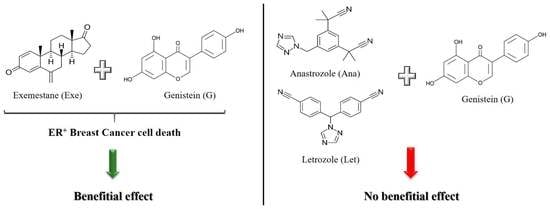
In Vitro Effects of Combining Genistein with Aromatase Inhibitors: Concerns Regarding Its Consumption during Breast Cancer Treatment
Abstract
1. Introduction
2. Results
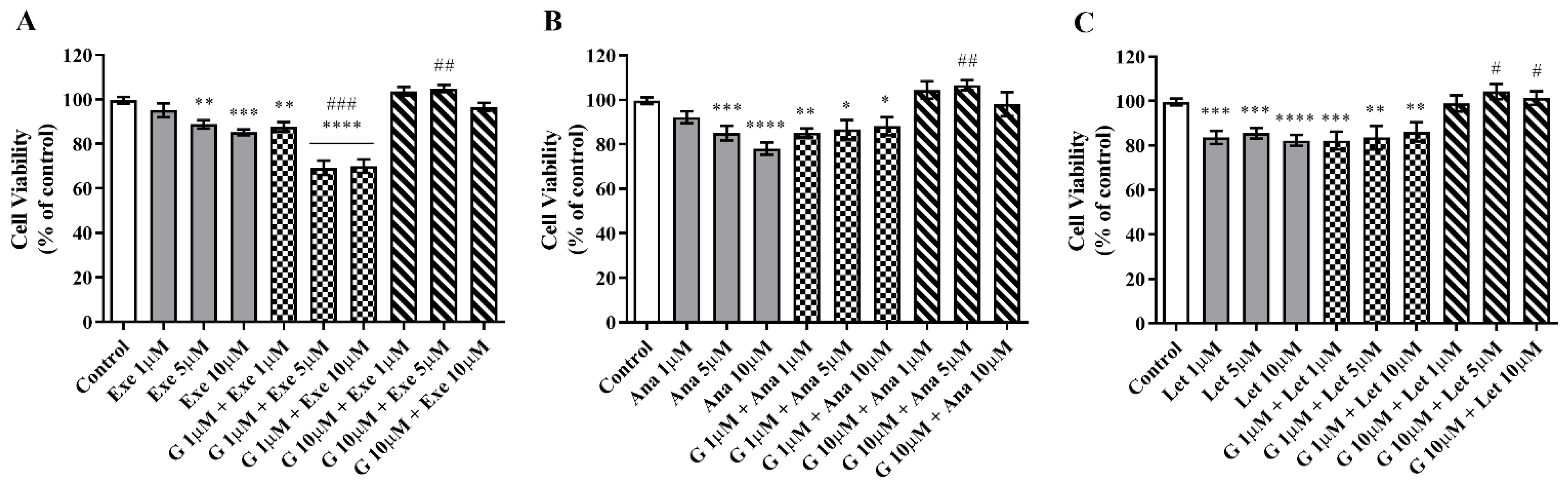
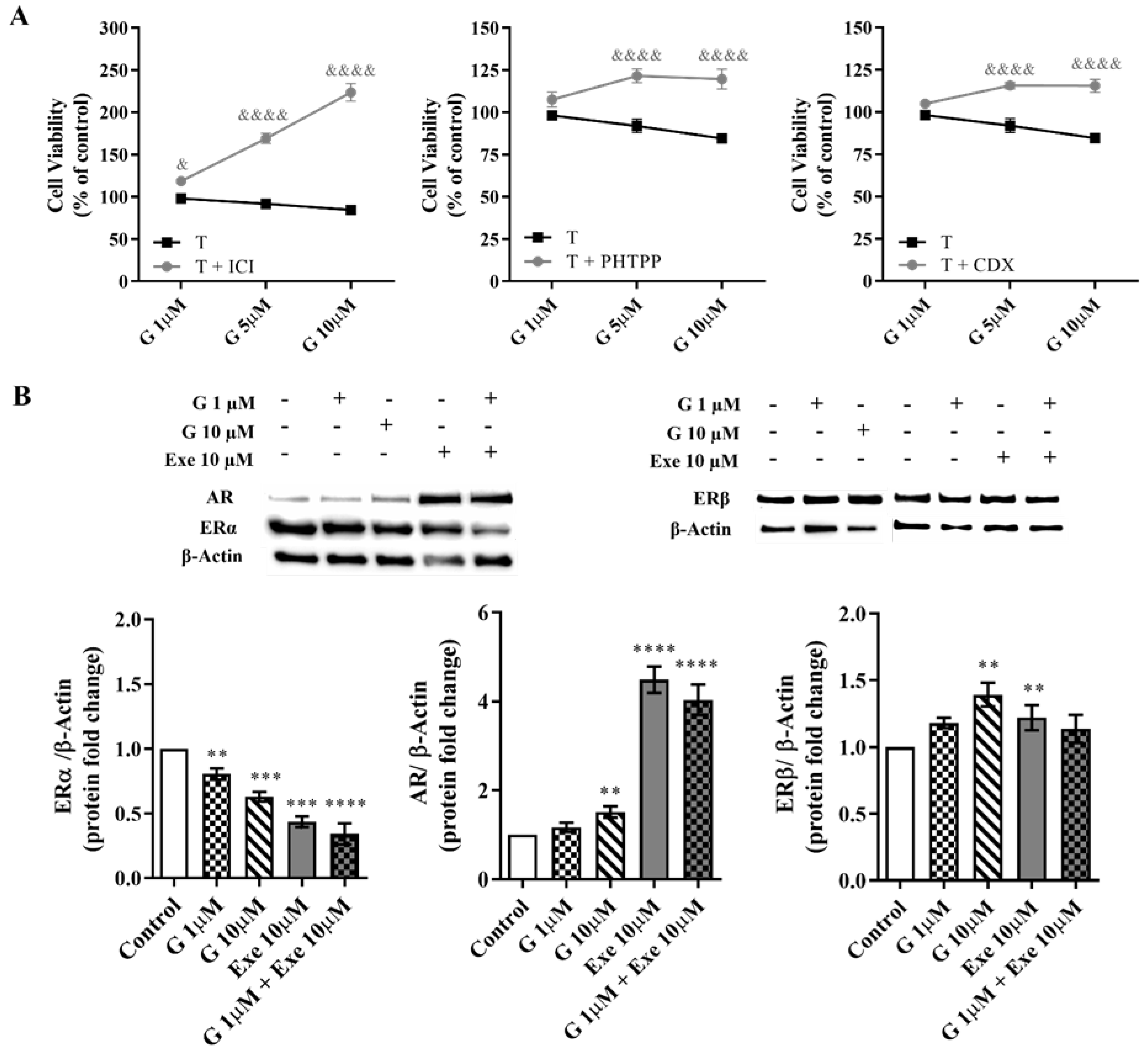
2.1. Genistein Decreases Cell Viability of ER+ Breast Cancer Cells
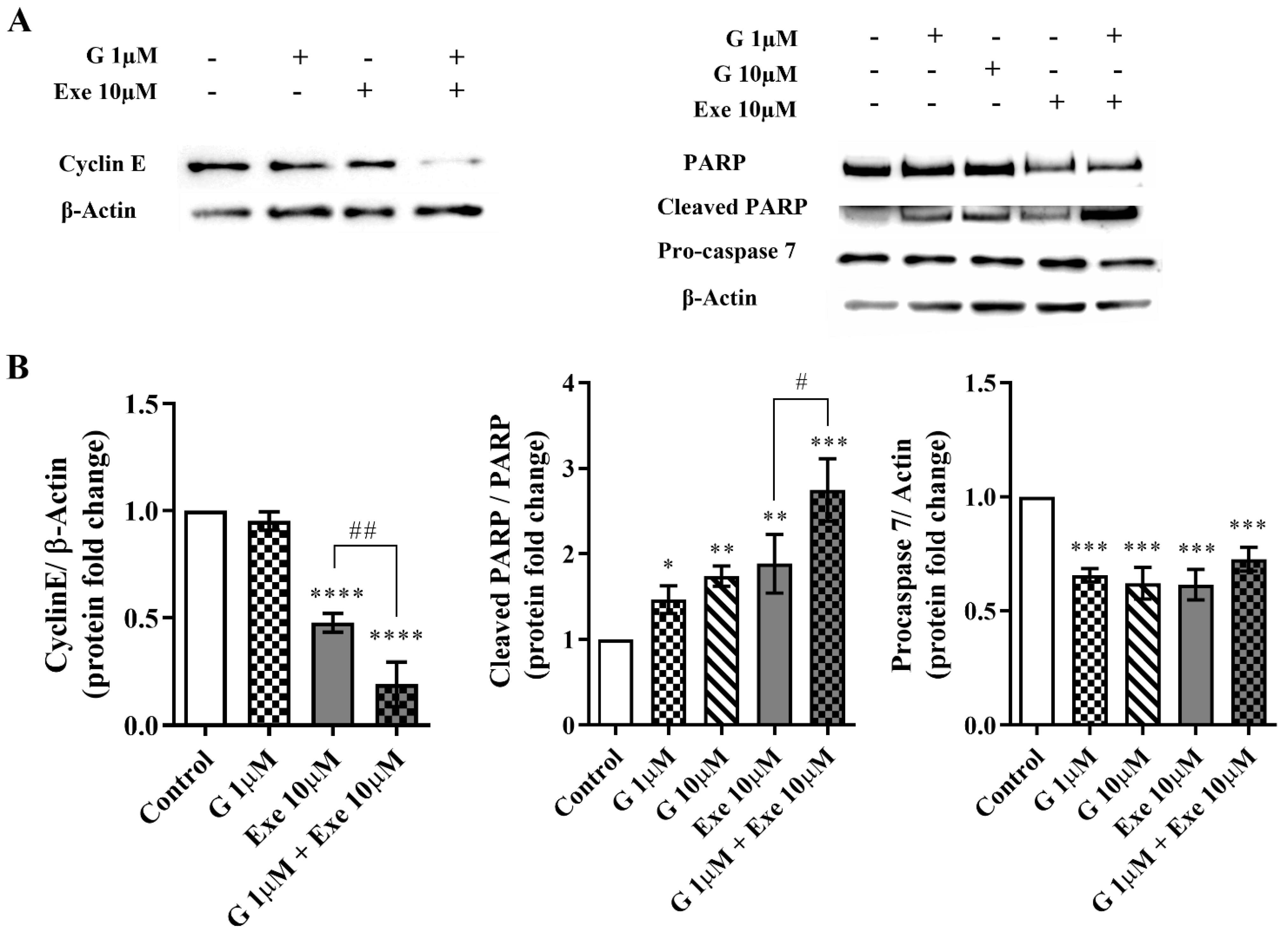
2.2. Genistein Potentiates Exemestane Anticancer Effect
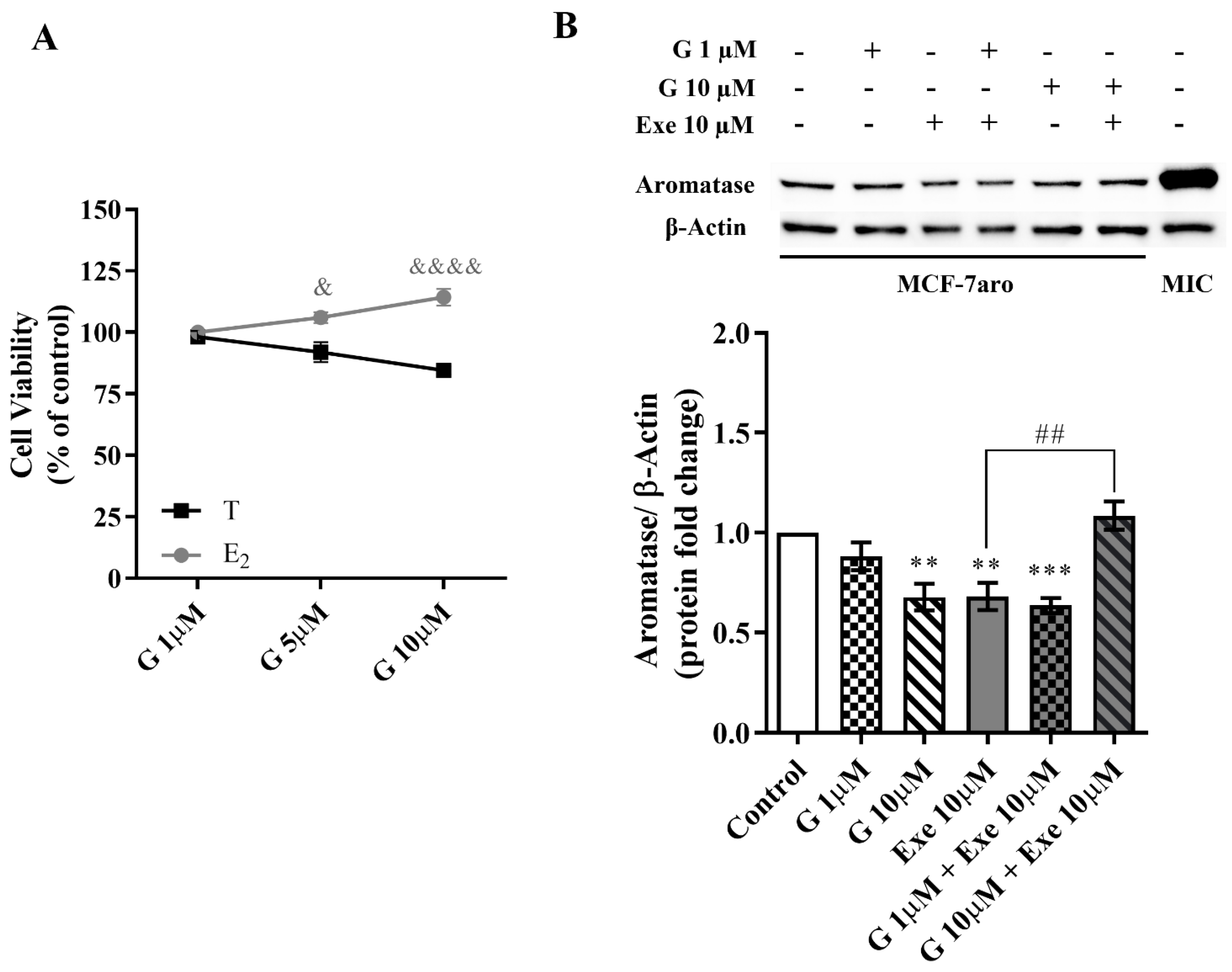
2.3. Genistein Affects Aromatase and Hormone Receptors’ Expressions
3. Methods
3.1. Cell Culture
3.2. Cell Viability Assay
3.3. Cell Cycle Progression Assay
3.4. Protein Expression
3.5. Statistical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makki, J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, F.; Crispino, S. Factors Predictive of Response to Hormone Therapy in Breast Cancer. Tumori J. 2008, 94, 370–383. [Google Scholar] [CrossRef]
- Jordan, V.C. New insights into the metabolism of tamoxifen and its role in the treatment and prevention of breast cancer. Steroids 2007, 72, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.F.; Oliveira, A.; Ramos, M.J.; Fernandes, P.A.; Teixeira, N.; Amaral, C. Estrogen receptor-positive (ER+) breast cancer treatment: Are multi-target compounds the next promising approach? Biochem. Pharmacol. 2020, 177, 113989. [Google Scholar] [CrossRef] [PubMed]
- Augusto, T.V.; Correia-Da-Silva, G.; Rodrigues, C.M.; Teixeira, N.; Amaral, C. Acquired resistance to aromatase inhibitors: Where we stand! Endocr. Relat. Cancer 2018, 25, R283–R301. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220, Erratum in: Ann. Oncol. 2021, 32, 284. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; Andre, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Chan, H.J.; Petrossian, K.; Chen, S. Structural and functional characterization of aromatase, estrogen receptor, and their genes in endocrine-responsive and –resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2015, 161, 73–83. [Google Scholar] [CrossRef]
