Armchair Janus WSSe Nanotube Designed with Selenium Vacancy as a Promising Photocatalyst for CO2 Reduction
Abstract
:1. Introduction
2. Results and Discussion
2.1. The CO2 Adsorption on Pristine Janus WSSe Nanotube
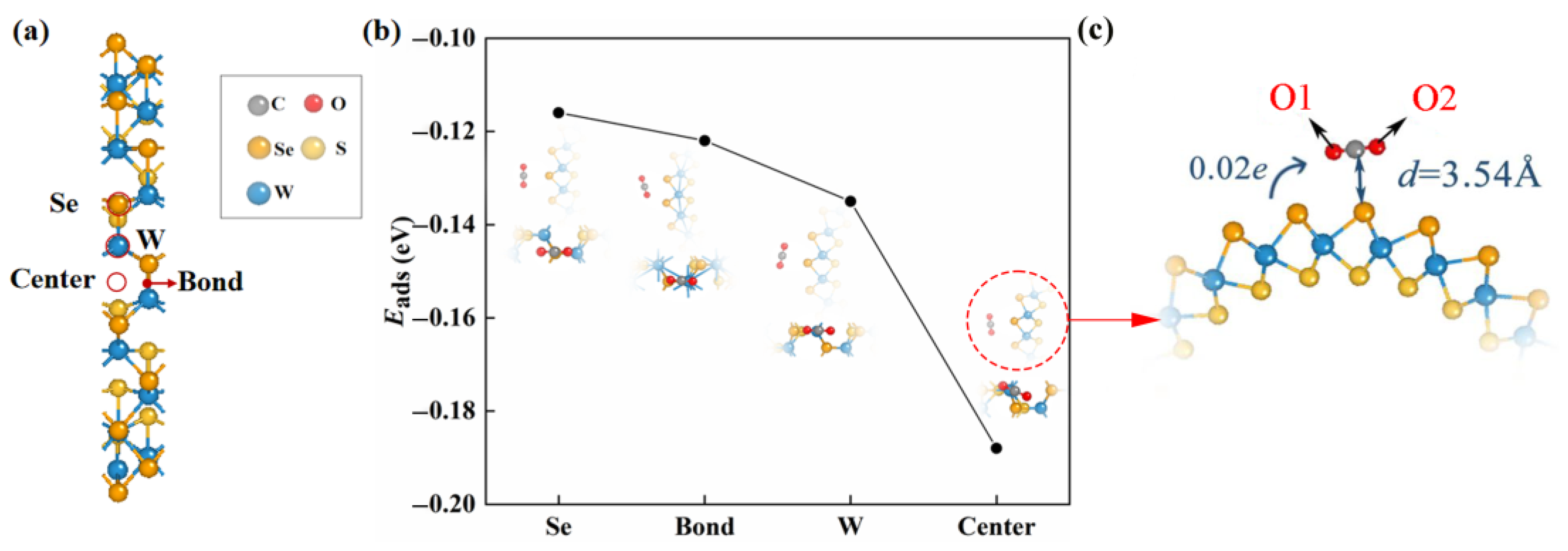
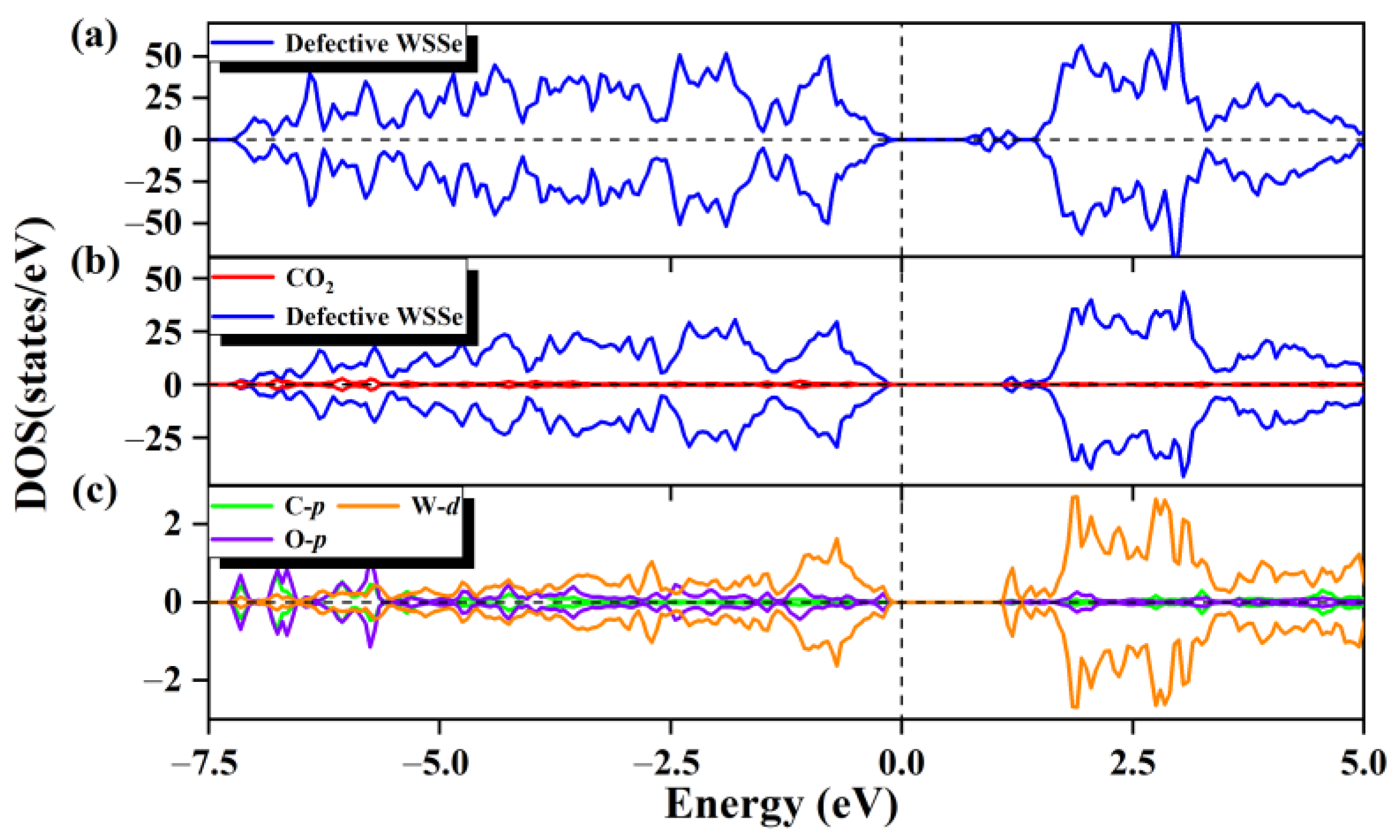
2.2. The CO2 Adsorption on Defective Janus Wsse Nanotube
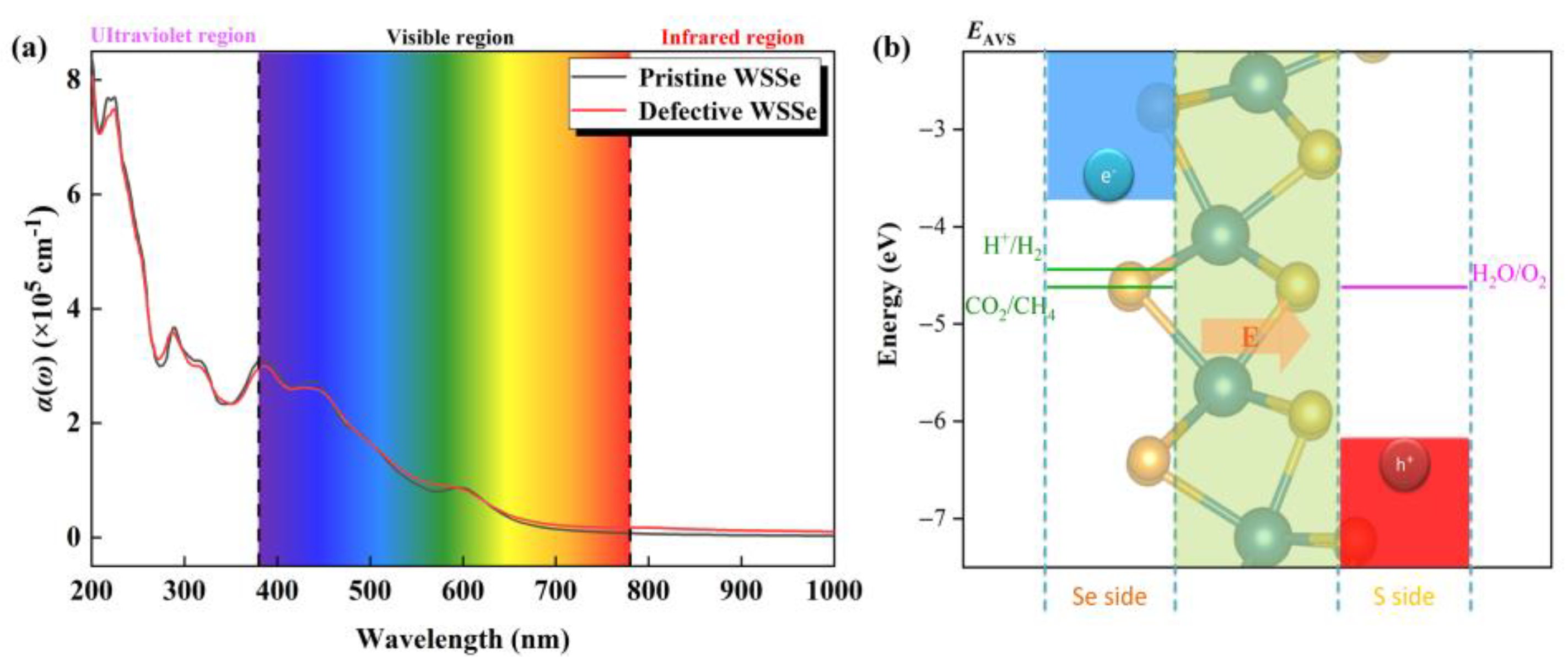
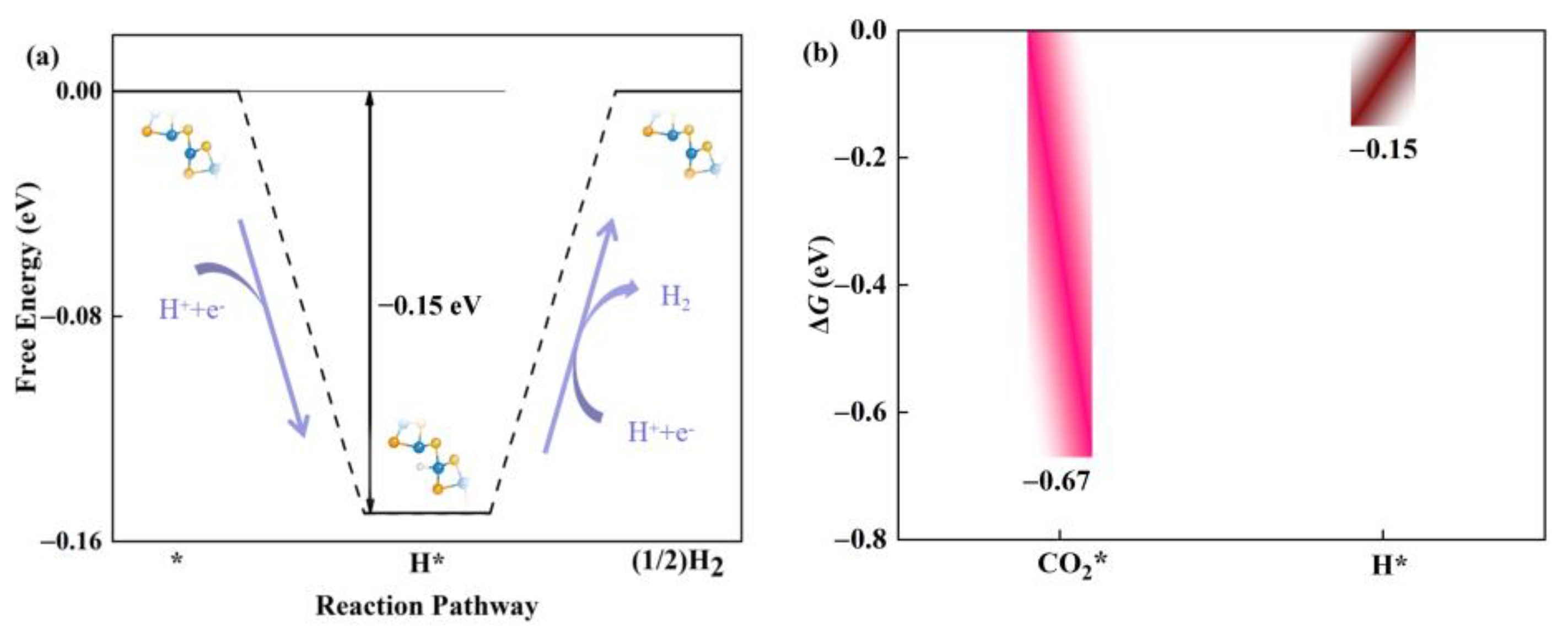
2.3. Photocatalytic Performance of Defective WSSe Nanotube for CO2RR
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gattrell, M.; Gupta, N.; Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Gattrell, M.; Gupta, N.; Co, A. Electrochemical reduction of CO2 to hydrocarbons to store renewable electrical energy and upgrade biogas. Energy Convers. Manag. 2007, 48, 1255–1265. [Google Scholar] [CrossRef]
- Wageh, S.; Al-Hartomy, O.A.; Alotaibi, M.F.; Liu, L.-J. Ionized cocatalyst to promote CO2 photoreduction activity over core–triple-shell ZnO hollow spheres. Rare Met. 2022, 41, 1077–1079. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Q.; Jena, P. SiTe monolayers: Si-based analogues of phosphorene. J. Mater. Chem. C 2016, 4, 6353–6361. [Google Scholar] [CrossRef]
- Modestra, J.A.; Mohan, S.V. Microbial electrosynthesis of carboxylic acids through CO2 reduction with selectively enriched biocatalyst: Microbial dynamics. J. CO2 Util. 2017, 20, 190–199. [Google Scholar] [CrossRef]
