Abstract
Photocatalytic conversion of carbon dioxide into chemical fuels offers a promising way to not only settle growing environmental problems but also provide a renewable energy source. In this study, through first-principles calculation, we found that the Se vacancy introduction can lead to the transition of physical-to-chemical CO2 adsorption on Janus WSSe nanotube. Se vacancies work at the adsorption site, which significantly improves the amount of transferred electrons at the interface, resulting in the enhanced electron orbital hybridization between adsorbents and substrates, and promising the high activity and selectivity for carbon dioxide reduction reaction (CO2RR). Under the condition of illumination, due to the adequate driving forces of photoexcited holes and electrons, oxygen generation reaction (OER) and CO2RR can occur spontaneously on the S and Se sides of the defective WSSe nanotube, respectively. The CO2 could be reduced into CH4, meanwhile, the O2 is produced by the water oxidation, which also provides the hydrogen and electron source for the CO2RR. Our finding reveals a candidate photocatalyst for obtaining efficient photocatalytic CO2 conversion.
1. Introduction
For the last few years, given the limitation of fossil fuel reserves and the growth of atmospheric CO2 levels, an urgent need has existed to create a sustainable option for converting unwanted CO2 into useful products in the form of chemicals and fuels [1,2,3], which will not only solve the greenhouse effect, melting glaciers, and other environmental problems caused by carbon dioxide, but also alleviate the current energy crisis [4]. The conversion of carbon dioxide could be operated through a variety of pathways, including biochemical [5], electrochemical [6,7], photochemical [8,9], and thermochemical [10] reactions. As sunlight is a theoretically unlimited power source, solar-powered CO2 reduction can be perceived as the best option among these promising approaches [11,12]. Until now, photocatalytic CO2RR has attracted great attentions and achieved many results [13,14,15,16]. Photocatalysis is widely believed to have three primary key steps, i.e., sunlight harvesting by the semiconductor (hν > Eg), photo-generated carrier separation and transport, and reactions on the surface [17,18,19,20]. While many solar active catalysts for CO2 photoreduction have been reported, they mostly suffer from instability, poor energy conversion rates, non-controllable selectivity, and failure to fully inhibit competing hydrogen evolution reactions (HER) in existence with water [21,22]. Consequently, it remains a great priority to design high-activity photocatalysts for CO2 reduction with great conversion efficiency and selectivity.
Soon after the Janus-structured MoSSe monolayer was fabricated by a modified chemical vapor deposition (CVD) method based on the sulfidation of MoSe2 monolayer [23] and the selenization of the MoS2 monolayer [24], the two-dimensional (2D) Janus transition metal dichalcogenides, such as Janus MoSSe and WSSe, have become candidates with great potential application for photocatalysis because of their excellent optical absorption, suitable band edge positions, and high carrier separation [19,23,24,25,26]. Our previous work demonstrated tha, the tubular Janus WSSe, obtained by rolling the planar Janus WSSe with an acceptable strain energy, possesses an enhanced electrostatic potential difference between the Se and S layers, resulting in a stronger built-in electric field than the planar structure. The stronger built-in electric field usually could help to strengthen the adsorption of small gas molecules, even to activate them. For CO2RR, due to the inertness of the CO2 gas molecule induced by the strong C=O bonds, effective activation of the CO2 molecule is key for the subsequent reduction. Therefore, to explore the photocatalytic CO2RR performance of the Janus WSSe nanotube is meaningful for developing a highly efficient photocatalyst.
In our research, the CO2 adsorption on the Janus WSSe nanotube in pristine and defective states had been studied using DFT calculations. Adsorption energy (), charge density difference (CDD), and density of state (DOS) were employed to explain the coupling between the substrate and adsorbate. It was found that the introduction of Se vacancies on Janus WSSe could brilliantly change the physical adsorption of CO2 into chemical adsorption, which effectively activates the CO2 gas molecules and makes CO2RR possible. The semiconducting property of the defective Janus WSSe nanotube is confirmed by the electronic band structure. We studied its photocatalytic CO2RR performance by analyzing the absorption spectrum, redox capacity, and reaction driving force of photo-excited carriers. Furthermore, in order to keep the CO2RR sustained and stable, we also consider the OER reaction on the S side of the defective Janus WSSe nanotube. Furthermore, competition from the CO2RR and hydrogen evolution reaction (HER) is addressed. We found that the defective WSSe nanotubes have excellent photocatalytic properties and can serve as a hopeful photocatalyst for light-driven CO2 reduction.
2. Results and Discussion
2.1. The CO2 Adsorption on Pristine Janus WSSe Nanotube
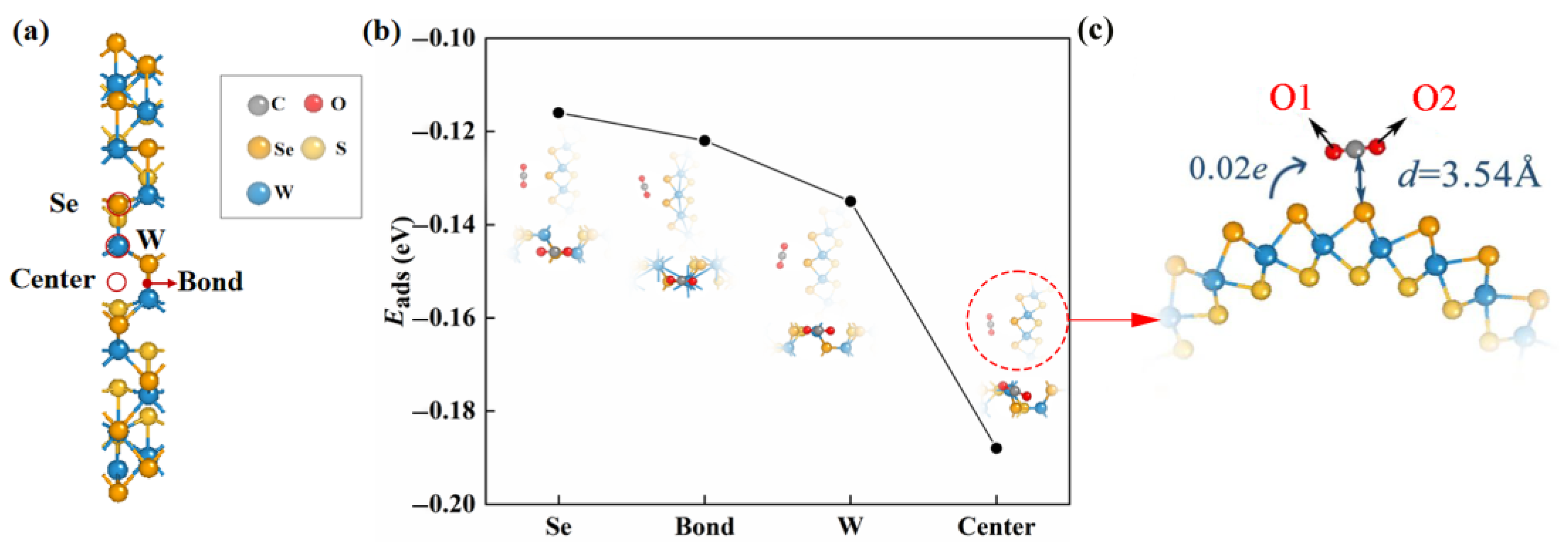
Janus WSSe nanotubes are constructed by scrolling Janus WSSe monolayers, whereby the W layer is interposed between the Se and S layers. Our previous work reported that the strain energy for the formation (0.10 eV/atom) of the Janus WSSe nanotubes with a structure of Se layer on the outside and S layer on the inside is lower than the one (0.23 eV/atom) with a contrary structure, indicating relatively more stability [27]. Herein, we chose the (12, 12) armchair Janus WSSe nanotube as the substrate in the adsorption system. As shown in Figure S1, the diameter is 21.86 Å and the height of Se-S is 3.22 Å. The W-S and W-Se bond lengths are 2.38 and 2.60 Å, respectively, separately a little shorter and larger than the corresponding ones (2.41 and 2.52 Å) in the planar structure [28]. In this study, we only considered the CO2 adsorption on the outer side (Se side) of the nanotube, and the case of the adsorption on the inner side is neglected because the CO2 gas molecules are difficult to pass through the nanotube walls to arrive on the inner side (the barrier is up to 28.33 eV, see Figure S2). We put a CO2 gas molecule on the Se side of the nanotube to build the adsorption system and completely relax it. As shown in Figure 1, there are four adsorption sites taken into consideration, namely center (above center of the hexagon), bond (above W-Se bond), and W/Se (above W/Se atom).
