Post-Ripening and Key Glycosyltransferase Catalysis to Promote Sweet Mogrosides Accumulation of Siraitia grosvenorii Fruits
Abstract
:1. Introduction
2. Results
2.1. Mogrosides Metabolic Profile in Post-Ripening Samples
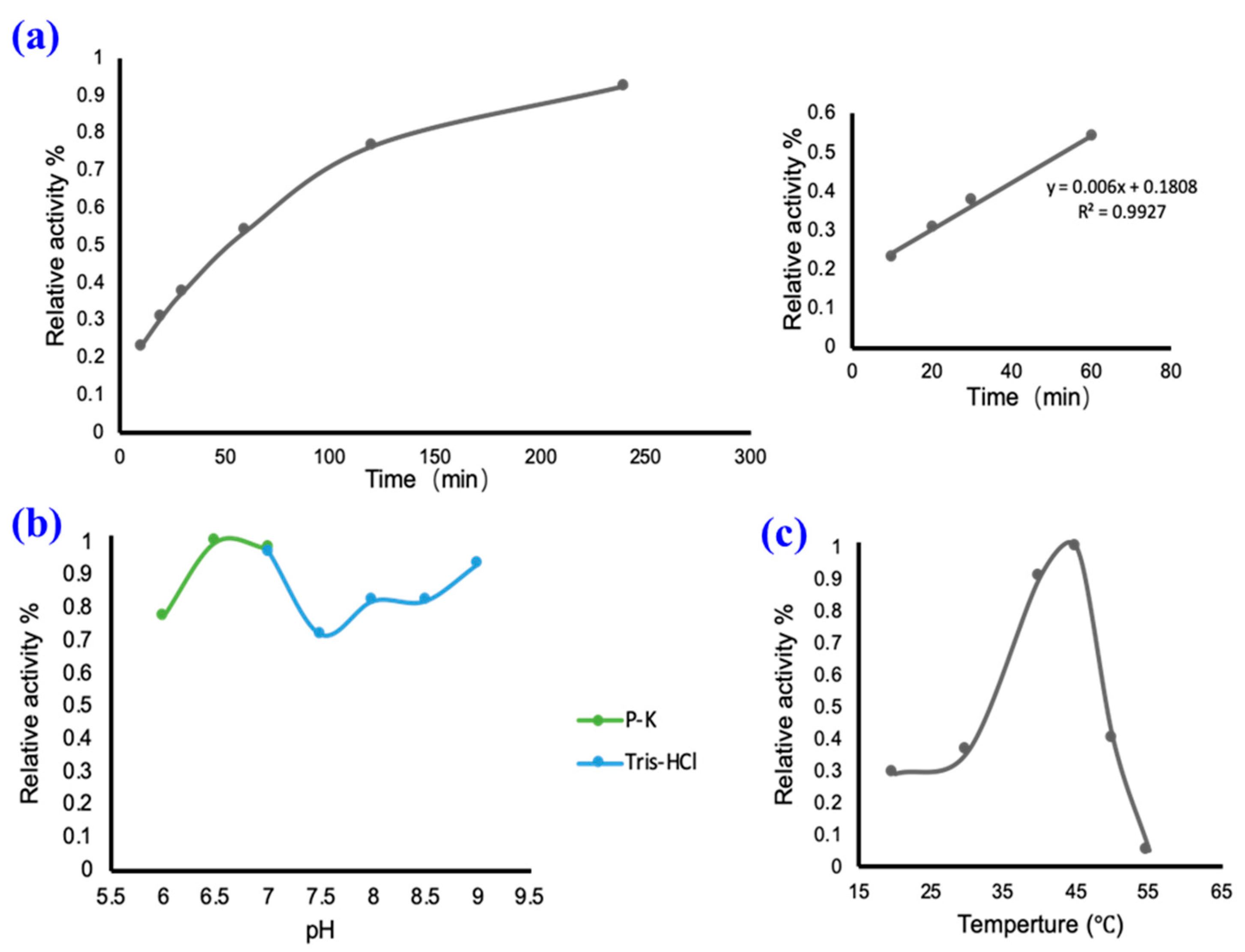
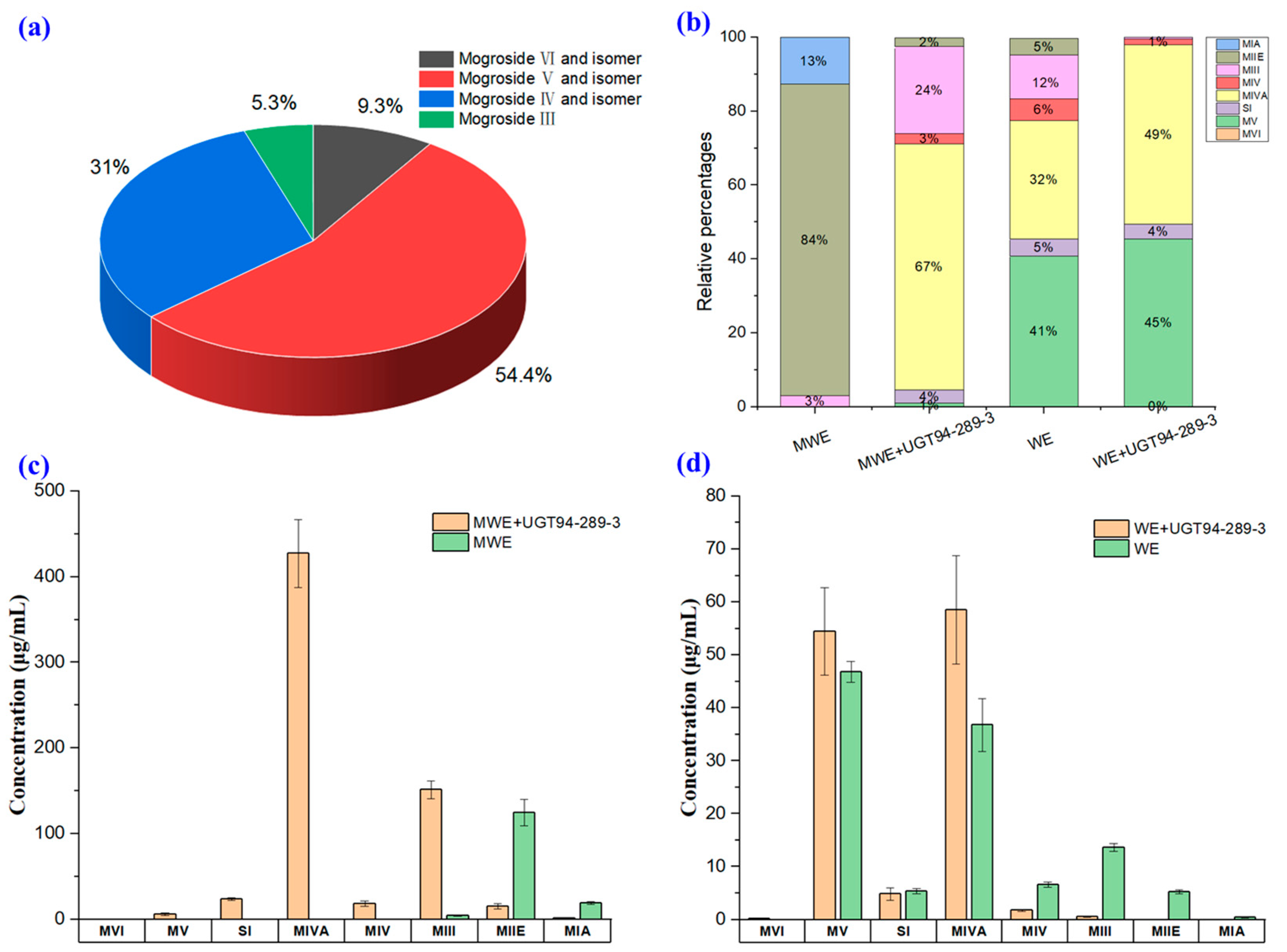
2.2. In Vitro Catalysis of UGT94-289-3
3. Materials and Methods
3.1. Chemicals, Reagents, and Plant Materials
3.2. Instruments
3.3. Sample Preparation for Metabolic Analysis
3.4. In Vitro Catalytic Experiment of UGT94-289-3
3.5. Metabolic Analysis Based on LC-MS
3.6. Statistical Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gong, X.; Chen, N.; Ren, K.; Jia, J.; Wei, K.; Zhang, L.; Lv, Y.; Wang, J.; Li, M. The fruits of Siraitia grosvenorii: A review of a Chinese food-medicine. Front. Pharmacol. 2019, 10, 1400. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Dai, L.; Liu, Y.; Dou, D.; Sun, Y.; Ma, L. Pharmacological activities of mogrosides. Future Med. Chem. 2018, 10, 845–850. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Jian, Y.; Wang, H.; Huang, H.; Gong, L.; Liu, G.; Yang, Y.; Wang, W. A Review of the Phytochemistry and Pharmacology of the Fruit of Siraitia grosvenorii (Swingle): A Traditional Chinese Medicinal Food. Molecules 2022, 27, 6618. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Peng, Y.; Wang, M.; Li, X. Anti-hyperglycemic and anti-hyperlipidemic effects of a special fraction of Luohanguo extract on obese T2DM rats. J. Ethnopharmacol. 2019, 247, 112273. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Shen, D.; Tang, Q.; Li, Z.; Jin, X.; Li, C. Mogroside V exerts anti-inflammatory effects on fine particulate matter-induced inflammation in porcine alveolar macrophages. Toxicol. Vitr. 2022, 80, 105326. [Google Scholar] [CrossRef]
- Lu, R.; Hu, J.; Liu, X.; Yu, L.; Hu, J.; Jiang, H.; Liu, S.; Li, M.; He, J.; Yang, X.; et al. Mogroside-rich extract from Siraitia grosvenorii fruits protects against heat stress-induced intestinal damage by ameliorating oxidative stress and inflammation in mice. Food Funct. 2023, 14, 1238–1247. