Analytical Quality by Design-Compliant Development of a Cyclodextrin-Modified Micellar ElectroKinetic Chromatography Method for the Determination of Trimecaine and Its Impurities
Abstract
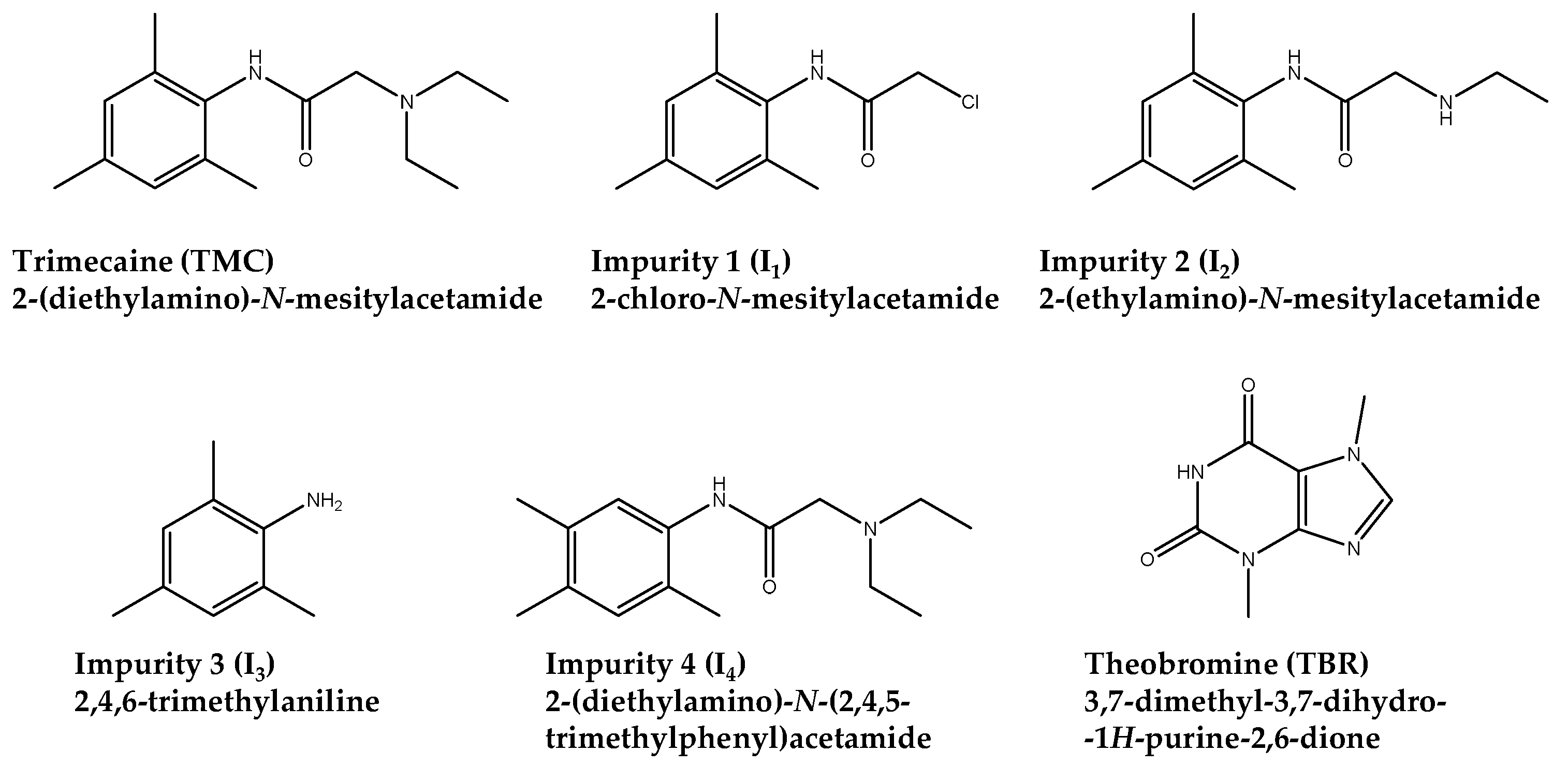
1. Introduction
2. Results and Discussion
2.1. Analytical Target Profile
- for Selectivity, a complete resolution of the peaks of the analytes and no interference with the injection solution’s excipient;
- Quantitation Limit (QL) for the impurities ≤0.1% w/w with respect to the API;
- minimum Working Range for the impurities, from the QL to 1% with respect to the API test concentration, and minimum Working Range for the API, from 80 to 120% of the test concentration;
- with respect to Accuracy, measured recovery values included in the range of 98 to 102% for the API and in the range of 95 to 105% for the impurities;
- concerning Precision, evaluated in terms of Repeatability, RSD values within 2% for the API and within 5% for the impurities, with a higher accepted value at the QL (RSD ≤ 15%).
