Anti-Inflammatory and Cytotoxic Activities of Clerodane-Type Diterpenes
Abstract
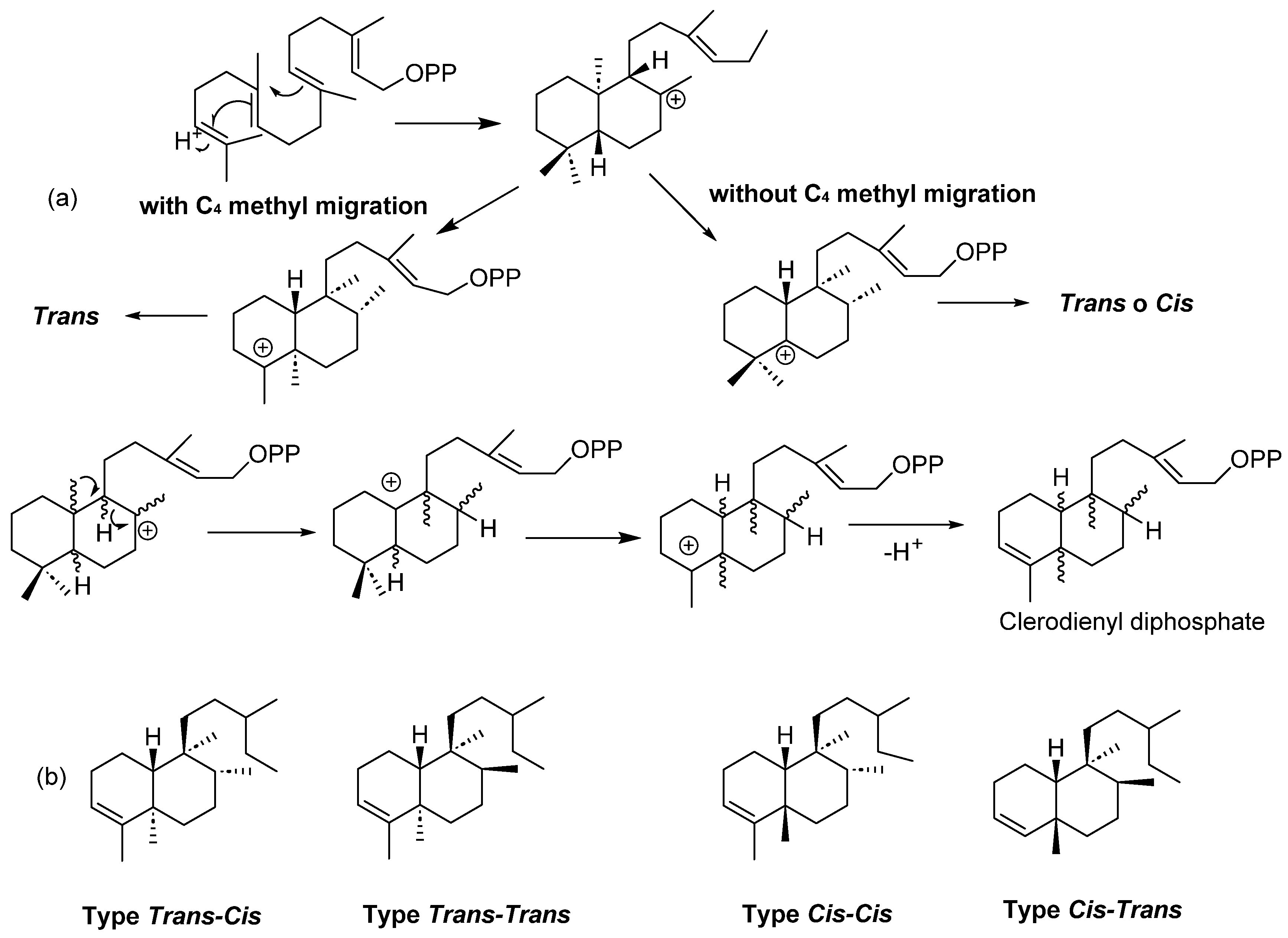
1. Introduction
2. Discussion
2.1. Cytotoxic Activity
2.2. Anti-Inflammatory Activity
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acquaviva, R.; Malfa, G.A.; Loizzo, M.R.; Xiao, J.; Bianchi, S.; Tundis, R. Advances on Natural Abietane, Labdane and Clerodane Diterpenes as Anti-Cancer Agents: Sources and Mechanisms of Action. Molecules 2022, 27, 4791. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Cao, J.; Zhang, Q.; Lin, L. The Drug Likeness Analysis of Anti-Inflammatory Clerodane Diterpenoids. Chin. Med. 2020, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Morris-Natschke, S.L.; Lee, K.-H. Clerodane Diterpenes: Sources, Structures, and Biological Activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef] [PubMed]
- dos Lima, J.; Marinho, E.M.; Alencar de Menezes, J.E.; Mendes, F.R.S.; da Silva Antonio, W.; Maria, K.A.F.; Emmanuel, S.M.; Marinho, M.M.; Bandeira, P.N.; dos Santos, H.S. Biological Properties of Clerodane-Type Diterpenes. J. Anal. Pharm. Res. 2022, 11, 56–64. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, R.; Chaudhary, A. Evaluation of Antiulcerogenic Activity of Clerodendron Infortunatum Extract on Albino Rat Gastric Ulceration. J. Drug Deliv. Ther. 2019, 9, 57–62. [Google Scholar] [CrossRef]
- Ranganathan, M.; Schnakenberg, A.; Skosnik, P.D.; Cohen, B.M.; Pittman, B.; Sewell, R.A.; D’Souza, D.C. Dose-Related Behavioral, Subjective, Endocrine, and Psychophysiological Effects of the κ Opioid Agonist Salvinorin A in Humans. Biol. Psychiatry 2012, 72, 871–879. [Google Scholar] [CrossRef]
- Cichon, J.; Liu, R.; Le, H.V. Therapeutic Potential of Salvinorin A and Its Analogues in Various Neurological Disorders. Transl. Perioper. Pain Med. 2022, 9, 452–457. [Google Scholar]
- Jiang, H.; Zhang, G.-J.; Liu, Y.-F.; Wang, H.-S.; Liang, D. Clerodane Diterpenoid Glucosides from the Stems of Tinospora sinensis. J. Nat. Prod. 2017, 80, 975–982. [Google Scholar] [CrossRef]
- Tokoroyama, T. Synthesis of Clerodane Diterpenoids and Related Compounds—Stereoselective Construction of the Decalin Skeleton with Multiple Contiguous Stereogenic Centers. Synthesis 2000, 2000, 611–633. [Google Scholar] [CrossRef]
- Hagiwara, H. Total Syntheses of Clerodane Diterpenoids—A Review. Nat. Prod. Commun. 2019, 14, 1934578X19843613. [Google Scholar] [CrossRef]
- Soerjomataram, I.; Bray, F. Planning for Tomorrow: Global Cancer Incidence and the Role of Prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of Precision Cancer Medicine: Evolution of the Treatment Paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Y.; Jian, M.; Fu, X.; Cheng, Y.; He, Y.; Fang, J.; Li, L.; Zhang, D. Breaking the Bottleneck in Anticancer Drug Development: Efficient Utilization of Synthetic Biology. Molecules 2022, 27, 7480. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A Compressive Review about Taxol®: History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef]
- Li, D.; Xue, M.; Geng, Z.; Chen, P. The Suppressive Effects of Bursopentine (BP5) on Oxidative Stress and NF-ĸB Activation in Lipopolysaccharide-Activated Murine Peritoneal Macrophages. Cell Physiol. Biochem. 2012, 29, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B. Role of PGE-2 and Other Inflammatory Mediators in Skin Aging and Their Inhibition by Topical Natural Anti-Inflammatories. Cosmetics 2019, 6, 6. [Google Scholar] [CrossRef]
- Araruna, M.E.; Serafim, C.; Alves Júnior, E.; Hiruma-Lima, C.; Diniz, M.; Batista, L. Intestinal Anti-Inflammatory Activity of Terpenes in Experimental Models (2010–2020): A Review. Molecules 2020, 25, 5430. [Google Scholar] [CrossRef]
- Olatunde, O.Z.; Yong, J.; Lu, C. New Neo-Clerodane Diterpenoids Isolated from Ajuga decumbens Thunb., Planted at Pingtan Island of Fujian Province with the Potent Anticancer Activity. Anti-Cancer Agents Med. Chem. 2023, 23, 237–244. [Google Scholar]
- Cai, S.; Risinger, A.L.; Petersen, C.L.; Grkovic, T.; O’Keefe, B.R.; Mooberry, S.L.; Cichewicz, R.H. Anacolosins A-F and Corymbulosins X and Y, Clerodane Diterpenes from Anacolosa clarkii Exhibiting Cytotoxicity toward Pediatric Cancer Cell Lines. J. Nat. Prod. 2019, 82, 928–936. [Google Scholar] [CrossRef]
- Vila-Luna, M.L.; Moo-Puc, R.E.; Torres-Tapia, L.W.; Peraza-Sánchez, S.R. Cytotoxic Activity of Casearborin c Isolated from Casearia corymbosa. J. Mex. Chem Soc. 2018, 62, 24–28. [Google Scholar] [CrossRef]
- Meesakul, P.; Ritthiwigrom, T.; Cheenpracha, S.; Sripisut, T.; Maneerat, W.; Machan, T.; Laphookhieo, S. A New Cytotoxic Clerodane Diterpene from Casearia graveolens Twigs. Nat. Prod. Commun. 2016, 11, 13–15. [Google Scholar] [CrossRef]
- Nuanyai, T.; Chailap, B.; Buakeaw, A.; Puthong, S. Cytotoxicity of Clerodane Diterpenoids from Fresh Ripe Fruits of Casearia grewiifolia. J. Sci. Technol. 2017, 39, 517–521. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Truong, N.B.; Doan, H.T.M.; Litaudon, M.; Retailleau, P.; Do, T.T.; Nguyen, H.V.; Chau, M.V.; Pham, C.V. Cytotoxic Clerodane Diterpenoids from the Leaves of Casearia grewiifolia. J. Nat. Prod. 2015, 78, 2726–2730. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, Q.; Yang, X.; Li, Y.; Zhang, X.; Li, Y.; Du, Q.; Jin, D.-Q.; Cui, J.; Lall, N.; et al. Diterpenoids from the Leaves of Casearia kurzii Showing Cytotoxic Activities. Bioorg. Chem. 2020, 98, 103741. [Google Scholar] [CrossRef]
- Zhang, L.-T.; Wang, X.-L.; Wang, T.; Zhang, J.-S.; Huang, Z.-Q.; Shen, T.; Lou, H.-X.; Ren, D.-M.; Wang, X.-N. Dolabellane and Clerodane Diterpenoids from the Twigs and Leaves of Casearia kurzii. J. Nat. Prod. 2020, 83, 2817–2830. [Google Scholar] [CrossRef]