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, X.; Wang, T.; Xu, R.; Shayiranbieke, A.; Zheng, X.; Jia, X.; Xiao, C.; Zhao, X. Screening of bioactive flavour compounds targeting muscarinic-3 acetylcholine receptor from Siraitia grosvenorii and evaluation of their synergistic anti-asthmatic activity. Food Chem. 2022, 395, 133593. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Qi, X.; Zou, J.; Sun, Z. Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J. Food Sci. Technol. 2018, 55, 1880–1888. [Google Scholar] [CrossRef]
- Chen, J.; Jiao, D.; Li, Y.; Jiang, C.; Tang, X.; Song, J.; Chen, Q. Mogroside V inhibits hyperglycemia-induced lung cancer cells metastasis through reversing EMT and damaging cytoskeleton. Curr. Cancer Drug Targets 2019, 19, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Yang, J.; Cao, F.; Xie, H.; Zhang, M.; Gong, Y.; Zhang, C. Mogroside IIIE, a novel anti-fibrotic compound, reduces pulmonary fibrosis through Toll-like receptor 4 pathways. J. Pharmacol. Exp. Ther. 2017, 361, 268–279. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Liu, J.; Liu, S.; Hu, J.; Wang, S.; Cui, K.; Yan, K.; Liu, X.; Wu, N.R.; Yang, X. Mogroside-rich extract from Siraitia grosvenorii fruits protects against the depletion of ovarian reserves in aging mice by ameliorating inflammatory stress. Food Funct. 2022, 13, 121–130. [Google Scholar] [CrossRef]
- Liu, H.; Qi, X.; Yu, K.; Lu, A.; Lin, K.; Zhu, J.; Zhang, M.; Sun, Z. AMPK activation is involved in hypoglycemic and hypolipidemic activities of mogroside-rich extract from Siraitia grosvenorii (Swingle) fruits on high-fat diet/streptozotocin-induced diabetic mice. Food Funct. 2019, 10, 151–162. [Google Scholar] [CrossRef]
- Xiao, R.; Liao, W.; Luo, G.; Qin, Z.; Han, S.; Lin, Y. Modulation of gut microbiota composition and short-chain fatty acid synthesis by mogroside V in an in vitro incubation system. ACS Omega 2021, 6, 25486–25496. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, Y.; Ebersole, J.; Huang, C.F. Insulin secretion stimulating effects of mogroside V and fruit extract of luo han kuo (Siraitia grosvenori Swingle) fruit extract. Acta Pharm. Sin. 2009, 44, 1252. [Google Scholar]
