Differential Mobility Spectrometry-Tandem Mass Spectrometry with Multiple Ion Monitoring Coupled with in Source-Collision Induced Dissociation: A New Strategy for the Quantitative Analysis of Pharmaceutical Polymer Excipients in Rat Plasma
Abstract
:1. Introduction
2. Results and Discussions
2.1. MS Analysis of PLAs
2.1.1. Fragmentation of PLA 20,000 by in Source-CID
2.1.2. Fragmentation Efficiency of the Sodium Adduct Ions [M + Na]+
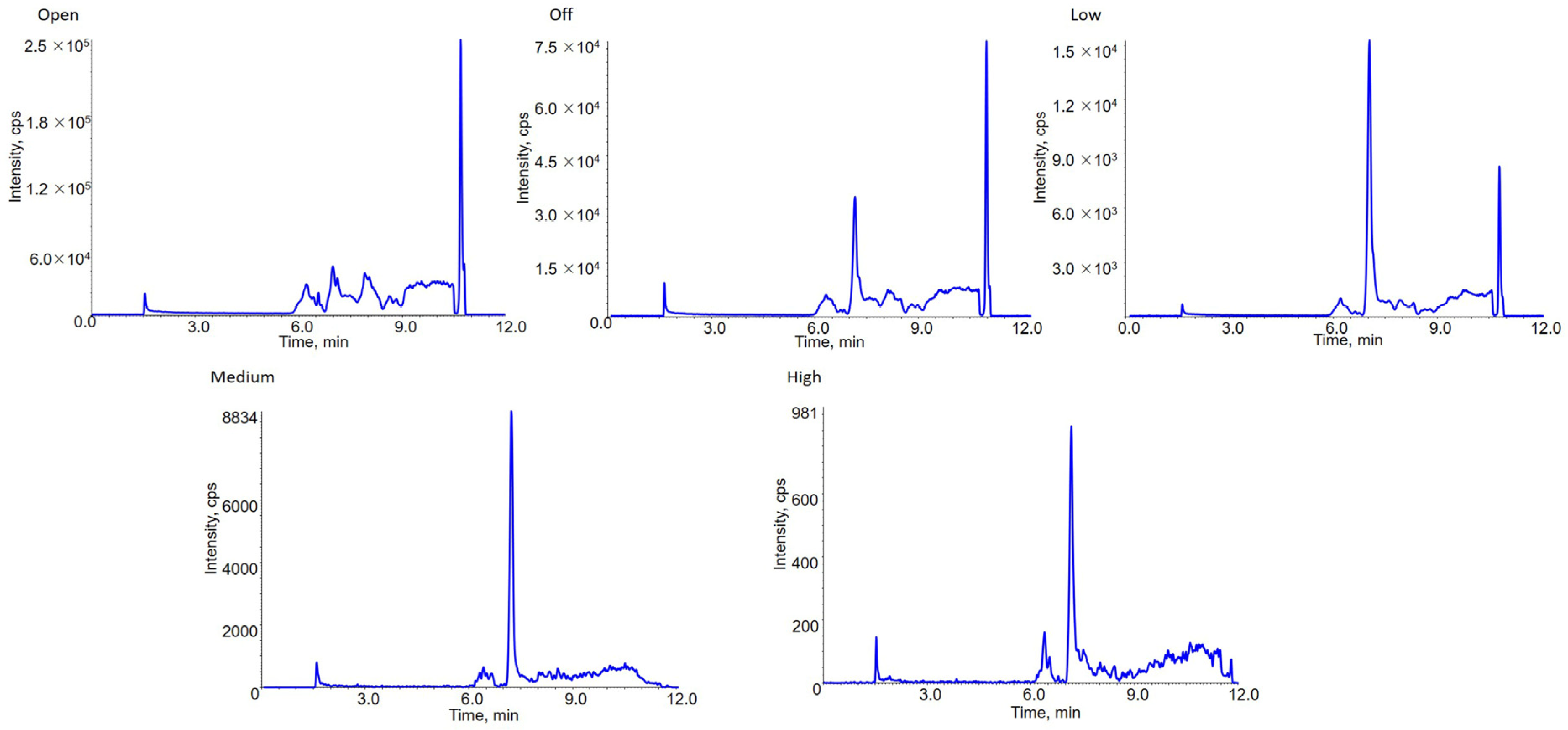
2.2. Application of DMS
2.3. Comparation between LC-MRM, LC-MIM and LC-DMS-MIM
2.4. Optimization of LC Condition
2.5. Sample Preparation
2.6. Assay Validation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. LC Condition
3.3. DMS and MS Conditions
3.4. Preparation of Calibration Standards and Quanlity Control (QC)
3.5. Sample Preparation
3.6. Assay Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Mu, W.; Chu, Q.; Liu, Y.; Zhang, N. A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy. Nanomicro Lett. 2020, 12, 142. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef] [PubMed]
- Vaid, R.; Yildirim, E.; Pasquinelli, M.A.; King, M.W. Hydrolytic Degradation of Polylactic Acid Fibers as a Function of pH and Exposure Time. Molecules 2021, 26, 7554. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.; Hennink, W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Yang, Z.; Sun, R.; Mo, Z.; Jin, G.; Wei, F.; Hu, J.; Guan, W.; Zhong, N. Preparation of lung-targeting, emodin-loaded polylactic acid microspheres and their properties. Int. J. Mol. Sci. 2014, 15, 6241–6251. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, Y.; Asawa, Y.; Takato, T.; Hoshi, K. Tissue reactions to engineered cartilage based on poly-L-lactic acid scaffolds. Tissue Eng. Part A 2009, 15, 1565–1577. [Google Scholar] [CrossRef]
- Lee, J.W.; Gardella, J.A., Jr. Simultaneous time-of-flight secondary ion MS quantitative analysis of drug surface concentration and polymer degradation kinetics in biodegradable poly(L-lactic acid) blends. Anal. Chem. 2003, 75, 2950–2958. [Google Scholar] [CrossRef]
- Pavot, V.; Berthet, M.; Rességuier, J.; Legaz, S.; Handké, N.; Gilbert, S.C.; Paul, S.; Verrier, B. Poly(lactic acid) and poly(lactic-co-glycolic acid) particles as versatile carrier platforms forvaccine delivery. Nanomedicine 2014, 17, 2703–2718. [Google Scholar] [CrossRef]
- Stankevich, K.S.; Gudima, A.; Filimonov, V.D.; Klüter, H.; Mamontova, E.M.; Tverdokhlebov, S.I.; Kzhyshkowska, J. Surface modification of biomaterials based on high-molecular polylactic acid and their effect on inflammatory reactions of primary human monocyte-derived macrophages: Perspective for personalized therapy. Mater. Sci. Eng. Mater. Biol. Appl. 2015, 51, 117–1126. [Google Scholar] [CrossRef]
- Tegnander, A.; Engebretsen, L.; Bergh, K.; Eide, E.; Holen, K.; Iversen, O. Activation of the complement system and adverse effects of biodegradable pins of polylactic acid (Biofix) in osteochondritis dissecans. Acta Orthop. Scand. 1994, 65, 472–475. [Google Scholar] [CrossRef] [Green Version]
