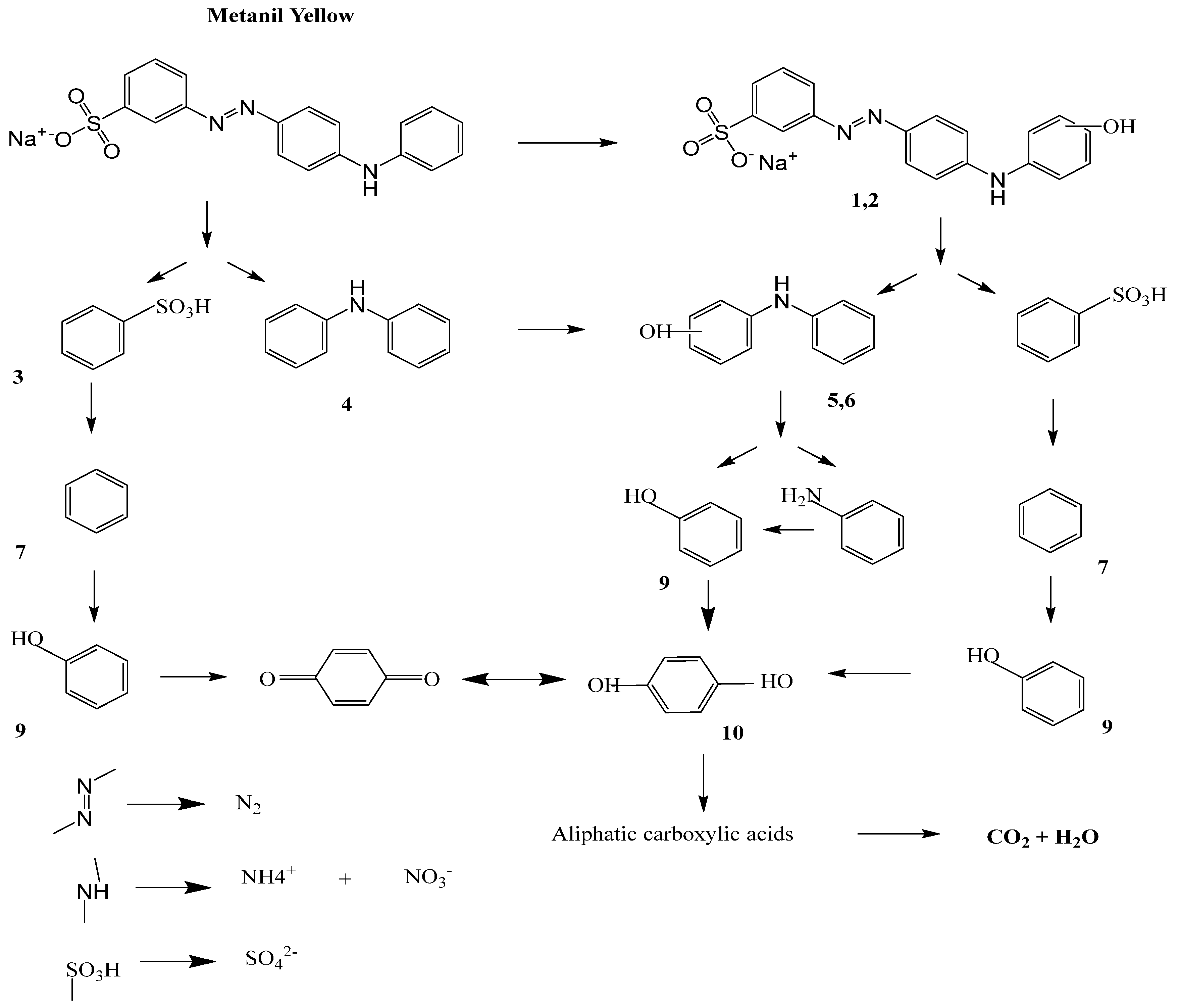
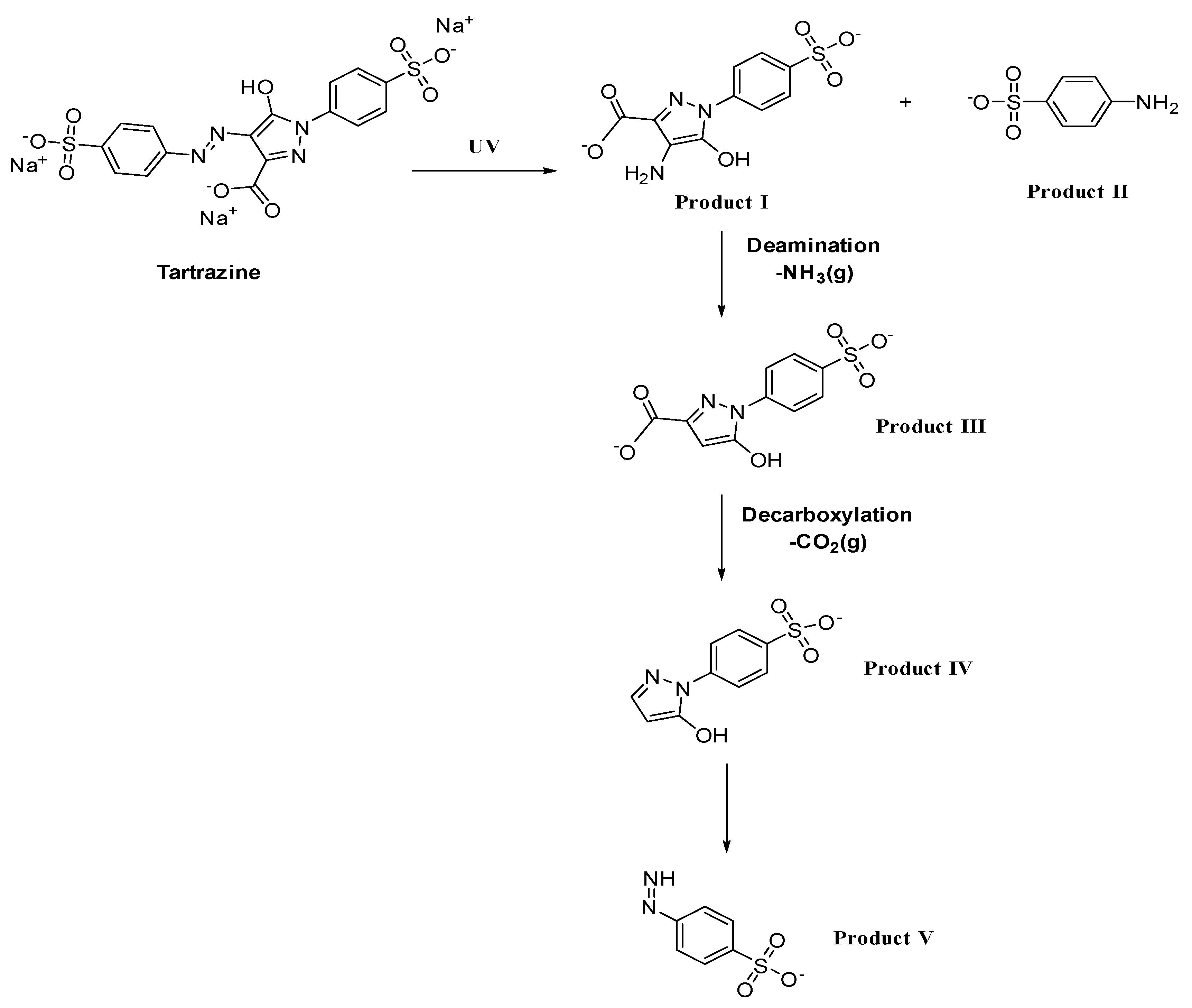
 ) takes place, which results in the formation of subsequent degradation products, i.e., product I and product II. Deamination in product I results in formation of product III, followed by decarboxylation, resulting in product IV. Finally, ring opening takes place with the removal of acrylaldehyde, forming product V [57].