- Sung, Y.Y.; Kim, S.H.; Yuk, H.J.; Yang, W.K.; Lee, Y.M.; Son, E.; Kim, D.S. Siraitia grosvenorii residual extract attenuates ovalbumin-induced lung inflammation by down-regulating IL-4, IL-5, IL-13, IL-17, and MUC5AC expression in mice. Phytomedicine 2019, 61, 152835. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.H.; Li, C.; Zhang, Y.; Wu, G.L.; Li, D.C.; Li, C.Y. Study on the antitussive, expectorant and antispasmodic effects of saponin from Momordica grosvenori. Chin. Pharm. J. 2007, 42, 1534–1536. [Google Scholar]
- Liu, C.; Dai, L.H.; Dou, D.Q.; Ma, L.Q.; Sun, Y.X. A natural food sweetener with anti-pancreatic cancer properties. Oncogenesis 2016, 5, e217. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Ding, Y.; Wang, W.; Liao, L.; Zhong, J.; Sun, P.; Lei, F.; Zhang, Y.; Xie, W. Effects of mogrosides on high-fat-diet-induced obesity and nonalcoholic fatty liver disease in mice. Molecules 2018, 23, 1894. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Chen, W.; Liu, L.; Ping, Y.; Xie, B. Effect of a Siraitia grosvenori extract containing mogrosides on the cellular immune system of type 1 diabetes mellitus mice. Mol. Nutr. Food Res. 2006, 50, 732. [Google Scholar]
- Murata, Y.; Yoshikawa, S.; Suzuki, Y.A.; Sugiura, M.; Inui, H.; Nakano, Y. Sweetness characteristics of the triterpene glycosides in Siraitia grosvenori. Nippon. Shokuhin Kagaku Kogaku Kaishi 2006, 53, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Yang, X. A minor, sweet cucurbitane glycoside from Siraitia Grosvenorii. Nat. Prod. Commun. 2009, 4, 769–772. [Google Scholar] [CrossRef] [Green Version]
- Pawar, R.S.; Krynitsky, A.J.; Rader, J.I. Sweeteners from plants—With emphasis on Stevia rebaudiana (Bertoni) and Siraitia grosvenorii (Swingle). Anal. Bioanal. Chem. 2013, 405, 4397–4407. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Muguruma, M.; Moto, M.; Okamura, M.; Kashida, Y.; Mitsumori, K. Thirteen-week repeated dose toxicity of Siraitia grosvenori extract in Wistar Hannover (GALAS) rats. Food Chem. Toxicol. 2007, 45, 1231–1237. [Google Scholar] [CrossRef]
- Jakinovich, W.; Moon, C.; Choi, Y.H.; Kinghorn, A.D. Evaluation of plant extracts for sweetness using the Mongolian gerbil. J. Nat. Prod. 1990, 53, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Marone, P.A.; Borzelleca, J.F.; Merkel, D.; Heimbach, J.T.; Kennepohl, E. Twenty eight-day dietary toxicity study of Luo Han fruit concentrate in Hsd: SD rats. Food Chem. Toxicol. 2008, 46, 910–919. [Google Scholar] [CrossRef]
