Phytochemical Profiling, Antioxidant and Tyrosinase Regulatory Activities of Extracts from Herb, Leaf and In Vitro Culture of Achillea millefolium (Yarrow)
Abstract
:1. Introduction
2. Results and Discussion
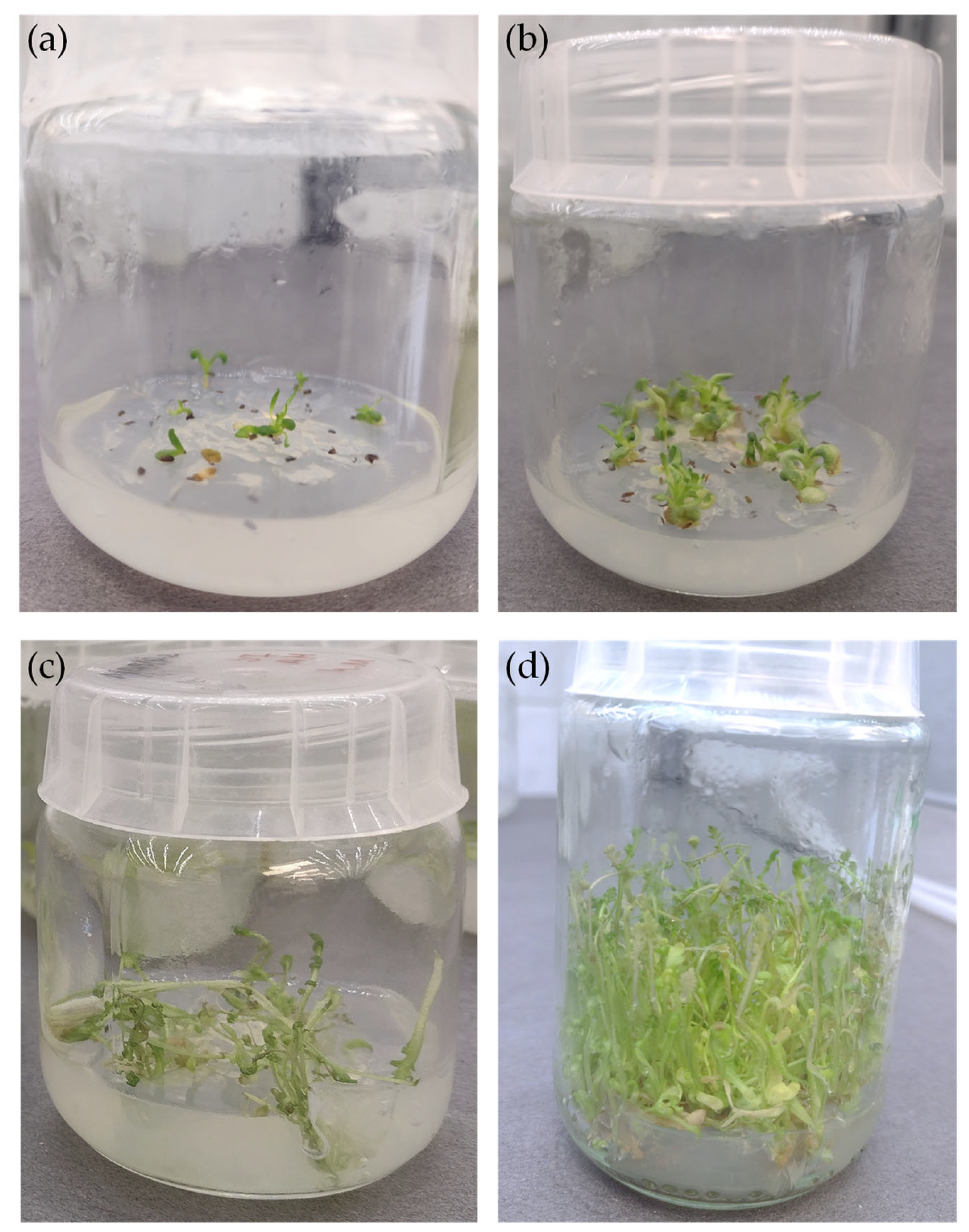
2.1. Achillea millefolium Microshoot Cultures
2.2. Comparative Study on Total Polyphenols and Antioxidant Activity
2.3. Phytochemical Profiling
2.4. Tyrosinase Inhibitory Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and Extraction
3.2.1. Achillea millefolium In Vitro Cultures
3.2.2. Achillea millefolium Herbs and Leaves from the Field Condition
3.2.3. Extraction Procedure
3.3. Sample Preparation and UHPLC-UV-hr-qTOF-MS/MS Analysis
3.4. Calibration, Data Cleansing, Peak Picking and Multivariate Analysis of the Recorded Spectral Data
3.5. Total Polyphenolic Content
3.6. DPPH Scavenging Activity Assay
3.7. Mushroom and Murine Tyrosinase Inhibitory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Saeidnia, S.; Gohari, A.; Mokhber-Dezfuli, N.; Kiuchi, F. A review on phytochemistry and medicinal properties of the genus Achillea. Daru 2011, 19, 173–186. [Google Scholar]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Kukula-Koch, W. Achillea species as sources of active phytochemicals for dermatological and cosmetic applications. Oxid. Med. Cell Longev. 2021, 25, 6643827. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/growth/tools-databases/cosing/index (accessed on 13 October 2022).
- Zengin, G.; Bulut, G.; Mollica, A.; Haznedaroglu, M.Z.; Dogan, A.; Aktumsek, A. Bioactivities of Achillea phrygia and Bupleurum croceum based on the composition of phenolic compounds: In vitro and in silico approaches. Food Chem. Toxicol. 2017, 107, 597–608. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Czop, M.; Sakipova, Z.; Głowniak, K.; Kukula-Koch, W. Achillea millefolium L. and Achillea biebersteinii Afan. hydroglycolic extracts–bioactive ingredients for cosmetic use. Molecules 2020, 25, 3368. [Google Scholar] [CrossRef]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: A Review. Phytother. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef]
- Lee, H.J.; Sim, M.O.; Woo, K.W.; Jeong, D.E.; Jung, H.K.; An, B.; Cho, H.W. Antioxidant and Antimelanogenic Activities of Compounds Isolated from the Aerial Parts of Achillea alpina L. Chem. Biodivers. 2019, 16, e1900033. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A.; Ceylan, R.; Uysal, S.; Mocan, A.; Guler, G.O.; Mahomoodally, M.F.; Glamočlija, J.; Ćirićf, A.; Soković, M. Shedding light on the biological and chemical fingerprints of three Achillea species (A. biebersteinii, A. millefolium and A. teretifolia). Food Funct. 2017, 8, 1152–1165. [Google Scholar] [CrossRef]