2.2. Knowledge Management
2.3. Risk Assessment and Critical Method Parameters
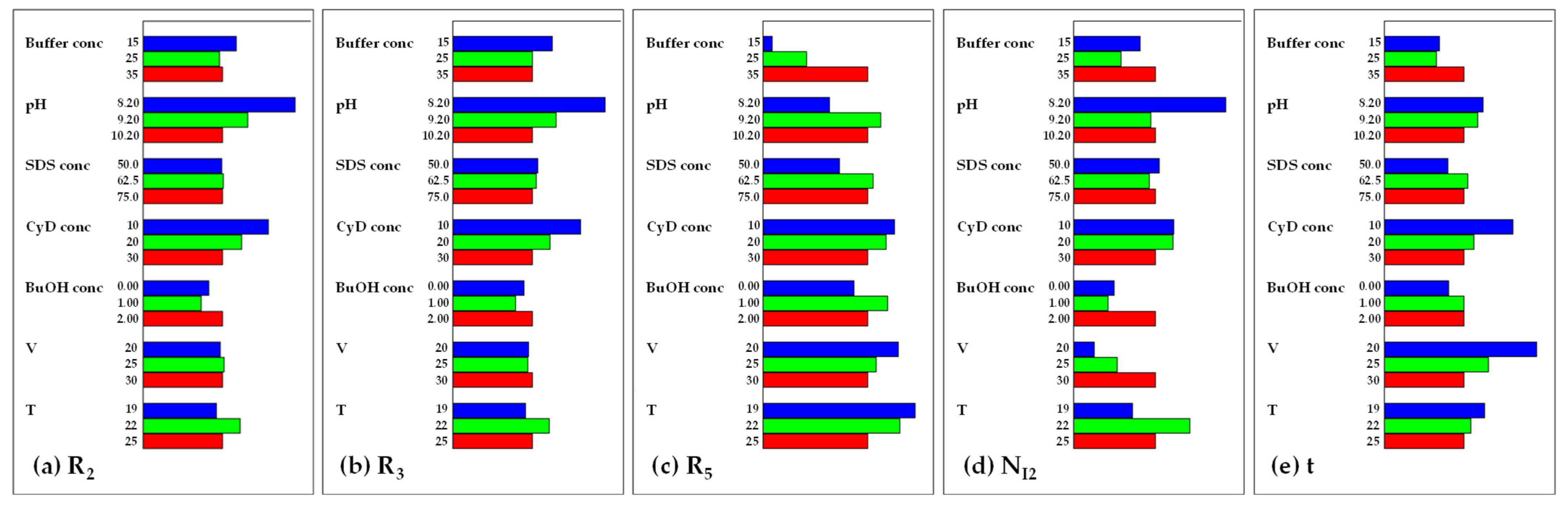
2.4. Screening DoE
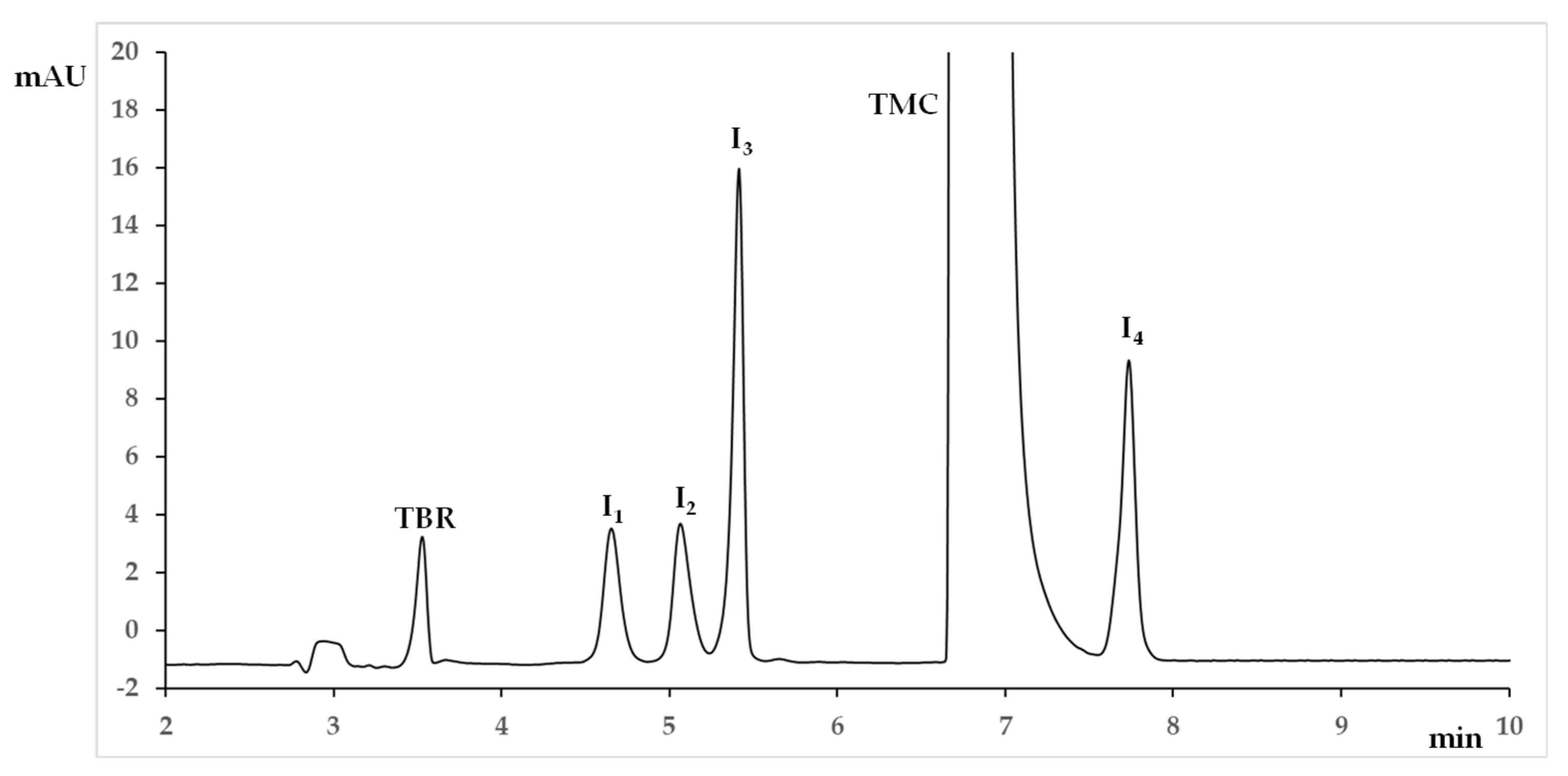
- the internal standard TBR and I1 were always the first and the second migrating peaks, respectively, and presented no separation issues;
- I2 was the third migrating peak in all the experiments, except for runs no. 1, nos. 3–6, and no. 10, where the inversion of the migration order occurred with respect to I3. Consequently, the resolution between I2 and I3 assumed both positive and negative values: a positive value when I2 was the first migrating peak (lower migration time) and I3 was the second migrating peak (higher migration time), a negative value when a change in migration order occurred, with I3 migrating before I2;
- Resolution between TMC and the first adjacent migrating peak (I2 or I3) was never critical and could be discarded from the data treatment;
- Efficiency of the I2 peak was critical with respect to the other peaks so it was included in data treatment;
- I4 was the last migrating peak in all the experiments and its migration time corresponded to analysis time.
- To obtain a baseline resolution between all the peak pairs: R1 (TBR/I1); R2 (I1/I2); R3 (I2/I3); R4 (I3/TMC); R5 (TMC/I4). As mentioned above, for R1 and R4 no critical issues were observed and these responses were discarded from the data treatment;
- To constrain the R3 (I2/I3) resolution towards positive values, so that the final migration order is first I2 and then I3;
- To maximize I2 efficiency NI2 (I2 number of theoretical plates);
- To minimize analysis time t (calculated as I4 migration time).
2.5. Response Surface Methodology
- pH was significant on all the CMAs and its most important linear effects were exerted on R2 and R3, although with opposite signs (negative for R2 and positive for R3, respectively);