- Chumsri, S.; Howes, T.; Bao, T.; Sabnis, G.; Brodie, A. Aromatase, aromatase inhibitors, and breast cancer. J. Steroid Biochem. Mol. Biol. 2011, 125, 13–22. [Google Scholar] [CrossRef]
- Sobral, A.F.; Amaral, C.; Correia-Da-Silva, G.; Teixeira, N. Unravelling exemestane: From biology to clinical prospects. J. Steroid Biochem. Mol. Biol. 2016, 163, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S. Aromatase Destabilizer: Novel Action of Exemestane, a Food and Drug Administration–Approved Aro-matase Inhibitor. Cancer Res. 2006, 66, 10281–10286. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Borges, M.; Melo, S.; da Silva, E.T.; Correia-Da-Silva, G.; Teixeira, N. Apoptosis and Autophagy in Breast Cancer Cells following Exemestane Treatment. PLoS ONE 2012, 7, e42398. [Google Scholar] [CrossRef]
- Amaral, C.; Augusto, T.V.; Almada, M.; Cunha, S.C.; Correia-Da-Silva, G.; Teixeira, N. The potential clinical benefit of targeting androgen receptor (AR) in estrogen-receptor positive breast cancer cells treated with Exemestane. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1866, 165661. [Google Scholar] [CrossRef] [PubMed]
- Augusto, T.V.; Amaral, C.; Almeida, C.F.; Teixeira, N.; Correia-Da-Silva, G. Differential biological effects of aromatase inhibitors: Apoptosis, autophagy, senescence and modulation of the hormonal status in breast cancer cells. Mol. Cell. Endocrinol. 2021, 537, 111426. [Google Scholar] [CrossRef]
- Kelly, C.M.; Buzdar, A.U. Anastrozole. Expert Opin. Drug Saf. 2010, 9, 995–1003. [Google Scholar] [CrossRef]
- Macedo, L.F.; Guo, Z.; Tilghman, S.L.; Sabnis, G.J.; Qiu, Y.; Brodie, A. Role of Androgens on MCF-7 Breast Cancer Cell Growth and on the Inhibitory Effect of Letrozole. Cancer Res. 2006, 66, 7775–7782. [Google Scholar] [CrossRef]
- Chen, R.; Cui, J.; Wang, Q.; Li, P.; Liu, X.; Hu, H.; Wei, W. Antiproliferative effects of anastrozole on MCF-7 human breast cancer cells in vitro are significantly enhanced by combined treatment with testosterone undecanoate. Mol. Med. Rep. 2012, 12, 769–775. [Google Scholar] [CrossRef]