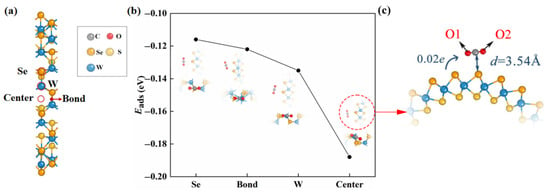
Figure 1.
(a) The adsorption sites (marked with red circles) in consideration of the pristine Janus WSSe nanotube. (b) The adsorption energy as well as the top (upper) and side (lower) views of the optimized configurations of CO2 gas molecule adsorbing on pristine WSSe nanotube with different adsorption sites. The gray, red, orange, yellow, and blue balls each represent C, O, Se, S, and W atoms. (c) The enlarged view for the top view of center adsorption site optimized structure. The adsorption distance between the substrate and the adsorbate is represented by the dark blue, d. Transfer of charge from substrate to CO2 molecule identified by the blue arrow.
According to Equation (1), we obtained Eads values of various adsorption sites, which were used to explore the most stable adsorption configuration. As shown in Figure 1b, we found that the Eads arrived the smallest (−0.19 eV) when the adsorbed CO2 gas molecule was located at the center site, which was the most stable adsorption configuration. The small absolute value of Eads of this adsorption configuration revealed that the adsorption is physical adsorption (usually, eV [29,30,31,32]).
We studied the mechanism of the CO2 physisorption on the pristine Janus WSSe nanotube in detail based on the adsorption distance and Bader charge results. The CO2 gas molecule kept the linear morphology after adsorption (see Figure 1c), and the distance from the C atom of the CO2 gas molecule to its nearest Se atom of the pristine Janus WSSe nanotube is as high as 3.54 Å, which greatly exceeds the Se-C bond length (2.29 Å). In addition, the amount of transfer electron, moving from the pristine Janus WSSe nanotube to the CO2 molecule, is only 0.02 e, indicating the weak interaction between the substrate and the CO2 molecule.
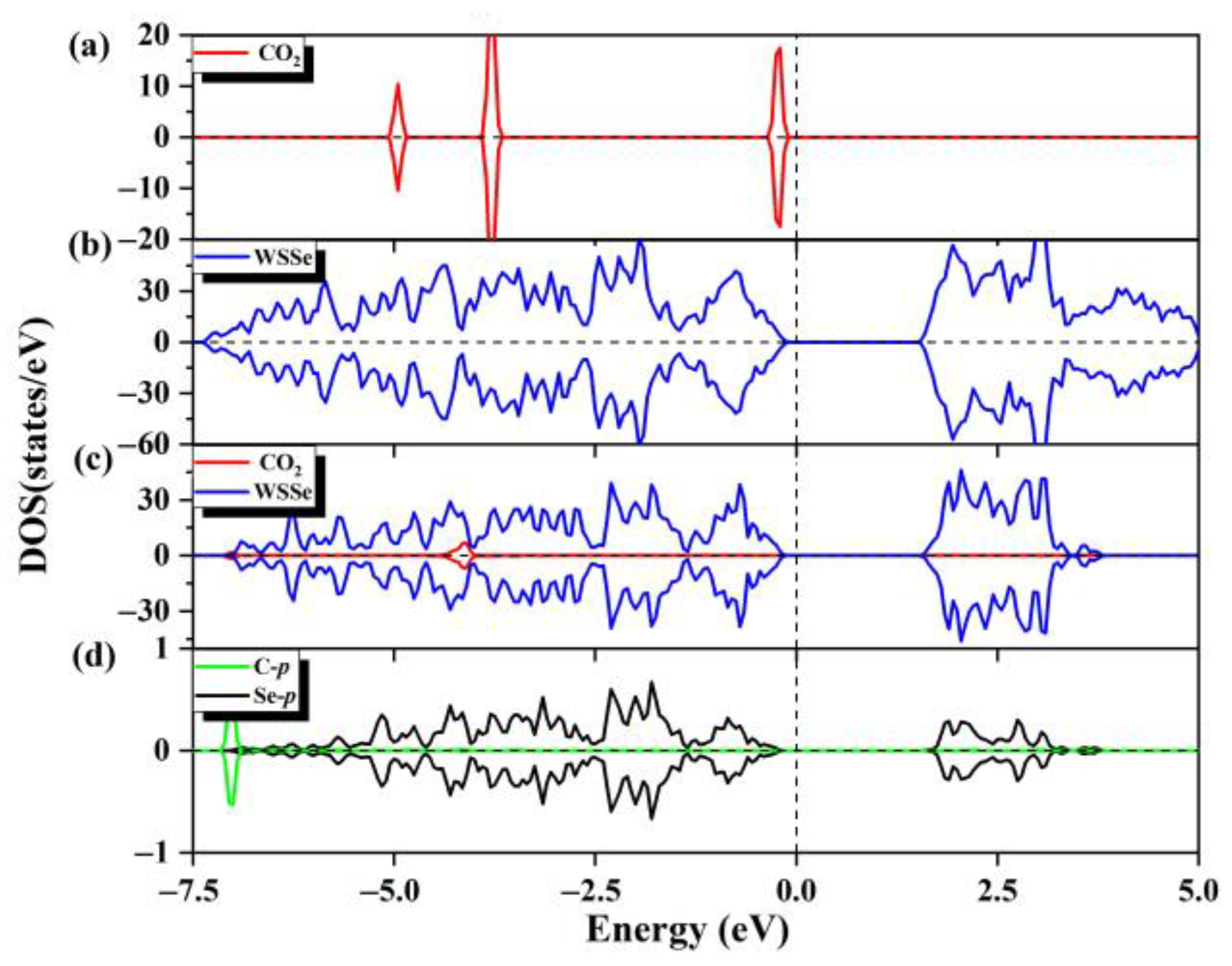
At the same time, we also calculated the DOS values of the adsorption configurations. As can be seen from Figure 2a–c, the projected DOS of the WSSe nanotube has a negligible change compared with those of the corresponding pristine WSSe nanotube, indicating that the electronic properties of the WSSe nanotube remain. However, there is a significant difference of the DOS between the adsorbed gas molecule and the pristine gas molecule, which is due to charge rearrangement after adsorption; that is, the O atoms gain electrons, while the C atom loses electrons (as listed in Table S1). The little orbital hybridization between the WSSe nanotube and CO2, mainly composed of the Se p and CO2 O p orbitals, is consistent with the tiny interfacial electron transfer, demonstrating that the interaction between the WSSe nanotube and molecules is weak. According to the above analysis, it can be determined that the adsorption of CO2 by the pristine WSSe nanotube is physisorption.
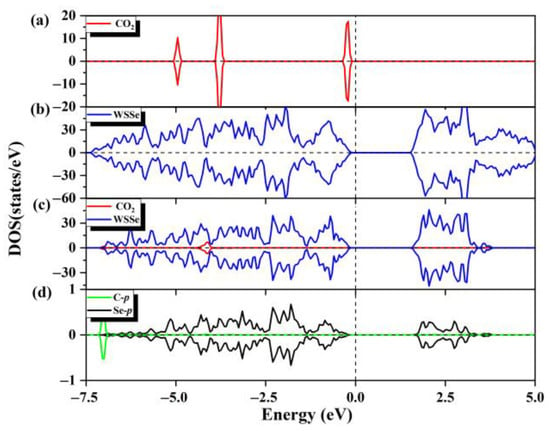
Figure 2.
(a) The total state density of the pristine CO2 gas molecules and (b) the total state density of the pristine WSSe nanotube. (c) The partial state density of the adsorption system, where WSSe nanotube is shown in dark blue and CO2 is shown in red. (d) Partial state densities of the C p orbitals (cyan) of the adsorbed CO2 gas molecule and the Se p orbitals (black) of the Se atom most nearby the adsorbed CO2 molecule. Fermi level is expressed by the vertical dashed line.
2.2. The CO2 Adsorption on Defective Janus Wsse Nanotube
The pristine WSSe nanotube can be used as a gas collection system for physical CO2 adsorption. However, in order to convert the CO2 gas into value-added industrial raw materials through chemical reactions, chemical adsorption of CO2 is required, which requires the substrate to have a stronger adsorption capacity. Our earlier results have reported that introducing vacancy defects could effectively improve the stability of the geometric structures for some gas adsorption systems, making the adsorption capacity of the substrate increase [33,34,35].