- Thakur, K.A.; Kean, R.T.; Hall, E.S.; Doscotch, M.A.; Munson, E.J. A Quantitative Method for Determination of Lactide Composition in Poly(lactide) Using (1)H NMR. Anal. Chem. 1997, 69, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Hanton, S.D.; Liu, X.M. GPC separation of polymer samples for MALDI analysis. Anal. Chem. 2000, 72, 4550–4554. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sargent, J.R.; Weber, W.P. Fractionation of moderate molecular weight polysiloxanes by centrifugal TLC. Anal. Chem. 2001, 73, 3781–3783. [Google Scholar] [CrossRef] [PubMed]
- Aaserud, D.J.; Prokai, L.; Simonsick, W.J. Gel permeation chromatography coupled to fourier transform mass spectrometry for polymer characterization. Anal. Chem. 1999, 71, 4793–4799. [Google Scholar] [CrossRef] [PubMed]
- Michelle Byrd, H.C.; McEwen, C.N. The limitations of MALDI-TOF mass spectrometry in the analysis of wide polydisperse polymers. Anal. Chem. 2000, 72, 4568–4576. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Cheng, T.C.; Wang, H.E.; Li, J.J.; Lin, W.W.; Huang, C.C.; Chuang, C.H.; Wang, Y.T.; Wang, J.Y.; Roffler, S.R.; et al. Using anti-poly(ethylene glycol) bioparticles for the quantitation of PEGylated nanoparticles. Sci. Rep. 2016, 6, 39119. [Google Scholar] [CrossRef]
- Cheng, T.C.; Chuang, K.H.; Chen, M.; Wang, H.E.; Tzou, S.C.; Su, Y.C.; Chuang, C.H.; Kao, C.H.; Chen, B.M.; Chang, L.S.; et al. Sensitivity of PEGylated interferon detection by anti-polyethylene glycol (PEG) antibodies depends on PEG length. Bioconjug Chem. 2013, 24, 1408–1413. [Google Scholar] [CrossRef]
- Liu, Y.; Reidler, H.; Pan, J.; Milunic, D.; Qin, D.; Chen, D.; Vallejo, Y.R.; Yin, R. A double antigen bridging immunogenicity ELISA for the detection of antibodies to polyethylene glycol polymers. J. Pharmacol. Tox. Met. 2011, 64, 238–245. [Google Scholar] [CrossRef]
- Baumann, A.; Piel, I.; Hucke, F.; Sandmann, S.; Hetzel, T.; Schwarz, T. Pharmacokinetics, excretion, distribution, and metabolism of 60-kDa polyethylene glycol used in BAY 94-9027 in rats and its value for human prediction. Eur. J. Pharm. Sci. 2019, 130, 11–20. [Google Scholar] [CrossRef]
- Hinton, T.M.; Monaghan, P.; Green, D.; Kooijmans, S.A.; Shi, S.; Breheney, K.; Tizard, M.; Nicolazzo, J.A.; Zelikin, A.N.; Wark, K. Biodistribution of polymer hydrogel capsules for the delivery of therapeutics. Acta Biomater. 2012, 8, 3251–3260. [Google Scholar] [CrossRef]
- Pirali, T.; Serafini, M.; Cargnin, S.; Genazzani, A.A. Applications of Deuterium in Medicinal Chemistry. J. Med. Chem. 2019, 62, 5276–5297. [Google Scholar] [CrossRef] [PubMed]