- Buffer conc had a notable positive linear effect both on R5 and analysis time;
- CyD conc was significant in all the responses apart from NI2. The effect on resolution was the opposite: an increase in CyD conc leads to a minimization of R2 and a maximization of R3 and R5;
- Voltage has a predominantly negative effect on analysis time, as expected, but also a significant influence on R2;
- The effect of BuOH conc is significant, even if quite limited, on both R3, R5, and analysis time;
- Quadratic effects were highlighted for pH, CyD conc, and V. The most important of them was the negative one, exerted by pH, on NI2;
- Significant interaction effects were found on R3, NI2, and analysis time, but were all limited in their extent.
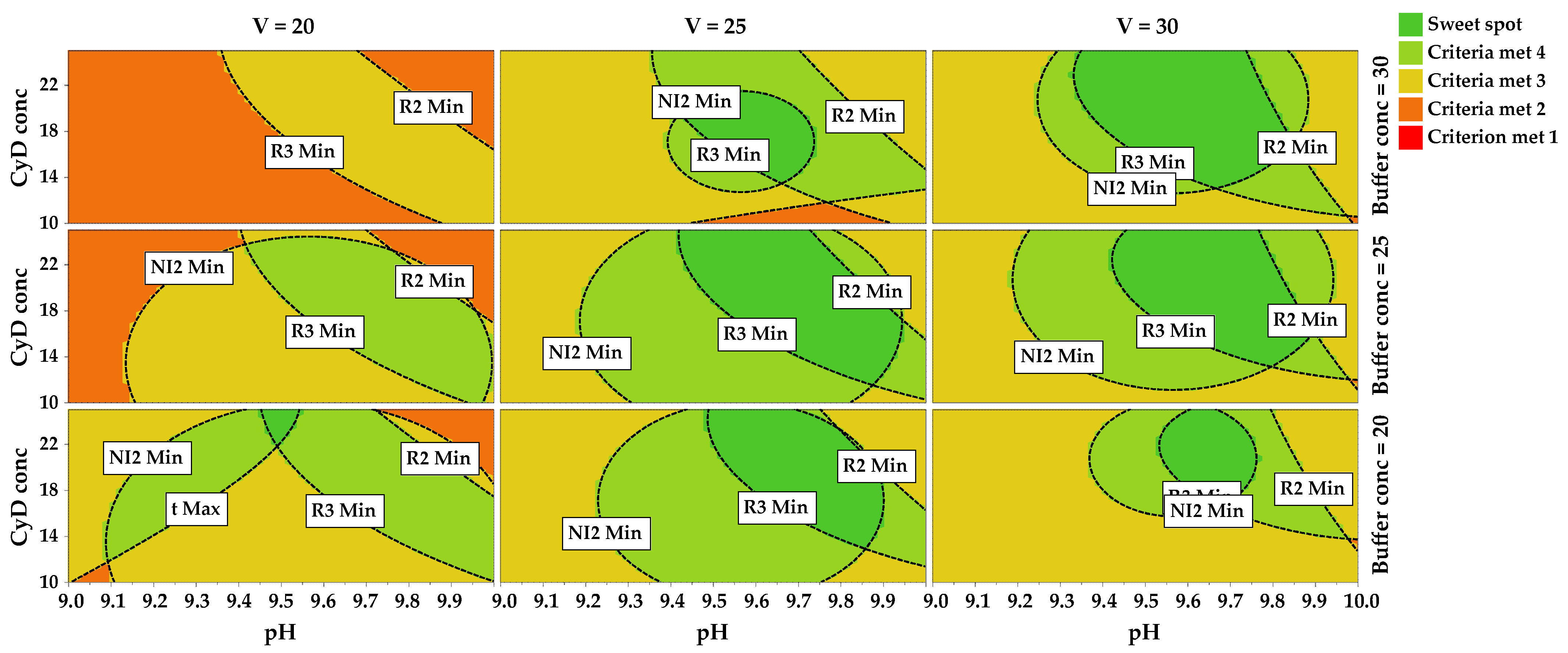
- For R2, a wide zone of the domain led to acceptable predicted values, excluding the area corresponding to high pH values and high CyD conc (Supplementary Figure S4);
- Concerning R3, the plot was divided into distinct zones corresponding to negative or positive values of predicted response; the desired zone corresponded to high values of pH and high values of CyD conc, and the higher values of this CMA were obtained at low values of V (Supplementary Figure S5);
- Looking at R5, the target value was obtained throughout all the experimental domains. A curvature of the isoresponse lines was observed due to the presence of quadratic effects of both pH and CyD conc (Supplementary Figure S6);
- The same curvature was also observed for NI2, for which the target approximately corresponded to the zone located at the center of the domain of CyD conc and V (Supplementary Figure S7);
- For analysis time, as expected, the best results were located at high values of V, but the target could be achieved also at medium values (Supplementary Figure S8).