Since the CO2 is more easily adsorbed on the Se side of WSSe nanotube, hereby, we applied the Se vacancy defects into the Janus WSSe nanotube to enhance its CO2 adsorption capacity, which also has been demonstrated to be more easily formed than the S and W vacancy defects in the WSSe layered material [33]. Based on the analysis on the elastic modulus, we find that a low Se vacancy concentration does not affect the mechanical property of the Janus WSSe nanotube drastically. (More details can be found in the Supporting Information and Figure S3). The calculated of CO2 molecule adsorbing on defective Janus WSSe nanotube is −1.41 eV, greatly exceeding the one (−0.19 eV) on the pristine Janus WSSe nanotube, indicating that the introduction of Se vacancy strengthens the CO2 adsorption. More interesting, as displayed in Figure 3a, the adsorbed CO2 molecule undergoes an obvious deformation from the initial linear shape into the bending one (∠OCO = 114.17°). Additionally, one of the C=O bonds in the adsorbed CO2 molecule (C-O2 bond) transforms into the C-O bond, and the C and O2 atoms bond to different W atoms, respectively. The obvious deformation demonstrates that the CO2 molecule could be activated by the defective Janus WSSe nanotube. However, the defective planar WSSe monolayer does not have such high activity. The adsorbed CO2 molecule on the defective planar WSSe monolayer keeps its linear shape (see Figure S4), and the adsorption energy in this case is only −0.20 eV. This phenomenon can be explained by the following reasons: (I) bending the planar structure allows more of the W atom area to be exposed, enlarging the contact surface of the CO2 molecule on the W atom; (II) the W atoms near the Se vacancy in the tubular structure WSSe have more electrons (0.15 e/atom) than the ones in the planar structure, according to the Bader charge results, which leads to easier electron transfer from W atoms on the WSSe nanotubes to the CO2 molecule and facilitates the formation of strong bonds.
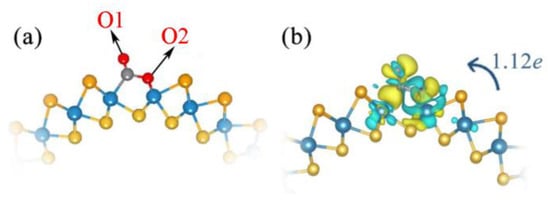
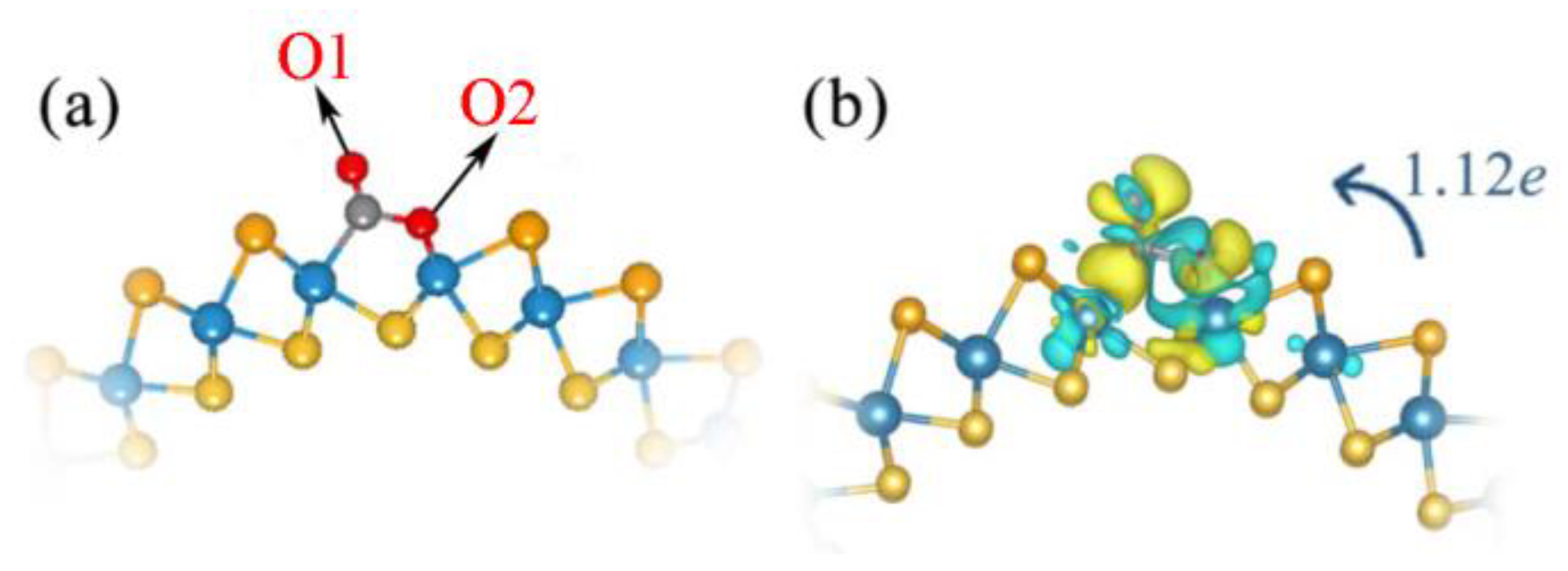
Figure 3.
Top view (a) of the optimized structure and CDD (b) of Janus WSSe nanotube with Se vacancy adsorbed CO2 gas molecules. Cyan (yellow) areas indicate charge depletion (accumulation). The isosurface level is 0.002 eÅ−3. Transfer of charge from substrate to CO2 molecule identified by the blue arrow.
In the following, we further discuss the enhanced adsorption of CO2 on WSSe nanotubes with the introduction of Se vacancies, from the aspects of CDD, electron transfer, and DOS. As mentioned before, after CO2 adsorption at the Se vacancy site, C and O2 atoms separately bond to W atoms. As plotted in Figure 3b, the electron transfer amount from the defective Janus WSSe nanotube to the CO2 molecules is up to 1.12 e. The formation of C-W and O-W bonds indicate that on the defective Janus WSSe nanotube, the CO2 adsorption is chemical adsorption.
