Synthesis and Assessment of Antimicrobial Composites of Ag Nanoparticles or AgNO3 and Egg Shell Membranes
Abstract
1. Introduction
2. Results and Discussion
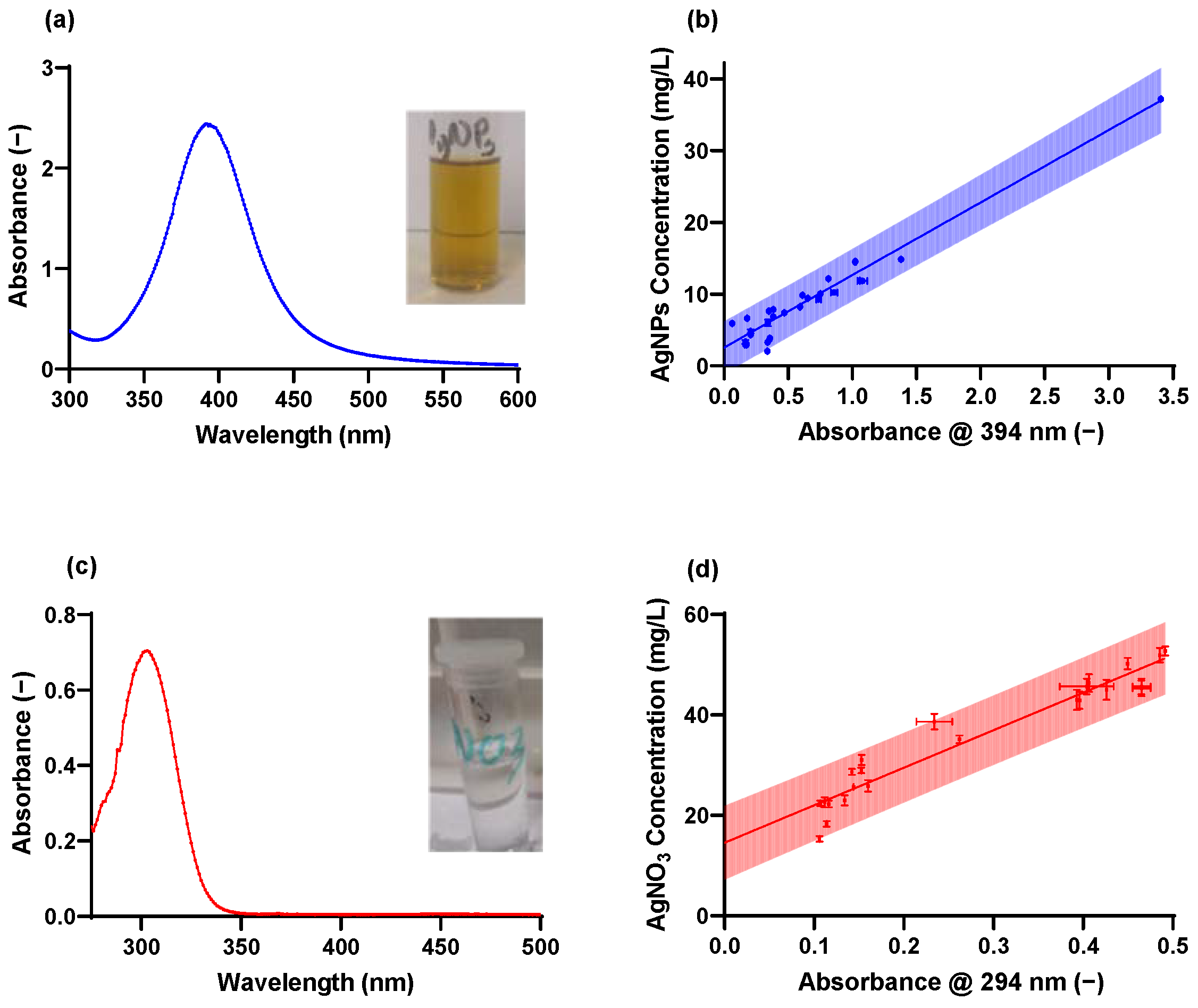
2.1. Synthesis and Characterization of AgNPs
2.2. Ag/ESM Composite Adsorption Optimization, Kinetics, and Equilibrium Behaviour
| Kinetic Law | Differential Form * | Analytical Form * |
|---|---|---|
| Pseudo-first order (PFO) [65] | ||
| Pseudo-second order (PSO) [65] | ||
| Two-phase adsorption (2-PA) [58,59,66,67] | [68] | |
| Crank internal mass transfer model (CIMT) [65] | [68] | |
| Weber and Morris (W&M) [65,69] | [70] |
| Kinetic Law | AgNPs | AgNO3 | ||
|---|---|---|---|---|
| Fitted Parameters | R2/RMSE | Fitted Parameters | R2/RMSE | |
| PFO | k1 = 0.339 min−1 | 0.922/0.0316 mg·g−1 | k1 = 0.848 min−1 | 0.990/0.261 mg·g−1 |
| PSO | k2 = 0.921 g·mg−1·min−1 | 0.970/0.0196 mg·g−1 | k2 = 0.113 g·mg−1·min−1 | 0.942/0.634 mg·g−1 |
| 2-PA | kfast = 0.540 min−1 | 0.982/0.0154 mg·g−1 | kfast = kslow = 0.848 min−1 ϕ = N/A | 0.990/0.261 mg·g−1 |
| kslow = 0.0297 min−1 | ||||
| ϕ = 0.616 | ||||
| CIMT | = 0.0111 h−1 | 0.940/0.0278 mg·g−1 | = 0.0565 h−1 De = 1.41 × 10−10 m2·s−1 | 0.963/0.509 mg·g−1 |
| De = 2.78 × 10−11 m2·s−1 | ||||
| W&M | De1 = 2.78 × 10−11 m2·s−1 | 0.986/0.0133 mg·g−1 | De1 = 1.41 × 10−10 m2·s−1 | 0.989/0.276 mg·g−1 |
| De2 = 1.24 × 10−12 m2·s−1 | De2 = 2.80 × 10−14 m2·s−1 | |||
| De3 = 0 m2·s−1 | De3 = 0 m2·s−1 | |||

2.3. Characterization of Ag/ESM Composites
2.4. Antimicrobial Activity of Ag/ESM Composites
3. Materials and Methods
3.1. Preparation of Eggshell Membranes (ESM)
3.2. Synthesis of AgNPs
3.3. Detection and Characterization of AgNPs
3.4. Production of ESM Composites
3.5. Characterization of ESM Composites
3.6. Antimicrobial Activity of AgNPs, AgNO3, and ESM Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Egbo, M.K. A fundamental review on composite materials and some of their applications in biomedical engineering. J. King Saud Univ. Eng. Sci. 2021, 33, 557–568. [Google Scholar] [CrossRef]