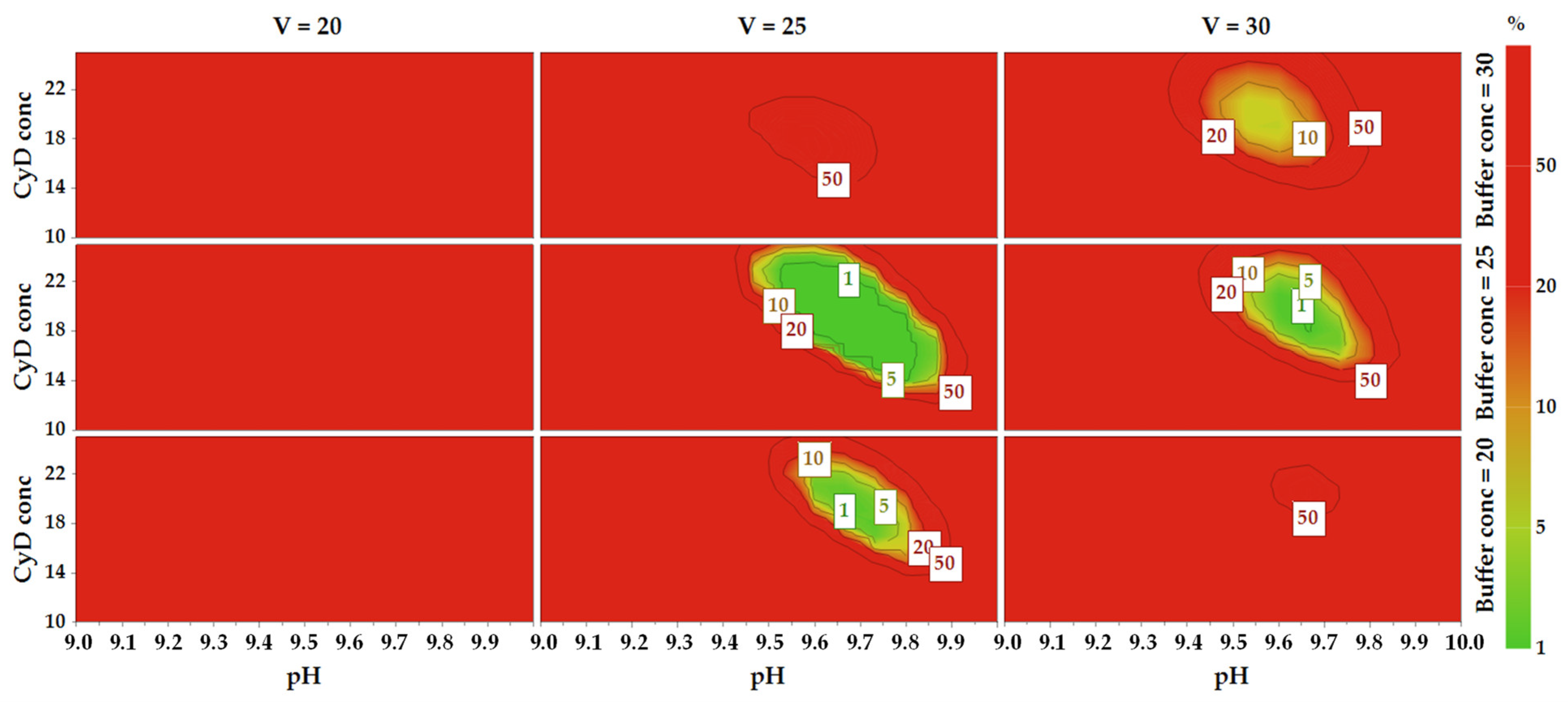
2.6. Method Operable Design Region
2.7. Robustness and Method Control
2.8. Method Validation and Application
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Solutions and Sample Preparation
3.3. CE Instrumentation and Analysis
3.4. Data Analysis and Software
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Řemínek, R.; Foret, F. Capillary electrophoretic methods for quality control analyses of pharmaceuticals: A review. Electrophoresis 2021, 42, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Aturki, Z.; Rocco, A.; Rocchi, S.; Fanali, S. Current applications of miniaturized chromatographic and electrophoretic techniques in drug analysis. J. Pharm. Biomed. Anal. 2014, 101, 194–220. [Google Scholar] [CrossRef] [PubMed]
- Görög, S. Critical review of reports on impurity and degradation product profiling in the last decade. Trends Anal. Chem. 2018, 101, 2–16. [Google Scholar] [CrossRef]
- Jouyban, A.; Kenndler, E. Impurity analysis of pharmaceuticals using capillary electromigration methods. Electrophoresis 2008, 29, 3531–3551. [Google Scholar] [CrossRef]
- Stěpánová, S.; Kašička, V. Determination of impurities and counterions of pharmaceuticals by capillary electromigration methods. J. Sep. Sci. 2014, 37, 2039–2055. [Google Scholar] [CrossRef]
- El Deeb, S.; Wätzig, H.; Abd El-Hady, D.; Sänger-van de Griend, C.; Scriba, G.K. Recent advances in capillary electrophoretic migration techniques for pharmaceutical analysis (2013–2015). Electrophoresis 2016, 37, 1591–1608. [Google Scholar] [CrossRef]
- Shah, M.; Patel, N.; Tripathi, N.; Vyas, V.K. Capillary electrophoresis methods for impurity profiling of drugs: A review of the past decade. J. Pharm. Anal. 2022, 12, 15–28. [Google Scholar] [CrossRef]