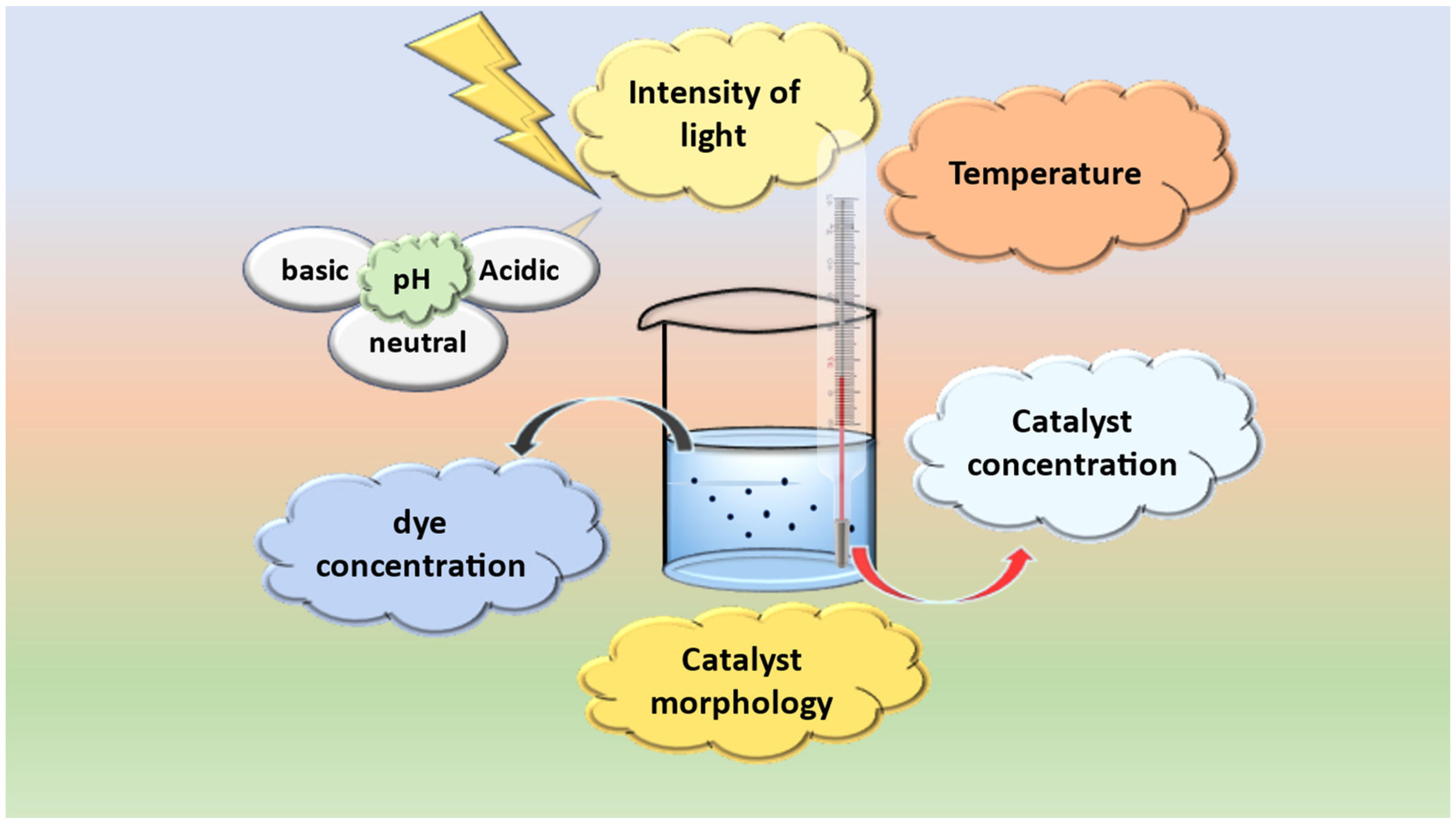
) takes place, which results in the formation of subsequent degradation products, i.e., product I and product II. Deamination in product I results in formation of product III, followed by decarboxylation, resulting in product IV. Finally, ring opening takes place with the removal of acrylaldehyde, forming product V [57].3.1. Factors Influencing Degradation of the Dyes
3.2. Implementation of Photocatalytic NPs in Dye Degradation
4. Advantages and Disadvantages
4.1. Advantages
- Environmentally friendly: green synthesis of NPs uses natural materials, such as plant extracts, which are in most cases eco-friendly and non-toxic. Therefore, the use of green-synthesized NPs for photocatalytic degradation of food dyes is an environmentally friendly process.
- Low cost: The green synthesis of NPs is a low-cost process that does not require any expensive equipment or chemicals, making it an affordable process and promising step towards the photocatalytic degradation of food dyes.
- High efficiency: Green-synthesized NPs have been reported to exhibit high photocatalytic activity due to their unique properties, such as high surface area, small particle size, and high reactivity.
- Selectivity: The use of green-synthesized NPs allows for selective degradation of food dyes, as they can be tuned to target specific types of dyes.
4.2. Disadvantages
- Lack of standardization: The green synthesis of NPs is still a developing field, and there is a lack of standardization in the methods used to synthesize NPs. This can lead to variability in the properties and performance of the NPs.
- Limited stability: Green-synthesized NPs may have limited stability and can be prone to agglomeration or oxidation, which can affect their performance.