- Kelly, A.; Mortensen, A. Composite Materials: Overview. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1361–1371. ISBN 978-0-08-043152-9. [Google Scholar]
- Haleem, A.; Pan, J.-M.; Shah, A.; Hussain, H.; He, W. A systematic review on new advancement and assessment of emerging polymeric cryogels for environmental sustainability and energy production. Sep. Purif. Technol. 2023, 316, 123678. [Google Scholar] [CrossRef]
- Chermant, J.-L.; Coster, M. Composites, Microstructure of: Quantitative Description. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1396–1402. ISBN 978-0-08-043152-9. [Google Scholar]
- Haleem, A.; Shafiq, A.; Chen, S.-Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Chen, S.; Pan, J.; Weidong, H. Gamma radiation induced synthesis of double network hydrophilic cryogels at low pH loaded with AuNPs for fast and efficient degradation of Congo red. J. Hazard. Mater. Adv. 2023, 10, 100299. [Google Scholar] [CrossRef]
- Hymavathi, A. Materials Today: Proceedings A green synthetic approach of silver nanoparticles using Premna tomentosa leaf extract and their anti-microbial study. Mater. Today Proc. 2022, 62, 6776–6779. [Google Scholar] [CrossRef]
- Matar, G.H.; Akyüz, G.; Kaymazlar, E.; Andac, M. An Investigation of Green Synthesis of Silver Nanoparticles Using Turkish Honey against Pathogenic Bacterial Strains. Nature 2022, 19, 29. [Google Scholar]
- Zhang, J.; Liu, L.; Wang, Y.; Wang, C.; Guo, Y.; Yuan, Z.; Jia, Y.; Li, P.; Sun, S.; Zhao, G. A highly selective red-emitting fluorescent probe and its micro-nano-assembly for imaging endogenous peroxynitrite (ONOO−) in living cells. Anal. Chim. Acta 2023, 1241, 340778. [Google Scholar] [CrossRef] [PubMed]
- Abass Sofi, M.; Sunitha, S.; Ashaq Sofi, M.; Khadheer Pasha, S.K.; Choi, D. An overview of antimicrobial and anticancer potential of silver nanoparticles. J. King Saud Univ. Sci. 2022, 34, 101791. [Google Scholar] [CrossRef]
- Swidan, N.S.; Hashem, Y.A.; Elkhatib, W.F.; Yassien, M.A. Antibiofilm activity of green synthesized silver nanoparticles against biofilm associated enterococcal urinary pathogens. Sci. Rep. 2022, 12, 3869. [Google Scholar] [CrossRef] [PubMed]