- Osaka, I.; Yoshimoto, A.; Watanabe, M.; Takama, M.; Murakami, M.; Kawasaki, H.; Arakawa, R. Quantitative determination of cyclic polylactic acid oligomers in serum by direct injection liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2008, 870, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Gu, X.; Achanzar, W.E.; Chadwick, K.D.; Gan, J.; Brock, B.J.; Kishnani, N.S.; Humphreys, W.G.; Iyer, R.A. Quantitative analysis of polyethylene glycol (PEG) and PEGylated proteins in animal tissues by LC-MS/MS coupled with in-source CID. Anal. Chem. 2014, 86, 7642–7649. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, Y.; He, Y.; Ren, T.; Yin, L.; Fawcett, J.P.; Gu, J.; Sun, H. Impact of molecular weight on the mechanism of cellular uptake of polyethylene glycols (PEGs) with particular reference to P-glycoprotein. Acta. Pharm. Sin. 2020, 10, 2002–2009. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Z.; Tong, J.; Sun, H.; Fawcett, J.P.; Gu, J. The biological fate of the polymer nanocarrier material monomethoxy poly(ethylene glycol)-block-poly(d,l-lactic acid) in rat. Acta. Pharm. Sin. 2021, 11, 1003–1009. [Google Scholar] [CrossRef]
- Ren, T.; Li, R.; Zhao, L.; Fawcett, J.P.; Sun, D.; Gu, J. Biological fate and interaction with cytochromes P450 of the nanocarrier material, d-alpha-tocopheryl polyethylene glycol 1000 succinate. Acta. Pharm. Sin. 2022, 12, 3156–3166. [Google Scholar] [CrossRef]
- Ruifeng, B.; Dong, S.; Yuqin, S.; Zhiqiong, G.; Dafeng, C.; John Paul, F.; Jingkai, G. Disposition and fate of polyoxyethylene glycerol ricinoleate as determined by LC-Q-TOF MS coupled with MSALL, SWATH and HR MS/MS techniques. Chin. Chem. Lett. 2021, 32, 3237–3240. [Google Scholar]
- Meng, X.J.; Xu, H.T.; Zhang, Z.; Fawcett, J.P.; Li, J.; Yang, Y.; Gu, J.K. Differential mobility spectrometry tandem mass spectrometry with multiple ion monitoring for the bioanalysis of liraglutide. Anal. Bioanal. Chem. 2017, 409, 4885–4891. [Google Scholar] [CrossRef]
- Fu, Y.; Xia, Y.Q.; Flarakos, J.; Tse, F.L.; Miller, J.D.; Jones, E.B.; Li, W. Differential mobility spectrometry coupled with multiple ion monitoring in regulated LC-MS/MS bioanalysis of a therapeutic cyclic peptide in human plasma. Anal. Chem. 2016, 88, 3655–3661. [Google Scholar] [CrossRef]
- Levin, D.S.; Miller, R.A.; Nazarov, E.G.; Vouros, P. Rapid separation and quantitative analysis of peptides using a new nanoelectrospray differential mobility spectrometer-mass spectrometer system. Anal. Chem. 2006, 78, 5443–5452. [Google Scholar] [CrossRef]
- Beach, D.G.; Kerrin, E.S.; Quilliam, M.A. Selective quantitation of the neurotoxin BMAA by use of hydrophilic-interaction liquid chromatography-differential mobility spectrometry-tandem mass spectrometry (HILIC-DMS-MS/MS). Anal. Bioanal. Chem. 2015, 407, 8397–8409. [Google Scholar] [CrossRef] [PubMed]
- Kolakowski, B.M.; Mester, Z. Review of applications of high-field asymmetric waveform ion mobility spectrometry (FAIMS) and differential mobility spectrometry (DMS). Analyst 2007, 132, 842–864. [Google Scholar] [CrossRef] [PubMed]
- Lapthorn, C.; Pullen, F.; Chowdhry, B.Z. Ion mobility spectrometry-mass spectrometry (IMS-MS) of small molecules: Separating and assigning structures to ions. Mass. Spectrom. Rev. 2013, 32, 43–71. [Google Scholar] [CrossRef] [Green Version]