- Toxicity: Although green-synthesized NPs are generally considered to be non-toxic, some plant extracts used for their synthesis may contain toxic compounds. Therefore, it is important to ensure that the NPs are thoroughly characterized and tested for toxicity before use.
- Scale-up challenges: generally, green synthesis of NPs is carried out on smaller scales, so introducing it at industrial levels might be challenging. This can limit the practical application of the NPs for industrial-scale photocatalytic degradation of food dyes.
5. Economic Cost
6. Challenges
- Selection of appropriate NPs: The choice of NPs used for the photodegradation process is critical. The NPs should be stable, non-toxic, and efficient in absorbing light in the visible range. The stability of NPs synthesized using green methods can be a challenge as they may be prone to aggregation, leading to reduced efficiency in dye degradation.
- Scalability: The scalability of the green-synthesis method may be a challenge as it may not be possible to produce the large quantities of NPs needed for industrial applications.
- Photocatalytic activity: The photocatalytic activity of the NPs should be high enough to ensure efficient degradation of the dyes. The photocatalytic activity of nanoparticles can be improved by optimizing their size, shape, and surface area.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brudzyńska, P.; Sionkowska, A.; Grisel, M. Plant-Derived Colorants for Food, Cosmetic and Textile Industries: A Review. Materials 2021, 14, 3484. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety Assessment of Personal Care Products/Cosmetics and Their Ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [CrossRef]
- Padervand, M.; Rhimi, B.; Wang, C. One-Pot Synthesis of Novel Ternary Fe3N/Fe2O3/C3N4 Photocatalyst for Efficient Removal of Rhodamine B and CO2 Reduction. J. Alloys Compd. 2021, 852, 156955. [Google Scholar] [CrossRef]
- Chequer, F.D.; De Oliveira, G.R.; Ferraz, E.A.; Cardoso, J.C.; Zanoni, M.B.; de Oliveira, D.P. Textile Dyes: Dyeing Process and Environmental Impact. Eco-Friendly Text. Dye. Finish. 2013, 6, 151–176. [Google Scholar]
- Khan, A.D.; Alam, M.N. Cosmetics and Their Associated Adverse Effects: A Review. J. Appl. Pharm. Sci. Res. 2019, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sakin Omer, O.; Hussein, M.A.; Hussein, B.H.M.; Mgaidi, A. Adsorption Thermodynamics of Cationic Dyes (Methylene Blue and Crystal Violet) to a Natural Clay Mineral from Aqueous Solution between 293.15 and 323.15 K. Arab. J. Chem. 2018, 11, 615–623. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Varjani, S.J.; Suganya, S. Treatment of Dye Wastewater Using an Ultrasonic Aided Nanoparticle Stacked Activated Carbon: Kinetic and Isotherm Modelling. Bioresour. Technol. 2018, 250, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Shafiq, A.; Chen, S.Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef] [PubMed]
- Pimol, P.; Khanidtha, M.; Prasert, P. Influence of Particle Size and Salinity on Adsorption of Basic Dyes by Agricultural Waste: Dried Seagrape (Caulerpa lentillifera). J. Environ. Sci. 2008, 20, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A Review on Classifications, Recent Synthesis and Applications of Textile Dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Arami, M.; Limaee, N.Y.; Mahmoodi, N.M. Evaluation of the Adsorption Kinetics and Equilibrium for the Potential Removal of Acid Dyes Using a Biosorbent. Chem. Eng. J. 2008, 139, 2–10. [Google Scholar] [CrossRef]
- Safarik, I.; Mullerova, S.; Pospiskova, K. Semiquantitative Determination of Food Acid Dyes by Magnetic Textile Solid Phase Extraction Followed by Image Analysis. Food Chem. 2019, 274, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Eisenbrand, G.; Pool-Zobel, B.; Baker, V.; Balls, M.; Blaauboer, B.; Boobis, A.; Carere, A.; Kevekordes, S.; Lhuguenot, J.-C.; Pieters, R. Methods of in Vitro Toxicology. Food Chem. Toxicol. 2002, 40, 193–236. [Google Scholar] [CrossRef] [PubMed]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health Safety Issues of Synthetic Food Colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Kobun, R.; Siddiquee, S.; Shaarani, S.M. Rapid Detection of Allura Red (E129) Based on Chitosan/Nanoparticles/MWCNTs Modified Gold Electrode in Food Products. Trans. Sci. Technol. 2015, 2, 56–64. [Google Scholar]
- Wu, D.; Yan, J.; Wang, J.; Wang, Q.; Li, H. Characterisation of Interaction between Food Colourant Allura Red AC and Human Serum Albumin: Multispectroscopic Analyses and Docking Simulations. Food Chem. 2015, 170, 423–429. [Google Scholar] [CrossRef]