- Lui, A.; New, J.; Ogony, J.; Thomas, S.; Lewis-Wambi, J. Everolimus downregulates estrogen receptor and induces autophagy in aromatase inhibitor-resistant breast cancer cells. BMC Cancer 2016, 16, 487. [Google Scholar] [CrossRef] [PubMed]
- Taglieri, L.; De Iuliis, F.; Giuffrida, A.; Giantulli, S.; Silvestri, I.; Scarpa, S. Resistance to the mTOR inhibitor everolimus is reversed by the downregulation of survivin in breast cancer cells. Oncol. Lett. 2017, 14, 3832–3838. [Google Scholar] [CrossRef] [PubMed]
- Scheidemann, E.R.; Shajahan-Haq, A.N. Resistance to CDK4/6 Inhibitors in Estrogen Receptor-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 12292. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and Cancer: Current Status, Challenges, and Future Directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Prietsch, R.F.; Monte, L.G.; Da Silva, F.A.; Beira, F.T.; Del Pino, F.A.B.; Campos, V.F.; Collares, T.; Pinto, L.S.; Spanevello, R.M.; Gamaro, G.D.; et al. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol. Cell. Biochem. 2014, 390, 235–242. [Google Scholar] [CrossRef]
- Bhat, S.S.; Prasad, S.K.; Shivamallu, C.; Prasad, K.S.; Syed, A.; Reddy, P.; Cull, C.A.; Amachawadi, R.G. Genistein: A Potent Anti-Breast Cancer Agent. Curr. Issues Mol. Biol. 2021, 43, 1502–1517. [Google Scholar] [CrossRef]
- Brooks, J.D.; Thompson, L.U. Mammalian lignans and genistein decrease the activities of aromatase and 17β-hydroxysteroid dehydrogenase in MCF-7 cells. J. Steroid Biochem. Mol. Biol. 2005, 94, 461–467. [Google Scholar] [CrossRef]
- Le Bail, J.-C.; Champavier, Y.; Chulia, A.-J.; Habrioux, G. Effects of phytoestrogens on aromatase, 3β and 17β-hydroxysteroid dehydrogenase activities and human breast cancer cells. Life Sci. 2000, 66, 1281–1291. [Google Scholar] [CrossRef]
- Le Bail, J.; Laroche, T.; Marre-Fournier, F.; Habrioux, G. Aromatase and 17β-hydroxysteroid dehydrogenase inhibition by flavonoids. Cancer Lett. 1998, 133, 101–106. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Bannwart, C.; Wähälä, K.; Mäkelä, T.; Brunow, G.; Hase, T.; Arosemena, P.; Kellis, J.; Vickery, L.E. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J. Steroid Biochem. Mol. Biol. 1993, 44, 147–153. [Google Scholar] [CrossRef]