- Cai, F.; Gao, D.; Zhou, H.; Wang, G.; He, T.; Gong, H.; Miao, S.; Yang, F.; Wang, J.; Bao, X. Electrochemical promotion of catalysis over Pd nanoparticles for CO2 reduction. Chem. Sci. 2017, 8, 2569–2573. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-H.; Jiang, W.-J.; Yao, W.; Liu, Z.-L.; Liu, Z.; Yang, Y.; Gao, L.-Z. Advances in electrochemical reduction of carbon dioxide to formate over bismuth-based catalysts. Rare Met. 2021, 40, 2327–2353. [Google Scholar] [CrossRef]
- Qiao, L.; Song, M.; Geng, A.; Yao, S. Polyoxometalate-based high-nuclear cobalt–vanadium–oxo cluster as efficient catalyst for visible light-driven CO2 reduction. Chin. Chem. Lett. 2019, 30, 1273–1276. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Y.; Yan, L.; Su, Z. DFT study on sulfur-doped g-C3N4 nanosheets as a photocatalyst for CO2 reduction reaction. J. Phys. Chem. C 2018, 122, 7712–7719. [Google Scholar] [CrossRef]
- Tackett, B.M.; Gomez, E.; Chen, J.G. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2019, 2, 381–386. [Google Scholar] [CrossRef]
- Wang, X.-T.; Lin, X.-F.; Yu, D.-S. Metal-containing covalent organic framework: A new type of photo/electrocatalyst. Rare Met. 2021, 41, 1160–1175. [Google Scholar] [CrossRef]
- Zhou, A.-Q.; Yang, J.-M.; Zhu, X.-W.; Zhu, X.-L.; Liu, J.-Y.; Zhong, K.; Chen, H.-X.; Chu, J.-Y.; Du, Y.-S.; Song, Y.-H.; et al. Self-assembly construction of NiCo LDH/ultrathin g-C3N4 nanosheets photocatalyst for enhanced CO2 reduction and charge separation mechanism study. Rare Met. 2022, 41, 2118–2128. [Google Scholar] [CrossRef]
- Muiruri, J.K.; Ye, E.; Zhu, Q.; Loh, X.J.; Li, Z. Recent advance in nanostructured materials innovation towards photocatalytic CO2 reduction. Appl. Catal. A Gen. 2022, 648, 118927. [Google Scholar] [CrossRef]
- Luo, Z.; Li, Y.; Guo, F.; Zhang, K.; Liu, K.; Jia, W.; Zhao, Y.; Sun, Y. Carbon Dioxide Conversion with High-Performance Photocatalysis into Methanol on NiSe2/WSe2. Energies 2020, 13, 4330. [Google Scholar] [CrossRef]