- Tsuda, S.; Murakami, M.; Matsusaka, N.; Kano, K.; Taniguchi, K.; Sasaki, Y.F. DNA Damage Induced by Red Food Dyes Orally Administered to Pregnant and Male Mice. Toxicol. Sci. 2001, 61, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Shi, T.; Liu, Z.; Cheng, S.; Huang, Y.; Tao, X.; Liao, G.; Tang, Z. Ultrasensitive Non-Enzymatic Glucose Sensors Based on Different Copper Oxide Nanostructures by in-Situ Growth. Sens. Actuators B Chem. 2016, 236, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Journal, T.H.E.; Tropical, O.F.; Science, L. Survey on the use of synthetic food colors in food samples procured from. J. Trop. Life Sci. 2013, 3, 1–7. [Google Scholar]
- Motta, C.M.; Simoniello, P.; Arena, C.; Capriello, T.; Panzuto, R.; Vitale, E.; Agnisola, C.; Tizzano, M.; Avallone, B.; Ferrandino, I. Effects of Four Food Dyes on Development of Three Model Species, Cucumis sativus, Artemia salina and Danio rerio: Assessment of Potential Risk for the Environment. Environ. Pollut. 2019, 253, 1126–1135. [Google Scholar] [CrossRef]
- Shah, A. A Novel Electrochemical Nanosensor for the Simultaneous Sensing of Two Toxic Food Dyes. ACS Omega 2020, 5, 6187–6193. [Google Scholar] [CrossRef]
- Nayak, D.S.; Shetti, N.P. A Novel Sensor for a Food Dye Erythrosine at Glucose Modified Electrode. Sens. Actuators B Chem. 2016, 230, 140–148. [Google Scholar] [CrossRef]
- Arif, A.; Ahmad, A.; Ahmad, M. Toxicity Assessment of Carmine and Its Interaction with Calf Thymus DNA. J. Biomol. Struct. Dyn. 2021, 39, 5861–5871. [Google Scholar] [CrossRef] [PubMed]
- Guaraldo, T.T.; Zanoni, T.B.; de Torresi, S.I.; Gonçales, V.R.; Zocolo, G.J.; Oliveira, D.P.; Zanoni, M.V.B. On the Application of Nanostructured Electrodes Prepared by Ti/TiO2/WO3 “Template”: A Case Study of Removing Toxicity of Indigo Using Visible Irradiation. Chemosphere 2013, 91, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Asif Ahmed, M.; Al-Khalifa, A.S.; Al-Nouri, D.M.; El-din, M.F.S. Dietary Intake of Artificial Food Color Additives Containing Food Products by School-Going Children. Saudi J. Biol. Sci. 2021, 28, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.K.; Banerjee, T.S.; Talukder, G.; Sharma, A. Effects of Dyes (Indigo Carmine, Metanil Yellow, Fast Green FCF) and Nitrite in Vivo on Bone Marrow Chromosomes of Mice. Cancer Lett. 1986, 30, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, L.P.; Khanna, S.K.; Singh, G.B.; Murti, C.R.K. In Vitro Studies on the Biotransformation of Metanil Yellow. Environ. Res. 1982, 27, 185–189. [Google Scholar] [CrossRef]
- Gupta, S.; Sundarrajan, M.; Rao, K.V.K. Tumor Promotion by Metanil Yellow and Malachite Green during Rat Hepatocarcinogenesis Is Associated with Dysregulated Expression of Cell Cycle Regulatory Proteins. Teratog. Carcinog. Mutagen. 2003, 312, 301–312. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Saravanan, M.; Senthilkumar, P.; Gnanasekaran, K.; Shanmugavel, M.; Manikandan, E.; Pugazhendhi, A. Synthesis of Ecofriendly Copper Oxide Nanoparticles for Fabrication over Textile Fabrics: Characterization of Antibacterial Activity and Dye Degradation Potential. J. Photochem. Photobiol. B Biol. 2019, 191, 143–149. [Google Scholar] [CrossRef]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial Degradation of Dyes: An Overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef]
- Muniyasamy, A.; Sivaporul, G.; Gopinath, A.; Lakshmanan, R.; Altaee, A.; Achary, A.; Velayudhaperumal Chellam, P. Process Development for the Degradation of Textile Azo Dyes (Mono-, Di-, Poly-) by Advanced Oxidation Process—Ozonation: Experimental & Partial Derivative Modelling Approach. J. Environ. Manag. 2020, 265, 110397. [Google Scholar]
- Dhawle, R.; Frontistis, Z.; Mantzavinos, D.; Lianos, P. Production of Hydrogen Peroxide with a Photocatalytic Fuel Cell and Its Application to UV/H2O2 Degradation of Dyes. Chem. Eng. J. Adv. 2021, 6, 100109. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Yahyaoui, S.; Hanafy, H.; Seliem, M.K.; Bonilla-Petriciolet, A.; Luiz Dotto, G.; Sellaoui, L.; Li, Q. Effective Adsorption of Dyes on an Activated Carbon Prepared from Carboxymethyl Cellulose: Experiments, Characterization and Advanced Modelling. Chem. Eng. J. 2021, 417, 128116. [Google Scholar] [CrossRef]
- Kadam, J.; Dhawal, P.; Barve, S.; Kakodkar, S. Green Synthesis of Silver Nanoparticles Using Cauliflower Waste and Their Multifaceted Applications in Photocatalytic Degradation of Methylene Blue Dye and Hg2+ Biosensing. SN Appl. Sci. 2020, 2, 738. [Google Scholar] [CrossRef] [Green Version]
- Navia-Mendoza, J.M.; Filho, O.A.E.; Zambrano-Intriago, L.A.; Maddela, N.R.; Duarte, M.M.M.B.; Quiroz-Fernández, L.S.; Baquerizo-Crespo, R.J.; Rodríguez-Díaz, J.M. Advances in the Application of Nanocatalysts in Photocatalytic Processes for the Treatment of Food Dyes: A Review. Sustainability 2021, 13, 11676. [Google Scholar] [CrossRef]