- Pelissero, C.; Lenczowski, M.; Chinzi, D.; Davail-Cuisset, B.; Sumpter, J.; Fostier, A. Effects of flavonoids on aromatase activity, an in vitro study. J. Steroid Biochem. Mol. Biol. 1996, 57, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Toloi, M.R.T.; Vasconcelos, L.D.; Fonseca, M.J.V.; Correia-Da-Silva, G.; Teixeira, N. The role of soybean extracts and isoflavones in hormone-dependent breast cancer: Aromatase activity and biological effects. Food Funct. 2017, 8, 3064–3074. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Russo, G.L.; Daglia, M.; Kasi, P.D.; Ravi, S.; Nabavi, S.F. Understanding genistein in cancer: The “good” and the “bad” effects: A review. Food Chem. 2016, 196, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Stocco, B.; Toledo, K.; Fumagalli, H.F.; Bianchini, F.J.; Fortes, V.S.; Fonseca, M.J.V.; Toloi, M.R.T. Biotransformed Soybean Extract Induces Cell Death of Estrogen-Dependent Breast Cancer Cells by Modulation of Apoptotic Proteins. Nutr. Cancer 2015, 67, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cook, K.L.; Warri, A.; Cruz, I.M.; Rosim, M.; Riskin, J.; Helferich, W.; Doerge, D.; Clarke, R.; Hilakivi-Clarke, L. Lifetime Genistein Intake Increases the Response of Mammary Tumors to Tamoxifen in Rats. Clin. Cancer Res. 2017, 23, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Van Duursen, M.; Nijmeijer, S.; de Morree, E.; de Jong, P.C.; Berg, M.V.D. Genistein induces breast cancer-associated aromatase and stimulates estrogen-dependent tumor cell growth in in vitro breast cancer model. Toxicology 2011, 289, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Correia-Da-Silva, G.; Almeida, C.F.; Valente, M.J.; Varela, C.; Tavares-Da-Silva, E.; Vinggaard, A.M.; Teixeira, N.; Roleira, F.M.F. An Exemestane Derivative, Oxymestane-D1, as a New Multi-Target Steroidal Aromatase Inhibitor for Estrogen Receptor-Positive (ER+) Breast Cancer: Effects on Sensitive and Resistant Cell Lines. Molecules 2023, 28, 789. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Trouille, F.M.; Almeida, C.F.; Correia-Da-Silva, G.; Teixeira, N. Unveiling the mechanism of action behind the anti-cancer properties of cannabinoids in ER+ breast cancer cells: Impact on aromatase and steroid receptors. J. Steroid Biochem. Mol. Biol. 2021, 210, 105876. [Google Scholar] [CrossRef]