- Chun, L.I.; Lin, L.M.; Sui, F.; Wang, Z.M.; Huo, H.R.; Dai, L.; Jiang, T.L. Chemistry and pharmacology of Siraitia grosvenorii: A review. Chin. J. Nat. Med. 2014, 12, 89–102. [Google Scholar]
- Wang, L.; Liu, J.L.; Lu, F.L.; Song, Y.F.; Li, Y.Y.; Li, D.P. Improve the taste of bitter mogroside by enzymatic glycosylation method. Guihaia 2015, 35, 782–785. [Google Scholar]
- Matsumoto, K.; Kasai, R.; Ohtani, K.; Tanaka, O. Minor cucurbitane-glycosides from fruits of Siraitia grosvenori (Cucurbitaceae). Chem. Pharm. Bull. 2008, 38, 2030–2032. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, T.; Arihara, S.; Nakajima, T.; Okuhira, M. Studies on the constituents of fructus Momordicae. I. On the sweet princple. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1983, 103, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Ma, X.; Mo, C.; Wilson, I.W.; Song, C.; Zhao, H.; Yang, Y.; Fu, W.; Qiu, D. An efficient approach to finding Siraitia grosvenorii triterpene biosynthetic genes by RNA-seq and digital gene expression analysis. BMC Genom. 2011, 12, 343. [Google Scholar] [CrossRef] [Green Version]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena—An overview. Crit Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef]
- Itkin, M.; Davidovich-Rikanati, R.; Cohen, S.; Portnoy, V.; Doron-Faigenboim, A.; Oren, E.; Freilich, S.; Tzuri, G.; Baranes, N.; Shen, S. The biosynthetic pathway of the nonsugar, high-intensity sweetener mogroside V from Siraitia grosvenorii. Proc. Natl. Acad. Sci. USA 2016, 113, E7619. [Google Scholar] [CrossRef] [Green Version]
- Ukiya, M.; Akihisa, T.; Tokuda, H.; Toriumi, M.; Mukainaka, T.; Banno, N.; Kimura, Y.; Hasegawa, J.I.; Nishino, H. Inhibitory effects of cucurbitane glycosides and other triterpenoids from the fruit of Momordica grosvenori on epstein-barr virus early antigen induced by tumor promoter 12-O-tetradecanoylphorbol-13-acetate. J. Agric. Food Chem. 2002, 50, 6710. [Google Scholar] [CrossRef]
- Fang, C.; Wang, Q.; Liu, X.; Xu, G. Metabolic profiling analysis of Siraitia grosvenorii revealed different characteristics of green fruit and saccharified yellow fruit. J. Pharm. Biomed. Anal. 2017, 145, 158–168. [Google Scholar] [CrossRef]