- García, M.D.; Sáenz, M.T.; Gómez, M.A.; Fernández, M.A. Topical antiinflammatory activity of phytosterols isolated from Eryngium foetidum on chronic and acute inflammation models. Phytother. Res. 1999, 13, 78–80. [Google Scholar] [CrossRef]
- Gómez, M.A.; Sáenz, M.T.; García, M.D.; Fernández, M.A. Study of the topical anti-inflammatory activity of Achillea ageratum on chronic and acute inflammation models. Z Naturforsch. C J. Biosci. 1999, 54, 937–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausen, B.M.; Breuer, J.; Weglewski, J.; Rücker, G. alpha-Peroxyachifolid and other new sensitizing sesquiterpene lactones from yarrow (Achillea millefolium L., Compositae). Contact Dermat. 1991, 24, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of Achillea millefolium as used in cosmetics. Int. J. Toxicol. 2016, 35, 5S–15S. [Google Scholar] [CrossRef] [Green Version]
- Bilharz, M.; Schmitt, K.; Going Big with Big Matters. The Key Points Approach to Sustainable Consumption. GAIA 2011, 20, 232–235. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Barbulova, A.; Apone, F.; Colucci, G. Plant cell cultures as source of cosmetic active ingredients. Cosmetics 2014, 1, 94–104. [Google Scholar] [CrossRef]
- Krasteva, G.; Georgiev, V.; Pavlov, A. Recent applications of plant cell culture technology in cosmetics and foods. Eng. Life Sci. 2020, 18, 68–76. [Google Scholar] [CrossRef]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, I.C.A.; Pacheco, F.V.; Silva, S.T.; Bertolucci, S.K.V.; Pinto, J.E.B.P. In vitro culture of Achillea millefolium L.: Quality and intensity of light on growth and production of volatiles. Plant Cell Tissue Organ Cult. PCTOC 2015, 122, 299–308. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Pais, M.S.S.; Scheffer, J.J.C. Achillea millefolium L. ssp. millefolium (Yarrow): In vitro culture and production of essential oils. In Medicinal and Aromatic Plants VIII. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1995; p. 33. [Google Scholar] [CrossRef]
- Szopa, A.; Ekiert, H. Production of biologically active phenolic acids in Aronia melanocarpa (Michx.) Elliott in vitro cultures cultivated on different variants of the Murashige and Skoog medium. Plant Growth Regul. 2014, 72, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Turker, A.U.; Yucesan, B.; Gurel, E. In vitro regeneration of Achillea millefolium L from shoot-tips and root segments of seedlings. J. Plant Biochem. Biotechnol. 2009, 18, 65–69. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Maloupa, E. GIS-facilitated in vitro propagation and ex situ conservation of Achillea occulta. Plant Cell Tissue Organ. Cult. PCTOC 2011, 107, 531–540. [Google Scholar] [CrossRef]