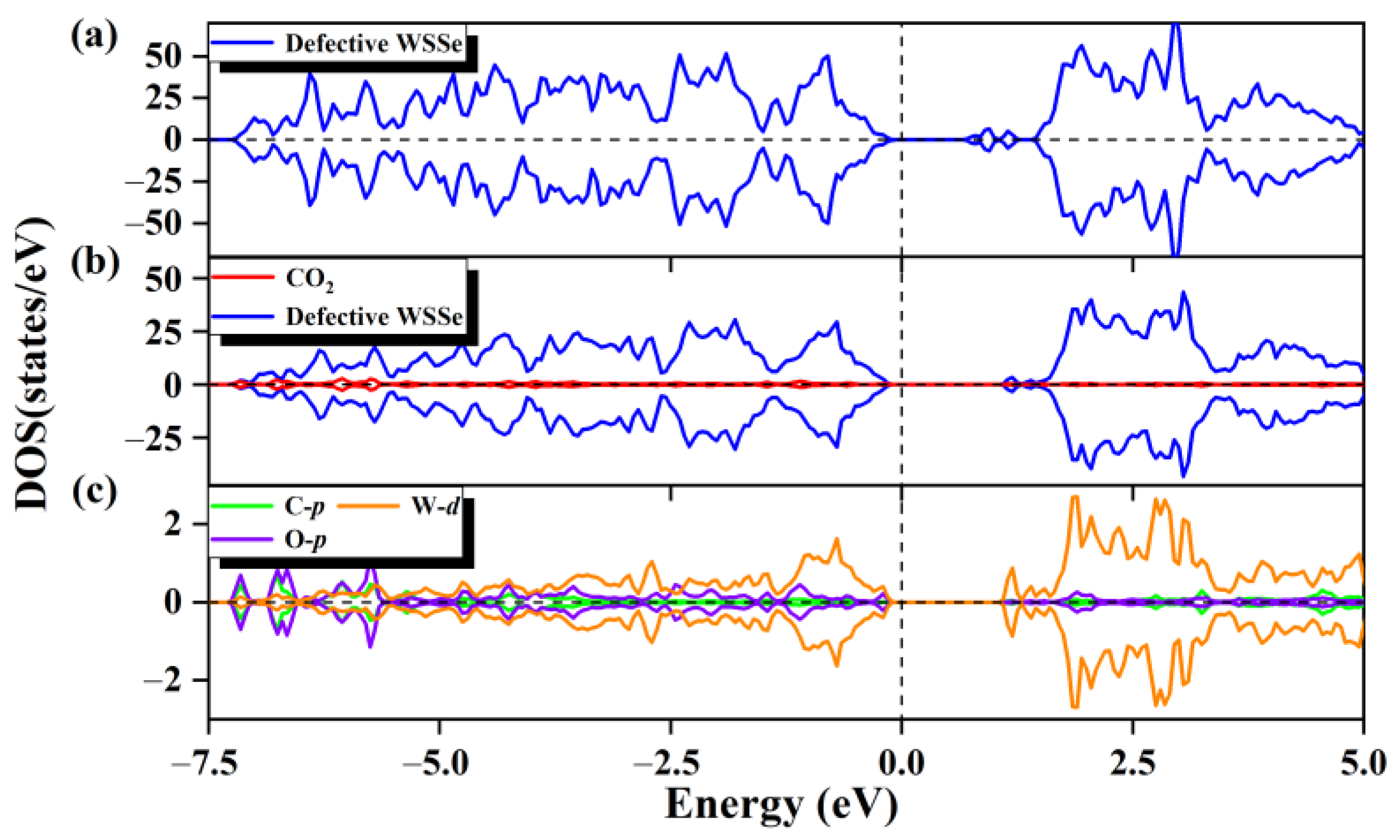
For the purpose of understanding the electronic origin of the chemisorption on the defective Janus WSSe nanotube, its corresponding DOS is calculated. For the defective Janus WSSe nanotube, its conduction band maximum (CBM) rises to a high level after the CO2 adsorption (see Figure 4a,b), which corresponds to the Bader charge result that the defective Janus WSSe nanotube loses 1.12 e. In addition, as shown in Figure 4c, there is an obvious orbital hybridization between the CO2 molecule and the defective Janus WSSe nanotube, which is mainly contributed by the O-p and C-p orbitals from the adsorbed molecule as well as the W-d orbitals from the W atoms in substrate bonding to the C and O2 atoms. This explains the phenomenon that the CO2 gas molecule is tightly attached to the defective Janus WSSe nanotube through the C-W and O-W bonds. In addition, the DOS of the CO2 molecules pre- and post-adsorption (see Figure 2a and Figure S5) shows that an obvious delocalization of DOS occurs after adsorption, which means a severe electron redistribution in the adsorbed CO2 gas molecule, caused by the gained electrons from the substrate. The results above provide more evidence that the adsorption of CO2 by the defective WSSe nanotubes is chemisorption. In other words, the introduction of Se vacancy can well convert the physical adsorption of CO2 into chemical adsorption on the Janus WSSe nanotube.
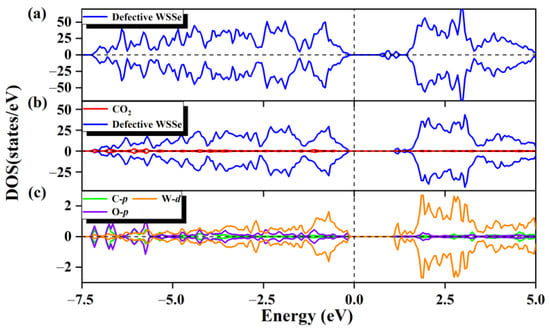
Figure 4.
The total state density of WSSe nanotube with Se vacancy (a). The partial state density (b) of the adsorption system, the defective WSSe nanotubes are shown in dark blue, and CO2 is shown in red. (c) Partial state densities of adsorbed CO2 gas molecules in C p orbitals (cyan), O p orbitals (purple), and W d orbitals of two W atoms in substrate (orange). The vertical dashed line shows the Fermi level.
2.3. Photocatalytic Performance of Defective WSSe Nanotube for CO2RR
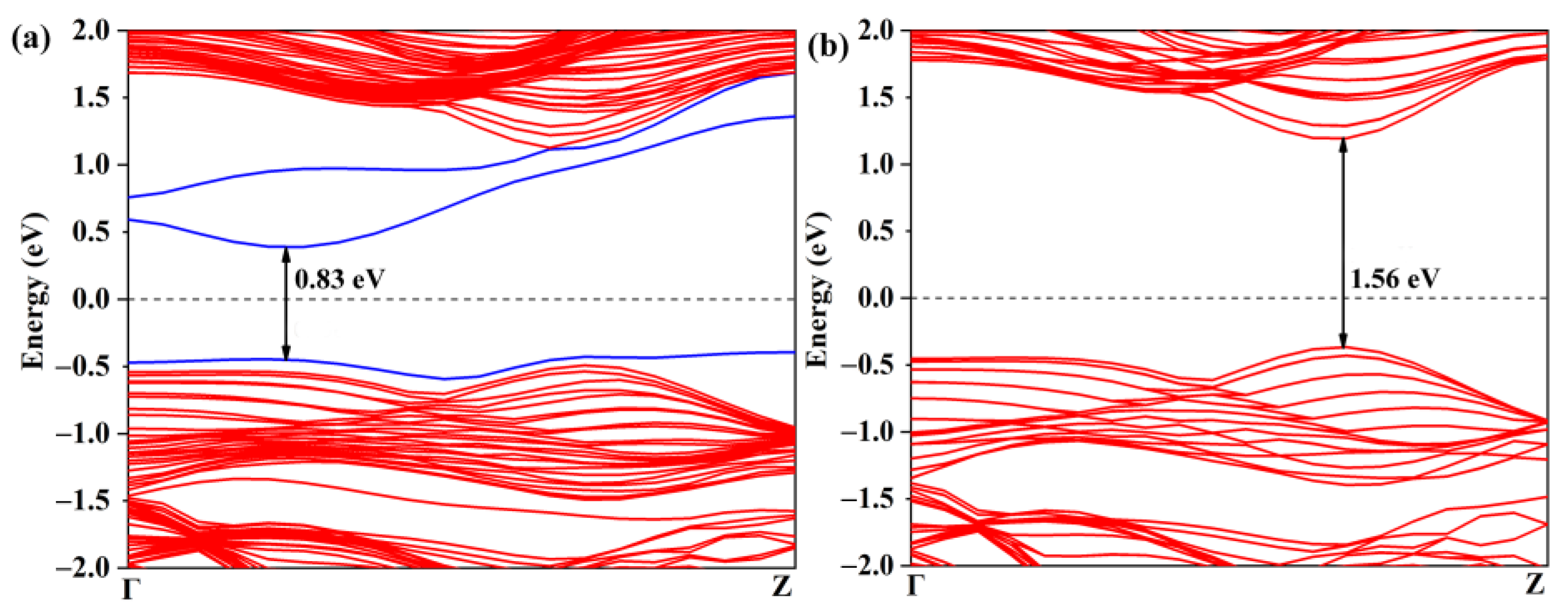
The activation of the CO2 gas molecule on the defective WSSe nanotube makes the further catalytic CO2 reduction reaction possible. As displayed in Figure 5a, though the Se vacancy bring about some gap states, the defective Janus WSSe nanotube still keeps the semiconductor character with a narrower band gap of 0.83 eV (the band gap of the pristine Janus WSSe nanotube is 1.56 eV, see Figure 5b). In the following, we studied the photocatalytic performance of the defective Janus WSSe nanotube.
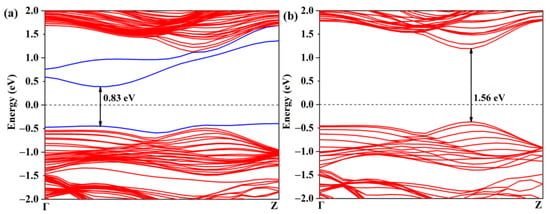
Figure 5.
Band structures of (a) defective and (b) pristine Janus WSSe nanotubes. The gap states caused by the Se vacancy are marked with blue lines. The black number represents the value of band gap.