- Ren, T.; Li, R.; Meng, X.; Fawcett, J.P.; Sun, D.; Gu, J. Differential mobility spectrometry followed by tandem mass spectrometry with multiple ion monitoring for bioanalysis of eptifibatide in rat plasma. J. Pharmaceut. Biomed. 2018, 151, 260–265. [Google Scholar] [CrossRef] [PubMed]






| Spiked Concentration (/μg·mL−1) | Day | Intra-Day Accuracy (DEV %) | Intra-Day Precision (CV %) | Inter-Day Accuracy (DEV %) | Inter-Day Precision (CV %) |
|---|---|---|---|---|---|
| 3.00 (LLOQ) | 1 | −0.94 | 5.31 | −2.46 | 5.86 |
| 2 | −3.56 | 6.65 | |||
| 3 | −2.89 | 6.34 | |||
| 4.00 | 1 | 4.58 | 3.88 | 6.18 | 5.24 |
| 2 | 4.38 | 6.61 | |||
| 3 | 9.58 | 4.12 | |||
| 24.00 | 1 | 11.39 | 4.02 | 9.07 | 4.28 |
| 2 | 10.42 | 2.93 | |||
| 3 | 5.42 | 4.12 | |||
| 90.00 | 1 | 4.65 | 3.68 | 3.98 | 3.52 |
| 2 | 5.24 | 2.13 | |||
| 3 | 2.06 | 4.25 |
| Storage Conditions | Low QC Samples (%) | High QC Samples (%) |
|---|---|---|
| Long terms (−80 °C, 14 days) | 94.4 ± 3.6 | 94.1 ± 4.5 |
| Three freeze-thaw cycles | 92.7 ± 6.0 | 95.5 ± 3.7 |
| Room temperature after sample preparation (25 °C, 3 h) | 93.4 ± 4.2 | 96.0 ± 1.8 |
| Autosampler (8 °C, 12 h) | 100.6 ± 5.7 | 101.5 ± 5.3 |
| Parameters | PLA 20,000 | PEG 2000 (IS) |
|---|---|---|
| Ionspray voltage (V) | 5500 | 5500 |
| Curtain gas (N2, psi) | 25 | 25 |
| Source temperature (°C) | 500 | 500 |
| Nebulizer gas (N2, psi) | 45 | 45 |
| Heater gas (N2, psi) | 45 | 45 |
| m/z transition | 671.2 → 671.2 | 133.0 → 89.1 |
| Declustering potential (V) | 180 | 210 |
| Collision energy (eV) | 20 | 11 |
| DMS temperature (°C) | 150 | 150 |
| DMS resolution enhancement | Medium | Medium |
| DMS separation voltage (V) | 3500 | 3500 |
| DMS compensation voltage (V) | 6 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, Z.; Liu, Y.; Cai, D.; Gu, J.; Sun, D. Differential Mobility Spectrometry-Tandem Mass Spectrometry with Multiple Ion Monitoring Coupled with in Source-Collision Induced Dissociation: A New Strategy for the Quantitative Analysis of Pharmaceutical Polymer Excipients in Rat Plasma. Molecules 2023, 28, 4782. https://doi.org/10.3390/molecules28124782
Zhang Y, Zhang Z, Liu Y, Cai D, Gu J, Sun D. Differential Mobility Spectrometry-Tandem Mass Spectrometry with Multiple Ion Monitoring Coupled with in Source-Collision Induced Dissociation: A New Strategy for the Quantitative Analysis of Pharmaceutical Polymer Excipients in Rat Plasma. Molecules. 2023; 28(12):4782. https://doi.org/10.3390/molecules28124782
Chicago/Turabian StyleZhang, Yuyao, Zhi Zhang, Yingze Liu, Deqi Cai, Jingkai Gu, and Dong Sun. 2023. "Differential Mobility Spectrometry-Tandem Mass Spectrometry with Multiple Ion Monitoring Coupled with in Source-Collision Induced Dissociation: A New Strategy for the Quantitative Analysis of Pharmaceutical Polymer Excipients in Rat Plasma" Molecules 28, no. 12: 4782. https://doi.org/10.3390/molecules28124782