- Rocha, T.T.; Araújo, D.X.; de Carvalho, A.A.; Germano, C.M.; de Fátima Santos, M.; Lameira, O.A.; Pinto, J.E.B.P. In vitro culture of Lippia dulcis (Trev.): Light intensity and wavelength effects on growth, antioxidant defense, and volatile compound production. Vitr. Cell Dev. Biol.-Plant 2022, 58, 636–652. [Google Scholar] [CrossRef]
- Ekiert, H.; Kubica, P.; Szopa, A. Successful cultivation and utilization of Aronia melanocarpa (Michx.) Elliott (Black chokeberry), a species of North-American origin, in Poland and the biosynthetic potential of cells from in vitro cultures. Med. Plants Domest. Biotechnol. Reg. Importance 2021, 69–111. [Google Scholar] [CrossRef]
- Georgieva, L.; Gadjalova, A.; Mihaylova, D.; Pavlov, A. Antioxidant activity, total phenolic and flavonoid content of water and ethanol extracts from Achillea millefolium L. Turk. J. Pharm. Sci. 2015, 10, 385–391. [Google Scholar]
- Georgieva, L.; Gadjalova, A.; Mihaylova, D.; Pavlov, A. Achillea millefolium L.-phytochemical profile and in vitro antioxidant activity. Int. Food Res. J. 2015, 22, 385–392. [Google Scholar]
- Mohaddab, M.; El Goumi, Y.; Gallo, M.; Montesano, D.; Zengin, G.; Bouyahya, A.; Fakiri, M. Biotechnology and in vitro culture as an alternative system for secondary metabolite production. Molecules 2022, 27, 8093. [Google Scholar] [CrossRef]
- Ramawat, K.G.; Mathur, M. Factors affecting the production of secondary metabolites. In Biotechnology: Secondary Metabolites, Plants and Microbes; Science Publishers: Enfield, NH, USA, 2007; pp. 59–102. [Google Scholar]
- Bruni, R.; Sacchetti, G. Factors affecting polyphenol biosynthesis in wild and field grown St. John’s Wort (Hypericum perforatum L. Hypericaceae/Guttiferae). Molecules 2009, 14, 682–725. [Google Scholar] [CrossRef] [Green Version]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.; Thiem, B.; Szopa, A.; Ekiert, H. Accumulation of valuable secondary metabolites: Phenolic acids and flavonoids in different in vitro systems of shoot cultures of the endangered plant species—Eryngium alpinum L. Plant Cell Tissue Organ. Cult. (PCTOC) 2020, 141, 381–391. [Google Scholar] [CrossRef] [Green Version]
- Kikowska, M.; Kedziora, J.; Krawczyk, A.; Thiem, B. Methyl jasmonate, yeast extract and sucrose stimulate phenolic acids accumulation in Eryngium planum L. shoot cultures. Acta Biochim. Pol. 2015, 62, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekiert, H.; Gomółka, E. Effect of light on contents of coumarin compounds in shoots of Ruta graveolens L. cultivated in vitro. Acta Soc. Bot Pol. 1999, 68, 197–200. [Google Scholar] [CrossRef]