- Dohnal, J.; Vytras, K. A determination of local anaesthetics in parenteral preparations by potentiometric titration with ion selective electrode. Farm. Obz. 1985, 54, 405–411. [Google Scholar]
- Kereichuk, A.S.; Pantsurkin, V.I.; Ptukha, E.V.; Potemkin, K.D.; Chekryshkina, L.A. Determination of trimecaine and lidocaine using an ion-sensitive electrode. J. Anal. Chem. USSR 1990, 45, 409–413. [Google Scholar]
- Netesina, I.P.; Tkach, V.I.; Tsyganok, L.P.; Kopytin, A.V.; Politov, Y.A. Determination of novacaine, sovcaine, and trimecaine using ion-sensitive electrodes. J. Anal. Chem. 1992, 47, 523–526. [Google Scholar]
- Plotycya, S.; Dubenska, L.; Blazheyeyskiy, M.; Pysarevska, S.; Sarahman, O. Determination of local anesthetics of amide group in pharmaceutical preparations by cyclic voltammetry. Electroanalysis 2016, 28, 2575–2581. [Google Scholar] [CrossRef]
- Matysová, L.; Koblová, P.; Galla, L.; Sklenářová, H.; Havlíková, L.; Solich, P. Chromatographic determination of active compounds in topical formulations. Anal. Methods 2011, 4, 1525–1529. [Google Scholar] [CrossRef]
- Piotrovskii, V.K.; Blagodatskikh, S.V. Determination of pyromecaine and trimecaine in blood serum by GLC method. Khim. Farm. Zh. 1983, 17, 1381–1383. [Google Scholar] [CrossRef]
- Björk, M.; Pettersson, K.-J.; Österlöf, G. Capillary gas chromatographic method for the simultaneous determination of local anaesthetic in plasma samples. J. Chromatogr. Biomed. 1990, 533, 229–234. [Google Scholar] [CrossRef]
- Stránský, Z.; Chmela, Z.; Pec, P.; Safarik, L. Determination of trimecaine metabolites in blood plasma by capillary isotachophoresis. J. Chromatogr. 1985, 342, 167–174. [Google Scholar] [CrossRef]
- Department of Health and Human Services, U.S Food and Drug Administration. Pharmaceutical CGMPs for the 21st Century—A Risk-Based Approach; Final Report; Department of Health and Human Services, U.S Food and Drug Administration: Siler Spring, MD, USA, 2004.