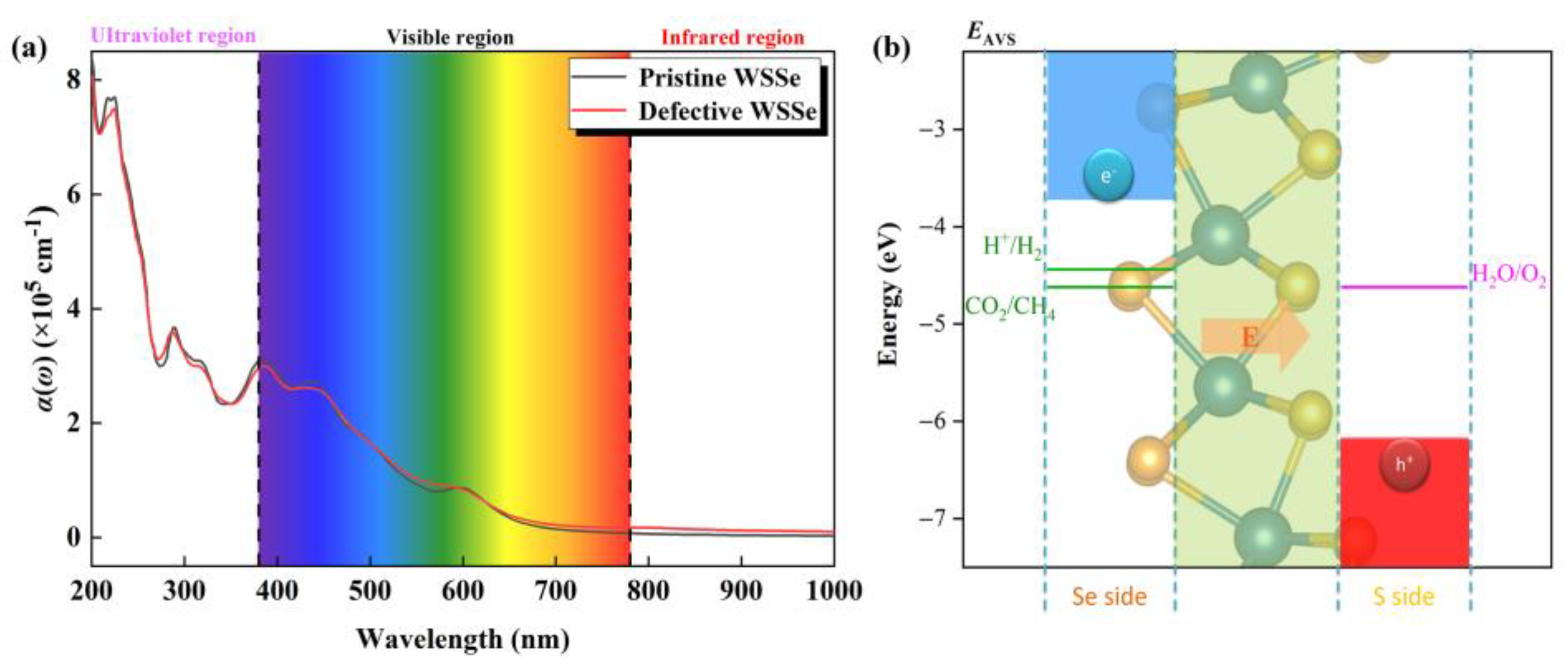
In order to initiate the photocatalytic conversion of CO2, an efficient photocatalyst must have a high photo-conversion efficiency. As shown in Figure 6a, there are several significant light absorption peaks (over 105 cm−1) among the visible light area for the pristine and defective Janus WSSe nanotubes, indicating they are promising catalyst candidates with visible-light responses. The highest absorption peak in the visible area for the pristine and defective Janus WSSe nanotubes arrive 3.10 × 105 cm−1 (at 380.00 nm, black line) and 2.96 × 105 cm−1 (at 380.00 nm, red line), which exceed the one of the planar Janus WSSe (1.30 × 105 cm−1 at 466.28 nm) [34] and are on par with some reported photocatalysts, namely, MoSSe/graphene (4.00 × 105 cm−1 at 500 nm) [36] and MoSSe/AlN (3.95 × 105 cm−1 at 412 nm) [37]. Although the difference between the light absorption spectra of the pristine and defective Janus WSSe nanotubes are not significant, as displayed in Figure S6, in the infrared and visible regions, the optical absorption coefficient of the defective Janus WSSe nanotube is higher than the one of the pristine Janus WSSe nanotube, which is consistent with the fact that the defective Janus WSSe nanotube has a smaller band gap than the pristine one. The non-zero absorbance value in the infrared region (IR) of the defective WSSe nanotube ensures the utilization of IR photons. Therefore, the introduction of Se vacancy defects makes the Janus WSSe nanotube use photons in a relatively larger energy range. Additionally, the negligible difference of light absorption spectra between these two kinds of nanotubes may be caused by the fact that the gap states are too weak in the defective Janus WSSe, where the concentration of Se vacancy is too low (just 4.17%). In the visible region, the reported optical absorption coefficient of defective Janus WSSe monolayer with a higher concentration of Se vacancy (6.25%) is more obviously higher than the pristine Janus WSSe monolayer [34], which agrees well with the results of nanotubes.
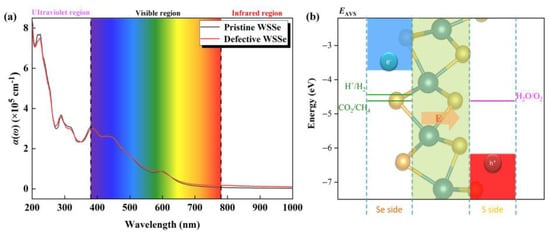
Figure 6.
(a) Optical absorbance of pristine and defective Janus WSSe nanotubes. (b) Schematic diagram of band edge position of Janus WSSe nanotube relative to normal hydrogen electrode (NHE) at pH = 0. EAVS represents the energy level relative to the absolute vacuum scale (AVS). The pink arrow represents the orientation of the built-in electric field.
In order for a semiconductor to be active for photo-reduction of CO2, the band edges must be aligned with the potentials of the reduction half-reactions [38]. On top of that, its band edge also needs to satisfy the oxidation potential of H2O/O2 because the oxygen evolution reaction (OER) could consume the redundant photo-excited holes and provide the necessary H+ + e− pair simultaneously. As shown in Figure 6b, the CBM in the photocatalytic redox capacity is above the CO2/CH4 reduction potential, and the VBM is below the H2O/O2 oxidation potential, indicating that the WSSe nanotubes have sufficient redox capacity for both photocatalytic CO2RR and OER. Furthermore, our previous work pointed out that [27] the dipole caused by the structural asymmetry introduces a built-in electric field with the direction from the Se layer to the S layer (see the pink arrow in Figure 6b). In this case, the photoexcited electron and hole will run fast in opposite directions, causing high spatial separation of the electron–hole pairs, which surely suppresses the recombination of photoexcited carriers.
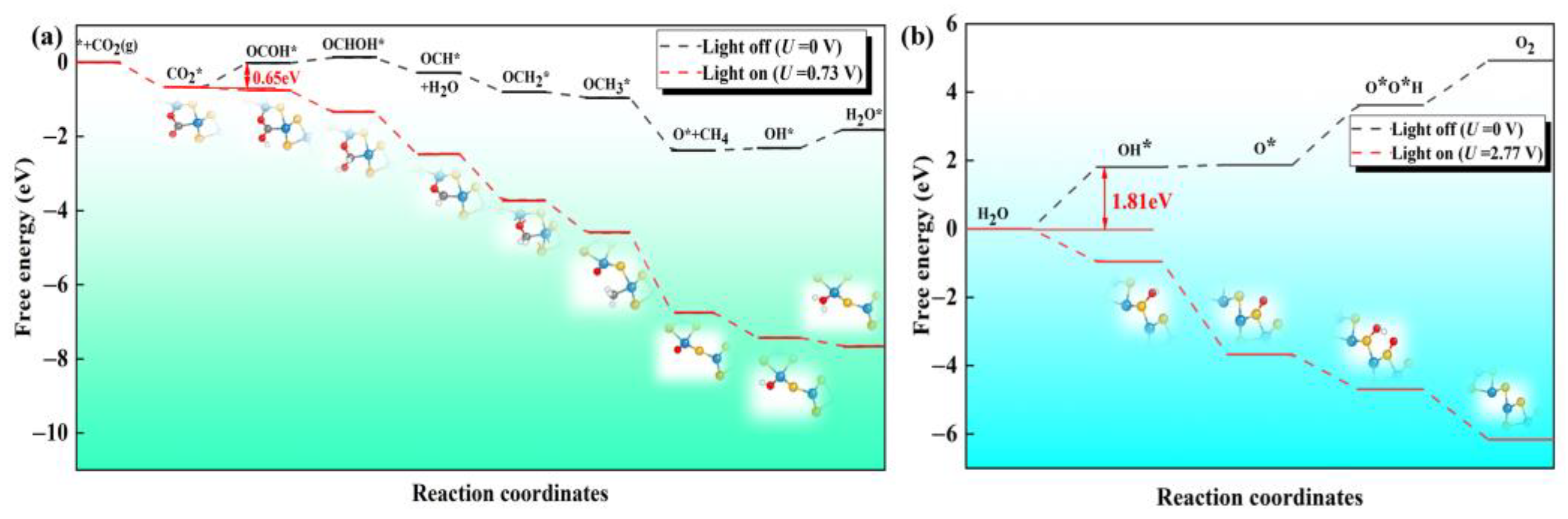
Next, we explore whether the reaction can be spontaneous under dynamic conditions. The case without any external potential (U = 0 V) is used to simulate the condition in darkness. We first screen the favorable reaction path of CO2RR on the defective Janus WSSe nanotube (see Figure S7). The CO2RR-to-CH4 process involves eight proton-coupled electron transfer steps (CO2 + 8H+ + 8e− → 2H2O + CH4). The free energy diagram and the corresponding intermediates for the CO2RR-to-CH4 are shown in Figure 7a. The most possible path is CO2 * → OCOH * → OCHOH * → OCH * → OCH2 * → OCH3 * → O * →OH * → H2O *. The electrocatalytic steps, i.e., OCHOH * → OCH *, OCH * → OCH2 *, OCH2 * → OCH3 *, and OCH3 * → O *, are exothermic by −0.41, −0.51, −0.15, and −1.43 eV, respectively; meanwhile, the other hydrogenation steps, i.e., CO2 * → OCOH *, OCOH * → OCHOH *, O * → OH *, and OH * → H2O *, are endothermic by 0.65, 0.15, 0.06, and 0.50 eV, respectively. The formation of OCOH * is the potential determining step (PDS) with a limiting potential (Ul) of −0.65 V. At the same time, we also investigated the OER process on the S side of the defective Janus WSSe nanotube along the 4 e transfer pathway, i.e., H2O → OH * → OOH * → O2 (see Figure 7b) [18,27]. The free energy changes (ΔG) for the four different steps are endothermic by 1.81, 0.06, 1.75, and 1.30 eV, respectively. The formation of OH * is the PDS with a Ul of −1.81 V.