- Wali, N.; Shabbir, A.; Wajid, N.; Abbas, N.; Naqvi, S.Z.H. Synergistic efficacy of colistin and silver nanoparticles impregnated human amniotic membrane in a burn wound infected rat model. Sci. Rep. 2022, 12, 6414. [Google Scholar] [CrossRef]
- Guerrero, D.S.; Bertani, R.P.; Ledesma, A.; de los Angeles Frías, M.; Romero, C.M.; Dávila Costa, J.S. Silver nanoparticles synthesized by the heavy metal resistant strain Amycolatopsis tucumanensis and its application in controlling red strip disease in sugarcane. Heliyon 2022, 8, e09472. [Google Scholar] [CrossRef]
- Li, P.; Guo, Y.; Jia, Y.; Guan, H.; Wang, C.; Wu, Z.; Sun, S.; Qu, Z.; Zhou, P.; Zhao, G. Achieving metal-free phosphorescence in dilute solutions for imaging hypoxia in cells and tumors. Mater. Chem. Front. 2021, 5, 7170–7175. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Fulazzaky, M.A.; Salmiati, S.; Kueh, A.B.H.; Fulazzaky, M.; Salim, M.R. Silver nanoparticles adsorption by the synthetic and natural adsorbent materials: An exclusive review. Nanotechnol. Environ. Eng. 2020, 5, 1. [Google Scholar] [CrossRef]
- Hassan, D.M.A.; Farghali, M.R.F. Adsorption of Silver Nanoparticles from Aqueous Solution by Multiwalled Carbon Nanotubes. Adv. Nanopart. 2017, 6, 22–32. [Google Scholar] [CrossRef]
- Islam, M.A.; Jacob, M.V.; Antunes, E. A critical review on silver nanoparticles: From synthesis and applications to its mitigation through low-cost adsorption by biochar. J. Environ. Manag. 2021, 281, 111918. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.A.; Benelli, P.; Amante, E.R. A literature review on adding value to solid residues: Egg shells. J. Clean. Prod. 2013, 46, 42–47. [Google Scholar] [CrossRef]
- Shahbandeh, M. Statista Global Egg Production from 1990 to 2018. Available online: https://www.statista.com/statistics/263972/egg-production-worldwide-since-1990/ (accessed on 27 April 2020).
- South African Poultry Association Egg Industry Production Report for November 2016. 2016. Available online: http://www.sapoultry.co.za/pdf-statistics/egg-industry.pdf (accessed on 10 March 2020).