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Pharmaceutical Development Q8(R2); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2009. [Google Scholar]
- Borman, P.; Truman, K.; Thompson, D.; Nethercote, P.; Chatfield, M. The application of Quality by Design to analytical methods. Pharm. Technol. 2007, 31, 142–152. [Google Scholar]
- Vogt, F.G.; Kord, A.S. Development of quality-by-design analytical methods. J. Pharm. Sci. 2011, 100, 797–812. [Google Scholar] [CrossRef]
- Monks, K.; Molnár, I.; Rieger, H.J.; Bogáti, B.; Szabó, E. Quality by Design: Multidimensional exploration of the design space in high performance liquid chromatography method development for better robustness before validation. J. Chromatogr. A 2012, 1232, 218–230. [Google Scholar] [CrossRef]
- Orlandini, S.; Pinzauti, S.; Furlanetto, S. Application of quality by design to the development of analytical separation methods. Anal. Bioanal. Chem. 2013, 405, 443–450. [Google Scholar] [CrossRef]
- Rozet, E.; Lebrun, P.; Hubert, P.; Debrus, B.; Boulanger, B. Design Spaces for analytical methods. Trends Anal. Chem. 2013, 42, 157–167. [Google Scholar] [CrossRef]
- Deidda, R.; Orlandini, S.; Hubert, P.; Hubert, C. Risk-based approach for method development in pharmaceutical quality control context: A critical review. J. Pharm. Biomed. Anal. 2018, 161, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Tome, T.; Žigart, N.; Časar, Z.; Obreza, A. Development and optimization of liquid chromatography analytical methods by using AQbD principles: Overview and recent advances. Org. Process Res. Dev. 2019, 23, 1784–1802. [Google Scholar] [CrossRef]
- Ermer, J.; Aguiar, D.; Boden, A.; Ding, B.; Obeng, D.; Rose, M.; Vokrot, J. Lifecycle management in pharmaceutical analysis: How to establish an efficient and relevant continued performance monitoring program. J. Pharm. Biomed. Anal. 2020, 181, 113051. [Google Scholar] [CrossRef] [PubMed]
- Volta e Sousa, L.; Gonçalves, R.; Menezes, J.C.; Ramos, A. Analytical method lifecycle management in pharmaceutical industry: A review. AAPS PharmSciTech 2021, 22, 128. [Google Scholar] [CrossRef] [PubMed]
- Borman, P.; Campa, C.; Delpierre, G.; Hook, E.; Jackson, P.; Kelley, W.; Protz, M.; Vandeputte, O. Selection of analytical technology and development of analytical procedures using the analytical target profile. Anal. Chem. 2022, 94, 559–570. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Analytical Procedure Development Q14; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2022. [Google Scholar]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures Q2(R2); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2022. [Google Scholar]
- Dispas, A.; Avohou, H.T.; Lebrun, P.; Hubert, P.; Hubert, C. Quality by Design’ approach for the analysis of impurities in pharmaceutical drug products and drug substances. Trends Anal. Chem. 2018, 101, 24–33. [Google Scholar] [CrossRef]
- Pasquini, B.; Gotti, R.; Villar-Navarro, M.; Douša, M.; Renai, L.; Del Bubba, M.; Orlandini, S.; Furlanetto, S. Analytical quality by design in the development of a solvent-modified micellar electrokinetic chromatography method for the determination of sitagliptin and its related compounds. J. Pharm. Biomed. Anal. 2021, 202, 114163. [Google Scholar] [CrossRef]
- Krait, S.; Schneidmadel, F.R.; Scriba, G.K.E. Quality by design-assisted development of a capillary electrophoresis method for the enantiomeric purity determination of tenofovir. Electrophoresis 2022, 43, 964–969. [Google Scholar] [CrossRef]
- Modroiu, A.; Krait, S.; Hancu, G.; Scriba, G.K.E. Quality by design-guided development of a capillary electrophoresis method for the chiral purity determination of silodosin. J. Pharm. Biomed. Anal. 2023, 222, 115117. [Google Scholar] [CrossRef]
- Orlandini, S.; Gotti, R.; Furlanetto, S. Multivariate optimization of capillary electrophoresis methods: A critical review. J. Pharm. Biomed. Anal. 2014, 87, 290–307. [Google Scholar] [CrossRef]