- Qiao, J.; Luo, Z.; Gu, Z.; Zhang, Y.; Ma, X. Identification of a novel specific cucurbitadienol synthase allele in Siraitia grosvenorii correlates with high catalytic efficiency. Molecules 2019, 24, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mou, J.F.; Wang, S.X.; Luo, Q.; Wang, P.C.; Huang, S.X.; Su, X.J.; Chen, X.; Liang, C.Q.; Zhou, X.L. Determination of content of 6 kinds of mogrosides in Siraitia grosvenorii by Using HPLC-MS/MS. Food Res. Dev. 2018, 38, 139–144. [Google Scholar]
- Smanski, M.J.; Zhou, H.; Claesen, J.; Shen, B.; Fischbach, M.A.; Voigt, C.A. Synthetic biology to access and expand nature’s chemical diversity. Nat. Rev. Microbiol. 2016, 14, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Mipeshwaree Devi, A.; Khedashwori Devi, K.; Premi Devi, P.; Lakshmipriyari Devi, M.; Das, S. Metabolic engineering of plant secondary metabolites: Prospects and its technological challenges. Front. Plant Sci 2023, 14, 1171154. [Google Scholar] [CrossRef]
- Qiao, J.; Luo, Z.; Cui, S.; Zhao, H.; Tang, Q.; Mo, C.; Ma, X.; Ding, Z. Modification of isoprene synthesis to enable production of curcurbitadienol synthesis in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2019, 46, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mu, S.; Yang, J.; Liu, C.; Zhang, Y.; Chen, P.; Zeng, Y.; Zhu, Y.; Sun, Y. Glycosyltransferase engineering and multi-glycosylation routes development facilitating synthesis of high-intensity sweetener mogrosides. iScience 2022, 25, 105222. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Liu, T.; Xie, L.; Mo, C.; Qiao, J.; Huang, X.; Cui, S.; Jia, X.; Luo, Z.; Ma, X. Heterologous mogrosides biosynthesis in cucumber and tomato by genetic manipulation. Commun. Biol. 2023, 6, 191. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Ogawa, T.; Suzuki, Y.A.; Yoshikawa, S.; Nakano, Y. Digestion and absorption of Siraitia grosvenori triterpenoids in the rat. Biosci. Biotechnol. Biochem. 