- Szopa, A.; Ekiert, H.; Szewczyk, A.; Fugas, E. Production of bioactive phenolic acids and furanocoumarins in vitro cultures of Ruta graveolens L. and Ruta graveolens ssp. divaricata (Tenore) Gams. Under different light conditions. Plant Cell Tissue Organ Cult. 2012, 110, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, S.S.; Akbar, F.; Kanwal, F. Correlation of different spectral lights with biomass accumulation and production of antioxidant secondary metabolites in callus cultures of medicinally important Prunella vulgaris L. J. Photochem. Photobiol. B Biol. 2016, 159, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Angelis, A.; Antosiewicz, B.; Sakipova, Z.; Kozhanova, K.; Głowniak, K.; Kukula-Koch, W. Identification of mushroom and murine tyrosinase inhibitors from Achillea biebersteinii Afan. Extract. Molecules 2021, 11, 964. [Google Scholar] [CrossRef] [PubMed]
- Gaweł-Bęben, K.; Czech, K.; Luca, S.V. Cannabidiol and Minor Phytocannabinoids: A Preliminary Study to Assess Their Anti-Melanoma, Anti-Melanogenic, and Anti-Tyrosinase Properties. Pharmaceuticals 2023, 16, 648. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lu, Y.; Tonissen, K.; Di Trapani, G.; Tang, W.; Feng, Y. Application of traditional Chinese medicine as skin depigmentation agents. Heliyon 2022, 24, e12571. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Yoshimori, A.; Ogawa, H.; Shirai, Y.; Abe, H.; Kamiya, T.; Tanuma, S. The structural differences between mushroom and human tyrosinase cleared by investigating the inhibitory activities of stilbenes. J. Mol. Struct. 2023, 1272, 134180. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell Mol. Life Sci. CMLS 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Kawakami, F.; Lwin, T.T.; Imai, M.; Shamsa, F. Biochemical characterization of ferulic acid and caffeic acid which effectively inhibit melanin synthesis via different mechanisms in B16 melanoma cells. Biol. Pharm. Bull. 2018, 41, 806–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.H.; Kim, J.K.; Kim, J.; Jung, S.H.; Lee, K. Characterization of caffeoylquinic acids from Lepisorus thunbergianus and their melanogenesis inhibitory activity. ACS Omega 2020, 5, 30946–30955. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Liu, M.; Xie, R.; Zang, Y.; Li, J.; Aisa, H.A. Guaianolide sesquiterpene lactones from Achillea millefolium L. Phytochemistry 2021, 186, 112733. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Wysokińska, H. Liquid shoot culture of Salvia officinalis L. for micropropagation and production of antioxidant compounds; effect of triacontanol. Acta Soc. Bot. Pol. 2008, 77, 99–104. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Matejic, J.S.; Dzamic, A.M.; Mihajilov-Krstev, T.; Randelovic, V.N.; Krivosej, Z.D.; Marin, P.D. Total phenolic content, flavonoid concentration, antioxidant and antimicrobial activity of methanol extracts from three Seseli L. Taxa. Cent. Eur. J. Biol. 2012, 7, 1116–1122. [Google Scholar] [CrossRef]