- Shuo, Y.; Zhang, C.; Yang, X.; Liu, F.; Zhang, Q.; Li, A.; Ma, J.; Lee, D.; Ohizumi, Y.; Guo, Y. Clerodane Diterpenoids from Casearia kurzii and Their Cytotoxic Activities. J. Nat. Med. 2019, 73, 826–833. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Q.; Yang, X.; Xi, Y.; Zhang, X.; Wang, H.; Zhang, J.; Tuerhong, M.; Jin, D.-Q.; Lee, D.; et al. Cytotoxic Diterpenoids as Potential Anticancer Agents from the Twigs of Casearia kurzii. Bioorg. Chem. 2019, 89, 102995. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; Bezerra, D.P.; do Nascimento Silva, J.; da Costa, M.P.; de Oliveira Ferreira, J.R.; Alencar, N.M.N.; de Figueiredo, I.S.T.; Cavalheiro, A.J.; Machado, C.M.L.; Chammas, R.; et al. Preclinical Anticancer Effectiveness of a Fraction from Casearia Sylvestris and Its Component Casearin X: In Vivo and Ex Vivo Methods and Microscopy Examinations. J. Ethnopharmacol. 2016, 186, 270–279. [Google Scholar] [CrossRef]
- Zou, M.-F.; Pan, Y.-H.; Hu, R.; Yuan, F.-Y.; Huang, D.; Tang, G.-H.; Li, W.; Yin, S. Highly Modified Nor-Clerodane Diterpenoids from Croton yanhuii. Fitoterapia 2021, 153, 104979. [Google Scholar] [CrossRef]
- Li, C.; Sun, X.; Yin, W.; Zhan, Z.; Tang, Q.; Wang, W.; Zhuo, X.; Wu, Z.; Zhang, H.; Li, Y.; et al. Crassifolins Q−W: Clerodane Diterpenoids From Croton crassifolius With Anti-Inflammatory and Anti-Angiogenesis Activities. Front. Chem. 2021, 9, 733350. [Google Scholar] [CrossRef]
- Tian, J.-L.; Yao, G.-D.; Wang, Y.-X.; Gao, P.-Y.; Wang, D.; Li, L.-Z.; Lin, B.; Huang, X.-X.; Song, S.-J. Cytotoxic Clerodane Diterpenoids from Croton crassifolius. Bioorg. Med. Chem. Lett. 2017, 27, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Cao, D.; Gao, Y.; Li, S.; Zhu, J.; Yang, B.; Zhou, L.; Zhou, Y.; Jin, J.; Zhao, Z. New Clerodane Diterpenoids from Croton crassifolius. Fitoterapia 2016, 108, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, I.; Venturella, S.R.T.; Bressiani, P.A.; Marco, I.G.; Novello, C.R.; Almeida, I.V.; Vicentini, V.E.P.; Mello, J.C.P.; Düsman, E. Cytotoxicity of Extracts and Compounds Isolated from Croton echioides in Animal Tumor Cell (HTC). Braz. J. Biol. 2022, 82, e264356. [Google Scholar] [CrossRef] [PubMed]
- Guetchueng, S.T.; Nahar, L.; Ritchie, K.J.; Ismail, F.M.D.; Evans, A.R.; Sarker, S.D. Ent-Clerodane Diterpenes from the Bark of Croton oligandrus Pierre Ex Hutch. and Assessment of Their Cytotoxicity against Human Cancer Cell Lines. Molecules 2018, 23, 410. [Google Scholar] [CrossRef]
- Ng, S.-Y.; Kamada, T.; Suleiman, M.; Vairappan, C.S. Two New Clerodane-Type Diterpenoids from Bornean Liverwort Gottschelia schizopleura and Their Cytotoxic Activity. Nat. Prod. Res. 2018, 32, 1832–1837. [Google Scholar] [CrossRef]
- Aimaiti, S.; Suzuki, A.; Saito, Y.; Fukuyoshi, S.; Goto, M.; Miyake, K.; Newman, D.J.; O’Keefe, B.R.; Lee, K.-H.; Nakagawa-Goto, K. Corymbulosins I–W, Cytotoxic Clerodane Diterpenes from the Bark of Laetia corymbulosa. J. Org. Chem. 2018, 83, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Widyowati, R.; Sugimoto, S.; Yamano, Y.; Sukardiman; Otsuka, H.; Matsunami, K. New Cis-Ent-Clerodanes from Linaria japonica. Phytochem. Lett. 2015, 14, 56–62. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Thonangi, C.V.; Dakal, T.C.; Vedula, G.S.; Dhabhai, B.; Polimati, H.; Akula, A.; Nguyen, H.T. Potential Anti-Hepatocellular Carcinoma Properties and Mechanisms of Action of Clerodane Diterpenes Isolated from Polyalthia longifolia Seeds. Sci. Rep. 2022, 12, 9267. [Google Scholar] [CrossRef]
- Yu, Z.-X.; Fu, Y.-H.; Chen, G.-Y.; Song, X.-P.; Han, C.-R.; Li, X.-B.; Song, X.-M.; Wu, A.-Z.; Chen, S.-C. New Clerodane Diterpenoids from the Roots of Polyalthia laui. Fitoterapia 2016, 111, 36–41. [Google Scholar] [CrossRef]
- Bautista, E.; Fragoso-Serrano, M.; Ortiz-Pastrana, N.; Toscano, R.A.; Ortega, A. Structural Elucidation and Evaluation of Multidrug-Resistance Modulatory Capability of Amarissinins A–C, Diterpenes Derived from Salvia amarissima. Fitoterapia 2016, 114, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fragoso-Serrano, M.; Ortiz-Pastrana, N.; Luna-Cruz, N.; Toscano, R.A.; Alpuche-Solís, A.G.; Ortega, A.; Bautista, E. Amarisolide F, an Acylated Diterpenoid Glucoside and Related Terpenoids from Salvia amarissima. J. Nat. Prod. 2019, 82, 631–635. [Google Scholar] [CrossRef]
- Bautista, E.; Fragoso-Serrano, M.; Toscano, R.A.; García-Peña, M.d.R.; Ortega, A. Teotihuacanin, a Diterpene with an Unusual Spiro-10/6 System from Salvia amarissima with Potent Modulatory Activity of Multidrug Resistance in Cancer Cells. Org. Lett. 2015, 17, 3280–3282. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Brito, C.; Pérez-Juanchi, D.; Rivera-Chávez, J.; Hernández-Herrera, A.D.; Bedolla-García, B.Y.; Zamudio, S.; Ramírez-Apan, T.; Quijano, L.; Esquivel, B. Clerodane and 5 10-Seco-Clerodane-Type Diterpenoids from Salvia involucrata. J. Mol. Struct. 2021, 1237, 130367. [Google Scholar] [CrossRef]
- Jiang, Y.-J.; Su, J.; Shi, X.; Wu, X.-D.; Chen, X.-Q.; He, J.; Shao, L.-D.; Li, X.-N.; Peng, L.-Y.; Li, R.-T.; et al. Neo-Clerodanes from the Aerial Parts of Salvia leucantha. Tetrahedron 2016, 72, 5507–5514. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Hu, P.; Ji, J.; Li, X.; Chen, J. Neoclerodane Diterpenoids from Scutellaria barbata with Cytotoxic Activities. Nat. Prod. Res. 2020, 34, 1345–1351. [Google Scholar] [CrossRef]
- Yang, G.-C.; Hu, J.-H.; Li, B.-L.; Liu, H.; Wang, J.-Y.; Sun, L.-X. Six New Neo-Clerodane Diterpenoids from Aerial Parts of Scutellaria barbata and Their Cytotoxic Activities. Planta Med. 2018, 84, 1292–1299. [Google Scholar] [CrossRef]