- Nazim, M.; Khan, A.A.P.; Asiri, A.M.; Kim, J.H. Exploring Rapid Photocatalytic Degradation of Organic Pollutants with Porous CuO Nanosheets: Synthesis, Dye Removal, and Kinetic Studies at Room Temperature. ACS Omega 2021, 6, 2601–2612. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.O.; Durosinmi, L.M.; Oluwafemi, O.S.; Olayanju, D.S.; Akinola, A.S.; Obisesan, O.R.; Akinyele, O.F.; Ajayeoba, T.A. Potential of Cobalt and Cobalt Oxide Nanoparticles as Nanocatalyst towards Dyes Degradation in Wastewater. Nano-Struct. Nano-Objects 2020, 21, 100405. [Google Scholar] [CrossRef]
- Hashmi, S.S.; Shah, M.; Muhammad, W.; Ahmad, A.; Ullah, M.A.; Nadeem, M.; Abbasi, B.H. Potentials of Phyto-Fabricated Nanoparticles as Ecofriendly Agents for Photocatalytic Degradation of Toxic Dyes and Waste Water Treatment, Risk Assessment and Probable Mechanism. J. Indian Chem. Soc. 2021, 98, 100019. [Google Scholar] [CrossRef]
- Nadeem, M.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Hashmi, S.S.; Ahmad, W.; Zahir, A. The Current Trends in the Green Syntheses of Titanium Oxide Nanoparticles and Their Applications. Green Chem. Lett. Rev. 2018, 11, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, B.H.; Shah, M.; Hashmi, S.S.; Nazir, M.; Naz, S.; Ahmad, W.; Khan, I.U.; Hano, C. Green Bio-Assisted Synthesis, Characterization and Biological Evaluation of Biocompatible Zno Nps Synthesized from Different Tissues of Milk Thistle (Silybum marianum). Nanomaterials 2019, 9, 1171. [Google Scholar] [CrossRef] [Green Version]
- Parveen, K.; Banse, V.; Ledwani, L. Green Synthesis of Nanoparticles: Their Advantages and Disadvantages. AIP Conf. Proc. 2016, 1724, 020048. [Google Scholar]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Eswaran, S.G.; Afridi, P.S. Effective Multi Toxic Dyes Degradation Using Bio-Fabricated Silver Nanoparticles as a Green Catalyst. Appl. Biochem. Biotechnol. 2023, 195, 3872–3887. [Google Scholar] [CrossRef] [PubMed]
- Geetha, R.; Ashokkumar, T.; Tamilselvan, S.; Govindaraju, K.; Sadiq, M.; Singaravelu, G. Green Synthesis of Gold Nanoparticles and Their Anticancer Activity. Cancer Nanotechnol. 2013, 4, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.V.; Jelastin Kala, S.M.; Prakash, K.S. Green Synthesis Derived Pt-Nanoparticles Using Xanthium strumarium Leaf Extract and Their Biological Studies. J. Environ. Chem. Eng. 2019, 7, 103146. [Google Scholar] [CrossRef]
- Jayarambabu, N.; Akshaykranth, A.; Venkatappa Rao, T.; Venkateswara Rao, K.; Rakesh Kumar, R. Green Synthesis of Cu Nanoparticles Using Curcuma longa Extract and Their Application in Antimicrobial Activity. Mater. Lett. 2020, 259, 126813. [Google Scholar] [CrossRef]
- Ezhilarasi, A.A.; Vijaya, J.J.; Kaviyarasu, K.; Maaza, M.; Ayeshamariam, A.; Kennedy, L.J. Green Synthesis of NiO Nanoparticles Using Moringa oleifera Extract and Their Biomedical Applications: Cytotoxicity Effect of Nanoparticles against HT-29 Cancer Cells. J. Photochem. Photobiol. B Biol. 2016, 164, 352–360. [Google Scholar] [CrossRef]
- Fatmarin, Z.; Dani Nandiyanto, A.B. Economic Evaluation of Zinc Oxide Nanoparticle Production through Green Synthesis Method Using Cassia fistula Plant Extract. Int. J. Energ. 2020, 5, 18–24. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Nachane, R.P.; Balasubramanya, R.H.; Varadarajan, P.V. A Novel One-Pot “green” Synthesis of Stable Silver Nanoparticles Using Soluble Starch. Carbohydr. Res. 2006, 341, 2012–2018. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B. Green Synthesis of Biogenic Silver Nanoparticles for E Ffi Cient Catalytic Removal of Harmful Organic Dyes. Nanomaterials 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-Mediated Photodegradation of Pollutants under Visible-Light Irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef]
- Selvaraj, V.; Karthika, T.S.; Mansiya, C.; Alagar, M. An over Review on Recently Developed Techniques, Mechanisms and Intermediate Involved in the Advanced Azo Dye Degradation for Industrial Applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Prabhakar Vattikuti, S.V.; Devarayapalli, K.C.; Yoo, K.; Shim, J.; Sreekanth, T.V.M. Green Synthesis: Photocatalytic Degradation of Textile Dyes Using Metal and Metal Oxide Nanoparticles-Latest Trends and Advancements. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2617–2723. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sleiman, M.; Vildozo, D.; Ferronato, C.; Chovelon, J.M. Photocatalytic Degradation of Azo Dye Metanil Yellow: Optimization and Kinetic Modeling Using a Chemometric Approach. Appl. Catal. B Environ. 2007, 77, 1–11. [Google Scholar] [CrossRef]
- Dos Santos, T.C.; Zocolo, G.J.; Morales, D.A.; Umbuzeiro, G.d.A.; Zanoni, M.V.B. Assessment of the Breakdown Products of Solar/UV Induced Photolytic Degradation of Food Dye Tartrazine. Food Chem. Toxicol. 2014, 68, 307–315. [Google Scholar] [CrossRef]