- Biswas, M.; Ali, A.; Cho, K.Y.; Oh, W.C. Novel synthesis of WSe2-Graphene-TiO2 ternary nanocomposite via ultrasonic technics for high photocatalytic reduction of CO2 into CH3OH. Ultrason. Sonochem. 2018, 42, 738–746. [Google Scholar] [CrossRef]
- Ali, A.; Oh, W.C. Preparation of Nanowire like WSe2-Graphene Nanocomposite for Photocatalytic Reduction of CO2 into CH3OH with the Presence of Sacrificial Agents. Sci. Rep. 2017, 7, 1867. [Google Scholar] [CrossRef] [Green Version]
- Ju, L.; Bie, M.; Shang, J.; Tang, X.; Kou, L. Janus transition metal dichalcogenides: A superior platform for photocatalytic water splitting. J. Phys. Mater. 2020, 3, 22004. [Google Scholar] [CrossRef]
- Ju, L.; Shang, J.; Tang, X.; Kou, L. Tunable Photocatalytic Water Splitting by the Ferroelectric Switch in a 2D AgBiP2Se6 Monolayer. J. Am. Chem. Soc. 2020, 142, 1492–1500. [Google Scholar] [CrossRef]
- Ju, L.; Bie, M.; Tang, X.; Shang, J.; Kou, L. Janus WSSe Monolayer: An Excellent Photocatalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 29335–29343. [Google Scholar] [CrossRef]
- Lingampalli, S.R.; Ayyub, M.M.; Rao, C.N.R. Recent Progress in the Photocatalytic Reduction of Carbon Dioxide. ACS Omega 2017, 2, 2740–2748. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wen, J.; Low, J.; Fang, Y.; Yu, J. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel. Sci. China Mater. 2014, 57, 70–100. [Google Scholar] [CrossRef] [Green Version]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, S.; Kholmanov, I.; Dong, L.; Er, D.; Chen, W.; Guo, H.; Jin, Z.; Shenoy, V.B.; Shi, L. Janus monolayer transition-metal dichalcogenides. ACS Nano 2017, 11, 8192–8198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, A.