- Yuan, Q.-Q.; Song, W.-B.; Wang, W.-Q.; Xuan, L.-J. Scubatines A–F, New Cytotoxic Neo-Clerodane Diterpenoids from Scutellaria barbata D. Don. Fitoterapia 2017, 119, 40–44. [Google Scholar] [CrossRef]
- Wang, M.; Ma, C.; Chen, Y.; Li, X.; Chen, J. Cytotoxic Neo-Clerodane Diterpenoids from Scutellaria barbata D.Don. Chem. Biodivers. 2019, 16, e1800499. [Google Scholar] [CrossRef]
- Dai, S.-J.; Xiao, K.; Zhang, L.; Han, Q.-T. New Neo-Clerodane Diterpenoids from Scutellaria strigillosa with Cytotoxic Activities. J. Asian Nat. Prod. Res. 2016, 18, 456–461. [Google Scholar] [CrossRef]
- Dai, S.-J.; Zhang, L.; Xiao, K.; Han, Q.-T. New Cytotoxic Neo-Clerodane Diterpenoids from Scutellaria strigillosa. Bioorg. Med. Chem. Lett. 2016, 26, 1750–1753. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Shen, J.; Zhang, F.; Liang, J.; Xia, Z. Sulfated Neo-Clerodane Diterpenoids and Triterpenoid Saponins from Sheareria nana S. Moore. Fitoterapia 2018, 124, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, P.-L.; Zhou, M.-X.; Shen, T.; Zou, Y.-X.; Lou, H.-X.; Wang, X.-N. New Nor-Clerodane-Type Furanoditerpenoids from the Rhizomes of Tinospora capillipes. Phytochem. Lett. 2016, 15, 225–229. [Google Scholar] [CrossRef]
- Subash-Babu, P.; Alshammari, G.M.; Ignacimuthu, S.; Alshatwi, A.A. Epoxy Clerodane Diterpene Inhibits MCF-7 Human Breast Cancer Cell Growth by Regulating the Expression of the Functional Apoptotic Genes Cdkn2A, Rb1, Mdm2 and P53. Biomed. Pharmacother. 2017, 87, 388–396. [Google Scholar] [CrossRef]
- Qin, N.; Wang, A.; Li, D.; Wang, K.; Lin, B.; Li, Z.; Hua, H. Cytotoxic Clerodane Furanoditerpenoids from the Root of Tinospora sagittata. Phytochem. Lett. 2015, 12, 173–176. [Google Scholar] [CrossRef]
- Liu, W.; Song, Z.; Wang, H.; Yang, X.; Joubert, E.; Zhang, J.; Li, S.; Tuerhong, M.; Abudukeremu, M.; Jin, J.; et al. Diterpenoids as Potential Anti-Inflammatory Agents from Ajuga pantantha. Bioorg. Chem. 2020, 101, 103966. [Google Scholar] [CrossRef]
- Dong, B.; Yang, X.; Liu, W.; An, L.; Zhang, X.; Tuerhong, M.; Du, Q.; Wang, C.; Abudukeremu, M.; Xu, J.; et al. Anti-Inflammatory Neo-Clerodane Diterpenoids from Ajuga pantantha. J. Nat. Prod. 2020, 83, 894–904. [Google Scholar] [CrossRef]
- Pu, D.-B.; Zhang, X.-J.; Bi, D.-W.; Gao, J.-B.; Yang, Y.; Li, X.-L.; Lin, J.; Li, X.-N.; Zhang, R.-H.; Xiao, W.-L. Callicarpins, Two Classes of Rearranged Ent-Clerodane Diterpenoids from Callicarpa Plants Blocking NLRP3 Inflammasome-Induced Pyroptosis. J. Nat. Prod. 2020, 83, 2191–2199. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.; Wang, Q.; Shang, K.; Pu, D.-B.; Zhang, R.-H.; Li, X.-L.; Dai, X.-C.; Zhang, X.-J.; Xiao, W.-L. Clerodane Diterpenoids with Potential Anti-Inflammatory Activity from the Leaves and Twigs of Callicarpa cathayana. Chin. J. Nat. Med. 2019, 17, 953–962. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lin, J.-J.; Chen, S.-R.; Hwang, T.-L.; Fang, S.-Y.; Korinek, M.; Chen, C.-Y.; Lin, Y.-S.; Wu, T.-Y.; Yen, M.-H.; et al. Clerodane Diterpenoids from Callicarpa hypoleucophylla and Their Anti-Inflammatory Activity. Molecules 2020, 25, 2288. [Google Scholar] [CrossRef]
- Ye, G.-H.; Xue, J.-J.; Liang, W.-L.; Yang, S.-J. Three New Bioactive Diterpenoids from the Roots of Croton crassifolius. Nat. Prod. Res. 2021, 35, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, S.A.S.; Pinto, M.E.F.; Bobey, A.F.; Russo, H.M.; Batista, A.N.L.; Batista, J.M.; Codo, A.C.; Medeiros, A.I.; Bolzani, V.S. Diterpenoids with Inhibitory Activity of Nitrite Production from Croton floribundus. J. Ethnopharmacol. 2020, 249, 112320. [Google Scholar] [CrossRef]
- Li, F.; Zhang, D.-B.; Li, J.-T.; He, F.-J.; Zhu, H.-L.; Li, N.; Xiao, X.-C.; Ren, L.; Zheng, W. Bioactive Terpenoids from Croton laui. Nat. Prod. Res. 2021, 35, 2849–2857. [Google Scholar] [CrossRef]
- Somteds, A.; Tantapakul, C.; Kanokmedhakul, K.; Laphookhieo, S.; Phukhatmuen, P.; Kanokmedhakul, S. Inhibition of Nitric Oxide Production by Clerodane Diterpenoids from Leaves and Stems of Croton poomae Esser. Nat. Prod. Res. 2021, 35, 2722–2729. [Google Scholar] [CrossRef]
- Salinas-Sánchez, D.O.; Zamilpa, A.; Pérez, S.; Herrera-Ruiz, M.; Tortoriello, J.; González-Cortazar, M.; Jiménez-Ferrer, E. Effect of Hautriwaic Acid Isolated from Dodonaea viscosa in a Model of Kaolin/Carrageenan-Induced Monoarthritis. Planta Med. 2015, 81, 1240–1247. [Google Scholar] [CrossRef]
- Zhang, P.-Z.; Zhang, Y.-M.; Lin, Y.; Wang, F.; Zhang, G.-L. Three New Diterpenes from Dysoxylum lukii and Their NO Production Inhibitory Activity. J. Asian Nat. Prod. Res. 2020, 22, 531–536. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, R.; Zhang, J.; Wu, X.; Shen, T.; Zhou, J.; Qiao, Y.; Gao, Y.; Lou, H. Clerodane Diterpenoids from the Chinese Liverwort Jamesoniella autumnalis and Their Anti-Inflammatory Activity. Phytochemistry 2018, 154, 85–93. [Google Scholar] [CrossRef]
- Polbuppha, I.; Suthiphasilp, V.; Maneerat, T.; Charoensup, R.; Limtharakul, T.; Cheenpracha, S.; Pyne, S.G.; Laphookhieo, S. Nitric Oxide Production Inhibitory Activity of Clerodane Diterpenes from Monoon membranifolium. Nat. Prod. Res. 2022, 36, 2513–2517. [Google Scholar] [CrossRef]
- ur Rehman, T.; Khan, A.; Abbas, A.; Hussain, J.; Khan, F.U.; Stieglitz, K.; Ali, S. Investigation of Nepetolide as a Novel Lead Compound: Antioxidant, Antimicrobial, Cytotoxic, Anticancer, Anti-Inflammatory, Analgesic Activities and Molecular Docking Evaluation. Saudi Pharm. J. 2018, 26, 422–429. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Vu, T.-Y.; Chandi, V.; Polimati, H.; Tatipamula, V.B. Dual COX and 5-LOX Inhibition by Clerodane Diterpenes from Seeds of Polyalthia longifolia (Sonn.) Thwaites. Sci. Rep. 2020, 10, 15965. [Google Scholar] [CrossRef]