- Nakano, T.; Ikawa, N.I.; Ozimek, L. Chemical composition of chicken eggshell and shell membranes. Poult. Sci. 2003, 82, 510–514. [Google Scholar] [CrossRef]
- Hussain, A. Delectric Properties and Microwave Assisted Separation of Eggshell and Membrane. Master’s Thesis, McGill University, Sainte-Anne-de-Bellevue, QC, Canada, 2009. [Google Scholar]
- Buksh, N.; Yun, C.; Ping, X.; Jhatial, G.H.; Yanhai, S. Chicken Eggshell as a Potential Eco-friendly, Low-cost Sorbent: A Mini Review. J. Environ. Earth Sci. 2018, 8, 28–39. [Google Scholar]
- Rose, M.L.H.; Hincke, M.T. Protein constituents of the eggshell: Eggshell-specific matrix proteins. Cell. Mol. Life Sci. 2009, 66, 2707–2719. [Google Scholar] [CrossRef]
- Hincke, M.T.; Nys, Y.; Gautron, J.; Mann, K.; Rodriguez-Navarro, A.B.; McKee, M.D. The eggshell: Structure, composition and mineralization. Front. Biosci. 2012, 17, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, Z.; Ahn, D.U.; Huang, X. Development of an antibacterial nanobiomaterial for wound-care based on the absorption of AgNPs on the eggshell membrane. Colloids Surf. B Biointerfaces 2019, 183, 110449. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, C.M.M.; Esmaili, H.; Ansah, G.; Hincke, M.T. Ovocalyxin-36 is a pattern recognition protein in chicken eggshell membranes. PLoS ONE 2013, 8, e84112. [Google Scholar] [CrossRef]
- Li, J.; Ng, D.H.L.; Ma, R.; Zuo, M.; Song, P. Eggshell membrane-derived MgFe2O4 for pharmaceutical antibiotics removal and recovery from water. Chem. Eng. Res. Des. 2017, 126, 123–133. [Google Scholar] [CrossRef]
- Hayajneh, M.T.; Almomani, M.A.; Al-Shrida, M.M. Effects of waste eggshells addition on microstructures, mechanical and tribological properties of green metal matrix composite. Sci. Eng. Compos. Mater. 2019, 26, 423–434. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Sharma, S.; Mishra, R.K. Characterization of waste eggshells and CaCO3 reinforced AA2014 green metal matrix composites: A green approach in the synthesis of composites. Int. J. Precis. Eng. Manuf. 2016, 17, 1383–1393. [Google Scholar] [CrossRef]
- Shin, L.J.; Barathi Dassan, E.G.; Zainol Abidin, M.S.; Anjang, A. Tensile and Compressive Properties of Glass Fiber-Reinforced Polymer Hybrid Composite with Eggshell Powder. Arab. J. Sci. Eng. 2020, 45, 5783–5791. [Google Scholar] [CrossRef]
- Bhagavatheswaran, E.S.; Das, A.; Rastin, H.; Saeidi, H.; Jafari, S.H.; Vahabi, H.; Najafi, F.; Khonakdar, H.A.; Formela, K.; Jouyandeh, M.; et al. The Taste of Waste: The Edge of Eggshell Over Calcium Carbonate in Acrylonitrile Butadiene Rubber. J. Polym. Environ. 2019, 27, 2478–2489. [Google Scholar] [CrossRef]
- Ding, Q.; Kang, Z.; He, X.; Wang, M.; Lin, M.; Lin, H.; Yang, D.P. Eggshell membrane-templated gold nanoparticles as a flexible SERS substrate for detection of thiabendazole. Microchim. Acta 2019, 186, 453. [Google Scholar] [CrossRef] [PubMed]
- Ofuyatan, O.M.; Adeniyi, A.G.; Ijie, D.; Ighalo, J.O.; Oluwafemi, J. Development of high-performance self compacting concrete using eggshell powder and blast furnace slag as partial cement replacement. Constr. Build. Mater. 2020, 256, 119403. [Google Scholar] [CrossRef]
- Venkata Krishnaiah, R.; Dayakar, P.; Mohan, S.J. Effect of egg shell powder on strength behaviour of concrete. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 562–564. [Google Scholar] [CrossRef]
- Raji, S.A.; Samuel, A.T. Egg Shell As A Fine Aggregate In Concrete For Sustainable Construction. Int. J. Sci. Technol. Res. 2015, 4, 8–13. [Google Scholar]
- Cree, D.; Pliya, P. Effect of elevated temperature on eggshell, eggshell powder and eggshell powder mortars for masonry applications. J. Build. Eng. 2019, 26, 100852. [Google Scholar] [CrossRef]
- Tiong, H.Y.; Lim, S.K.; Lee, Y.L.; Ong, C.F.; Yew, M.K. Environmental impact and quality assessment of using eggshell powder incorporated in lightweight foamed concrete. Constr. Build. Mater. 2020, 244, 118341. [Google Scholar] [CrossRef]