-Y.; Zhu, H.; Xiao, J.; Chuu, C.-P.; Han, Y.; Chiu, M.-H.; Cheng, C.-C.; Yang, C.-W.; Wei, K.-H.; Yang, Y. Janus monolayers of transition metal dichalcogenides. Nat. Nanotechnol. 2017, 12, 744–749. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Wu, X.; Wang, H.; Wang, Y. A Janus MoSSe monolayer: A potential wide solar-spectrum water-splitting photocatalyst with a low carrier recombination rate. J. Mater. Chem. A 2018, 6, 2295–2301. [Google Scholar] [CrossRef]
- Xia, C.; Xiong, W.; Du, J.; Wang, T.; Peng, Y.; Li, J. Universality of electronic characteristics and photocatalyst applications in the two-dimensional Janus transition metal dichalcogenides. Phys. Rev. B 2018, 98, 165424. [Google Scholar] [CrossRef]
- Ju, L.; Liu, P.; Yang, Y.; Shi, L.; Yang, G.; Sun, L. Tuning the photocatalytic water-splitting performance with the adjustment of diameter in an armchair WSSe nanotube. J. Energy Chem. 2021, 61, 228–235. [Google Scholar] [CrossRef]
- Evarestov, R.A.; Kovalenko, A.V.; Bandura, A.V. First-principles study on stability, structural and electronic properties of monolayers and nanotubes based on pure Mo(W)S(Se)2 and mixed (Janus) Mo(W)SSe dichalcogenides. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 115, 113681. [Google Scholar] [CrossRef]
- Ju, L.; Dai, Y.; Wei, W.; Li, M.; Huang, B. DFT investigation on two-dimensional GeS/WS2 van der Waals heterostructure for direct Z-scheme photocatalytic overall water splitting. Appl. Surf. Sci. 2018, 434, 365–374. [Google Scholar] [CrossRef]