- Orlandini, S.; Hancu, G.; Szabó, Z.-I.; Modroiu, A.; Papp, L.-A.; Gotti, R.; Furlanetto, S. New trends in the quality control of enantiomeric drugs: Quality by Design-compliant development of chiral capillary electrophoresis methods. Molecules 2022, 27, 7058. [Google Scholar] [CrossRef]
- Perovani, I.S.; Serpellone, C.O.; de Oliveira, A.R.M. An appraisal of experimental designs: Application to enantioselective capillary electromigration techniques. Electrophoresis 2021, 42, 1726–1743. [Google Scholar] [CrossRef]
- Caprini, C.; Pasquini, B.; Melani, F.; Del Bubba, M.; Giuffrida, A.; Calleri, E.; Orlandini, S.; Furlanetto, S. Exploring the intermolecular interactions acting in solvent-modified MEKC by Molecular Dynamics and NMR: The effect of n-butanol on the separation of diclofenac and its impurities. J. Pharm. Biomed. Anal. 2018, 149, 249–257. [Google Scholar] [CrossRef]
- Lewis, G.A.; Mathieu, D.; Phan-Tan-Luu, R. Pharmaceutical Experimental Design; Marcel Dekker: New York, NY, USA, 1999. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 4th ed.; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Wikström, C.; Wold, S. Design of Experiments-Principles and Applications, 3rd ed.; Umetrics AB: Umeå, Sweden, 2008. [Google Scholar]
- Herrador, M.A.; Asuero, A.G.; Gonzalez, A.G. Estimation of the uncertainty of indirect measurements from the propagation of distributions by using the Monte-Carlo method: An overview. Chemom. Intell. Lab. Syst. 2005, 79, 115–122. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q1(R2); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2005. [Google Scholar]
| CMPs | CMP Abbreviation | Screening Levels | RSM Levels and Domain 1 | Working Point with MODR |
|---|---|---|---|---|
| Buffer concentration | Buffer conc | 15–25–35 mM | 20–30 mM | 23 mM (21–26 mM) |
| Buffer pH | pH | 8.20–9.20–10.20 | 9.00–10.00 | 9.70 (9.50–9.77) |
| SDS concentration | SDS conc | 50.0–62.5–75.0 mM | 65.0 mM | 65.0 mM |
| DMβCyD concentration | CyD conc | 10–20–30 mM | 10–25 mM | 20 mM (17–23 mM) |
| n-butanol concentration | BuOH conc | 0.00–1.00–2.00% v/v | 0.20–1.80% v/v | 1.00% v/v (0.25–1.29% v/v) |
| Voltage | V | 20–25–30 kV | 20–30 kV | 25 kV (23–29 kV) |
| Temperature | T | 19–22–25 °C | 22 °C | 22 °C |
| No. Exp. | Buffer Conc (mM) | pH | SDS Conc (mM) | CyD Conc (mM) | BuOH Conc (% v/v) | V (kV) | T (°C) | R2 | R3 | R5 | NI2 | t (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 9.20 | 62.5 | 30 | 1.00 | 30 | 25 | 1.67 | −0.88 | 2.64 | 13,279 | 4.38 |
| 2 | 35 | 8.20 | 62.5 | 20 | 2.00 | 25 | 25 | 14.38 | 11.06 | 3.78 | 36,880 | 10.65 |
| 3 | 35 | 10.20 | 50.0 | 20 | 1.00 | 30 | 22 | 1.00 | −2.25 | 4.87 | 29,692 | 5.22 |
| 4 | 25 | 10.20 | 75.0 | 10 | 1.00 | 25 | 25 | 2.53 | −1.67 | 3.78 | 7550 | 9.51 |
| 5 | 35 | 9.20 | 75.0 | 30 | 0.00 | 25 | 22 | 3.83 | −1.48 | 4.82 | 18,090 | 8.04 |
| 6 | 25 | 10.20 | 62.5 | 30 | 2.00 | 20 | 22 | 0.87 | −3.64 | 4.09 | 14,510 | 10.56 |
| 7 | 25 | 9.20 | 75.0 | 20 | 2.00 | 30 | 19 | 4.58 | 0.00 | 4.32 | 19,078 | 6.81 |
| 8 | 25 | 9.20 | 62.5 | 20 | 1.00 | 25 | 22 | 5.65 | 1.38 | 4.78 | 12,229 | 8.35 |
| 9 | 35 | 9.20 | 62.5 | 10 | 1.00 | 20 | 19 | 5.81 | 2.74 | 7.14 | 3195 | 20.68 |
| 10 | 15 | 10.20 | 62.5 | 20 | 0.00 | 25 | 19 | 1.30 | −2.50 | 3.23 | 6706 | 6.99 |
| 11 | 15 | 8.20 | 75.0 | 20 | 1.00 | 20 | 22 | 15.37 | 14.58 | 3.16 | 27,893 | 13.32 |
| 12 | 25 | 8.20 | 50.0 | 30 | 1.00 | 25 | 19 | 5.94 | 3.16 | 2.74 | 14,257 | 7.11 |
| 13 | 15 | 9.20 | 50.0 | 10 | 2.00 | 25 | 22 | 16.04 | 15.22 | 2.93 | 27,987 | 10.38 |
| 14 | 25 | 8.20 | 62.5 | 10 | 0.00 | 30 | 22 | 18.87 | 19.85 | 2.80 | 36,535 | 8.61 |
| 15 | 25 | 9.20 | 50.0 | 20 | 0.00 | 20 | 25 | 2.83 | 0.00 | 2.99 | 3809 | 8.91 |
| 16 | 25 | 9.20 | 62.5 | 20 | 1.00 | 25 | 22 | 5.34 | 1.10 | 4.64 | 12,342 | 8.10 |