- Bensaifi, E.; Bouteldja, F.; Nouaouria, M.S.; Breul, P. Influence of crushed granulated blast furnace slag and calcined eggshell waste on mechanical properties of a compacted marl. Transp. Geotech. 2019, 20, 100244. [Google Scholar] [CrossRef]
- Abdelmalik, A.A.; Ogbodo, M.O.; Momoh, G.E. Investigating the mechanical and insulation performance of waste eggshell powder/epoxy polymer for power insulation application. SN Appl. Sci. 2019, 1, 1238. [Google Scholar] [CrossRef]
- Jaques, N.G.; William de Lima Souza, J.; Popp, M.; Kolbe, J.; Lia Fook, M.V.; Ramos Wellen, R.M. Kinetic investigation of eggshell powders as biobased epoxy catalyzer. Compos. Part B Eng. 2020, 183, 107651. [Google Scholar] [CrossRef]
- Odetoye, T.E.; Agu, J.O.; Ajala, E.O. Biodiesel production from poultry wastes: Waste chicken fat and eggshell. J. Environ. Chem. Eng. 2021, 9, 105654. [Google Scholar] [CrossRef]
- Ajala, E.O.; Ajala, M.A.; Odetoye, T.E.; Aderibigbe, F.A.; Osanyinpeju, H.O.; Ayanshola, M.A. Thermal modification of chicken eggshell as heterogeneous catalyst for palm kernel biodiesel production in an optimization process. Biomass Convers. Biorefinery 2020, 11, 2599–2615. [Google Scholar] [CrossRef]
- Kavitha, V.; Geetha, V.; Jacqueline, P.J. Production of biodiesel from dairy waste scum using eggshell waste. Process Saf. Environ. Prot. 2019, 125, 279–287. [Google Scholar] [CrossRef]
- Li, Z.; Yang, D.P.; Chen, Y.; Du, Z.; Guo, Y.; Huang, J.; Li, Q. Waste eggshells to valuable Co3O4/CaCO3 materials as efficient catalysts for VOCs oxidation. Mol. Catal. 2020, 483, 110766. [Google Scholar] [CrossRef]
- Murcia-Salvador, A.; Pellicer, J.A.; Rodríguez-López, M.I.; Gómez-López, V.M.; Núñez-Delicado, E.; Gabaldón, J.A. Egg by-products as a tool to remove direct blue 78 dye from wastewater: Kinetic, equilibrium modeling, thermodynamics and desorption properties. Materials 2020, 13, 1262. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Salih, N.R. Application of eggshell wastes for boron remediation from water. J. Mol. Liq. 2018, 256, 599–610. [Google Scholar] [CrossRef]
- Sree, G.V.; Nagaraaj, P.; Kalanidhi, K.; Aswathy, C.A.; Rajasekaran, P. Calcium oxide a sustainable photocatalyst derived from eggshell for efficient photo-degradation of organic pollutants. J. Clean. Prod. 2020, 270, 122294. [Google Scholar] [CrossRef]
- Tsai, W.T.; Yang, J.M.; Lai, C.W.; Cheng, Y.H.; Lin, C.C.; Yeh, C.W. Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour. Technol. 2006, 97, 488–493. [Google Scholar] [CrossRef]
- Brink, H.G.; Mahlangu, Z. Microbial Lead(II) precipitation: The influence of growth substrate. Chem. Eng. Trans. 2018, 64, 439–444. [Google Scholar] [CrossRef]
- Seo, S.B.; Dananjaya, S.H.S.; Nikapitiya, C.; Park, B.K.; Gooneratne, R.; Kim, T.Y.; Lee, J.; Kim, C.H.; De Zoysa, M. Silver nanoparticles enhance wound healing in zebrafish (Danio rerio). Fish Shellfish Immunol. 2017, 68, 536–545. [Google Scholar] [CrossRef]
- Nakamura, T.; Magara, H.; Herbani, Y.; Sato, S. Fabrication of silver nanoparticles by highly intense laser irradiation of aqueous solution. Appl. Phys. A 2011, 104, 1021–1024. [Google Scholar] [CrossRef]
- Oves, M.; Khan, M.S.; Zaidi, A.; Ahmed, A.S.; Ahmed, F.; Ahmad, E.; Sherwani, A.; Owais, M.; Azam, A. Antibacterial and Cytotoxic Efficacy of Extracellular Silver Nanoparticles Biofabricated from Chromium Reducing Novel OS4 Strain of Stenotrophomonas maltophilia. PLoS ONE 2013, 8, e59140. [Google Scholar] [CrossRef] [PubMed]
- Tendenedzai, J.T.; Chirwa, E.M.N.; Brink, H.G. Enterococcus spp. Cell-Free Extract: An Abiotic Route for Synthesis of Selenium Nanoparticles (SeNPs), Their Characterisation and Inhibition of Escherichia coli. Nanomaterials 2022, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Beckman. What Is Z-Average Size. Available online: https://www.beckman.com/support/faq/scientific/what-is-z-average-size (accessed on 15 March 2023).