- Almeida, C.F.; Teixeira, N.; Oliveira, A.; Augusto, T.V.; Correia-Da-Silva, G.; Ramos, M.J.; Fernandes, P.A.; Amaral, C. Discovery of a multi-target compound for estrogen receptor-positive (ER+) breast cancer: Involvement of aromatase and ERs. Biochimie 2020, 181, 65–76. [Google Scholar] [CrossRef]
- Amaral, C.; Varela, C.L.; Maurício, J.; Sobral, A.F.; Costa, S.C.; Roleira, F.M.; da Silva, E.T.; Correia-Da-Silva, G.; Teixeira, N. Anti-tumor efficacy of new 7α-substituted androstanes as aromatase inhibitors in hormone-sensitive and resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2017, 171, 218–228. [Google Scholar] [CrossRef]
- Augusto, T.V.; Amaral, C.; Varela, C.L.; Bernardo, F.; da Silva, E.T.; Roleira, F.F.; Costa, S.; Teixeira, N.; Correia-Da-Silva, G. Effects of new C6-substituted steroidal aromatase inhibitors in hormone-sensitive breast cancer cells: Cell death mechanisms and modulation of estrogen and androgen receptors. J. Steroid Biochem. Mol. Biol. 2019, 195, 105486. [Google Scholar] [CrossRef]
- Zhou, D.J.; Pompon, D.; Chen, S. Stable expression of human aromatase complementary DNA in mammalian cells: A useful system for aromatase inhibitor screening. Cancer Res. 1990, 50, 6949–6954. [Google Scholar]
- Sun, X.-Z.; Zhou, D.; Chen, S. Autocrine and paracrine actions of breast tumor aromatase. A three-dimensional cell culture study involving aromatase transfected MCF-7 and T-47D cells. J. Steroid Biochem. Mol. Biol. 1997, 63, 29–36. [Google Scholar] [CrossRef]
- Masri, S.; Phung, S.; Wang, X.; Chen, S. Molecular characterization of aromatase inhibitor-resistant, tamoxifen-resistant and LTEDaro cell lines. J. Steroid Biochem. Mol. Biol. 2010, 118, 277–282. [Google Scholar] [CrossRef]