- Kumar, A. A Review on the Factors Affecting the Photocatalytic Degradation of Hazardous Materials. Mater. Sci. Eng. Int. J. 2017, 1, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photocatalytic Production of H2O2 and Organic Peroxides in Aqueous Suspensions of TiO2, ZnO, and Desert Sand. Environ. Sci. Technol. 1988, 22, 798–806. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. Photocatalytic Degradation of Eriochrome Black-T by the Ni:TiO2 Nanocomposites. Desalin. Water Treat. 2017, 71, 406–419. [Google Scholar] [CrossRef]
- Pakzad, K.; Alinezhad, H.; Nasrollahzadeh, M. Green Synthesis of Ni@Fe3O4 and CuO Nanoparticles Using Euphorbia maculata Extract as Photocatalysts for the Degradation of Organic Pollutants under UV-Irradiation. Ceram. Int. 2019, 45, 17173–17182. [Google Scholar] [CrossRef]
- Lanjwani, M.F.; Khuhawar, M.Y.; Khuhawar, T.M.J.; Lanjwani, A.H.; Memon, S.Q.; Soomro, W.A.; Rind, I.K. Photocatalytic Degradation of Eriochrome Black T Dye by ZnO Nanoparticles Using Multivariate Factorial, Kinetics and Isotherm Models. J. Clust. Sci. 2022, 34, 1121–1132. [Google Scholar] [CrossRef]
- Kumar, A. Photodegradation of Methyl Orange in Aqueous Solution by the Visible Light Active Co:La:TiO2 Nanocomposite. Chem. Sci. J. 2017, 8, 1000164–1000174. [Google Scholar]
- Davis, R.J.; Gainer, J.L.; O’Neal, G.; Wu, I.-W. Photocatalytic Decolorization of Wastewater Dyes. Water Environ. Res. 1994, 66, 50–53. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Wouters, R.D.; Muraro, P.C.L.; Druzian, D.M.; Viana, A.R.; de Oliveira Pinto, E.; da Silva, J.K.L.; Vizzotto, B.S.; Ruiz, Y.P.M.; Galembeck, A.; Pavoski, G.; et al. Zinc Oxide Nanoparticles: Biosynthesis, Characterization, Biological Activity and Photocatalytic Degradation for Tartrazine Yellow Dye. J. Mol. Liq. 2023, 371, 121090. [Google Scholar] [CrossRef]
- Gupta, S.; Tejavath, K.K. Catalytic Reduction of Organic Dyes with Green Synthesized Silver Nanoparticles Using Aloe vera Leaf Extract. J. Nanosci. Nanoeng. Appl. 2019, 9, 9–21. [Google Scholar] [CrossRef]
- Karthik, K.V.; Raghu, A.V.; Reddy, K.R.; Ravishankar, R.; Sangeeta, M.; Shetti, N.P.; Reddy, C.V. Green Synthesis of Cu-Doped ZnO Nanoparticles and Its Application for the Photocatalytic Degradation of Hazardous Organic Pollutants. Chemosphere 2022, 287, 132081. [Google Scholar] [CrossRef]
- Faisal, S.; Khan, M.A.; Jan, H.; Shah, S.A.; Shah, S.A.; Rizwan, M.; Wajidullah, A.M.T.; Redaina. Edible Mushroom (Flammulina velutipes) as Biosource for Silver Nanoparticles: From Synthesis to Diverse Biomedical and Environmental Applications. Nanotechnology 2021, 32, 065101. [Google Scholar] [CrossRef]
- Kheibarian, Z.; Soleimani, E.; Mardani, H.R. Photocatalytic Activity of Cu@Ag BNCs Synthesized by the Green Method: Photodegradation Methyl Orange and Indigo Carmine. Inorg. Nano-Met. Chem. 2022, 53, 355–365. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green Synthesis of Zinc Oxide Nanoparticles Using Phoenix dactylifera Waste as Bioreductant for Effective Dye Degradation and Antibacterial Performance in Wastewater Treatment. J. Hazard. Mater. 2021, 402, 123560. [Google Scholar] [CrossRef] [PubMed]
- Bibi, I.; Nazar, N.; Ata, S.; Sultan, M.; Ali, A.; Abbas, A.; Jilani, K.; Kamal, S.; Sarim, F.M.; Khan, M.I.; et al. Green Synthesis of Iron Oxide Nanoparticles Using Pomegranate Seeds Extract and Photocatalytic Activity Evaluation for the Degradation of Textile Dye. J. Mater. Res. Technol. 2019, 8, 6115–6124. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.H.; Miah, M.Y.; Paul, S.C.; Aka, T.D.; Saha, O.; Rahaman, M.M.; Sharif, M.J.I.; Habiba, O.; Ashaduzzaman, M. Green Synthesis of Iron Oxide Nanoparticle Using Carica papaya Leaf Extract: Application for Photocatalytic Degradation of Remazol Yellow RR Dye and Antibacterial Activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef] [PubMed]
- Rajendrachari, S.; Taslimi, P.; Karaoglanli, A.C.; Uzun, O.; Alp, E.; Jayaprakash, G.K. Photocatalytic Degradation of Rhodamine B (RhB) Dye in Waste Water and Enzymatic Inhibition Study Using Cauliflower Shaped ZnO Nanoparticles Synthesized by a Novel One-Pot Green Synthesis Method. Arab. J. Chem. 2021, 14, 103180. [Google Scholar] [CrossRef]
- Jain, R.; Mendiratta, S.; Kumar, L.; Srivastava, A. Green Synthesis of Iron Nanoparticles Using Artocarpus heterophyllus Peel Extract and Their Application as a Heterogeneous Fenton-like Catalyst for the Degradation of Fuchsin Basic Dye. Curr. Res. Green Sustain. Chem. 2021, 4, 100086. [Google Scholar] [CrossRef]
- Kouhbanani, M.A.J.; Beheshtkhoo, N.; Taghizadeh, S.; Amani, A.M.; Alimardani, V. One-Step Green Synthesis and Characterization of Iron Oxide Nanoparticles Using Aqueous Leaf Extract of Teucrium polium and Their Catalytic Application in Dye Degradation. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 015007. [Google Scholar] [CrossRef]