- Feng, X.-S.; Yan, W.; Bai, L.-H.; Wang, K.; Chen, X.-Q. Neo-Clerodane Diterpenoids from the Aerial Parts of Scutellaria barbata with Anti-Inflammatory Activity. Chem. Biodivers. 2021, 18, e2100693. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.-W.; Luo, J.-G.; Zhu, M.-D.; Zhao, H.-J.; Kong, L.-Y. Neo-Clerodane Diterpenoids from the Aerial Parts of Teucrium fruticans Cultivated in China. Phytochemistry 2015, 119, 26–31. [Google Scholar] [CrossRef] [PubMed]
- You, J.-Q.; Liu, Y.-N.; Zhou, J.-S.; Sun, X.-Y.; Lei, C.; Mu, Q.; Li, J.-Y.; Hou, A.-J. Cis-Clerodane Diterpenoids with Structural Diversity and Anti-Inflammatory Activity from Tinospora crispa. Chin. J. Chem. 2022, 40, 2882–2892. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Deng, L.; Song, J.-Q.; Zhu, Y.; Yuan, R.-W.; Fan, X.-Z.; Zhou, H.; Huang, Y.-S.; Zhang, L.-J.; Liao, H.-B. Clerodane Diterpenoids with Anti-Inflammatory and Synergistic Antibacterial Activities from Tinospora crispa. Org. Chem. Front. 2022, 9, 6945–6957. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, H.; Hu, S.; Xu, H.; Yang, B.; Yang, Q.; Xue, Y.; Cheng, L.; Jiang, J.; Zhang, J.; et al. Clerodane-Type Diterpenoids from Tuberous Roots of Tinospora sagittata (Oliv.) Gagnep. Fitoterapia 2016, 110, 59–65. [Google Scholar] [CrossRef]
- Chang, H.-L.; Chang, F.-R.; Chen, J.-S.; Wang, H.-P.; Wu, Y.-H.; Wang, C.-C.; Wu, Y.-C.; Hwang, T.-L. Inhibitory Effects of 16-Hydroxycleroda-3,13(14)E-Dien-15-Oic Acid on Superoxide Anion and Elastase Release in Human Neutrophils through Multiple Mechanisms. Eur. J. Pharmacol. 2008, 586, 332–339. [Google Scholar] [CrossRef]



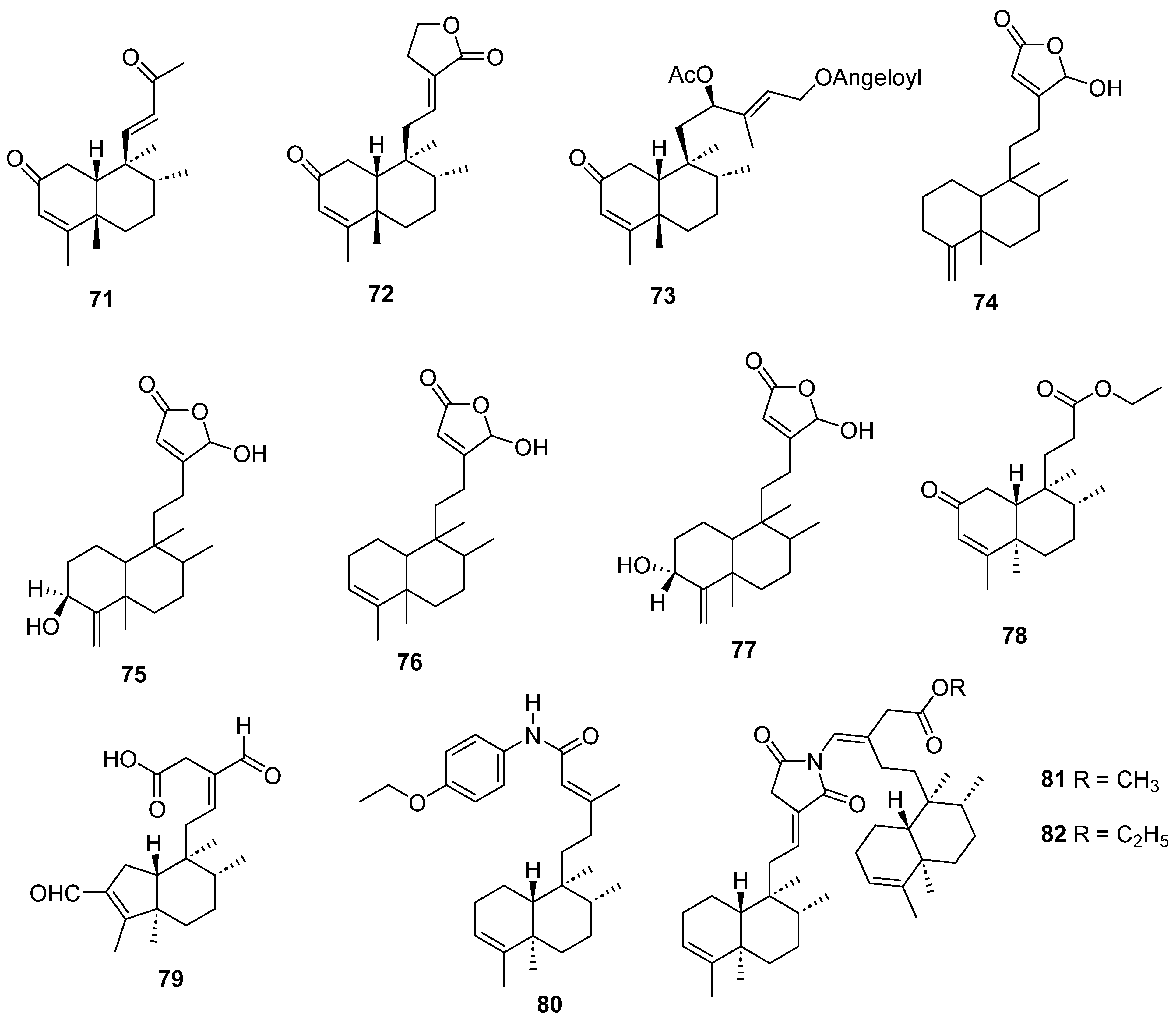

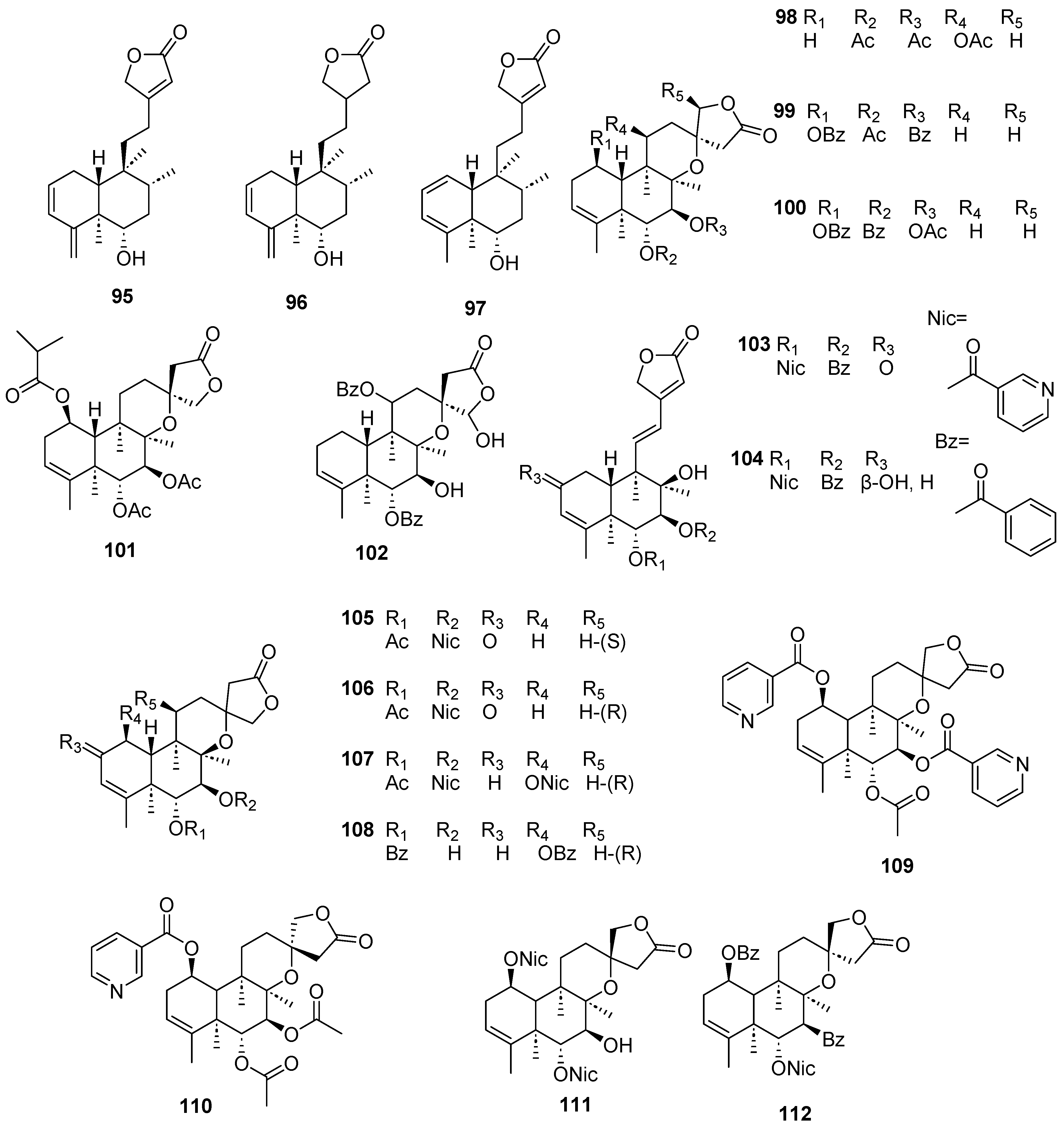
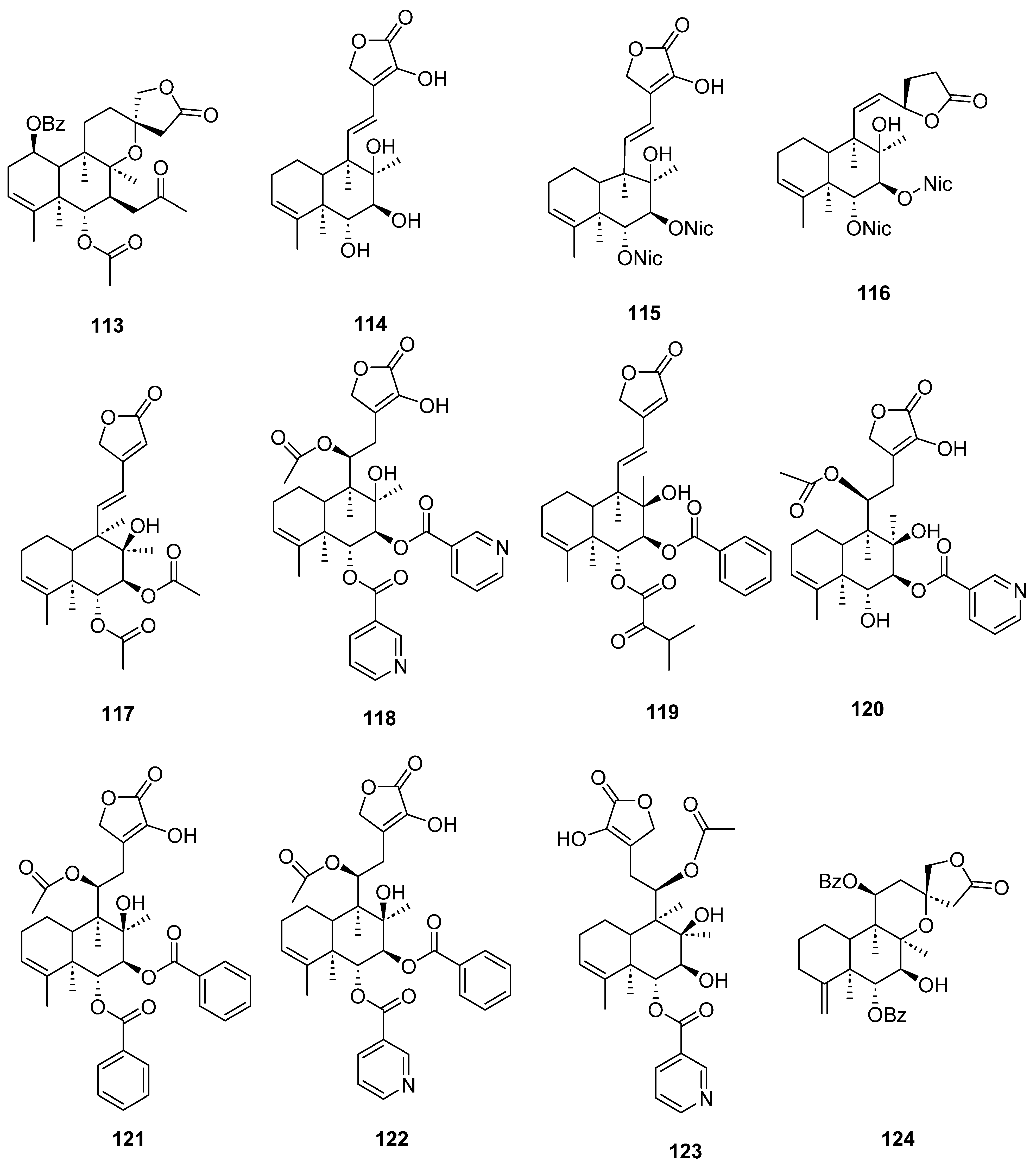

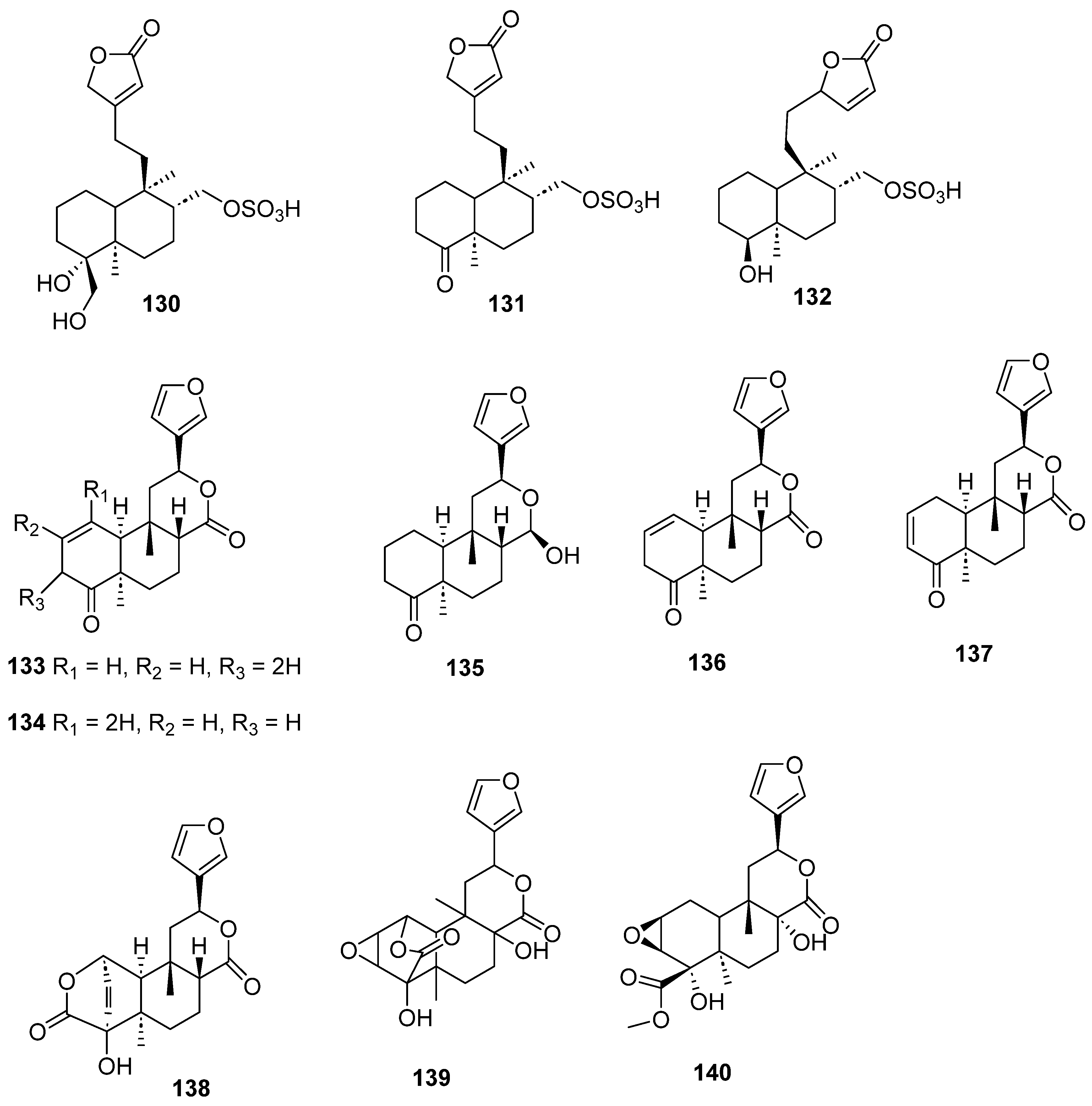


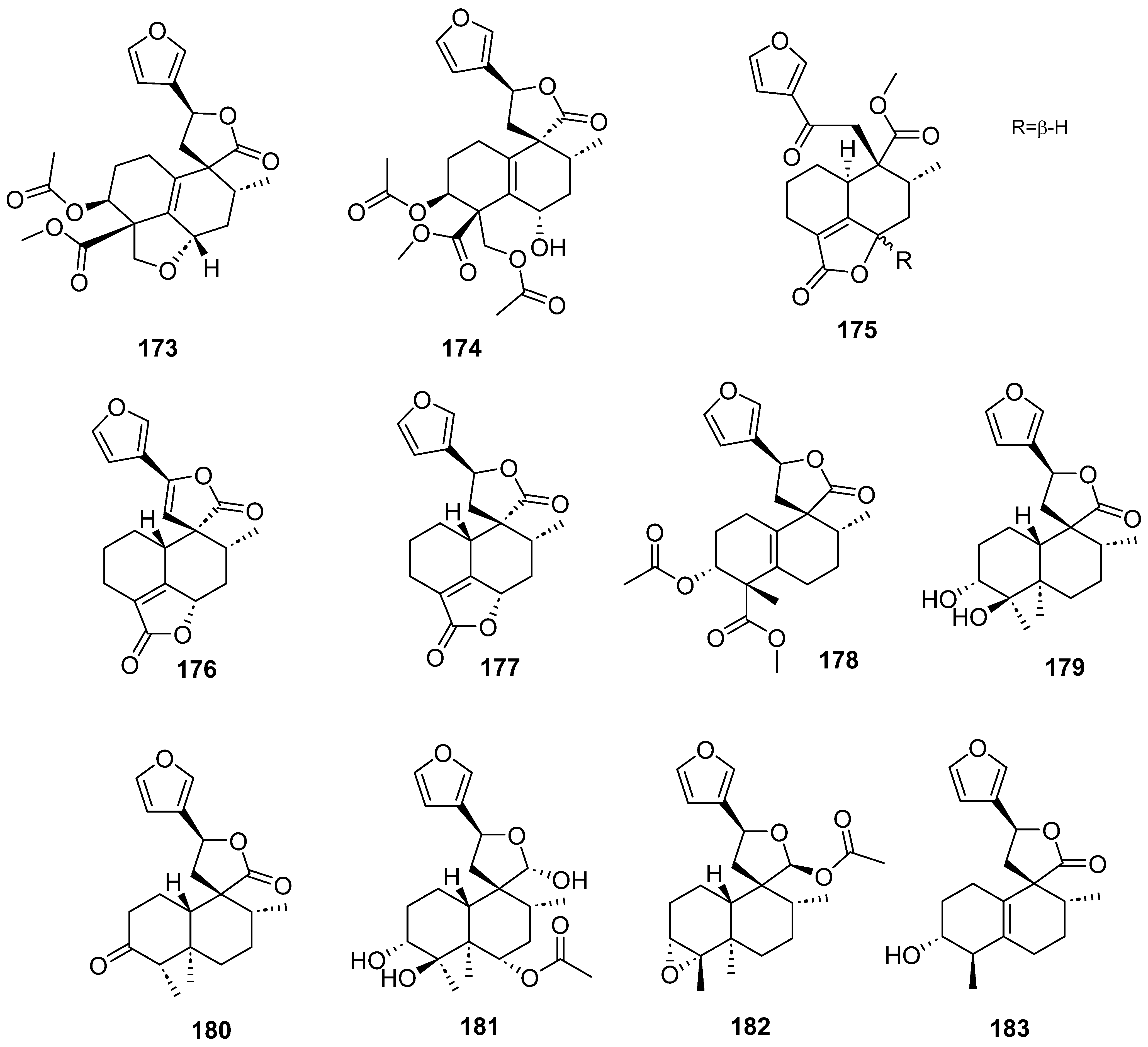
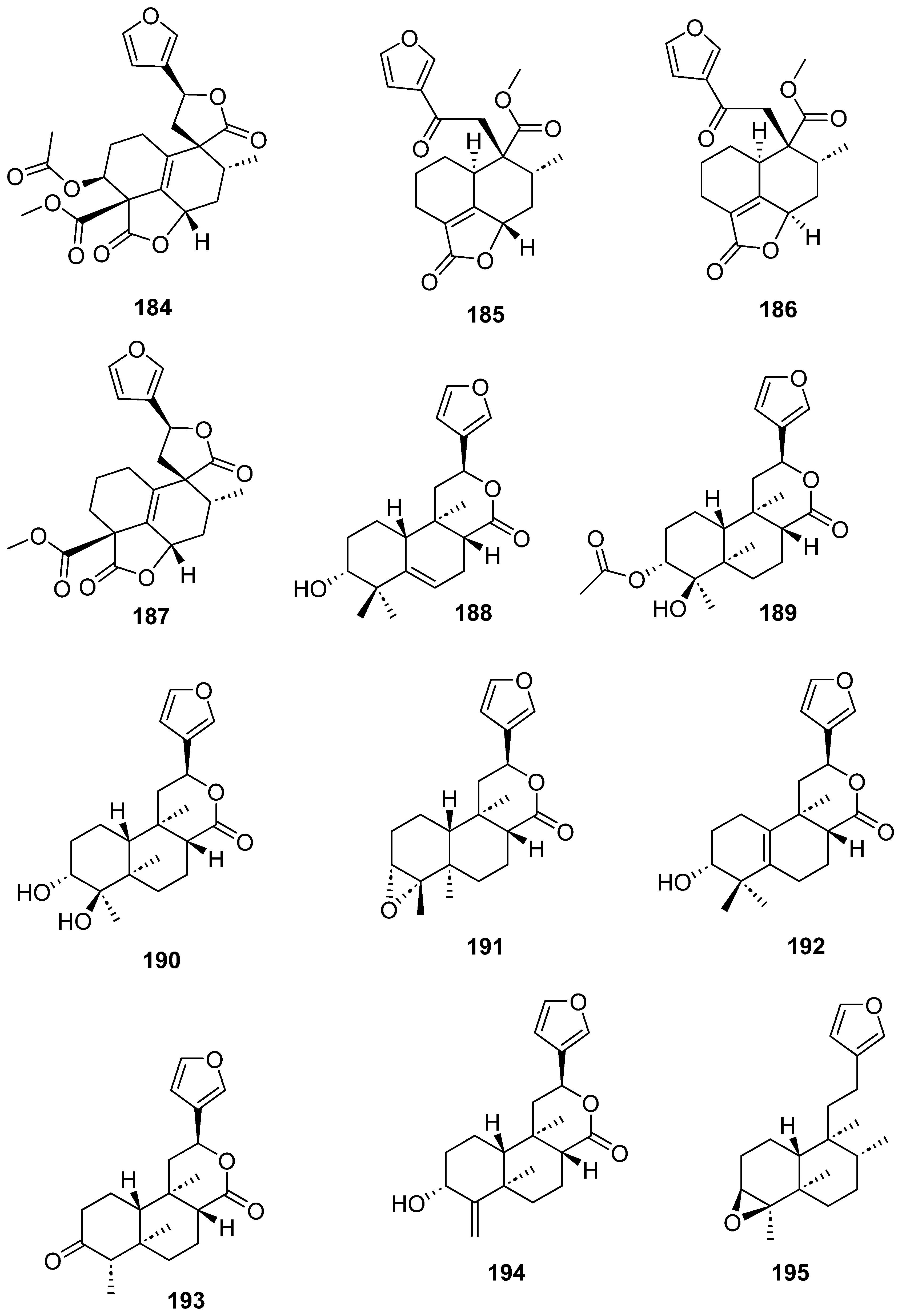

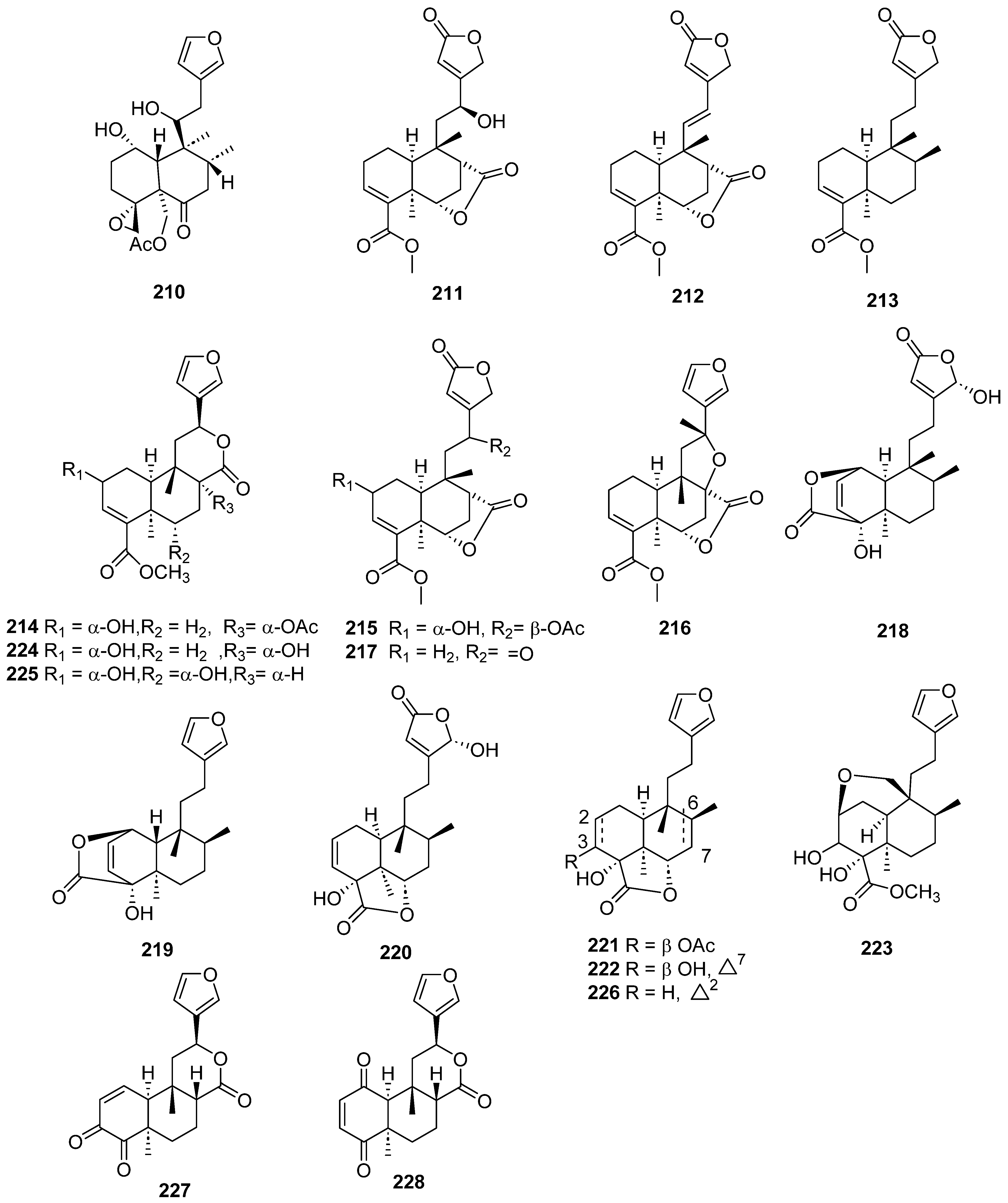
| Plant | Family | Part of Plant | Collection Place |
|---|---|---|---|
| Ajuga decumbens [20] | Lamiaceae | Aerial parts | Pingtan island of Fujian Province. |
| Anacolosa clarkia [21] | Olacaceae | Fruit, leaves and twigs of the plant | Bana Forest Preserve in Danang. NCI Natural Products Repository. |
| Casearia corymbosa [22] | Salicaceae | Stem bark | Othón P. Blanco, Quintana Roo, Mexico. |
| Casearia graveolens [23] | Salicaceae | Twigs | Chiang Rai Province, northern Thailand. |
| Casearia grewiifolia [24,25] | Salicaceae | Fresh fruits | Khon Kaen University campus. |
| Leaves | Phu Loc–Thua Thien Hue, Vietnam. | ||
| Casearia kurzii [26,27,28,29] | Salicaceae | Fruit, leaves and twigs | Bana Forest Preserve in Danang, Vietnam. |
| Twigs and leaves | Xishuangbanna County, Yunn an Province, P. R. China. | ||
| Casearia sylvestris [30] | Salicaceae | Leaves | Parque Estadual Carlos Botelho (São Miguel Arcanjo, São Paulo State.) |
| Croton caudatus [31] | Euphorbiaceae | Leaves and twigs | Xishuangbanna Prefecture, Yunnan Province, P. R. China. |
| Croton crassifolius [32,33,34] | Euphorbiaceae | Roots | Yulin City, Guangxi Province, China. |
| Southeast China, Thailand, Vietnam, and Laos. | |||
| Fujian Province, People’s Republic of China. | |||
| Croton echioides [35] | Euphorbiaceae | Stems | Brazil |
| Croton oligandrus [36] | Euphorbiaceae | Bark | Mount Eloundem, Central Region, Cameroon. |
| Gottschelia schizopleura [37] | Cephaloziellaceae | Aerial parts | Mount Alab, Sabah, North Borneo, Malaysia. |
| Laetia corymbulosa [38] | Salicaceae | Bark | The plant was provided by NCI/NIH (Frederick, MD, U.S.). |
| Linaria japonica [39] | Plantaginaceae | Whole plants | Hiroshima, Japan. |
| Polyalthia longifolia [40] | Annonaceae | Seeds | Tirupati, India. |
| Polyalthia laui [41] | Annonaceae | Roots | Hainan Province, China. |
| Salvia amarissima [42,43,44] | Lamiaceae | Leaves and flowers | Teotihuacan, State of Mexico. |
| Aerial portions | Teotihuacan Valley | ||
| Salvia involucrata [45] | Lamiaceae | Aerial parts | Municipality of Xilitla, State of San Luis Potosí, Mexico. |
| Salvia leucantha [46] | Lamiaceae | Aerial parts | Yunnan Province, China. |
| Scutellaria barbata [47,48,49,50] | Lamiaceae | Whole plant | Linyi district, Shandong Province, China. |
| Aerial parts | Purchased in a drugstore of Liaoning Guodayizhi Pharmaceutical Co., Ltd. China. | ||
| Aerial parts | Purchased from Bozhou Herbal Market in Anhui Province, China | ||
| Scutellaria strigillosa [51,52] | Lamiaceae | Whole plants | Yantai district, Shandong Province, China. |
| Whole plants | Hebei, Shandong, Zhejiang and Jilin Provinces, China | ||
| Sheareria nana [53] | Asteraceae | Whole herb | Jishou, Hunan Province, China. |
| Tinospora capillipes [54] | Menispermaceae | Whole herb | Xishuangbanna County, Yunnan Province, China. |
| Tinospora cordifolia [55] | Menispermaceae | Stems | India |
| Tinospora sagittata [56] | Menispermaceae | Roots | Anguo Medicine market in Hebei Province, China. |
| Ajuga pantantha [57,58] | Lamiaceae | Aerial parts | Yunnan Province, China. |
| Aerial parts | Purchased from Anhui Province, China. | ||
| Callicarpa arborea [59] | Lamiaceae | Twigs | Xishuangbanna and Yuanyang Prefectures. |
| Callicarpa cathayana [60] | Lamiaceae | Dried aerial parts | Bozhou Herbal Market in Anhui Province, China. |
| Callicarpa hypoleucophylla [61] | Lamiaceae | Leaves and twigs | Kaohsiung city, Taiwan. |
| Croton crassifolius [32,62] | Euphorbiaceae | Roots | Guangxi Province, China. |
| Croton floribundus [63] | Euphorbiaceae | Roots | Provided by the company Mudas Nativas e Exóticas. LTDA of CNPJ, Araraquara Brazil. |