- Ju, L.; Liu, C.; Shi, L.; Sun, L. The high-speed channel made of metal for interfacial charge transfer in Z-scheme g–C3N4/MoS2 water-splitting photocatalyst. Mater. Res. Express 2019, 6, 115545. [Google Scholar] [CrossRef]
- Lin, H.-F.; Liu, L.-M.; Zhao, J. 2D lateral heterostructures of monolayer and bilayer phosphorene. J. Mater. Chem. C 2017, 5, 2291–2300. [Google Scholar] [CrossRef]
- Ma, D.; Ju, W.; Li, T.; Zhang, X.; He, C.; Ma, B.; Lu, Z.; Yang, Z. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016, 383, 98–105. [Google Scholar] [CrossRef]
- Ju, L.; Tang, X.; Li, X.; Liu, B.; Qiao, X.; Wang, Z.; Yin, H. NO2 Physical-to-Chemical Adsorption Transition on Janus WSSe Monolayers Realized by Defect Introduction. Molecules 2023, 28, 1644. [Google Scholar] [CrossRef]
- Ju, L.; Tang, X.; Zhang, Y.; Li, X.; Cui, X.; Yang, G. Single Selenium Atomic Vacancy Enabled Efficient Visible-Light-Response Photocatalytic NO Reduction to NH3 on Janus WSSe Monolayer. Molecules 2023, 28, 2959. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Chen, M.; Ma, D.; Ju, L. Tunable Photocatalytic Water Splitting Performance of Armchair MoSSe Nanotubes Realized by Polarization Engineering. Inorg. Chem. 2022, 61, 17353–17361. [Google Scholar] [CrossRef]
- Deng, S.; Li, L.; Rees, P. Graphene/MoXY Heterostructures Adjusted by Interlayer Distance, External Electric Field, and Strain for Tunable Devices. ACS Appl. Nano Mater. 2019, 2, 3977–3988. [Google Scholar] [CrossRef]
- Ren, K.; Wang, S.; Luo, Y.; Chou, J.-P.; Yu, J.; Tang, W.; Sun, M. High-efficiency photocatalyst for water splitting: A Janus MoSSe/XN (X = Ga, Al) van der Waals heterostructure. J. Phys. D Appl. Phys. 2020, 53, 185504. [Google Scholar] [CrossRef]