2010, 74, 673–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.T.; Yang, J.T.; Chen, K.I.; Wang, T.Y.; Lu, T.J.; Cheng, K.C. Hydrolyzation of mogrosides: Immobilized β-glucosidase for mogrosides deglycosylation from Lo Han Kuo. Food Sci. Nutr. 2019, 7, 834–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Liu, S.; Bian, L.; Li, Z.; Luo, C.; Chen, Y.; Wu, X. Engineering of a UDP-Glycosyltransferase for the efficient whole-cell biosynthesis of siamenoside I in Escherichia coli. J. Agric. Food Chem. 2022, 70, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
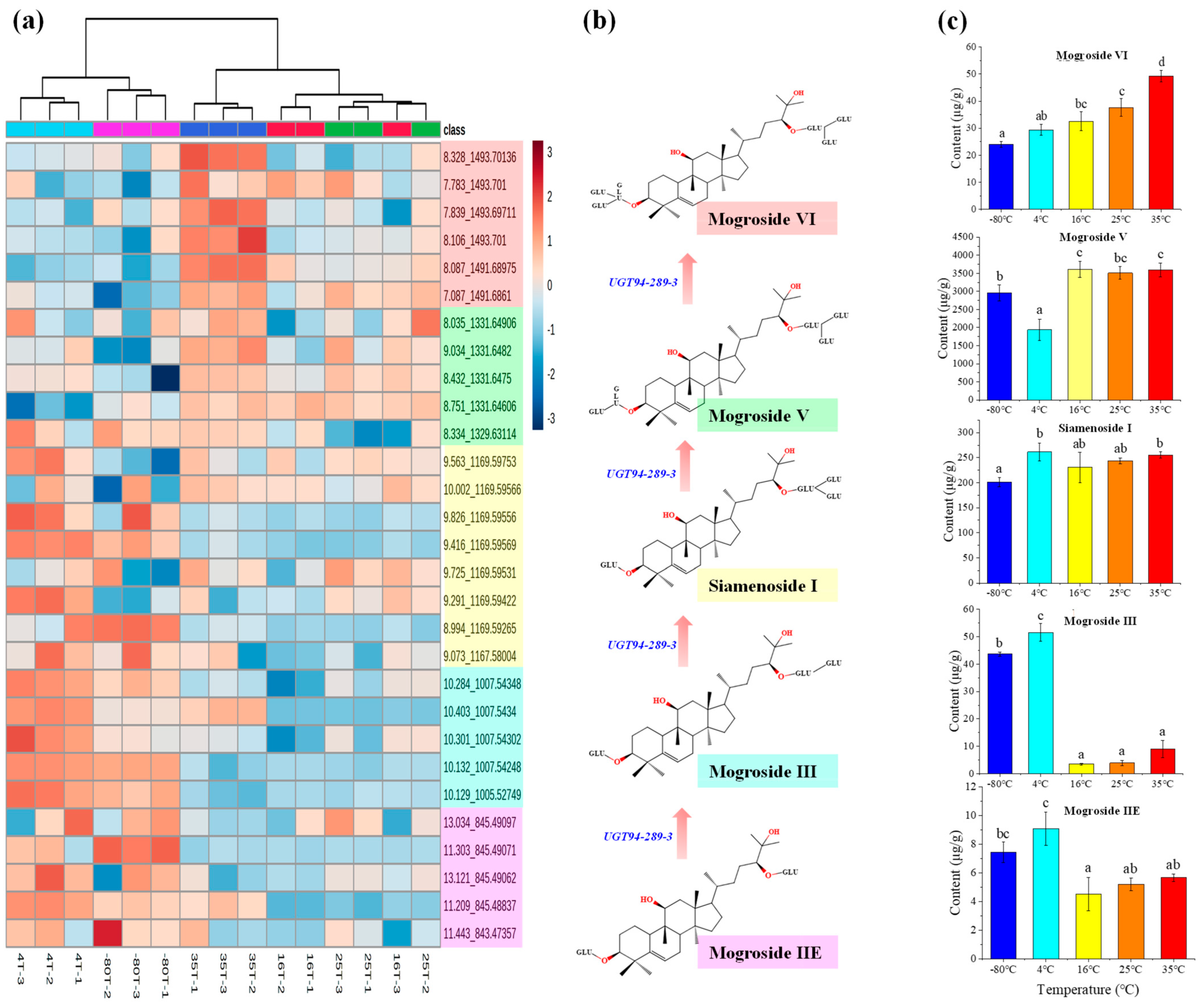
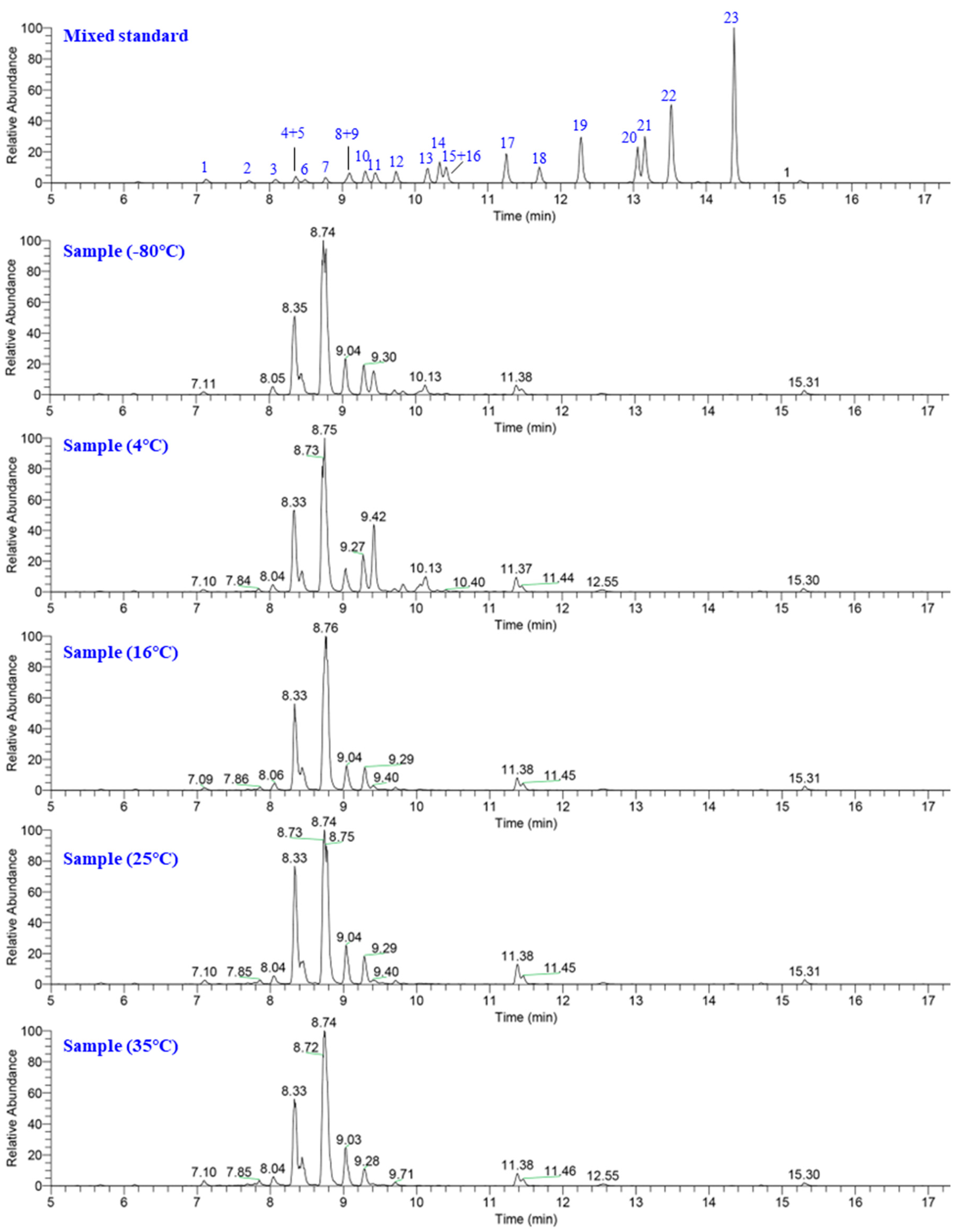
| Compound ID | Compound Name | Retention Time (min) | Molecular Formula | Calc. MW | MS (m/z) | MS2 Fragment (m/z) |
|---|---|---|---|---|---|---|
| 8.328_1493.70136 | Mogroside VIA | 8.328 | C66H112O34 | 1448.7035 | 1447.6924; 1493.7002 | 1447.6924; 1285.6514; 1123.5892; 961.5258; 799.4753; 637.4352 |
| 7.783_1493.701 | Isomer of mogroside VI | 7.783 | C66H112O34 | 1448.7035 | 1447.6924; 1493.7002 | 1447.6924; 1285.6514; 1123.5892; 961.5258; 799.4753; 637.4352 |
| 7.839_1493.69711 | Mogroside VI | 7.839 | C66H112O34 | 1448.7035 | 1447.6924; 1493.7002 | 1447.6924; 1285.6514; 1123.5892; 961.5258; 799.4753; 637.4352 |
| 8.106_1493.701 | Isomer of mogroside VI | 8.106 | C66H112O34 | 1448.7035 | 1447.6924; 1493.7002 | 1447.6924; 1285.6514; 1123.5892; 961.5258; 799.4753; 637.4352 |
| 8.087_1491.68975 | 11-oxo-mogroside VI | 8.087 | C66H110O34 | 1446.6878 | 1447.6924; 1493.7002 | 1447.6924; 1285.6514; 1123.5892; 961.5258; 799.4753; 637.4352 |
| 7.087_1491.6861 | Isomer of 11-oxo-mogroside VI | 7.087 | C66H110O34 | 1446.6878 | 1491.6869; 1445.6812 | 1445.6812; 1283.6067; 1121.5883; 797.4802 |
| 8.035_1331.64906 | 11-epi-morgroside V | 8.035 | C60H102O29 | 1286.6506 | 1331.6470; 1285.6514 | 1285.6514; 1123.5892; 961.5258; 799.4753; 637.4352 |
| 9.034_1331.6482 | Isomogroside V | 9.034 | C60H102O29 | 1286.6506 | 1331.6470; 1285.6514 | 1285.6514; 1123.5892; 961.5258; 799.4753; 637.4352 |
| 8.432_1331.6475 | Isomer of mogroside V | 8.432 | C60H102O29 | 1286.6506 | 1331.6470; 1285.6514 | 1285.6514; 1123.5892; 961.5258; 799.4753; 637.4352 |
| 8.751_1331.64606 | Mogroside V | 8.751 | C60H102O29 | 1286.6506 | 1331.6470; 1285.6514 | 1285.6514; 1123.5892; 961.5258; 799.4753; 637.4352 |
| 8.334_1329.63114 | 11-oxo-mogroside V | 8.334 | C60H100O29 | 1284.635 | 1329.6327; 1283.6267 | 1283.6267; 1121.5709; 959.5239; 797.4661; 635.4319 |