| Croton laui [64] | Euphorbiaceae | Leaves | Hainan Province, China. |
| Croton poomae [65] | Euphorbiaceae | Leaves and stems | Bung Kan Province, Thailand. |
| Dodonaea viscosa [66] | Sapindaceae | Leaves | Sierra de Huautla, Morelos State, Mexico. |
| Dysoxylum lukii. [67] | Meliaceae | Twigs and leaves | Xishuangbanna County, Yunnan Province, China. |
| Jamesoniella autumnalis [68] | Adelanthaceae | Whole plant | Wangtiane park, Changbaishan City, Jilin Province, China. |
| Monoon membranifolium [69] | Annonaceae | Twig extract | Thailand and Peninsula Malaysia. |
| Nepeta suavis [70] | Lamiaceae | Roots | Found in central and southern Europe, North Africa and southern Asia. |
| Polyalthia longifolia [71] | Annonaceae | Seeds | Seshachalam hills, Tirupati, India. |
| Scutellaria barbata [72] | Lamiaceae | Aerial parts | Baise city, Guangxi Province, China. |
| Teucrium fructicans [73] | Lamiaceae | Aerial parts | Jiansu Province, China. |
| Tinospora crispa [74,75] | Menispermaceae | Stems | Mengla County, Yunnan Province, China. |
| Vines and leaves | Longzhou County, Guangxi Province, China. | ||
| Tinospora sagittata [76] | Menispermaceae | Tuberous roots | Shiyan city of Hubei Province, China. |
| Plant Source | Compound Name | Methods | Results | References |
|---|---|---|---|---|
| Ajuga decumbens | Compound 1 | CCK8 method A549 HeLa | IC50 µM 71.4 71.6 | [20] |
| Ajugamarin A1 (2) | A549 HeLa | 76.7 5.39 × 10−7 | ||
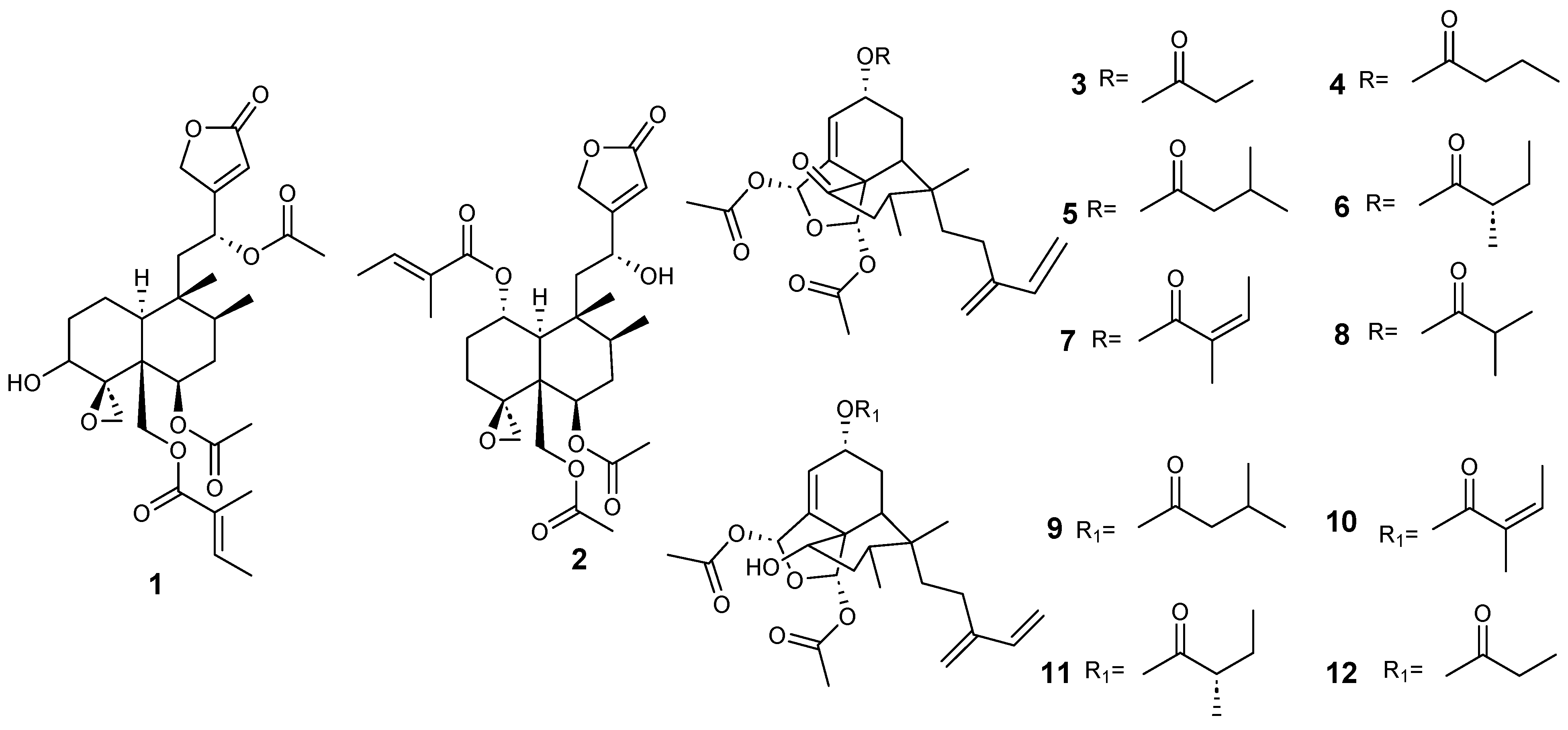
| Anacolosa clarkii | Anacolosin A (3) | SRB assay A-673 SJCRH30 D283 Hep293TT | TGI μM 1.10 0.52 0.70 1.00 | [21] |
| Anacolosin B (4) | A-673 SJCRH30 D283 Hep293TT | 1.00 0.50 0.60 0.90 | ||
| Anacolosin C (5) | A-673 SJCRH30 D283 Hep293TT | 1.10 0.67 0.66 1.00 | ||
| Anacolosin D (6) | A-673 SJCRH30 D283 Hep293TT | 1.20 0.73 0.66 0.80 | ||
| Anacolosin E (7) | A-673 SJCRH30 D283 Hep293TT | 3.10 1.90 2.00 1.80 | ||
| Anacolosin F (8) | A-673 SJCRH30 D283 Hep293TT | 4.10 2.30 2.30 3.20 | ||
| Corymbulosin X (9) | A-673 SJCRH30 D283 Hep293TT | 0.70 0.34 0.36 0.22 | ||
| Corymbulosin Y (10) | A-673 SJCRH30 D283 Hep293TT | 1.00 0.44 0.70 0.28 | ||
| Compound 11 | A-673 SJCRH30 D283 Hep293TT | 1.70 0.80 1.10 0.60 | ||
| Caseamembrin S (12) | A-673 SJCRH30 D283 Hep293TT | 0.90 0.36 0.50 0.30 | ||
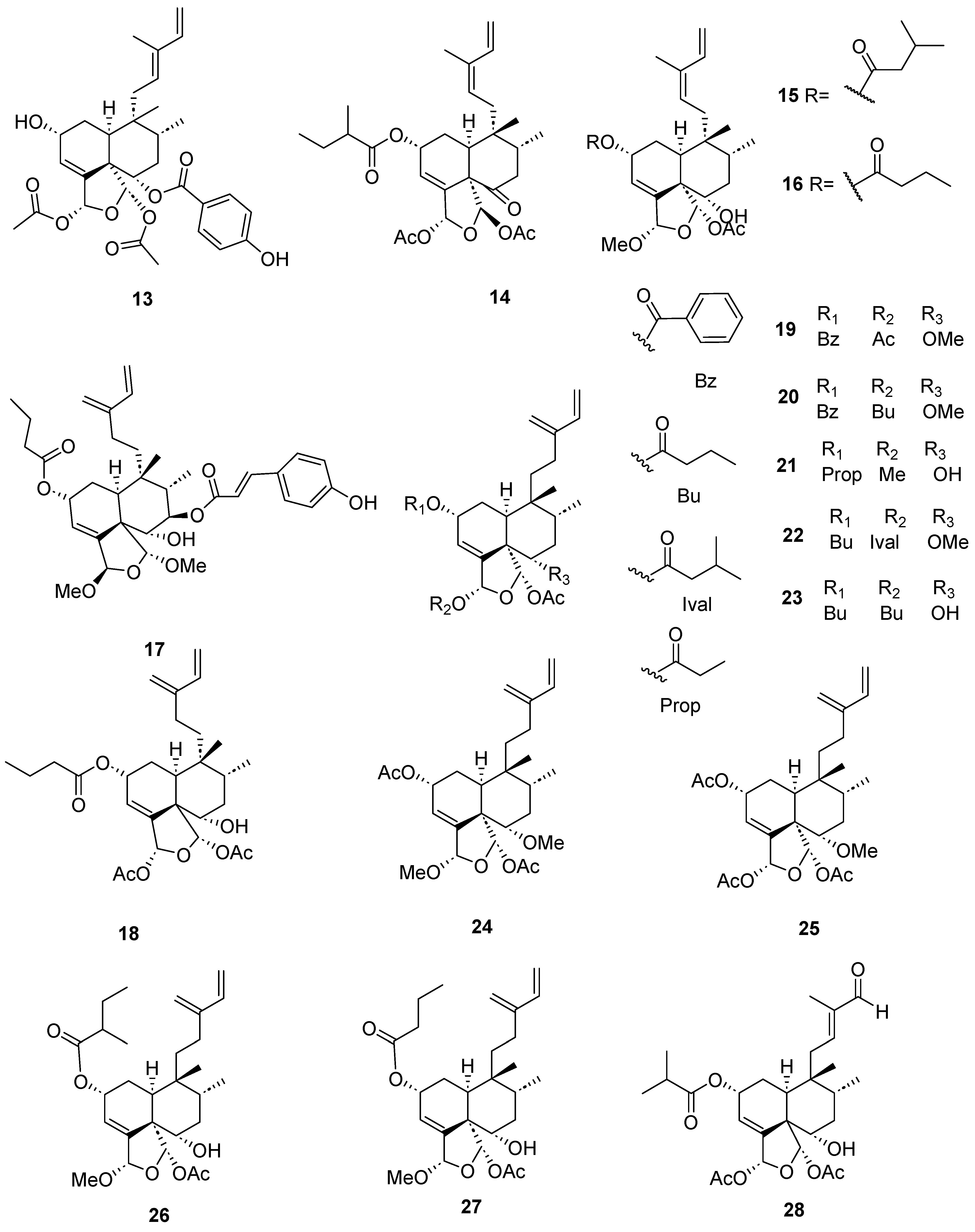
| Casearia corymbosa | Casearborin c (13) | SRB assay HeLa SiHa Vero | CC50µM (SI) 13.44 77.36 50.26 | [22] |
| Casearia graveolens | Caseariagraveolin (14) | REMA assay KB MCF-7 | IC50 μM 2.48 6.63 | [23] |
| Casearia grewiifolia | Caseargrewiin M (15) | MTT assay BT474 Chago-K1 Hep-G2 KATO-III SW620 | IC50 µg/mL 6.30 6.10 4.64 5.50 5.50 | [24,25] |
| Caseargrewiin G (16) | BT474 Chago-K1 Hep-G2 KATO-III SW620 | 5.67 6.10 0.90 5.46 3.85 | ||
| Caseagrewiifolin B (17) | WST-1 assay KB Hep-G2 | IC50 μM 6.2 7.0 | ||
| Caseanigrescen D (18) | KB Hep-G2 LU-1 MCF-7 NIH-3T3 | 0.5 0.3 0.9 0.8 0.3 | ||
| Casearia kurzii | Kurziterpene A (19) | MTT assay A549, HeLa HepG2 | IC50 μM 40.8 >60 >60 | [26,27,28,29] |
| Kurziterpene B (20) | A549 HeLa Hep-G2 | 19.7 12.1 49.3 | ||
| Kurziterpene C (21) | A549, HeLa Hep-G2 | >60 49.4 >60 | ||
| Kurziterpene D (22) | A549, HeLa Hep-G2 | 18.3 9.0 >60 | ||
| Kurziterpene E (23) | A549, HeLa Hep-G2 | 10.2 5.3 10.7 | ||
| Analysis via flow cytometry | Apoptosis of HeLa | |||
| (2R,5S,6S,8R,9R,10S,18S,19S)-2,19-diacetoyloxy-6,18-dimethoxy-18,19-epoxycleroda-3,13(16),14-triene (24) | MTT assay A549 HeLa Hep-G2 | IC50 μM >60 17.9 >60 | ||
| Corymbulosin M (25) | A549 HeLa Hep-G2 | 5.5 4.1 9.3 | ||
| Analysis via flow cytometry | Apoptosis of HeLa | |||
| Caseamembrin B (26) | MTT assay A549 HeLa Hep-G2 | IC50 μM 36.1 18.8 >60 | ||
| Caseamembrin U (27) | A549 HeLa Hep-G2 | 33.2 15.6 >60 | ||
| Caseakurzin A (28) | QIR assay A549 | IC50 μM 10.8 | ||
| Caseakurzin B (29) | QIR assay A549 | IC50 μM 4.4 | ||
| Cell apoptosis assay | Apoptosis of A549 | |||
| Caseakurzin C (30) | QIR assay A549 | IC50 μM 30.3 | ||
| Caseakurzin D (31) | 27.8 | |||
| Caseakurzin E (32) | 32.7 | |||
| Caseakurzin F (33) | 26.8 | |||
| Caseakurzin J (34) | QIR assay A549 | IC50 μM 4.6 | ||
| Cell apoptosis assay | Apoptosis of A549 | |||
| Kurzipene A (35) | MTT assay Hep-G2 A549 HeLa K562 | IC50 μM >60 >60 >60 >60 | ||
| Kurzipene B (36) | Hep-G2 A549 HeLa K562 | >60 32.6 54.6 >60 | ||
| Kurzipene C (37) | Hep-G2 A549 HeLa K562 | >60 >60 >60 >60 | ||
| Kurzipene D (38) | Hep-G2 A549 HeLa K562 | 9.7 10.9 12.4 7.2 | ||
| Flow cytometry | Apoptosis of Hep-G2 | |||
| Anti-tumor assay using zebrafish model | It blocked tumor cell invasion and metastasis | |||
| Kurzipene E (39) | Hep-G2 A549 HeLa K562 | >60 >60 >60 >60 | ||
| Kurzipene F (40) | Hep-G2 A549 HeLa K562 | >60 >60 33.1 >60 | ||
| Corymbulosin V (41) | Hep-G2 A549 HeLa K562 | 16.8 11.2 14.2 10.3 | ||
| Corymbulosin M (25) | Hep-G2 A549 HeLa K562 | 20.6 18.4 17.5 16.5 | ||
| Casearia sylvestris | Casearin X (42) | Induced sarcoma tumor 25 mg/kg/day | Tumor inhibition % 90.0 | [30] |
| Croton caudatus | Crocleropene A (43) | MTT assay MCF-7 | IC50 μM 35.8 | [31] |
| Crocleropene B (44) | MCF-7 | 40.2 | ||
| Croton crassifolius | Crassifolius A (45) | Morphology | Induced apoptosis | [32,33,34] |
| Western blot | Caspase activation | |||
| MTT assay Hep3B Hep-G2 | IC50 µM 17.91 42.04 | |||
| Crassifolin C (46) | Hep-G2 | 51.63 | ||
| Compound 47 | Hep-G2 | 45.22 | ||
| Crassifolin B (48) | CT26.WT | 96.6 | ||
| Crassifolin Q (49) | HUVEC assay | Compounds 49–51 and 53 inhibited angiogenesis | ||
| Crassifolin R (50) | ||||
| Crassifolin S (51) | ||||
| Crassifolin T (52) | HUVEC assay | Anti-angiogenesis effect | ||