- Uchida, R.; Ishikawa, S.; Tomoda, H. Inhibition of tyrosinase activity and melanine pigmentation by 2-hydroxytyrosol. Acta Pharm. Sin. B 2014, 4, 141–145. [Google Scholar] [CrossRef] [Green Version]


| Total Polyphenols (mg GAE/g Dried Extract) | Dpph Scavenging (EC50; µg/mL) | ||
|---|---|---|---|
| AmH | H2O | 0.87 ± 0.01 | 26.12 ± 0.86 |
| 50% EtOH | 1.71 ± 0.04 | 8.22 ± 2.32 | |
| 96% EtOH | 0.55 ± 0.01 | 20.49 ± 3.56 | |
| AmL | H2O | 1.51 ± 0.04 | 24.93 ± 0.69 |
| 50% EtOH | 2.63 ± 0.11 | 5.10 ± 0,04 | |
| 96% EtOH | 0.46 ± 0.01 | 38.89 ± 3.25 | |
| AmIV | H2O | 0.13 ± 0.01 | >500 |
| 50% EtOH | 0.24 ± 0.06 | 400.05 ± 45.47 | |
| 96% EtOH | 0.06 ± 0.01 | >500 | |
| L-ascorbic acid | - | 1.47 ± 0.29 | |
| No. | RT [min] | m/z Meas. | M Meas. | Ions | Name | Compound Class | AmL | AmH | AmIV |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.69 | 131.04573 | 132.05258 | [M-H]−, [M-H-H2O]− | D-Asparagine | Amino acids | ++ | +++ | +++ |
| 2 | 0.84 | 191.01948 | 192.02677 | [M-H]−, [M-H-H2O]− | Citric acid | AHA- alpha hydroxy acid | +++ | +++ | +++ |
| 3 | 1.28 | 164.07129 | 165.07857 | [M-H]− | 3-Phenyl-β-alanine | Amino acids | Tr | Tr | Tr |
| 4 | 1.41 | 315.07089 | 316.07808 | [M-H]− | Gentisic acid 5-O-glucoside | Phenolic acid | +++ | +++ | + |
| 5 | 1.84 | 353.08612 | 354.09339 | [M-H]− | Neochlorogenic acid | Phenolic acid | +++ | ++ | Tr |
| 6 | 2 | 203.08181 | 204.08908 | [M-H]− | L-Tryptophan | Amino acids | +++ | +++ | + |
| 7 | 2.6 | 137.02531 | 138.03258 | [M-H]− | 3,4-Dihydroxybenzaldehyde | Phenolic aldehyde | Tr | Tr | Tr |
| 8 | 3.27 | 353.08673 | 354.09401 | [M-H]− | Chlorogenic acid | Phenolic acid | +++ | ++ | Tr |
| 9 | 3.68 | 353.08617 | 354.09345 | [M-H]− | (1R,3R,4S,5S)-4-(((2E)-3-(3,4-Dihydroxyphenyl)prop-2-enoyl)oxy)- -1,3,5-trihydroxycyclohexanecarboxylic acid | Phenolic acid | Tr | Tr | Tr |
| 10 | 4.55 | 353.08609 | 354.09337 | [M-H]− | Chlorogenic acid | Phenolic acid | +++ | + | Tr |
| 11 | 4.74 | 311.07578 | 312.08306 | [M-H]− | 4-[(2E)-3-(3,4-Dihydroxyphenyl)prop-2-enoyl]oxy-2,3-dihydroxy- -2-methylbutanoic acid | Phenolic acid | Tr | Tr | Tr |
| 12 | 4.78 | 515.11701 | 516.12428 | [M-H]− | Cynarin | Phenolic acid | +++ | + | Tr |
| 13 | 5.07 | 367.10169 | 368.10897 | [M-H]− | 3-O-Feruloylquinic acid | Phenolic acid | +++ | +++ | Tr |
| 14 | 5.09 | 609.14408 | 610.15136 | [M-H]− | Luteolin-7,3′-di-O-glucoside | Flavonoid glucosides | +++ | +++ | Tr |
| 15 | 5.23 | 371.09659 | 372.10387 | [M-H]− | 3-(Benzoyloxy)-2-hydroxypropyl.beta.-D-glucopyranosiduronic acid | Phenolic acid | Tr | Tr | Tr |
| 16 | 5.29 | 563.13792 | 564.14519 | [M-H]− | Schaftoside | Flavonoids | + | +++ | Tr |
| 17 | 5.35 | 463.08594 | 464.09322 | [M-H]− | Hyperin | Flavonoid | +++ | +++ | Tr |