- Chen, S.; Masri, S.; Hong, Y.; Wang, X.; Phung, S.; Yuan, Y.-C.; Wu, X. New experimental models for aromatase inhibitor resistance. J. Steroid Biochem. Mol. Biol. 2007, 106, 8–15. [Google Scholar] [CrossRef]
- Sahin, I.; Bilir, B.; Ali, S.; Sahin, K.; Kucuk, O. Soy Isoflavones in Integrative Oncology: Increased Efficacy and Decreased Toxicity of Cancer Therapy. Integr. Cancer Ther. 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.-P.; Wang, G.; Zhao, Z.-B.; Wang, Q.; Shi, Y. Synergistic cytotoxic effect of genistein and doxorubicin on drug-resistant human breast cancer MCF-7/Adr cells. Oncol. Rep. 2014, 32, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Balapure, A.K.; Kaushik, S.; Shyam, H.; Sharma, R. Genistein synergizes centchroman action in human breast cancer cells. Indian J. Pharmacol. 2016, 48, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Yamamoto, D.; Kiyozuka, Y.; Tsuta, K.; Uemura, Y.; Hioki, K.; Tsutsui, Y.; Tsubura, A. Effects of genistein and synergistic action in combination with eicosapentaenoic acid on the growth of breast cancer cell lines. J. Cancer Res. Clin. Oncol. 2000, 126, 448–454. [Google Scholar] [CrossRef]
- Mai, Z.; Blackburn, G.L.; Zhou, J.-R. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol. Carcinog. 2007, 46, 534–542. [Google Scholar] [CrossRef]
- Ju, Y.H.; Doerge, D.R.; Woodling, K.A.; Hartman, J.A.; Kwak, J.; Helferich, W.G. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis 2008, 29, 2162–2168. [Google Scholar] [CrossRef]
- Hwang, H.C.; Clurman, B.E. Cyclin E in normal and neoplastic cell cycles. Oncogene 2005, 24, 2776–2786. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Mo, B.; Hu, C.; Liu, H.; Qi, H.; Wang, X.; Xu, J. Genistein induces G2/M cell cycle arrest via stable activation of ERK1/2 pathway in MDA-MB-231 breast cancer cells. Cell. Biol. Toxicol. 2008, 24, 401–409. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, M.; Parris, A.B.; Xing, Y.; Yang, X. Genistein targets the cancerous inhibitor of PP2A to induce growth inhibition and apoptosis in breast cancer cells. Int. J. Oncol. 2016, 49, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N. Genistein induces Ca2+-mediated, calpain/caspase-12-dependent apoptosis in breast cancer cells. Biochem. Biophys. Res. Commun. 2004, 321, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.M.; Wu, J.; Shen, Z.Z.; Barsky, S.H. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998, 58, 4851–4857. [Google Scholar]