| Crassifolin U (53) | HUVEC assay Junction densities Vessel areas Vessel lengths | IC50 μM 7.20 48.27 8.62 | ||
| Croton echioides | CEH-1 (54) | MTT assay HTC | Compound 54 diminished 67% cell viability and 55 < 76%. | [35] |
| CEH-4 (55) | ||||
| Croton oligandrus | Megalocarpoidolide D (56) | MTT assay A549 MCF-7 | IC50 µM 63.8 136.2. | [36] |
| 12-epi-megalocarpodolide D (57) | A549 MCF-7 | 138.6 171.3 | ||
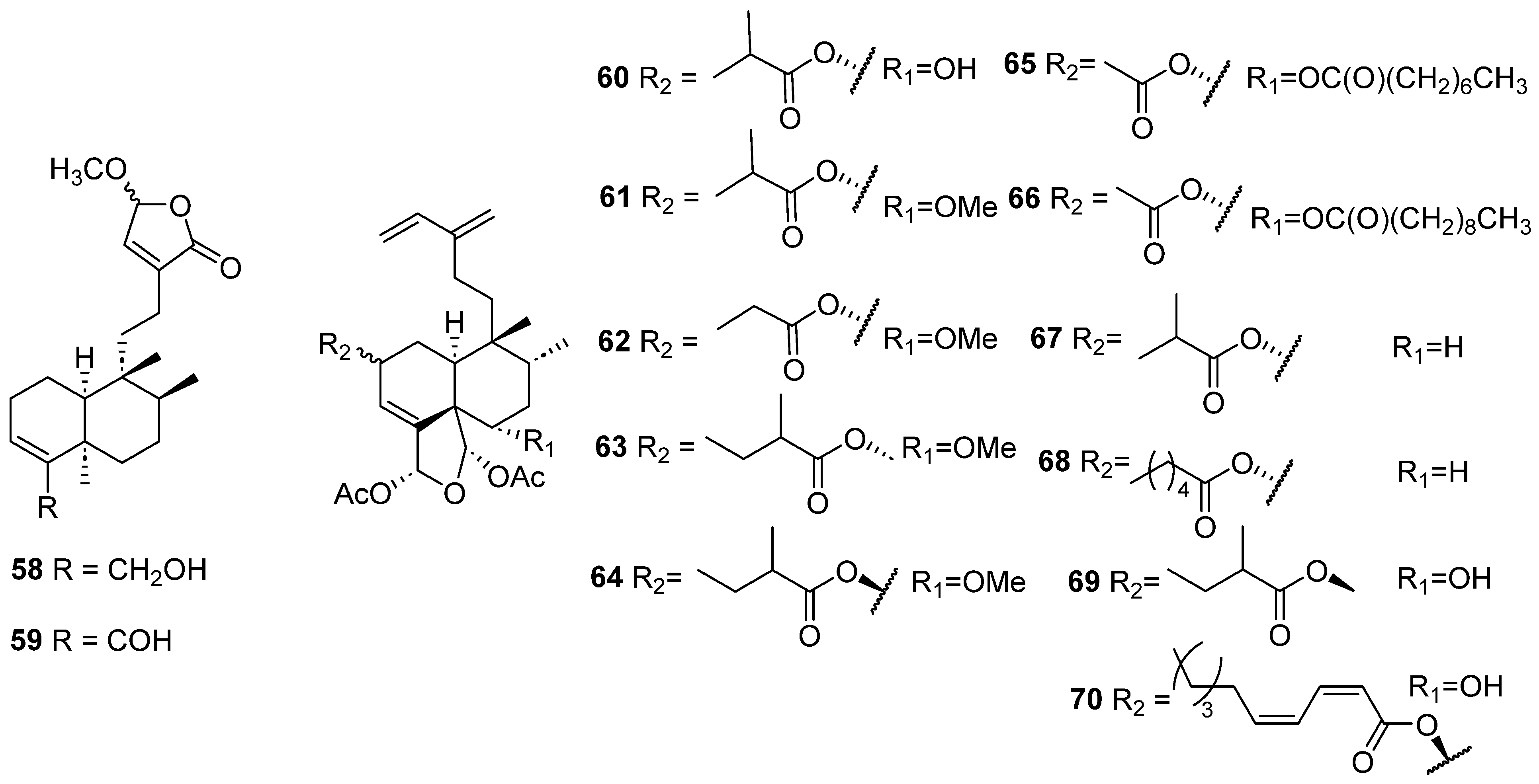
| Gottschelia schizopleura | Schizopleurolide A (58) | MTT assay HL-60 B16-F10 | IC50 µM 38.47 47.25 | [37] |
| Schizopleurolide B (59) | HL-60 B16-F10 | 36.13 44.33 | ||
| Laetia corymbulosa | Corymbulosin I (60) | Flow cytometry | Compounds 60, 61, 12 and 11 induced apoptosis in MDA-MB-231 | [38] |
| SRB assay A549 MDA-MB-231 MCF-7 KB KB-VIN | IC50 µM 0.66 0.48 0.68 0.56 0.98 | |||
| Corymbulosin K (61) | A549 MDA-MB-231 MCF-7 KB KB-VIN | 0.47 0.49 0.50 0.45 0.49 | ||
| Corymbulosin L (62) | A549 MDA-MB-231 MCF-7 KB KB-VIN | 4.60 4.95 4.94 5.19 4.92 | ||
| Corymbulosin N (63) | A549 MDA-MB-231 MCF-7 KB KB-VIN | 5.04 4.90 5.82 5.23 5.19 | ||
| Corymbulosin O (64) | A549 MDA-MB-231 MCF-7 KB KB-VIN | 4.75 3.31 4.65 4.25 4.76 | ||
| Corymbulosin P (65) | A549 MDA-MB-231 MCF-7 KB KB-VIN | 5.98 4.93 6.39 5.16 5.03 | ||
| Corymbulosin Q (66) | A549 MDA-MB-231 MCF-7 KB KB-VIN | 40.2 20.5 31.7 19.8 39.2 | ||
| Corymbulosin S (67) | A549 MDA-MB-231 MCF-7 KB KB-VIN | >40 22.9 26.2 25.1 26.6 | ||
| Corymbulosin T (68) | A549 MDA-MB-231 MCF-7 KB KB-VIN | 2.29 0.49 0.69 0.56 0.61 | ||
| Corymbulosin V (41) | A549 MDA-MB-231 MCF-7 KB KB-VIN | 4.76 4.73 5.19 4.74 4.88 | ||
| Caseamembrin S (12) | A549 MDA-MB-231 MCF-7 KB KB-VIN | 0.58 0.45 0.66 0.53 0.90 | ||
| Caseamembrin E (69) | A549 MDA-MB-231 MCF-7 KB KB-VIN | 0.53 0.40 0.55 0.43 0.51 | ||
| Corymbulosin A (70) | A549 MDA-MB-231 MCF-7 KB KB-VIN | 0.45 0.43 0.44 0.42 0.45 | ||
| Compound 11 | A549 MDA-MB-231 MCF-7 KB KB-VIN | 4.15 0.54 0.89 0.73 4.07 | ||
| Linaria japonica | Linarenone C (71) | MTT assay A549 | IC50 µM 51.2 | [39] |
| Linarenone E (72) | 86.5 | |||
| Linarienone (73) | 79.0 | |||
| Polyalthia longifolia | 16-hydroxy-cleroda-4(18),13-dien-16,15-olide (74) | Evaluation of morphometric liver and biochemical parameters in (NDEA+PB)-induced HCC rats | Compound 75 and 77 restored the parameters’ biochemical and liver morphology | [40] |
| MTT assay Hep-G2 | IC50 µg/mL 34.33 | |||
| 3α,16α-dihydroxy-cleroda-4(18),13(14)Z-dien-15,16-olide (75) | Hep-G2 HuH-7 | 14.34 47.32 | ||
| 16α-hydroxy-cleroda-3,13(14)Z-dien-15,16-olide (76) | Hep-G2 | 29.21 | ||
| 3β-16a-dihydroxy-cleroda-4(18),13(14)Z-dien-15,16-olide (77) | Hep-G2 HuH-7 | 24.91 48.57 | ||
| Polyalthia laui | Polylauiester A (78) | MTT assay HeLa MCF-7 A549 | IC50 μM 34.84 33.21 35.65 | [41] |
| (4→2)-abeo-2,13-diformyl-cleroda-2,12E-dien-14-oic acid (79) | HeLa MCF-7 A549 | 39.31 37.35 37.82 | ||
| Polylauiamide B (80) | HeLa MCF-7 A549 | 28.09 29.16 29.25 | ||
| Polylauiamide C (81) | HeLa MCF-7 A549 | 25.01 30.30 28.65 | ||
| Polylauiamide D (82) | HeLa MCF-7 A549 | 26.73 27.03 28.88 | ||
| Salvia amarissima | Teotihuacanin (83) | SRB assay MDA-MB-231 HeLa HCT-15 HCT-116 MCF-7 | IC50 μM 12.3 13.7 12.9 10.9 >20 | [42,43,44] |
| Amarissinin A (84) | MCF-7 MCF-7/Vin+ MDA-MB-231 HeLa | 18.2 0.27 19.3 14.0 | ||
| Amarissinin B (85) | SRB assay | 83, 84, 85, 86 and 87 exhibited MDR modulatory effects in mammalian cancer cells | ||
| Amarissinin C (86) | ||||
| Amarisolide F (87) | SRB assay MCF-7 HeLa HCT-15 HCT-116 MDA-MB-231 | IC50 μM 42.1 >42 >42 >42 >42 | ||
| Salvia involucrata | Involucratin A (88) | U251 PC-3 K562 SKLU-1 | 49.6 14.7 24.8 12.6 | [45] |
| Involucratin B (89) | U251 PC-3 K562 HCT-15 MCF-7 SKLU-1 COS-7 | 5.1 23.5 34.7 11.8 0.5 36.7 21.6 | ||
| Involucratin C (90) | PC-3 K562 HCT-15 SKLU-1 COS-7 | 11.0 19.4 9.7 16.8 11.9 | ||
| (-)-Hardwickiic acid (91) | U251 PC-3 K562 HCT-15 MCF-7 SKLU-1 COS-7 | 22.4 1.8 45.5 10.4 1.4 11.5 19.8 | ||
| 7α-hydroxybacchotricuneatin A (92) | U251 PC-3 K562 HCT-15 SKLU-1 COS-7 | 3.8 12.8 20.2 13.3 33.0 14.2 | ||
| Kingidiol (93) | SRB assay U251 PC-3 K562 HCT-15 MCF-7 SKLU-1 COS-7 | IC50 μM 22.4 13.0 51.6 15.5 0.8 22.9 19.7 | ||
| Salvia leucantha | Salvileucantholide (94) | MTT assay HCT-116 BT474 HepG2 Hsp90 | IC50 µM 32.61 25.02 37.35 6.78 | [46] |
| Scutellaria barbata | Scubatine A (95) | MTT assay HL-60 A549 | IC50 µM >20 >20 | [47,48,49,50] |
| Scubatine B (96) | HL-60 A549 | >20 >20 | ||
| Scubatine C (97) | HL-60 A549 | >20 >20 | ||
| Scubatine D (98) | HL-60 A549 | >20 >20 | ||
| Scubatine E (99) | HL-60 A549 | >20 >20 | ||
| Scubatine F (100) | HL-60 A549 | 15.3 10.4 | ||
| Scutebata E (101) | MTT assay HL-60 A549 LoVo | IC50 µM >20 >20 61.23 | ||
| Scutolide K (102) | HL-60 A549 | >20 >20 | ||
| Scutebata X (103) | SGC-7901 MCF-7 A549 | >40 37.2 >40 | ||
| Scutebata Y (104) | SGC-7901 MCF-7 A549 | >40 >40 >40 | ||
| Scutebata Z (105) | SGC-7901 MCF-7 A549 | >40 >40 >40 | ||
| Scutebata A1 (106) | SGC-7901 MCF-7 A549 | >40 >40 35.5 | ||
| Scutebata B1 (107) | SGC-7901 MCF-7 A549 | >40 >40 >40 | ||
| Scutebata C1 (108) | SGC-7901 MCF-7 A549 | 17.9 29.9 35.7 | ||
| Barbatin H. (109) | LoVo MCF-7 SMMC-7721 HCT-116 | 32.44 49.86 48.75 44.24 | ||
| Scuterbarbatine F (110) | LoVo MCF-7 SMMC-7721 HCT-116 | 23.32 49.19 58.12 78.83 | ||
| 6-O-nicotinoylscutebarbatine G (111) | LoVo SMMC-7721 HCT-116 | 29.44 65.51 54.44 | ||
| Scutebata G (112) | LoVo MCF-7 SMMC-7721 HCT-116 | 22.56 31.33 32.49 28.29 | ||
| Scutebata D (113) | LoVo MCF-7 SMMC-7721 HCT-116 | 20.75 31.42 29.24 62.66 | ||
| Barbatin C (114) | LoVo MCF-7 SMMC-7721 HCT-116 | 37.99 28.06 72.69 32.94 | ||