- Fan, Y.; Song, X.; Ai, H.; Li, W.; Zhao, M. Highly Efficient Photocatalytic CO2 Reduction in Two-Dimensional Ferroelectric CuInP2S6 Bilayers. ACS Appl Mater Interfaces 2021, 13, 34486–34494. [Google Scholar] [CrossRef]
- Qiao, M.; Liu, J.; Wang, Y.; Li, Y.; Chen, Z. PdSeO3 Monolayer: Promising Inorganic 2D Photocatalyst for Direct Overall Water Splitting Without Using Sacrificial Reagents and Cocatalysts. J. Am. Chem. Soc. 2018, 140, 12256–12262. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Qu, Z.W.; Zhu, H.; Kroes, G.J.; Nørskov, J.K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]
- Goyal, A.; Marcandalli, G.; Mints, V.A.; Koper, M.T.M. Competition between CO2 Reduction and Hydrogen Evolution on a Gold Electrode under Well-Defined Mass Transport Conditions. J. Am. Chem. Soc. 2020, 142, 4154–4161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooka, H.; Figueiredo, M.C.; Koper, M.T.M. Competition between Hydrogen Evolution and Carbon Dioxide Reduction on Copper Electrodes in Mildly Acidic Media. Langmuir 2017, 33, 9307–9313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronsted, J. Acid and Basic Catalysis. Chem. Rev. 1928, 5, 231–338. [Google Scholar] [CrossRef]
- Evans, M.; Polanyi, M. Inertia and driving force of chemical reactions. Trans. Faraday Soc. 1938, 34, 11–24. [Google Scholar] [CrossRef]
- Hongzhiwei Technology, D.S., Version 2022B, China. 2022. Available online: https://iresearch.net.cn/cloudSoftware (accessed on 14 December 2022).