| 18 | 5.38 | 221.04479 | 222.05207 | [M-H]− | Fraxidin | Coumarin | Tr | +++ | +++ |
| 19 | 5.43 | 337.09133 | 338.09861 | [M-H]− | 3-O-p-Coumaroylquinic acid | Phenolic acid | +++ | +++ | Tr |
| 20 | 5.46 | 595.12842 | 596.13568 | [M-H]− | Peltatoside | Flavonoids | +++ | + | Tr |
| 21 | 5.82 | 609.14383 | 610.15111 | [M-H]− | Rutin | Flavonoids | +++ | +++ | Tr |
| 22 | 6.13 | 447.09125 | 448.09853 | [M-H]− | Luteolin 7-glucoside | Flavonoid glucosides | +++ | +++ | Tr |
| 23 | 6.66 | 577.15398 | 578.16126 | [M-H]− | Spherobioside | Flavonoids | +++ | +++ | Tr |
| 24 | 6.74 | 447.09085 | 448.09813 | [M-H]− | Juncein | Flavonoid glucosides | +++ | +++ | Tr |
| 25 | 6.83 | 431.0972 | 432.10632 | [M-H]− | Dienin 7-O-beta-D-glucoside | Flavonoid glucosides | Tr | Tr | Tr |
| 26 | 6.88 | 445.07551 | 446.08279 | [M-H]− | Apigenin 7-glucuronide | Flavonoid glucosides | +++ | +++ | Tr |
| 27 | 6.92 | 607.16497 | 608.17225 | [M-H]− | Chrysoeriol 7-neohesperidoside | Flavonoids | +++ | ++ | Tr |
| 28 | 7.05 | 475.08592 | 476.09319 | [M-H]− | Hispidulin 7-glucuronide | Flavonoids | +++ | +++ | Tr |
| 29 | 7.63 | 269.04456 | 270.05183 | [M-H]− | Apigenin | Flavonoids | +++ | +++ | Tr |
| 30 | 7.73 | 417.08072 | 418.08799 | [M-H]− | Juglanin | Flavonoids | +++ | ++ | Tr |
| 31 | 8.06 | 285.03923 | 286.04651 | [M-H]− | Luteolin | Flavonoids | +++ | +++ | Tr |
| 32 | 8.28 | 591.16978 | 592.17734 | [M-H]−, [M + HCOO]− | 5-Hydroxy-3-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl 6-O-(6-deoxyhexopyranosyl)hexopyranoside | Flavonoid glucosides | Tr | Tr | Tr |
| 33 | 8.56 | 207.06537 | 208.07265 | [M-H]− | Ethyl trans-caffeate | Phenolic compound | +++ | +++ | + |
| 34 | 9.09 | 269.04456 | 270.05183 | [M-H]− | Trihydroxyflavone or Apigenin | Flavonoids | +++ | +++ | Tr |
| 35 | 9.34 | 329.06492 | 330.07224 | [M-H]− | 3,4′-Dimethoxy-5,7,3′-trihydroxyflavone | Flavonoids | Tr | Tr | Tr |
| 36 | 9.35 | 299.05464 | 300.06191 | [M-H]− | Luteolin 7-methyl ether | Flavonoids | +++ | +++ | Tr |
| 37 | 9.62 | 329.06515 | 330.07243 | [M-H]− | Cirsiliol | Flavonoids | +++ | +++ | Tr |
| 38 | 10.4 | 359.07595 | 360.08322 | [M-H]− | 3′,4′,6-Trihydroxy-3,5,7-trimethoxyflavone | Flavonoids | +++ | Tr | Tr |
| 39 | 11.65 | 329.23187 | 330.23915 | [M-H]− | (9Z)-5,8,11-Trihydroxyoctadec-9-enoic acid | Fatty acid | ++ | +++ | +++ |
| 40 | 12.17 | 283.05974 | 284.06702 | [M-H]− | Genkwanin | Flavonoids | +++ | +++ | Tr |
| 41 | 12.2 | 373.09123 | 374.09854 | [M-H]− | Pulicarin | Flavonoids | +++ | + | Tr |
| 42 | 12.48 | 313.07013 | 314.07741 | [M-H]− | Pectolinarigenin | Flavonoids | +++ | +++ | Tr |
| 43 | 13.69 | 343.08068 | 344.08796 | [M-H]− | 5,3′-Dihydroxy-6,7,4′-trimethoxyflavone | Flavonoids | Tr | Tr | Tr |