| Scutebarbatine A (115) | LoVo | 67.77 | ||
| Scutebarbatine G (116) | LoVo SMMC-7721 HCT-116 | 56.46 70.16 44.25 | ||
| 6,7-di-O-acetoxybarbatin A (117) | LoVo MCF-7 SMMC-7721 HCT-116 | 60.33 37.31 77.93 32.28 | ||
| Scutebarbatine X (118) | LoVo MCF-7 SMMC-7721 HCT-116 | 43.21 74.83 46.14 62.11 | ||
| Barbatin F (119) | LoVo HCT-116 | 56.46 44.25 | ||
| Barbatin G (120) | LoVo SMMC-7721 MCF-7 HCT-116 | 60.33 37.31 77.93 32.28 | ||
| Scutebata A (121) | LoVo SMMC-7721 MCF-7 HCT-116 HL-60 A549 | 4.57 7.68 5.31 6.23 >20 >20 | ||
| Scutebata B (122) | LoVo SMMC-7721 MCF-7 HCT-116 | 10.73 18.96 10.27 28.48 | ||
| Scutebata C (123) | LoVo SMMC-7721 MCF-7 | 47.15 33.18 38.79 | ||
| Scutebata P (124) | LoVo SMMC-7721 MCF-7 HCT-116 HL-60 A549 HCT-116 | 15.17 42.63 32.49 23.97 5.6 21.7 23.97 | ||
| Scutellaria strigillosa | Scutestrigillosin A (125) | REMA assay P-388 HONE-1, HT-29 MCF-7 | IC50 μM 5.8 3.5 4.7 5.7 | [51,52] |
| Scutestrigillosin B (126) | P-388 HONE-1 HT-29 MCF-7 | 5.2 4.2 4.1 6.0 | ||
| Scutestrigillosin C (127) | P-388 HONE-1, HT-29 MCF-7 | 7.1 3.9 6.4 7.7 | ||
| Scutestrigillosin D (128) | P388 HONE 1 HT-29 MCF-7 | 5.6 3.4 4.7 5.2 | ||
| Scutestrigillosin E (129) | P388 HONE 1 HT-29 MCF-7 | 8.9 7.3 8.1 7.4 | ||
| Sheareria nana | Sheareria A (130) | CCK8 assay HeLa PANC-1 A549 | IC50 µM 11.6 7.1 9.3 | [53] |
| Sheareria B (131) | HeLa PANC-1 A549 | 9.4 5.6 6.8 | ||
| Sheareria C (132) | HeLa PANC-1 A549 | 17.2 9.8 12.5 | ||
| Tinospora cordifolia | Tinocapillin A (133) | MTT assay A549 HepG2 HeLa OS-RC-2 | IC50 µM 14.0 9.9 9.7 10.6 | [54] |
| Tinocapillin B (134) | A549 HepG2 HeLa OS-RC-2 | 9.6 10.1 12.0 19.1 | ||
| Tinocapillin C (135) | A549 HeLa | 53.2 67.5 | ||
| Tinocallone A (136) | A549 HepG2 HeLa | 67.8 68.4 79.3 | ||
| Tinocallone C (137) | A549 HepG2 HeLa OS-RC-2 | 16.3 13.8 17.5 12.8 | ||
| Columbin (138) | A549 HeLa | 77.3 58.4 | ||
| Tinospora capillipes | ECD (epoxy clerodane diterpene) (139) | MTT assay V79 MCF-7 Vero | IC50 µM 52.7 3.2 45.8 | [55] |
| qPCR analysis | Inhibited MCF-7 grow by regulation the expression of genes such Cdkn2A, Rb1, Mdm2 y p53 | |||
| Tinospora sagittata | Tinosporin A (140) | MTT assay HL-60 MCF-7 | IC50 µM 18.63 23.58 | [56] |
| Plant Source | Compound Name | Methods | Results | References |
|---|---|---|---|---|
| Ajuga pantantha | Ajugapantin C (141) | Western Blot Analysis | Compounds 141, 142 and 146 downregulated iNOS and COX-2 protein levels | [57,58] |
| Docking Analysis | Compounds 141, 142 and 146 have strong interactions with the iNOS and COX-2 proteins | |||
| Griess assay BV-2 cells stimulated LPS | IC50 µM 20.2 | |||
| Ajugapantin E (142) | Griess assay BV-2 cells stimulated LPS | IC50 µM 45.5. | ||
| Ajugapantin F (143) | 34.0 | |||
| Ajugapantin G (144) | 27.0 | |||
| Ajugapantin H (145) | 45.0 | |||
| Ajugapantin I (146) | 25.8 | |||
| Pantanpene α (147) | Griess assay BV-2 cells stimulated LPS | IC50 μM 65.7 | ||
| Pantanpene B (148) | 37.7 | |||
| Pantanpene C (149) | 61.7 | |||
| Pantanpene d (150) | >50% inhibition at 30 μM | |||
| Pantanpene E (151) | Griess assay BV-2 cells stimulated LPS | IC50 μM 21.7 | ||
| Anti-inflammatory assay in zebrafish model | The anti-inflammatory effect was confirmed | |||
| Docking Analysis | Compounds 148 and 151 have strong interactions with the iNOS and COX-2 proteins | |||
| Callicarpa arborea | Callicarpin A (152) | NLRP3 Inflammasome activation assay J774A.1 cells were primed with LPS | IC50 μM 16.6 | [59] |
| Callicarpin B (153) | 4.0 | |||
| Callicarpin C (154) | 25.4 | |||
| (16S)-Tris-O-Acetylcallicarpin C (155) | 5.3 | |||
| Callicarpin E (156) | 24.7 | |||
| Callicarpin F (157) | 1.5 | |||
| Callicarpin G (158) | NLRP3 Inflammasome activation assay J774A.1 cells were primed with LPS | IC50 μM 1.4 | ||
| Pyroptosis fluorescence microscopy | The compound 153 inhibited pyroptosis and blocked NLRP3 inflammasome activation by hampering Casp-1 cleavage and IL-1β secretion | |||
| Callicarpa cathayana | Cathayanalactone A (159) | Griess assay RAW264.7 macrophages stimulated LPS | IC50 µM 22.92 | [60] |
| Cathayanalactone B (160) | 13.25 | |||
| Cathayanalactone C (161) | Griess assay RAW264.7 macrophages stimulated LPS | IC50 µM 82.82 | ||
| 15-methoxypatagonic acid (162) | 35.35 | |||
| 16-hydroxycleroda-3, 13-dien-16, 15-olide-18-oic acid (163) | Griess assay RAW264.7 macrophages stimulated LPS | IC50 µM 17.49 | ||
| ELISA assay Quantification of TNF-α, IL-6 and IL-1β | Compounds 161–163 inhibited IL-1β, IL-6 and TNF-α | |||
| Callicarpa hypoleucophylla | Callihypolin A (164) | Inhibitory activities in - superoxide anion generation and - elastase release in formyl-methionyl-leucyl-phenylalanine (fMLF)/cytochalasin (CB)-induced human neutrophils | % of inhibition 20.28 8.26 | [61] |
| Callihypolin B (165) | 32.19 17.55 | |||
| Compound 166 | 31.19 12.15 | |||
| Patagonic acid (167) | 32.88 13.57 | |||
| Limbatolide F (168) | 23.65 7.33 | |||
| Limbatolide A (169) | 8.44 10.50 | |||
| Compound 170 | 7.93 9.30 | |||
| Clerodermic acid (171) | 15.23 11.80 | |||
| Visclerodol acid (172) | 18.80 16.30 | |||
| Croton crassifolius | Crassifolin Q (49) | ELISA assay IL-6 TNF-α | % of production 72.23 89.38 | [32,62] |
| Crassifolin R (50) | 77.88 77.73 | |||
| Crassifolin S (51) | 73.36 79.23 | |||
| Crassifolin T (52) | 35.48 54.14 | |||
| Crassifolin U (53) | 32.78 12.53 | |||
| Compound 173 | Griess assay RAW264.7 macrophages stimulated LPS | IC50 μM 25.8 | ||
| Compound 174 | 173 at 178 < 50% inhibition at 50 µM | |||
| C-6 epimer of crotoeuricin C (175) | ||||
| Crotocaudin (176) | ||||
| Teucvin (177) | ||||
| Crassifolin F (178) | ||||