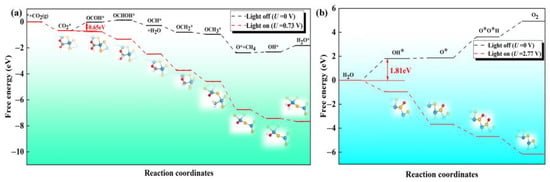
Figure 7.
The Gibbs free energy diagrams for the (a) 8 e pathway of CO2RR and (b) 4 e pathway of OER on the defective Janus WSSe nanotube under different light conditions. The extra potentials provided by photogenerated electrons and holes are 0.73 and 2.77 V, respectively.
According to the free energy calculations mentioned above, it could be found that both the CO2RR and OER have endothermic steps; thus, they could not take place spontaneously without photo-irradiation. However, the high enough external potential supplied by the photo-excited carriers helps to overcome the Ul of these redox half-reactions, making the redox half-reactions proceed spontaneously [39]. The extra potential of the photogenerated electrons/holes (Ue/Uh) is defined as the energy difference between H+/H2 reduction potential and the CBM/VBM [18,39,40,41]. According to our previous work [27], the Ue and Uh of the defective Janus WSSe nanotube at pH = 0 are 0.73 and 2.77 V, respectively, which are sufficient enough to separately cover the Ul of CO2RR and OER. Therefore, in consideration of Ue and Uh, all the reduction and oxidation steps become downhill (red dash lines in Figure 7a,b). That is to say, under the light irradiation, both CO2RR and OER can operate spontaneously.
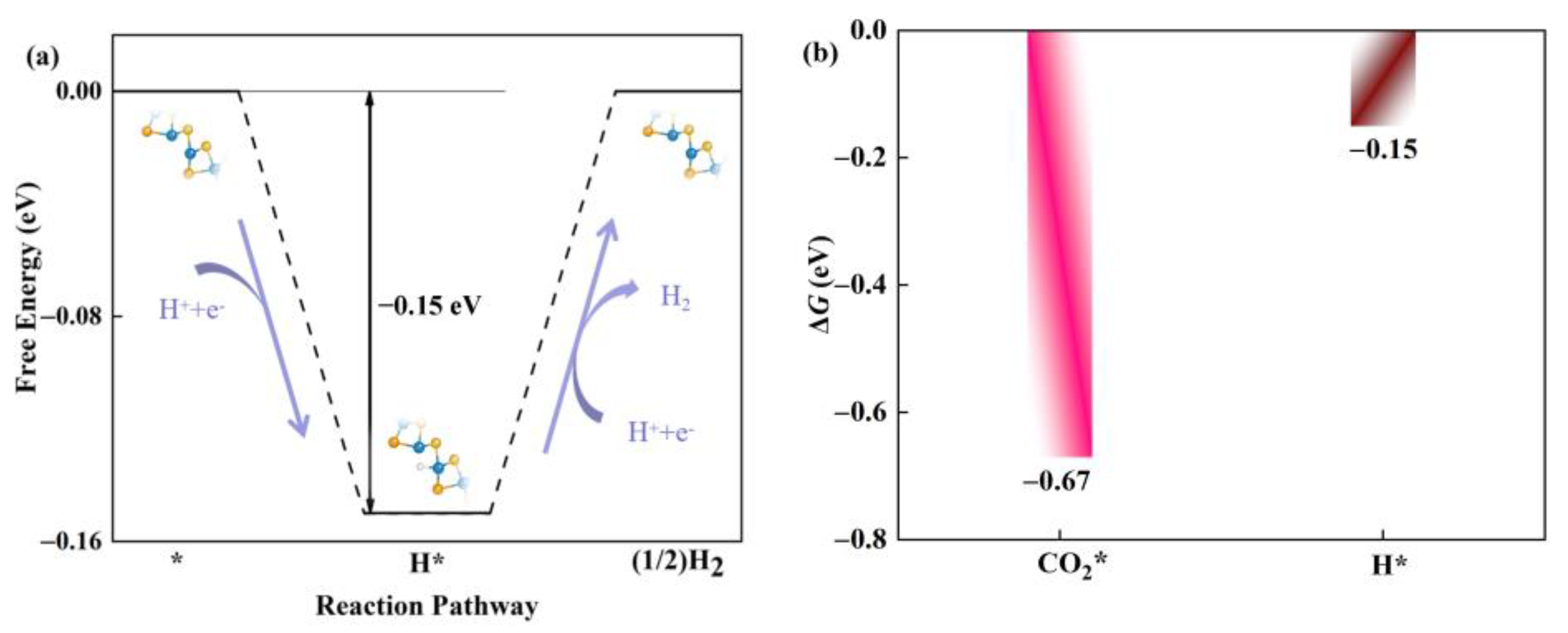
Usually, the hydrogen evolution reaction (HER) is considered to be an important competitive side reaction in the catalytic CO2RR [42,43]. Next, we investigated the competitive relationship between CO2RR and HER in the defective Janus WSSe nanotubes. Based on the Brønsted-Evans-Polanyi relation [44,45], the reaction with lower Gibbs, ΔG, values has a smaller reaction barrier; thus, it is more favorable for kinetics. Accordingly, the ΔG for H * formation energy (ΔGH*) is calculated (Figure 8a) and compared with the one for CO2 * formation energy (ΔGCO2*). As shown in Figure 8b, ΔGCO2* (−0.67 eV) is more negative than ΔGH* (−0.15 eV), which ensures that the active sites are preferred to be occupied by CO2 *. Therefore, the defective Janus WSSe nanotube is more selective for CO2RR over HER.
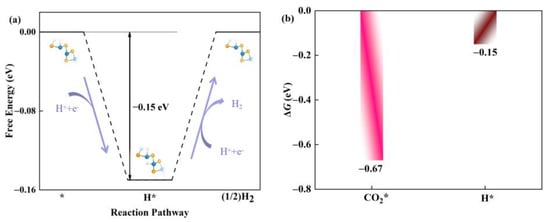
Figure 8.
(a) Gibbs free energy diagram of HER on defective Janus WSSe nanotube. (b) ΔGco2* (pink bar) vs. ΔGH* (brown bar) of defective Janus WSSe nanotube. * means the adsorption site.