- Li, Y.; Upadhyay, S.; Bhuiyan, M.; Sarkar, F.H. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene 1999, 18, 3166–3172. [Google Scholar] [CrossRef]
- Chen, J.; Duan, Y.; Zhang, X.; Ye, Y.; Ge, B.; Chen, J. Genistein induces apoptosis by the inactivation of the IGF-1R/p-Akt signaling pathway in MCF-7 human breast cancer cells. Food Funct. 2015, 6, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Pucci, B.; Kasten, M.; Giordano, A. Cell Cycle and Apoptosis. Neoplasia 2000, 2, 291–299. [Google Scholar] [CrossRef]
- Ye, R.; Bodero, A.; Zhou, B.-B.; Khanna, K.K.; Lavin, M.F.; Lees-Miller, S.P. The Plant Isoflavenoid Genistein Activates p53 and Chk2 in an ATM-dependent Manner. J. Biol. Chem. 2001, 276, 4828–4833. [Google Scholar] [CrossRef]
- Ouyang, G.; Yao, L.; Ruan, K.; Song, G.; Mao, Y.; Bao, S. Genistein induces G2/M cell cycle arrest and apoptosis of human ovarian cancer cells via activation of DNA damage checkpoint pathways. Cell Biol. Int. 2009, 33, 1237–1244. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.-Z.; Du, G.-J.; Qi, L.-W.; Calway, T.; He, T.-C.; Du, W.; Yuan, C.-S. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int. J. Oncol. 2013, 43, 289–296. [Google Scholar] [CrossRef]
- Romagnolo, D.F.; Donovan, M.G.; Papoutsis, A.J.; Doetschman, T.C.; Selmin, O.I. Genistein Prevents BRCA1 CpG Methylation and Proliferation in Human Breast Cancer Cells with Activated Aromatic Hydrocarbon Receptor. Curr. Dev. Nutr. 2017, 1, e000562. [Google Scholar] [CrossRef]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.Å. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Rechoum, Y.; Rovito, D.; Iacopetta, D.; Barone, I.; Andò, S.; Weigel, N.L.; O’malley, B.W.; Brown, P.H.; Fuqua, S.A.W. AR collaborates with ERα in aromatase inhibitor-resistant breast cancer. Breast Cancer Res. Treat. 2014, 147, 473–485. [Google Scholar] [CrossRef]