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Li, D.-H.; Li, Q.-M.; Qi, S.-L.; Qin, H.-C.; Liang, X.-Q.; Li, L. Theoretical Study of Hydrogen Production from Ammonia Borane Catalyzed by Metal and Non-Metal Diatom-Doped Cobalt Phosphide. Molecules 2022, 27, 8206. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Sheng, L. Al-Decorated C2N Monolayer as a Potential Catalyst for NO Reduction with CO Molecules: A DFT Investigation. Molecules 2022, 27, 5790. [Google Scholar] [CrossRef]
- Li, X.; Dai, Y.; Ma, Y.; Li, M.; Yu, L.; Huang, B. Landscape of DNA-like inorganic metal free double helical semiconductors and potential applications in photocatalytic water splitting. J. Mater. Chem. A 2017, 5, 8484–8492. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, L.; Tang, X.; Li, J.; Dong, H.; Yang, S.; Gao, Y.; Liu, W. Armchair Janus WSSe Nanotube Designed with Selenium Vacancy as a Promising Photocatalyst for CO2 Reduction. Molecules 2023, 28, 4602. https://doi.org/10.3390/molecules28124602
Ju L, Tang X, Li J, Dong H, Yang S, Gao Y, Liu W. Armchair Janus WSSe Nanotube Designed with Selenium Vacancy as a Promising Photocatalyst for CO2 Reduction. Molecules. 2023; 28(12):4602. https://doi.org/10.3390/molecules28124602
Chicago/Turabian StyleJu, Lin, Xiao Tang, Jingli Li, Hao Dong, Shenbo Yang, Yajie Gao, and Wenhao Liu. 2023. "Armchair Janus WSSe Nanotube Designed with Selenium Vacancy as a Promising Photocatalyst for CO2 Reduction" Molecules 28, no. 12: 4602. https://doi.org/10.3390/molecules28124602
APA StyleJu, L., Tang, X., Li, J., Dong, H., Yang, S., Gao, Y., & Liu, W. (2023). Armchair Janus WSSe Nanotube Designed with Selenium Vacancy as a Promising Photocatalyst for CO2 Reduction. Molecules, 28(12), 4602. https://doi.org/10.3390/molecules28124602