- Al-Zaqri, N.; Umamakeshvari, K.; Mohana, V.; Muthuvel, A.; Boshaala, A. Green Synthesis of Nickel Oxide Nanoparticles and Its Photocatalytic Degradation and Antibacterial Activity. J. Mater. Sci. Mater. Electron. 2022, 33, 11864–11880. [Google Scholar] [CrossRef]
- Shimi, A.K.; Ahmed, H.M.; Wahab, M.; Katheria, S.; Wabaidur, S.M.; Eldesoky, G.E.; Islam, M.A.; Rane, K.P. Synthesis and Applications of Green Synthesized TiO2 Nanoparticles for Photocatalytic Dye Degradation and Antibacterial Activity. J. Nanomater. 2022, 2022, 7060388. [Google Scholar] [CrossRef]
- Devi, T.A.; Sivaraman, R.M.; Sheeba Thavamani, S.; Peter Amaladhas, T.; AlSalhi, M.S.; Devanesan, S.; Kannan, M.M. Green Synthesis of Plasmonic Nanoparticles Using Sargassum ilicifolium and Application in Photocatalytic Degradation of Cationic Dyes. Environ. Res. 2022, 208, 112642. [Google Scholar] [CrossRef]
- Kumar, S.A.; Jarvin, M.; Inbanathan, S.S.R.; Umar, A.; Lalla, N.P.; Dzade, N.Y.; Algadi, H.; Rahman, Q.I.; Baskoutas, S. Facile Green Synthesis of Magnesium Oxide Nanoparticles Using Tea (Camellia sinensis) Extract for Efficient Photocatalytic Degradation of Methylene Blue Dye. Environ. Technol. Innov. 2022, 28, 102746. [Google Scholar] [CrossRef]
- Raghavendra, V.B.; Shankar, S.; Govindappa, M.; Pugazhendhi, A.; Sharma, M.; Nayaka, S.C. Green Synthesis of Zinc Oxide Nanoparticles (ZnO NPs) for Effective Degradation of Dye, Polyethylene and Antibacterial Performance in Waste Water Treatment. J. Inorg. Organomet. Polym. Mater. 2022, 32, 614–630. [Google Scholar] [CrossRef]
- Prabavathi, A.; Balu, A.R.; Nagarethinam, V.S.; Raja, N.; Suganya, M.; Usharani, K.; Balamurugan, S.; Karthika, M.; Kayathiri, C. Cauliflower Shaped CdO:Mo Nanostructures with Enhanced Photocatalytic Activity against the Degradation of Metanil Yellow Dye. Nano-Struct. Nano-Objects 2020, 22, 100458. [Google Scholar] [CrossRef]
- Biju, R.; Ravikumar, R.; Thomas, C.; Indulal, C.R. Enhanced Photocatalytic Degradation of Metanil Yellow Dye Using Polypyrrole-Based Copper Oxide–Zinc Oxide Nanocomposites under Visible Light. J. Nanoparticle Res. 2022, 24, 117. [Google Scholar] [CrossRef]
- Hakeem, M.K.; Shah, A.; Nisar, J.; Iftikhar, F.J.; Khan, S.B.; Shah, I. Electrochemical Sensing Platform for the Detection and Degradation Studies of Metanil Yellow. J. Electrochem. Soc. 2022, 169, 056503. [Google Scholar] [CrossRef]
- Nazim, M.; Kim, J.H.; Lee, H.Y.; Cho, S.K. Development of Three-Dimensional Nickel-Cobalt Oxide Nanoflowers for Superior Photocatalytic Degradation of Food Colorant Dyes: Catalyst Properties and Reaction Kinetic Study. Langmuir 2021, 37, 12929–12939. [Google Scholar] [CrossRef]
- Khan, A.A.P.; Nazim, M.; Asiri, A.M. Efficient Catalytic Degradation of Organic Pollutants with Cupric Oxide Nanomaterials in Aqueous Medium. J. Environ. Chem. Eng. 2021, 9, 106305. [Google Scholar] [CrossRef]
- Jamwal, D. Tungsten Oxide Nanostructures Peculiarity and Photocatalytic Activity for the Efficient Elimination of the Organic Pollutant; Research Square Platform LLC: Durham, NC, USA, 2023; pp. 1–18. [Google Scholar]
- Tang, W.R.; Wu, T.; Lin, Y.W. Salicylic Acid-Sensitised Titanium Dioxide for Photocatalytic Degradation of Fast Green FCF under Visible Light Irradiation. Micro Nano Lett. 2019, 14, 359–362. [Google Scholar] [CrossRef]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment: A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Malati, M.F.; Ganjali, F.; Zamiri, E.; Shokat, S.Z.; Jalali, F.; Padevand, M.; Ledri, R.T.; Maleki, A. Efficient Photodegradation of Eriochrome Black-T by a Trimetallic Magnetic Self-Synthesized Nanophotocatalyst Based on Zn/Au/Fe-Embedded Poly (vinyl alcohol). Langmuir 2020, 38, 13728–13743. [Google Scholar] [CrossRef]
- Shah, L.A.; Malik, T.; Siddiq, M.; Haleem, A.; Sayed, M. TiO2 nanotubes doped poly(vinylidene fluoride) polymer membranes(PVDF/TNT) for efficient photocatalytic degradation of brilliant green dye. J. Environ. Chem. Eng. 2019, 7, 103291. [Google Scholar] [CrossRef]
- Bibi, S.; Ahmad, A.; Anjum, M.A.R.; Haleem, A.; Siddiq, A.; Shah, S.S.; Kahtani, A.A. Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105580. [Google Scholar] [CrossRef]
- Haleem, A.; Pan, J.M.; Shah, A.; Hussain, H.; He, W.D. A systematic review on new advancement and assessment of emerging polymeric cryogels for environmental sustainability and energy production. Sep. Purf. Tech. 2023, 316, 123678. [Google Scholar] [CrossRef]
- Haleem, A.; Chen, S.Q.; Pan, J.M.; He, W.D. Gamma radiation induced synthesis of double network hydrophilic cryogels at low pH loaded with AuNPs for fast and efficient degradation of Congo red. J. Hazard. Mater. Adv. 2023, 10, 100299. [Google Scholar] [CrossRef]