| No. Exp. | pH | Buffer Conc (mM) | CyD Conc (mM) | V (kV) | BuOH Conc (% v/v) | R2 | R3 | R5 | NI2 | t (min) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9.20 | 22 | 13.0 | 22 | 1.48 | 6.35 | −3.43 | 2.47 | 8400 | 9.23 |
| 2 | 9.80 | 22 | 13.0 | 22 | 0.52 | 3.70 | 1.45 | 3.05 | 18,908 | 10.17 |
| 3 | 9.20 | 28 | 13.0 | 22 | 0.52 | 5.82 | −2.60 | 3.37 | 7954 | 10.41 |
| 4 | 9.80 | 28 | 13.0 | 22 | 1.48 | 2.60 | 2.43 | 4.08 | 8604 | 11.89 |
| 5 | 9.20 | 22 | 22.0 | 22 | 0.52 | 4.47 | −0.86 | 3.31 | 6884 | 8.68 |
| 6 | 9.80 | 22 | 22.0 | 22 | 1.48 | 1.81 | 3.32 | 4.74 | 12,511 | 9.54 |
| 7 | 9.20 | 28 | 22.0 | 22 | 1.48 | 5.38 | 0.00 | 5.20 | n.d.1 | 9.79 |
| 8 | 9.80 | 28 | 22.0 | 22 | 0.52 | 1.63 | 3.11 | 4.79 | 8061 | 9.84 |
| 9 | 9.20 | 22 | 13.0 | 28 | 0.52 | 4.10 | −1.77 | 2.94 | 4379 | 6.43 |
| 10 | 9.80 | 22 | 13.0 | 28 | 1.48 | 2.226 | 1.73 | 3.67 | 9325 | 6.86 |
| 11 | 9.20 | 28 | 13.0 | 28 | 1.48 | 4.09 | −1.03 | 4.00 | 5713 | 7.35 |
| 12 | 9.80 | 28 | 13.0 | 28 | 0.52 | 2.54 | 1.37 | 3.06 | 8342 | 7.26 |
| 13 | 9.20 | 22 | 22.0 | 28 | 1.48 | 4.34 | 0.00 | 4.46 | n.d.1 | 6.17 |
| 14 | 9.80 | 22 | 22.0 | 28 | 0.52 | 1.59 | 2.34 | 3.80 | 9707 | 5.80 |
| 15 | 9.20 | 28 | 22.0 | 28 | 0.52 | 4.48 | 0.00 | 3.41 | n.d.1 | 6.32 |
| 16 | 9.80 | 28 | 22.0 | 28 | 1.48 | 1.42 | 3.13 | 4.92 | 13,364 | 6.66 |
| 17 | 9.00 | 25 | 17.5 | 25 | 1.00 | 4.40 | −1.66 | 3.61 | 5076 | 7.79 |
| 18 | 10.00 | 25 | 17.5 | 25 | 1.00 | 1.51 | 2.73 | 4.53 | 6878 | 8.25 |
| 19 | 9.50 | 20 | 17.5 | 25 | 1.00 | 3.71 | 1.15 | 2.26 | 25,460 | 7.41 |
| 20 | 9.50 | 30 | 17.5 | 25 | 1.00 | 2.97 | 1.59 | 3.90 | 10,100 | 8.66 |
| 21 | 9.50 | 25 | 10.0 | 25 | 1.00 | 5.31 | −0.95 | 4.31 | 17,255 | 9.29 |
| 22 | 9.50 | 25 | 25.0 | 25 | 1.00 | 2.26 | 2.29 | 3.96 | 12,609 | 7.41 |
| 23 | 9.50 | 25 | 17.5 | 20 | 1.00 | 4.51 | 1.33 | 3.20 | 26,097 | 11.39 |
| 24 | 9.50 | 25 | 17.5 | 30 | 1.00 | 2.92 | 1.27 | 3.77 | 19,239 | 5.62 |
| 25 | 9.50 | 25 | 17.5 | 25 | 0.20 | 4.02 | 0.89 | 2.36 | 26,779 | 7.47 |
| 26 | 9.50 | 25 | 17.5 | 25 | 1.80 | 2.87 | 1.63 | 3.91 | 10,783 | 8.37 |
| 27 | 9.50 | 25 | 17.5 | 25 | 1.00 | 3.35 | 1.32 | 3.06 | 17,716 | 7.91 |
| 28 | 9.50 | 25 | 17.5 | 25 | 1.00 | 3.56 | 1.36 | 2.98 | 18,518 | 8.27 |
| 29 | 9.50 | 25 | 17.5 | 25 | 1.00 | 3.85 | 1.27 | 3.06 | 23,917 | 8.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzullo, L.; Gotti, R.; Orlandini, S.; Slavíčková, P.; Jireš, J.; Zapadlo, M.; Douša, M.; Nekvapilová, P.; Řezanka, P.; Furlanetto, S. Analytical Quality by Design-Compliant Development of a Cyclodextrin-Modified Micellar ElectroKinetic Chromatography Method for the Determination of Trimecaine and Its Impurities. Molecules 2023, 28, 4747. https://doi.org/10.3390/molecules28124747
Marzullo L, Gotti R, Orlandini S, Slavíčková P, Jireš J, Zapadlo M, Douša M, Nekvapilová P, Řezanka P, Furlanetto S. Analytical Quality by Design-Compliant Development of a Cyclodextrin-Modified Micellar ElectroKinetic Chromatography Method for the Determination of Trimecaine and Its Impurities. Molecules. 2023; 28(12):4747. https://doi.org/10.3390/molecules28124747
Chicago/Turabian StyleMarzullo, Luca, Roberto Gotti, Serena Orlandini, Patricie Slavíčková, Jakub Jireš, Michal Zapadlo, Michal Douša, Pavla Nekvapilová, Pavel Řezanka, and Sandra Furlanetto. 2023. "Analytical Quality by Design-Compliant Development of a Cyclodextrin-Modified Micellar ElectroKinetic Chromatography Method for the Determination of Trimecaine and Its Impurities" Molecules 28, no. 12: 4747. https://doi.org/10.3390/molecules28124747
APA StyleMarzullo, L., Gotti, R., Orlandini, S., Slavíčková, P., Jireš, J., Zapadlo, M., Douša, M., Nekvapilová, P., Řezanka, P., & Furlanetto, S. (2023). Analytical Quality by Design-Compliant Development of a Cyclodextrin-Modified Micellar ElectroKinetic Chromatography Method for the Determination of Trimecaine and Its Impurities. Molecules, 28(12), 4747. https://doi.org/10.3390/molecules28124747