- Li, J.; Zhai, D.; Lv, F.; Yu, Q.; Ma, H.; Yin, J.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016, 36, 254–266. [Google Scholar] [CrossRef]
- Velgosova, O.; Čižmárová, E.; Málek, J.; Kavuličova, J. Effect of storage conditions on long-term stability of Ag nanoparticles formed via green synthesis. Int. J. Miner. Metall. Mater. 2017, 24, 1177–1182. [Google Scholar] [CrossRef]
- Brusseau, M.L.; Rao, P.S.C.; Gillham, R.W. Sorption nonideality during organic contaminant transport in porous media. Crit. Rev. Environ. Control 1989, 19, 33–99. [Google Scholar] [CrossRef]
- Brusseau, M.L.; Jessup, R.E.; Suresh, P.; Rao, C. Nonequilibrium Sorption of Organic Chemicals: Elucidation of Rate-Limiting Processes. Environ. Sci. Technol. 1991, 25, 134–142. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Largitte, L.; Pasquier, R. New models for kinetics and equilibrium homogeneous adsorption. Chem. Eng. Res. Des. 2016, 112, 289–297. [Google Scholar] [CrossRef]
- Myslitskaya, N.A.; Khitrin, A.V.; Ivanov, A.M.; Samusev, I.G.; Bryukhanov, V.V. Dynamics of colloid silver nanoparticles in an evaporating water drop. Russ. Phys. J. 2012, 54, 1280–1285. [Google Scholar] [CrossRef]
- Palencia, M.S.; Berrio, M.E.; Palencia, S.L. Effect of capping agent and diffusivity of different silver nanoparticles on their antibacterial properties. J. Nanosci. Nanotechnol. 2017, 17, 5197–5204. [Google Scholar] [CrossRef]
- Aliofkhazraei, M. Diffusion of Nanoparticles in Gases and Liquids. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–1426. ISBN 978-3-319-15337-7. [Google Scholar]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Cornelissen, G.; Van Noort, P.C.M.; Parsons, J.R.; Govers, H.A.J. Temperature dependence of slow adsorption and desorption kinetics of organic compounds in sediments. Environ. Sci. Technol. 1997, 31, 454–460. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Song, L.; Mashayekhi, H.; Chefetz, B.; Xing, B. Adsorption and desorption of phenanthrene on carbon nanotubes in simulated gastrointestinal fluids. Environ. Sci. Technol. 2011, 45, 6018–6024. [Google Scholar] [CrossRef]
- van Veenhuyzen, B.; Tichapondwa, S.; Hörstmann, C.; Chirwa, E.; Brink, H.G. High capacity Pb(II) adsorption characteristics onto raw- and chemically activated waste activated sludge. J. Hazard. Mater. 2021, 416, 125943. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Muedi, K.L.L.; Brink, H.G.G.; Masindi, V.; Maree, J.P.P. Effective removal of arsenate from wastewater using aluminium enriched ferric oxide-hydroxide recovered from authentic acid mine drainage. J. Hazard. Mater. 2021, 414, 125491. [Google Scholar] [CrossRef] [PubMed]
- Scott Fogler, H. Elements of Chemical Reaction Engineering, 4th ed.; Pearson Education Limited: London, UK, 2013; ISBN 0130473944. [Google Scholar]
- Harned, H.S.; Hildreth, C.L. The Diffusion Coefficient of Silver Nitrate in Dilute Aqueous Solution at 25°. J. Am. Chem. Soc. 1951, 73, 3292–3293. [Google Scholar] [CrossRef]