- Yu, L.; Rios, E.; Castro, L.; Liu, J.; Yan, Y.; Dixon, D. Genistein: Dual Role in Women’s Health. Nutrients 2021, 13, 3048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, T.T.; Cunnick, J.E.; Murphy, P.A.; Hendrich, S. Daidzein and Genistein Glucuronides In Vitro Are Weakly Estrogenic and Activate Human Natural Killer Cells at Nutritionally Relevant Concentrations. J. Nutr. 1999, 129, 399–405. [Google Scholar] [CrossRef] [PubMed]

| Treatment | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|
| Control | 76.71 ± 0.42 | 8.26 ± 0.23 | 14.94 ± 0.36 |
| Gen 1 µM | 78.82 ± 0.41 | 3.54 ± 0.11 **** | 17.01 ± 0.39 * |
| Exe 10 µM | 85.79 ± 0.29 **** | 2.61 ± 0.23 **** | 11.18 ± 0.42 *** |
| Gen 1 µM + Exe 10 µM | 88.00 ± 0.39 | 1.40 ± 0.13 # | 10.37 ± 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezerra, P.H.A.; Amaral, C.; Almeida, C.F.; Correia-da-Silva, G.; Torqueti, M.R.; Teixeira, N. In Vitro Effects of Combining Genistein with Aromatase Inhibitors: Concerns Regarding Its Consumption during Breast Cancer Treatment. Molecules 2023, 28, 4893. https://doi.org/10.3390/molecules28134893
Bezerra PHA, Amaral C, Almeida CF, Correia-da-Silva G, Torqueti MR, Teixeira N. In Vitro Effects of Combining Genistein with Aromatase Inhibitors: Concerns Regarding Its Consumption during Breast Cancer Treatment. Molecules. 2023; 28(13):4893. https://doi.org/10.3390/molecules28134893
Chicago/Turabian StyleBezerra, Patrícia H. A., Cristina Amaral, Cristina F. Almeida, Georgina Correia-da-Silva, Maria Regina Torqueti, and Natércia Teixeira. 2023. "In Vitro Effects of Combining Genistein with Aromatase Inhibitors: Concerns Regarding Its Consumption during Breast Cancer Treatment" Molecules 28, no. 13: 4893. https://doi.org/10.3390/molecules28134893
APA StyleBezerra, P. H. A., Amaral, C., Almeida, C. F., Correia-da-Silva, G., Torqueti, M. R., & Teixeira, N. (2023). In Vitro Effects of Combining Genistein with Aromatase Inhibitors: Concerns Regarding Its Consumption during Breast Cancer Treatment. Molecules, 28(13), 4893. https://doi.org/10.3390/molecules28134893