- Amreen, J.; Haleem, A.; Shah, A.A.; Mushtaq, F.; Siddiq, M.; Bhatti, M.A.; Bukhari, S.N.U.S.; Chandio, A.D.; Mahdi, W.A.; Alshehri, S. Facile Synthesis and Fabrication of NIPAM-Based Cryogels for Environmental Remediation. Gels 2023, 9, 64. [Google Scholar] [CrossRef]


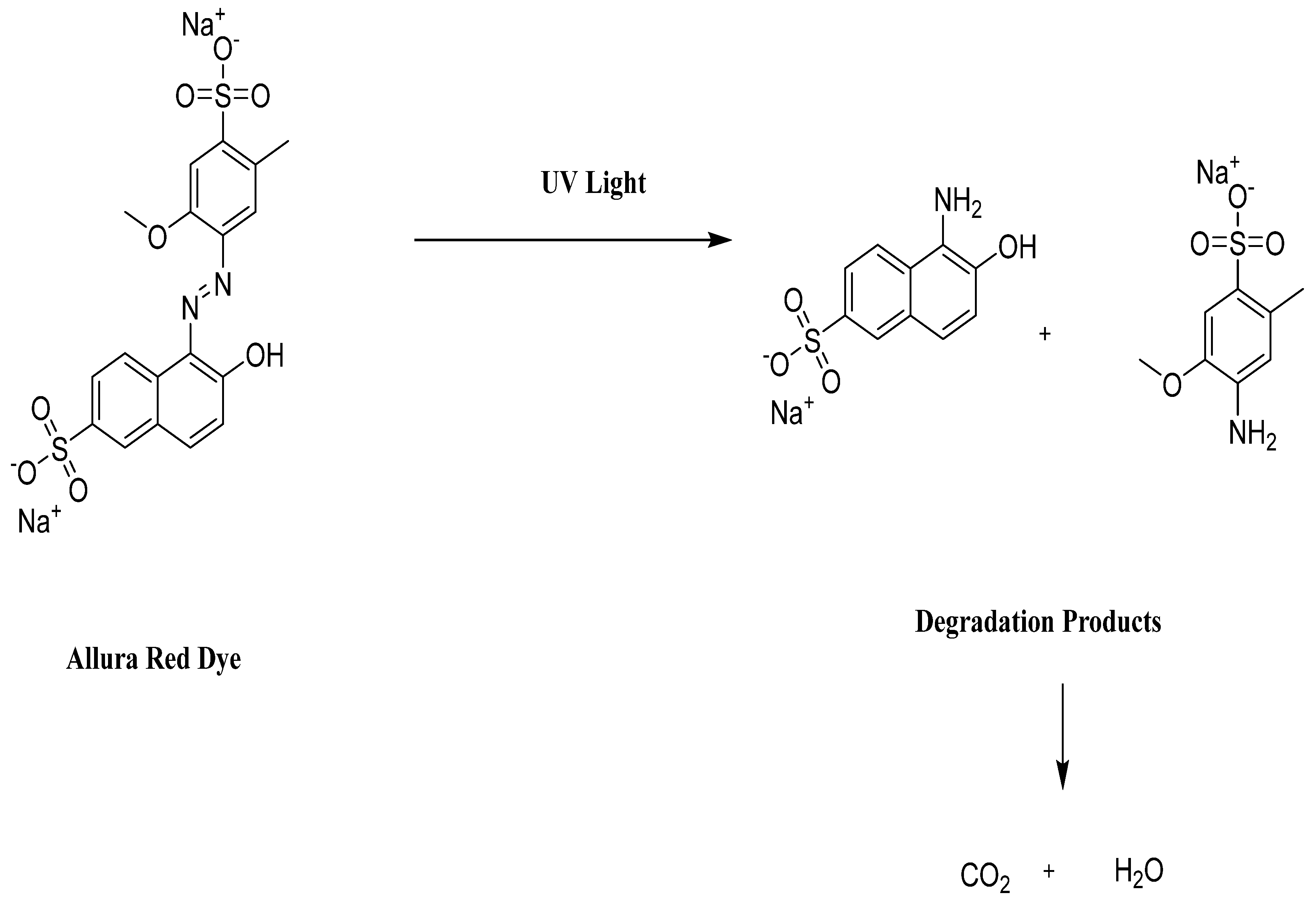
| S. No | Name of the Dye | Synonyms | Chemical Structure | Uses | Toxic Effects |
|---|---|---|---|---|---|
| 1 | Allura Red AC | Red 40 | 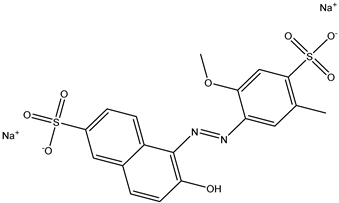 | used in candy, baked goods, cereals, and other processed foods [16,17]. | causes hyperactivity in children, DNA damage in animal studies, and may be carcinogenic [14,18]. |
| 2 | Tartrazine | Yellow 5 | 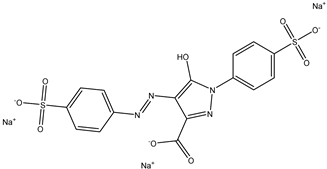 | used in processed foods, soft drinks, snacks, and candies [19]. | associated with allergic reactions, asthma, hyperactivity in children, kidney tumors in animal studies [20]. |
| 3 | Brilliant Blue FCF | Blue 1 |  | used in sports drinks, candies, baked goods, and other processed foods [21]. | causes hyperactivity in children, and it may be carcinogenic [22]. |
| 4 | Carmine | Red 4 | 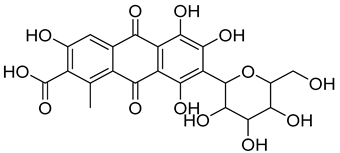 | used in many food products, including juices, ice cream, and baked goods [23]. | associated with allergic reactions and can cause severe allergic reactions in some individuals [24]. |
| 5 | Fast green FCF | Green 3 | 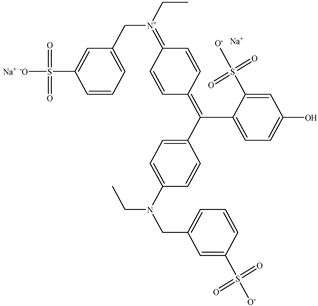 | used in processed foods, including candy, ice cream, and baked goods [25]. | affects bone marrow chromosomes in animal studies [26]. |
| 6 | Metanil yellow | Acid yellow 36 | 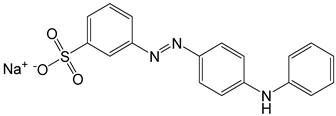 | used in a lot of basic food stuffs, sauces, spicy products, ice creams, cold drinks, and juices [21]. | mutagenic effects [27], causes damage to gastric mucosa, and often tends to be carcinogenic [28]. |
| S. No | Nanoparticles Synthesized | Plant Source | Dye Type | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|
| 1 | ZnO NPs | E. grandis leaves extract | Tartrazine yellow | 76.1% | [66] |
| 2 | Ag NPs | Aloe vera leaf extract | Tartrazine yellow | 55–60% | [67] |
| 3 | Ag NPs | Aloe vera leaf extract | Brilliant blue FCF | 95% | [67] |
| 4 | Ag NPs | Kalanchoe brasiliensis leaves extract | Carmine | 84.08% | [44] |
| 5 | Ag NPs | Flammulina velutipes as biosource | Carmine | 98.2% | [69] |
| 6 | Cu and Ag bimetallic nanocomposites | Citrus paradisi extract | Carmine | 100% | [70] |
| 7 | ZnO NPs | Phoenix dactylifera waste | Methylene blue and eosin yellow dyes | 90% | [71] |
| 8 | Fe2O3 NPs | pomegranate seeds extract | Reactive blue | 95.08% | [72] |
| 9 | Fe2O3 NPs | Carica papaya leaf extract | Remazol yellow RR dye | 76.6% | [73] |
| 10 | Cu-ZnO NPs | Synadium grantii leaf extract | Methylene blue | 91.3% | [68] |
| 11 | Cu-ZnO NPs | Synadium grantii leaf extract | Indigo carmine | 92.2% | [68] |
| 12 | Cu-ZnO NPs | Synadium grantii leaf extract | Rhodamine B | 90.1% | [68] |
| 13 | ZnO NPs | Alchemilla vulgaris (Lady’s mantle) leaves extract | Rhodamine B | 75% | [74] |
| 14 | Fe2O3 NPs | Artocarpus heterophyllus peel extract | Fuchsin Basic dye | 87.5% | [75] |
| 15 | Fe2O3 NPs | Teucrium polium leaf extract | Methyl orange | 73.6% | [76] |
| 16 | Ni@Fe3O4 and CuO NPs | Euphorbia maculata extract | Congo red | 88.8% | [61] |
| 17 | NiO NPs | Senna auriculata aqueous flower extract | Methylene blue | 90% | [77] |
| 18 | TiO2 NPs | mulberry plant extract | Methylene blue | 96% | [78] |
| 19 | Ag and Au NPs | Sargassum ilicifolium | Malachite green | 82.9% | [79] |
| 20 | MgO NPs | Camellia sinensis extract | Methylene blue | 97% | [80] |
| 21 | ZnO NPs | Areca catechu extract | Nigrosin | 96% | [81] |
| 22 | ZnO NPs | Areca catechu extract | Rhodamine B | 97% | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, K.A.; Shah, A.; Nisar, J.; Haleem, A.; Shah, I. Photocatalytic Degradation of Food and Juices Dyes via Photocatalytic Nanomaterials Synthesized through Green Synthetic Route: A Systematic Review. Molecules 2023, 28, 4600. https://doi.org/10.3390/molecules28124600
Khan KA, Shah A, Nisar J, Haleem A, Shah I. Photocatalytic Degradation of Food and Juices Dyes via Photocatalytic Nanomaterials Synthesized through Green Synthetic Route: A Systematic Review. Molecules. 2023; 28(12):4600. https://doi.org/10.3390/molecules28124600
Chicago/Turabian StyleKhan, Kashif Ali, Afzal Shah, Jan Nisar, Abdul Haleem, and Iltaf Shah. 2023. "Photocatalytic Degradation of Food and Juices Dyes via Photocatalytic Nanomaterials Synthesized through Green Synthetic Route: A Systematic Review" Molecules 28, no. 12: 4600. https://doi.org/10.3390/molecules28124600
APA StyleKhan, K. A., Shah, A., Nisar, J., Haleem, A., & Shah, I. (2023). Photocatalytic Degradation of Food and Juices Dyes via Photocatalytic Nanomaterials Synthesized through Green Synthetic Route: A Systematic Review. Molecules, 28(12), 4600. https://doi.org/10.3390/molecules28124600