- Muedi, K.L.; Masindi, V.; Maree, J.P.; Haneklaus, N.; Brink, H.G. Effective Adsorption of Congo Red from Aqueous Solution Using Fe/Al Di-Metal Nanostructured Composite Synthesised from Fe(III) and Al(III) Recovered from Real Acid Mine Drainage. Nanomaterials 2022, 12, 776. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Girods, P.; Dufour, A.; Fierro, V.; Rogaume, Y.; Rogaume, C.; Zoulalian, A.; Celzard, A. Activated carbons prepared from wood particleboard wastes: Characterisation and phenol adsorption capacities. J. Hazard. Mater. 2009, 166, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Wales, D.S.; Keane, M.A. Copper removal from aqueous systems: Biosorption by Pseudomonas syringae. Sep. Sci. Technol. 2001, 36, 223–240. [Google Scholar] [CrossRef]
- Daraei, H.; Mittal, A.; Mittal, J.; Kamali, H. Optimization of Cr(VI) removal onto biosorbent eggshell membrane: Experimental & theoretical approaches. Desalin. Water Treat. 2014, 52, 1307–1315. [Google Scholar] [CrossRef]
- Huang, L.; Chen, R.; Luo, J.; Hasan, M.; Shu, X. Synthesis of phytonic silver nanoparticles as bacterial and ATP energy silencer. J. Inorg. Biochem. 2022, 231, 111802. [Google Scholar] [CrossRef]
- Xin, Y.; Li, C.; Liu, J.; Liu, J.; Liu, Y.; He, W.; Gao, Y. Adsorption of heavy metal with modified eggshell membrane and the in situ synthesis of Cu-Ag/modified eggshell membrane composites. R. Soc. Open Sci. 2018, 5, 180532. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jin, Y.; Bai, F.; Jin, S. Pseudomonas Aeruginosa; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; ISBN 9780123971692. [Google Scholar]
- Poole, K. Pseudomonas aeruginosa: Resistance to the max. Front. Microbiol. 2011, 2, 65. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Á.T. Bacillus subtilis . Trends Microbiol. 2019, 27, 724–725. [Google Scholar] [CrossRef]
- Earl, A.M.; Losick, R.; Kolter, R. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 2008, 16, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Yassin, N.; Ahmad, A. Incidence and Resistotyping Profiles of Bacillus subtilis Isolated from Azadi Teaching Hospital in Duhok City, Iraq. Mater. Socio Med. 2012, 24, 194. [Google Scholar] [CrossRef]
- Midway, S.; Robertson, M.; Flinn, S.; Kaller, M. Comparing multiple comparisons: Practical guidance for choosing the best multiple comparisons test. PeerJ 2020, 8, e10387. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Aina, S.; Du Plessis, B.; Mjimba, V.; Brink, H. Eggshell valorization: Membrane removal, calcium oxide synthesis, and biochemical compound recovery towards cleaner productions. Biointerface Res. Appl. Chem. 2022, 12, 5870–5883. [Google Scholar] [CrossRef]
- Gavanji, S.; Bakhtari, A.; Famurewa, A.C.; Othman, E.M. Cytotoxic Activity of Herbal Medicines as Assessed in Vitro: A Review. Chem. Biodivers. 2023, 20, e202201098. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Patravale, V.; Dandekar, P.; Jain, R. Nanotoxicology: Evaluating toxicity potential of drug-nanoparticles. In Nanoparticulate Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2012; pp. 123–155. [Google Scholar]