| 9.563_1169.59753 | Isomer of mogroside IV | 9.563 | C54H92O24 | 1124.5978 | 1169.5940; 1123.5892 | 1123.5892; 961.5366; 799.4835; 637.4275 |
| 10.002_1169.59566 | Isomer of mogroside IV | 10.002 | C54H92O24 | 1124.5978 | 1169.5940; 1123.5892 | 1123.5892; 961.5366; 799.4835; 637.4275 |
| 9.826_1169.59556 | Isomer of mogroside IV | 9.826 | C54H92O24 | 1124.5978 | 1169.5940; 1123.5892 | 1123.5892; 961.5366; 799.4835; 637.4275 |
| 9.416_1169.59569 | Mogroside IVA | 9.416 | C54H92O24 | 1124.5978 | 1169.5940; 1123.5892 | 1123.5892; 961.5366; 799.4835; 637.4275 |
| 9.725_1169.59531 | Mogroside IV | 9.725 | C54H92O24 | 1124.5978 | 1169.5940; 1123.5892 | 1123.5892; 961.5366; 799.4835; 637.4275 |
| 9.291_1169.59422 | Siamenoside I | 9.291 | C54H92O24 | 1124.5978 | 1169.5940; 1123.5892 | 1123.5892; 961.5366; 799.4835; 637.4275 |
| 8.994_1169.59265 | Isomer of mogroside IV | 8.994 | C54H92O24 | 1124.5978 | 1169.5940; 1123.5892 | 1123.5892; 961.5366; 799.4835; 637.4275 |
| 9.073_1167.58004 | 11-O-SiamenosideⅠ | 9.073 | C54H90O24 | 1122.5822 | 1167.5787; 1121.5725 | 1121.5725; 959.5148; 797.3799; 635.4319 |
| 10.284_1007.54348 | Mogroside IIIE | 10.284 | C48H82O19 | 962.545 | 1007.5423; 961.5368 | 961.5368; 799.4839; 637.4294; 475.3731 |
| 10.403_1007.5434 | Isomer of mogroside III | 10.403 | C48H82O19 | 962.545 | 1007.5423; 961.5368 | 961.5368; 799.4839; 637.4294; 475.3731 |
| 10.301_1007.54302 | Mogroside IIIA2 | 10.301 | C48H82O19 | 962.545 | 1007.5423; 961.5368 | 961.5368; 799.4839; 637.4294; 475.3731 |
| 10.132_1007.54248 | Mogroside III | 10.132 | C48H82O19 | 962.545 | 1007.5423; 961.5368 | 961.5368; 799.4839; 637.4294; 475.3731 |
| 10.129_1005.52749 | 11-oxo-mogroside III | 10.129 | C48H80O19 | 960.5294 | 1005.5281; 959.5336 | 959.5336; 797.4736; 635.4147; 161.0452 |
| 13.034_845.49097 | Mogroside IIa | 13.034 | C42H72O14 | 800.4922 | 845.4894; 799.4837 | 799.4837; 637.4323; 161.0454 |
| 11.303_845.49071 | Isomer of mogroside IIE | 11.303 | C42H72O14 | 800.4922 | 845.4894; 799.4837 | 799.4837; 637.4323; 161.0454 |
| 13.121_845.49062 | Mogroside IIA1 | 13.121 | C42H72O14 | 800.4922 | 845.4894; 799.4837 | 799.4837; 637.4323; 161.0454 |