3. Computational Methods
In our work, all the computational models are constructed with the DeviceStudio software [46]. In addition, the Geometric relaxation and electronic structure were conducted based on DFT simulations employing DS-PAW software [47]. The exchange-correlation energy of Perdew–Burke–Ernzerhof (PBE) was employed [48]. To depict the van der Waals (vdW) coupling in the adsorption system, we used the zero-damping DFT-D3 method suggested from Grimme [49]. All internal coordinates with fixed lattice constants were permitted to relax during the optimization process. The sampling integration of the Brillouin zone was performed in accordance with the Monkhorst-Pack scheme [50], and the structure optimization and electronic properties are calculated with a 1 × 1 × 4 K-point. The value of 500 eV was chosen as the cutoff energy of plane-wave basis. We set the periodic boundary condition along the z-axis and put more than 10.8 Å vacuum spaces along the x and y directions to avoid the interaction between adjacent nanotubes. Periodic boundary conditions were set on the z-axis and a vacuum space of more than 10.8 Å was applied on the x- and y-axes to evade adjacent nanotubes from interacting with each other. Moreover, the ΔG of CO2RR and OER were calculated using the computational hydrogen electrode (CHE) model [51]. Additional details of the Gibbs free energy simulations are available in the Supporting Information.
The of the CO2 on the WSSe nanotube was obtained from the following equation [52,53],
where was the total energy of the adsorption system, while and separately were the total energies of the isolated CO2 molecule and the clean Janus WSSe nanotube. A higher negative indicated a more favorable exothermic adsorption.
The plane-integrated CDD was carried out in accordance with the equation,
where , , and separately were the charge density of the adsorption system, adsorbed CO2 molecule, and substrate.
The absorption coefficient, , used to estimate the solar energy gathering capacity was given by the following equation [27],
where ε1 and ε2 frequently were the real and imaginary parts of the frequency-dependent dielectric function, while c was the speed of light under vacuum.
4. Conclusions
In this paper, based on the first-principles calculations, we investigate the performance of the defective Janus WSSe nanotube for the photocatalytic CO2RR. The introduction of Se vacancy could significantly increase the amount of interfacial transferred electrons and lead to obvious electron orbital hybridization between adsorbates and substrates, making the CO2 adsorption on the Janus WSSe nanotube transform into chemisorption from physisorption. Strong chemisorption enables defective Janus WSSe nanotubes to be highly active and selective against CO2RR. In addition, the extra potential from photo-produced carriers is high enough to trigger spontaneous CO2RR and OER simultaneously on the defective Janus WSSe nanotube. For the first time, our work theoretically predicts the high photocatalytic performance of the defective Janus WSSe nanotube on CO2RR, which promisingly will stimulate extensive interests from material science and chemistry communities to realize our vision.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28124602/s1, Figure S1: Diameter, W-S bond length, W-S bond length and Se-S height of the pristine Janus WSSe nanotube; Figure S2: The relative adsorption energy and the location for the CO2 gas molecule passing through the pristine WSSe nanotube wall; Figure S3: Relative value of total energy variations as well as their corresponding fittings for the pristine and defective Janus WSSe nanotubes with respect to strain ε along the tube axis; Figure S4: Top view and side view of CO2 gas molecules adsorbed at the Se vacancy of Janus WSSe monolayer; Figure S5: The enlarged view for the partial density of states of CO2 portion from the adsorption system; Figure S6: The enlarged view the optical absorbance of pristine and defective Janus WSSe nanotubes at wavelength of 500–900 nm; Figure S7: The search process for the minimum energy reaction pathways of the CO2 reduction reactions on defective Janus WSSe nanotube; Table S1: The amount of charge transfer for C and O atoms of CO2 gas molecules adsorbed in pristine and defective Janus WSSe nanotubes, respectively; Free energy difference in the CO2RR and OER; Table S2: Zero-pint energy correction and entropy contribution of molecules and adsorbates in this study. Refs. [51,54] are cited in Supplementary Materials.
Author Contributions
Software, H.D. and S.Y.; Validation, J.L. and Y.G.; Formal analysis, L.J. and X.T.; Investigation, X.T., J.L., Y.G. and W.L.; Resources, L.J.; Data curation, X.T. and H.D.; Writing—original draft, L.J., X.T. and J.L.; Supervision, L.J.; Funding acquisition, L.J. All authors have read and agreed to the published version of the manuscript.
Funding
Our work is funded by the Natural Science Foundation of Henan Province (Grant No. 232300420128), National College Students Innovation and Entrepreneurship Training Program (Grant No. 202210479032), Open Project of Key Laboratory of Functional Materials and Devices for Informatics of Anhui Higher Education Institutes (Grant No. FSKFKT002), College Students Innovation Fund of Anyang Normal University (Grant No. 202210479049), and Key Scientific and Technological Projects in Anyang City (Grant No. 2022C01GX019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Gattrell, M.; Gupta, N.; Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Gattrell, M.; Gupta, N.; Co, A. Electrochemical reduction of CO2 to hydrocarbons to store renewable electrical energy and upgrade biogas. Energy Convers. Manag. 2007, 48, 1255–1265. [Google Scholar] [CrossRef]
- Wageh, S.; Al-Hartomy, O.A.; Alotaibi, M.F.; Liu, L.-J. Ionized cocatalyst to promote CO2 photoreduction activity over core–triple-shell ZnO hollow spheres. Rare Met. 2022, 41, 1077–1079. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Q.; Jena, P. SiTe monolayers: Si-based analogues of phosphorene. J. Mater. Chem. C 2016, 4, 6353–6361. [Google Scholar] [CrossRef]
- Modestra, J.A.; Mohan, S.V. Microbial electrosynthesis of carboxylic acids through CO2 reduction with selectively enriched biocatalyst: Microbial dynamics. J. CO2 Util. 2017, 20, 190–199. [Google Scholar] [CrossRef]
- Cai, F.; Gao, D.; Zhou, H.; Wang, G.; He, T.; Gong, H.; Miao, S.; Yang, F.; Wang, J.; Bao, X. Electrochemical promotion of catalysis over Pd nanoparticles for CO2 reduction. Chem. Sci. 2017, 8, 2569–2573. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Jiang, W.-J.; Yao, W.; Liu, Z.-L.; Liu, Z.; Yang, Y.; Gao, L.-Z. Advances in electrochemical reduction of carbon dioxide to formate over bismuth-based catalysts. Rare Met. 2021, 40, 2327–2353. [Google Scholar] [CrossRef]
- Qiao, L.; Song, M.; Geng, A.; Yao, S. Polyoxometalate-based high-nuclear cobalt–vanadium–oxo cluster as efficient catalyst for visible light-driven CO2 reduction. Chin. Chem. Lett. 2019, 30, 1273–1276. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Y.; Yan, L.; Su, Z. DFT study on sulfur-doped g-C3N4 nanosheets as a photocatalyst for CO2 reduction reaction. J. Phys. Chem. C 2018, 122, 7712–7719. [Google Scholar] [CrossRef]
- Tackett, B.M.; Gomez, E.; Chen, J.G. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2019, 2, 381–386. [Google Scholar] [CrossRef]
- Wang, X.-T.; Lin, X.-F.; Yu, D.-S. Metal-containing covalent organic framework: A new type of photo/electrocatalyst. Rare Met. 2021, 41, 1160–1175. [Google Scholar] [CrossRef]