| Distribution 1 (Red Peak) | Distribution 2 (Green Peak) | Distribution 3 (Blue Peak) | Total Distribution (Dashed Line/Bars) | ||||
|---|---|---|---|---|---|---|---|
| % of Total | d50 (nm) Predicted 1 | % of Total | d50 (nm) Predicted 1 | % of Total | d50 (nm) Predicted 1 | d50 (nm) Predicted 1 | Z-Average (nm) Measured 2 |
| 1.62 | 0.82 | 18.7 | 3.15 | 79.4 | 37.1 | 29.8 | 14.3 |
| Isotherm | AgNPs | AgNO3 | |||
|---|---|---|---|---|---|
| Non-Linear Form * | Fitted Parameters | R2/RMSE | Fitted Parameters | R2/RMSE | |
| Langmuir [65,74,75] | 0.867/ 0.060 mg·g−1 | 0.947/ 0.935 mg·g−1 | |||
| Freundlich [75] | 0.884/ 0.067 mg·g−1 | 0.942/ 0.977 mg·g−1 | |||
| Element | ESM (Wt%) | AgNO3/ESM (Wt%) | AgNPs/ESM (Wt%) |
|---|---|---|---|
| Ag | - | 15.68 | 4.87 |
| S | 46.42 | 30.52 | 36.05 |
| O | 49.97 | 53.19 | 57.62 |
| Si | 0.85 | - | - |
| Ca | 2.77 | 0.61 | 1.45 |
| Species | O_H | N_H | C=O (Amide I) | CN/NH (Amide II) | CN/NH (Amide III) | C_O | C_O Stretching | CH2 Deformation |
|---|---|---|---|---|---|---|---|---|
| ESM | 3274.36 | 2926.90 | 1637.21 | 1517.58 | 1439.67 | 1235.67 | 1080.33 | 874.94 |
| AgNO3/ESM | 3269.37 | 2927.48 | 1637.56 | 1518.81 | 1444.05 | 1235.26 | 1078.68 | 875.19 |
| AgNPs/ESM | 3275.56 | 2927.09 | 1638.14 | 1517.33 | 1442.24 | 1235.33 | 1077.26 | 875.50 |
| ESM | AgNO3/ESM | AgNPs/ESM | |
|---|---|---|---|
| O1s | 531.40 and 532.35 | 531.79 and 533.48 | 531.32 and 532.15 |
| N1s | 399.76 | 399.86 | 399.70 |
| 3d Ag0 | - | 368.08 and 374.77 | 367.89 and 373.91 |
| 3d AgO | - | 364.41 and 370.41 | - |
| Ca 2p | 347.22 and 350.80 | 347.19 and 350.75 | 347.21 and 350.78 |
| C1s | 284.57, 285.50, 287.75, and 288.57 | 284.56, 285.31, 287.45, and 288.74 | 284.54, 285.11, 287.5, and 288.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aina, S.T.; Kyomuhimbo, H.D.; Ramjee, S.; Du Plessis, B.; Mjimba, V.; Maged, A.; Haneklaus, N.; Brink, H.G. Synthesis and Assessment of Antimicrobial Composites of Ag Nanoparticles or AgNO3 and Egg Shell Membranes. Molecules 2023, 28, 4654. https://doi.org/10.3390/molecules28124654
Aina ST, Kyomuhimbo HD, Ramjee S, Du Plessis B, Mjimba V, Maged A, Haneklaus N, Brink HG. Synthesis and Assessment of Antimicrobial Composites of Ag Nanoparticles or AgNO3 and Egg Shell Membranes. Molecules. 2023; 28(12):4654. https://doi.org/10.3390/molecules28124654
Chicago/Turabian StyleAina, Samuel Tomi, Hilda Dinah Kyomuhimbo, Shatish Ramjee, Barend Du Plessis, Vuyo Mjimba, Ali Maged, Nils Haneklaus, and Hendrik Gideon Brink. 2023. "Synthesis and Assessment of Antimicrobial Composites of Ag Nanoparticles or AgNO3 and Egg Shell Membranes" Molecules 28, no. 12: 4654. https://doi.org/10.3390/molecules28124654
APA StyleAina, S. T., Kyomuhimbo, H. D., Ramjee, S., Du Plessis, B., Mjimba, V., Maged, A., Haneklaus, N., & Brink, H. G. (2023). Synthesis and Assessment of Antimicrobial Composites of Ag Nanoparticles or AgNO3 and Egg Shell Membranes. Molecules, 28(12), 4654. https://doi.org/10.3390/molecules28124654