| 11.209_845.48837 | mogroside IIE | 11.209 | C42H72O14 | 800.4922 | 845.4894; 799.4837 | 799.4837; 637.4323; 161.0454 |
| 11.443_843.47357 | 11-oxo-mogroside II | 11.443 | C42H70O14 | 798.4765 | 843.4745; 797.4689 | 797.4689; 635.4147 + B1:G30; 161.0454 |
| Post-Ripening Temperature | Content of Mogrosides (μg/g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mogroside Ⅵ | Mogroside Ⅴ | Miamenoside Ⅰ | Mogroside ⅣA | Mogroside Ⅳ | Mogroside Ⅲ | Mogroside ⅡE | Mogroside IA | |
| −80 °C | 24.21 ± 2.45 | 2959.34 ± 222.72 | 200.99 ± 8.72 | 158.84 ± 9.98 | 23.08 ± 3.7 | 43.64 ± 0.75 | 7.44 ± 0.71 | ND |
| 4 °C | 29.42 ± 2.11 | 1940.63 ± 296.75 | 261.41 ± 17.95 | 570.51 ± 59.09 | 42.58 ± 5.36 | 51.58 ± 3.21 | 9.09 ± 1.16 | ND |
| 16 °C | 32.56 ± 3.48 | 3606.5 ± 219.61 | 230.52 ± 30.05 | 135.66 ± 32.65 | 30.79 ± 4.97 | 3.48 ± 0.37 | 4.53 ± 1.17 | ND |
| 25 °C | 37.64 ± 3.25 | 3513.02 ± 175.61 | 243.55 ± 6.18 | 92.46 ± 25.95 | 42.99 ± 4.23 | 3.89 ± 0.94 | 5.21 ± 0.43 | ND |
| 35 °C | 49.3 ± 2.1 | 3593.92 ± 191.57 | 255.52 ± 5.92 | 73.05 ± 11.45 | 71.03 ± 16.46 | 8.99 ± 3.18 | 5.68 ± 0.26 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.; Zang, Y.; Xie, L.; Mo, C.; Su, J.; Jia, X.; Luo, Z.; Ma, X. Post-Ripening and Key Glycosyltransferase Catalysis to Promote Sweet Mogrosides Accumulation of Siraitia grosvenorii Fruits. Molecules 2023, 28, 4697. https://doi.org/10.3390/molecules28124697
Cui S, Zang Y, Xie L, Mo C, Su J, Jia X, Luo Z, Ma X. Post-Ripening and Key Glycosyltransferase Catalysis to Promote Sweet Mogrosides Accumulation of Siraitia grosvenorii Fruits. Molecules. 2023; 28(12):4697. https://doi.org/10.3390/molecules28124697
Chicago/Turabian StyleCui, Shengrong, Yimei Zang, Lei Xie, Changming Mo, Jiaxian Su, Xunli Jia, Zuliang Luo, and Xiaojun Ma. 2023. "Post-Ripening and Key Glycosyltransferase Catalysis to Promote Sweet Mogrosides Accumulation of Siraitia grosvenorii Fruits" Molecules 28, no. 12: 4697. https://doi.org/10.3390/molecules28124697
APA StyleCui, S., Zang, Y., Xie, L., Mo, C., Su, J., Jia, X., Luo, Z., & Ma, X. (2023). Post-Ripening and Key Glycosyltransferase Catalysis to Promote Sweet Mogrosides Accumulation of Siraitia grosvenorii Fruits. Molecules, 28(12), 4697. https://doi.org/10.3390/molecules28124697