| Croton floribundus | Croflorin A (179) | Griess assay RAW264.7 macrophages stimulated LPS | IC50 μM 28.52 | [63] |
| Croflorin B (180) | 40.26 | |||
| Croflorin C (181) | 25.47 | |||
| Croflorin D (182) | 35.78 | |||
| 3α-hydroxy-5,10-didehydrochiliolide (183) | 40.58 | |||
| Croton laui | 3S-acetoxyl-mollotucin D dilactone ester (184) | Griess assay RAW264.7 macrophages stimulated LPS | IC50 µM weak activity | [64] |
| 6S-crotoeurin C (185) | 1.2 | |||
| Crotoeurin C (186) | 1.6 | |||
| Mollotucin D dilactone ester (187) | weak activity | |||
| Crassifolin F compound 178 | weak activity | |||
| Croton poomae | Crotonolide K (188) | Griess assay RAW264.7 macrophages stimulated LPS | IC50 µM 46.43 | [65] |
| Furocrotinsulolide A acetate (189) | 31.99 | |||
| Furocrotinsulolide A (190) | 81.97 | |||
| Compound 191 | 86.98 | |||
| Compound 192 | 48.85 | |||
| Crotonolide E (193) | 74.78 | |||
| Crotonolide F (194) | 42.04 | |||
| Compound 195 | 32.19 | |||
| Dodonaea viscosa | Hautriwaic acid (196) | Arthritis in mice induced by caolin/carrageenan Doses mg/kg 5 10 20 | % inflammation of edema after 15 days 27 20 13 | [66] |
| ELISA assay Quantification of IL-10, TNF-α, IL-6 and IL-1β | Compound 196 diminished TNF-α, IL-6 and IL-1β and increased IL-10 | |||
| Dysoxylum lukii. | neoclerod-13Z-ene-3α, 4β, 15-triol (197) | Griess assay RAW264.7 macrophages stimulated LPS | IC50 µM. 25.5 | [67] |
| Jamesoniella autumnalis | Jamesoniellide Q (198) | Griess assay RAW264.7 macrophages stimulated LPS | IC50 µM 45.10 | [68] |
| Jamesoniellide R (199) | 82.98 | |||
| Monoon membranifolium | 2β-Methoxyhardwickiic acid (200) | Griess assay RAW264.7 macrophages stimulated LPS | IC50 µM 65.4 | [69] |
| (-)-hardwickiic acid (91) | 38.9 | |||
| 2β-acetoxyhardwickiic acid (201) | 16.1 | |||
| 2β-hydroxyhardwickiic acid (202) | 82.4 | |||
| 15-methoxypatagonic acid (203) | 28.9 | |||
| Nepeta suavis. | Nepetolide (204) | Carrageenan-induced hind paw edema Docking Analysis In silico evaluation | Compound 204 inhibited hind paw edema Target Cox-2 EGFR and Lox-2 | [70] |
| Polyalthia longifolia | 16-oxo-cleroda-3,13(14)E-dien-15-oic acid (205) | Cyclooxygenase inhibitory assay 5-LOX kit COX-1 COX-2 5-LOX | IC50 µM 8.00 8.41 8.41 | [40,71] |
| 16-hydroxy-cleroda-3,13-dien-15-oic acid (206) | COX-1 COX-2 5-LOX | 9.75 4.07 9.78 | ||
| 16-hydroxy-cleroda-4(18),13-dien-16,15-olide (74) | COX-1 COX-2 5-LOX | 3.77 2.71 4.06 | ||
| 3α,16α-dihydroxy-cleroda-4(18),13(14)Z-dien-15,16-olide (75) | COX-1 COX-2 5-LOX | 3.63 4.29 5.67 | ||
| 16α-hydroxy-cleroda-3,13(14)Z-dien-15,16-olide (76) | COX-1 COX-2 5-LOX | 3.01 3.29 4.58 | ||
| Docking Analysis In silico evaluation | Compounds 74–76 have interactions with COX-1/2 and LOX enzymes | |||
| 3β-16a-dihydroxy-cleroda-4(18),13(14)Z-dien-15,16-olide (77) | ELISA assay Quantification of cytokines such as TNF-α, TGF-β, IL-6, IL-10 and IL-1β | Compounds 74 and 77 inhibited production of proinfammatory cytokines and increased IL-10 and TGF-β | ||
| Docking Analysis In silico evaluation | Compound 74 docked into the active sites of MDM2, TNF-α, FAK and IL-6 Compound 77 docked into the active sites of MDM2, TNF-α, TGF-β and FAK | |||
| Scutellaria barbata | Scuttenline C (207) | Griess assay RAW264.7 macrophages stimulated LPS | IC50 μM 1.9 | [72] |
| Barbatin A (208) | 12.6 | |||
| Scutebarbatine F (209) | 3.7 | |||
| Teucrium fructicans | 11-hidroxyfruticolone (210) | Griess assay RAW264.7 macrophages stimulated LPS | IC50 μM 39.3 | [73] |
| Tinospora crispa | Crispinoid D (211) | qPCR assay IL-1β, IL-6, TNF-α, iNOs, CCL12 and COX-2 | Compounds 211–213 diminish the production of pro-inflammatory mediators | [74,75] |
| Luciferase assay: Inhibition of NF-κB | IC50 μM 5.94 | |||
| Tinosporol C (212) | Inhibition of NF-κB | 6.32 | ||
| marrubiagenin-methylester (213) | Inhibition of NF-κB | 25.20 | ||
| Tinopanoid A (214) | Griess assay BV-2 cells stimulated LPS | IC50 μM >60 | ||
| Tinopanoid B (215) | >60 | |||
| Tinopanoid C (216) | 24.1 | |||
| Tinopanoid D (217) | 41.1 | |||
| Tinopanoid E (218) | 7.5 | |||
| Tinopanoid F (219) | 50.8 | |||
| Tinopanoid G (220) | 10.6 | |||
| Tinopanoid H (221) | 39.4 | |||
| Tinopanoid I (222) | 59.1 | |||
| Tinopanoid J (223) | 45.9 | |||
| Tinospin C (224) | >60 | |||
| borapetol B (225) | >60 | |||
| Tinotufolin D (226) | 14.5 | |||
| Tinospora sagittata | Fibaruretin H (227) | Griess assay RAW264.7 macrophages stimulated LPS | % inhibition at 24 µM 27.0% | [76] |
| Fibaruretin I (228) | 33.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Casares, R.M.; Hernández-Vázquez, L.; Mandujano, A.; Sánchez-Pérez, L.; Pérez-Gutiérrez, S.; Pérez-Ramos, J. Anti-Inflammatory and Cytotoxic Activities of Clerodane-Type Diterpenes. Molecules 2023, 28, 4744. https://doi.org/10.3390/molecules28124744
Martínez-Casares RM, Hernández-Vázquez L, Mandujano A, Sánchez-Pérez L, Pérez-Gutiérrez S, Pérez-Ramos J. Anti-Inflammatory and Cytotoxic Activities of Clerodane-Type Diterpenes. Molecules. 2023; 28(12):4744. https://doi.org/10.3390/molecules28124744
Chicago/Turabian StyleMartínez-Casares, Rubria Marlen, Liliana Hernández-Vázquez, Angelica Mandujano, Leonor Sánchez-Pérez, Salud Pérez-Gutiérrez, and Julia Pérez-Ramos. 2023. "Anti-Inflammatory and Cytotoxic Activities of Clerodane-Type Diterpenes" Molecules 28, no. 12: 4744. https://doi.org/10.3390/molecules28124744
APA StyleMartínez-Casares, R. M., Hernández-Vázquez, L., Mandujano, A., Sánchez-Pérez, L., Pérez-Gutiérrez, S., & Pérez-Ramos, J. (2023). Anti-Inflammatory and Cytotoxic Activities of Clerodane-Type Diterpenes. Molecules, 28(12), 4744. https://doi.org/10.3390/molecules28124744