| 44 | 18.81 | 2293.2041 | 294.21944 | [M-H]− | Hydroxylinolenic acid | Fatty acid | Tr | Tr | Tr |
| 45 | 19.93 | 221.15373 | 222.16132 | [M-H]− | 3,6-Ditert-butyl-1,2-benzenediol | Phenolic compound | ++ | +++ | +++ |
| 46 | 20 | 295.21923 | 296.23522 | [M-H]− | Hydroxyoctadecadienoic acid | Fatty acid | ++ | +++ | +++ |
| 47 | 20.24 | 452.27606 | 453.28333 | [M-H]− | 1-Palmitoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine | Phospholipid | Tr | Tr | Tr |
| 48 | 20.95 | 265.14661 | 266.15389 | [M-H]− | Laurylsulfuric acid | Organic acid | + | +++ | + |
| 49 | 23.7 | 271.22659 | 272.23386 | [M-H]− | 2-Hydroxypalmitic acid | Fatty acid | +++ | ++ | ++ |
| 50 | 24.47 | 277.21072 | 278.22335 | [M-H]− | Octadecatrienoic acid | Fatty acid | Tr | Tr | Tr |
| 51 | 25.17 | 483.27031 | 484.27759 | [M-H]− | 1-Palmitoyl-2-hydroxy-sn-glycero-3-phospho-(1′-rac-glycerol) | Phospholipid | Tr | Tr | Tr |
| 52 | 26.02 | 279.22581 | 280.239 | [M-H]− | Octadecahenate | Organic acid | Tr | Tr | Tr |
| 53 | 26.96 | 255.22641 | 256.239 | [M-H]− | Hexadecanoic acid | Fatty acid | Tr | Tr | Tr |
| 54 | 27.2 | 339.23123 | 340.2385 | [M-H]− | 2,2′-Methylene-bis(6-tert-butyl-4 methylphenol) | Phenolic compound | Tr | Tr | Tr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czech, K.; Gaweł-Bęben, K.; Szopa, A.; Kukula-Koch, W.; Jakschitz, T.; Bonn, G.; Hussain, S.; Kubica, P.; Ekiert, H.; Głowniak, K. Phytochemical Profiling, Antioxidant and Tyrosinase Regulatory Activities of Extracts from Herb, Leaf and In Vitro Culture of Achillea millefolium (Yarrow). Molecules 2023, 28, 4791. https://doi.org/10.3390/molecules28124791
Czech K, Gaweł-Bęben K, Szopa A, Kukula-Koch W, Jakschitz T, Bonn G, Hussain S, Kubica P, Ekiert H, Głowniak K. Phytochemical Profiling, Antioxidant and Tyrosinase Regulatory Activities of Extracts from Herb, Leaf and In Vitro Culture of Achillea millefolium (Yarrow). Molecules. 2023; 28(12):4791. https://doi.org/10.3390/molecules28124791
Chicago/Turabian StyleCzech, Karolina, Katarzyna Gaweł-Bęben, Agnieszka Szopa, Wirginia Kukula-Koch, Thomas Jakschitz, Günther Bonn, Shah Hussain, Paweł Kubica, Halina Ekiert, and Kazimierz Głowniak. 2023. "Phytochemical Profiling, Antioxidant and Tyrosinase Regulatory Activities of Extracts from Herb, Leaf and In Vitro Culture of Achillea millefolium (Yarrow)" Molecules 28, no. 12: 4791. https://doi.org/10.3390/molecules28124791
APA StyleCzech, K., Gaweł-Bęben, K., Szopa, A., Kukula-Koch, W., Jakschitz, T., Bonn, G., Hussain, S., Kubica, P., Ekiert, H., & Głowniak, K. (2023). Phytochemical Profiling, Antioxidant and Tyrosinase Regulatory Activities of Extracts from Herb, Leaf and In Vitro Culture of Achillea millefolium (Yarrow). Molecules, 28(12), 4791. https://doi.org/10.3390/molecules28124791