- Zhou, A.-Q.; Yang, J.-M.; Zhu, X.-W.; Zhu, X.-L.; Liu, J.-Y.; Zhong, K.; Chen, H.-X.; Chu, J.-Y.; Du, Y.-S.; Song, Y.-H.; et al. Self-assembly construction of NiCo LDH/ultrathin g-C3N4 nanosheets photocatalyst for enhanced CO2 reduction and charge separation mechanism study. Rare Met. 2022, 41, 2118–2128. [Google Scholar] [CrossRef]
- Muiruri, J.K.; Ye, E.; Zhu, Q.; Loh, X.J.; Li, Z. Recent advance in nanostructured materials innovation towards photocatalytic CO2 reduction. Appl. Catal. A Gen. 2022, 648, 118927. [Google Scholar] [CrossRef]
- Luo, Z.; Li, Y.; Guo, F.; Zhang, K.; Liu, K.; Jia, W.; Zhao, Y.; Sun, Y. Carbon Dioxide Conversion with High-Performance Photocatalysis into Methanol on NiSe2/WSe2. Energies 2020, 13, 4330. [Google Scholar] [CrossRef]
- Biswas, M.; Ali, A.; Cho, K.Y.; Oh, W.C. Novel synthesis of WSe2-Graphene-TiO2 ternary nanocomposite via ultrasonic technics for high photocatalytic reduction of CO2 into CH3OH. Ultrason. Sonochem. 2018, 42, 738–746. [Google Scholar] [CrossRef]
- Ali, A.; Oh, W.C. Preparation of Nanowire like WSe2-Graphene Nanocomposite for Photocatalytic Reduction of CO2 into CH3OH with the Presence of Sacrificial Agents. Sci. Rep. 2017, 7, 1867. [Google Scholar] [CrossRef]
- Ju, L.; Bie, M.; Shang, J.; Tang, X.; Kou, L. Janus transition metal dichalcogenides: A superior platform for photocatalytic water splitting. J. Phys. Mater. 2020, 3, 22004. [Google Scholar] [CrossRef]
- Ju, L.; Shang, J.; Tang, X.; Kou, L. Tunable Photocatalytic Water Splitting by the Ferroelectric Switch in a 2D AgBiP2Se6 Monolayer. J. Am. Chem. Soc. 2020, 142, 1492–1500. [Google Scholar] [CrossRef]
- Ju, L.; Bie, M.; Tang, X.; Shang, J.; Kou, L. Janus WSSe Monolayer: An Excellent Photocatalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 29335–29343. [Google Scholar] [CrossRef]
- Lingampalli, S.R.; Ayyub, M.M.; Rao, C.N.R. Recent Progress in the Photocatalytic Reduction of Carbon Dioxide. ACS Omega 2017, 2, 2740–2748. [Google Scholar] [CrossRef]
- Li, X.; Wen, J.; Low, J.; Fang, Y.; Yu, J. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel. Sci. China Mater. 2014, 57, 70–100. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, S.; Kholmanov, I.; Dong, L.; Er, D.; Chen, W.; Guo, H.; Jin, Z.; Shenoy, V.B.; Shi, L. Janus monolayer transition-metal dichalcogenides. ACS Nano 2017, 11, 8192–8198. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.-Y.; Zhu, H.; Xiao, J.; Chuu, C.-P.; Han, Y.; Chiu, M.-H.; Cheng, C.-C.; Yang, C.-W.; Wei, K.-H.; Yang, Y. Janus monolayers of transition metal dichalcogenides. Nat. Nanotechnol. 2017, 12, 744–749. [Google Scholar] [CrossRef]
- Ma, X.; Wu, X.; Wang, H.; Wang, Y. A Janus MoSSe monolayer: A potential wide solar-spectrum water-splitting photocatalyst with a low carrier recombination rate. J. Mater. Chem. A 2018, 6, 2295–2301. [Google Scholar] [CrossRef]
- Xia, C.; Xiong, W.; Du, J.; Wang, T.; Peng, Y.; Li, J. Universality of electronic characteristics and photocatalyst applications in the two-dimensional Janus transition metal dichalcogenides. Phys. Rev. B 2018, 98, 165424. [Google Scholar] [CrossRef]
- Ju, L.; Liu, P.; Yang, Y.; Shi, L.; Yang, G.; Sun, L. Tuning the photocatalytic water-splitting performance with the adjustment of diameter in an armchair WSSe nanotube. J. Energy Chem. 2021, 61, 228–235. [Google Scholar] [CrossRef]
- Evarestov, R.A.; Kovalenko, A.V.; Bandura, A.V. First-principles study on stability, structural and electronic properties of monolayers and nanotubes based on pure Mo(W)S(Se)2 and mixed (Janus) Mo(W)SSe dichalcogenides. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 115, 113681. [Google Scholar] [CrossRef]
- Ju, L.; Dai, Y.; Wei, W.; Li, M.; Huang, B. DFT investigation on two-dimensional GeS/WS2 van der Waals heterostructure for direct Z-scheme photocatalytic overall water splitting. Appl. Surf. Sci. 2018, 434, 365–374. [Google Scholar] [CrossRef]
- Ju, L.; Liu, C.; Shi, L.; Sun, L. The high-speed channel made of metal for interfacial charge transfer in Z-scheme g–C3N4/MoS2 water-splitting photocatalyst. Mater. Res. Express 2019, 6, 115545. [Google Scholar] [CrossRef]
- Lin, H.-F.; Liu, L.-M.; Zhao, J. 2D lateral heterostructures of monolayer and bilayer phosphorene. J. Mater. Chem. C 2017, 5, 2291–2300. [Google Scholar] [CrossRef]
- Ma, D.; Ju, W.; Li, T.; Zhang, X.; He, C.; Ma, B.; Lu, Z.; Yang, Z. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016, 383, 98–105. [Google Scholar] [CrossRef]
- Ju, L.; Tang, X.; Li, X.; Liu, B.; Qiao, X.; Wang, Z.; Yin, H. NO2 Physical-to-Chemical Adsorption Transition on Janus WSSe Monolayers Realized by Defect Introduction. Molecules 2023, 28, 1644. [Google Scholar] [CrossRef]
- Ju, L.; Tang, X.; Zhang, Y.; Li, X.; Cui, X.; Yang, G. Single Selenium Atomic Vacancy Enabled Efficient Visible-Light-Response Photocatalytic NO Reduction to NH3 on Janus WSSe Monolayer. Molecules 2023, 28, 2959. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Chen, M.; Ma, D.; Ju, L. Tunable Photocatalytic Water Splitting Performance of Armchair MoSSe Nanotubes Realized by Polarization Engineering. Inorg. Chem. 2022, 61, 17353–17361. [Google Scholar] [CrossRef]
- Deng, S.; Li, L.; Rees, P. Graphene/MoXY Heterostructures Adjusted by Interlayer Distance, External Electric Field, and Strain for Tunable Devices. ACS Appl. Nano Mater. 2019, 2, 3977–3988. [Google Scholar] [CrossRef]
- Ren, K.; Wang, S.; Luo, Y.; Chou, J.-P.; Yu, J.; Tang, W.; Sun, M. High-efficiency photocatalyst for water splitting: A Janus MoSSe/XN (X = Ga, Al) van der Waals heterostructure. J. Phys. D Appl. Phys. 2020, 53, 185504. [Google Scholar] [CrossRef]
- Fan, Y.; Song, X.; Ai, H.; Li, W.; Zhao, M. Highly Efficient Photocatalytic CO2 Reduction in Two-Dimensional Ferroelectric CuInP2S6 Bilayers. ACS Appl Mater Interfaces 2021, 13, 34486–34494. [Google Scholar] [CrossRef]
- Qiao, M.; Liu, J.; Wang, Y.; Li, Y.; Chen, Z. PdSeO3 Monolayer: Promising Inorganic 2D Photocatalyst for Direct Overall Water Splitting Without Using Sacrificial Reagents and Cocatalysts. J. Am. Chem. Soc. 2018, 140, 12256–12262. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Qu, Z.W.; Zhu, H.; Kroes, G.J.; Nørskov, J.K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]
- Goyal, A.; Marcandalli, G.; Mints, V.A.; Koper, M.T.M. Competition between CO2 Reduction and Hydrogen Evolution on a Gold Electrode under Well-Defined Mass Transport Conditions. J. Am. Chem. Soc. 2020, 142, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Ooka, H.; Figueiredo, M.C.; Koper, M.T.M. Competition between Hydrogen Evolution and Carbon Dioxide Reduction on Copper Electrodes in Mildly Acidic Media. Langmuir 2017, 33, 9307–9313. [Google Scholar] [CrossRef] [PubMed]
- Bronsted, J. Acid and Basic Catalysis. Chem. Rev. 1928, 5, 231–338. [Google Scholar] [CrossRef]
- Evans, M.; Polanyi, M. Inertia and driving force of chemical reactions. Trans. Faraday Soc. 1938, 34, 11–24. [Google Scholar] [CrossRef]
- Hongzhiwei Technology, D.S., Version 2022B, China. 2022. Available online: https://iresearch.net.cn/cloudSoftware (accessed on 14 December 2022).
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Li, D.-H.; Li, Q.-M.; Qi, S.-L.; Qin, H.-C.; Liang, X.-Q.; Li, L. Theoretical Study of Hydrogen Production from Ammonia Borane Catalyzed by Metal and Non-Metal Diatom-Doped Cobalt Phosphide. Molecules 2022, 27, 8206. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Sheng, L. Al-Decorated C2N Monolayer as a Potential Catalyst for NO Reduction with CO Molecules: A DFT Investigation. Molecules 2022, 27, 5790. [Google Scholar] [CrossRef]
- Li, X.; Dai, Y.; Ma, Y.; Li, M.; Yu, L.; Huang, B. Landscape of DNA-like inorganic metal free double helical semiconductors and potential applications in photocatalytic water splitting. J. Mater. Chem. A 2017, 5, 8484–8492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).