Lipophilic Fe(III)-Complex with Potent Broad-Spectrum Anticancer Activity and Ability to Overcome Pt Resistance in A2780cis Cancer Cells
Abstract
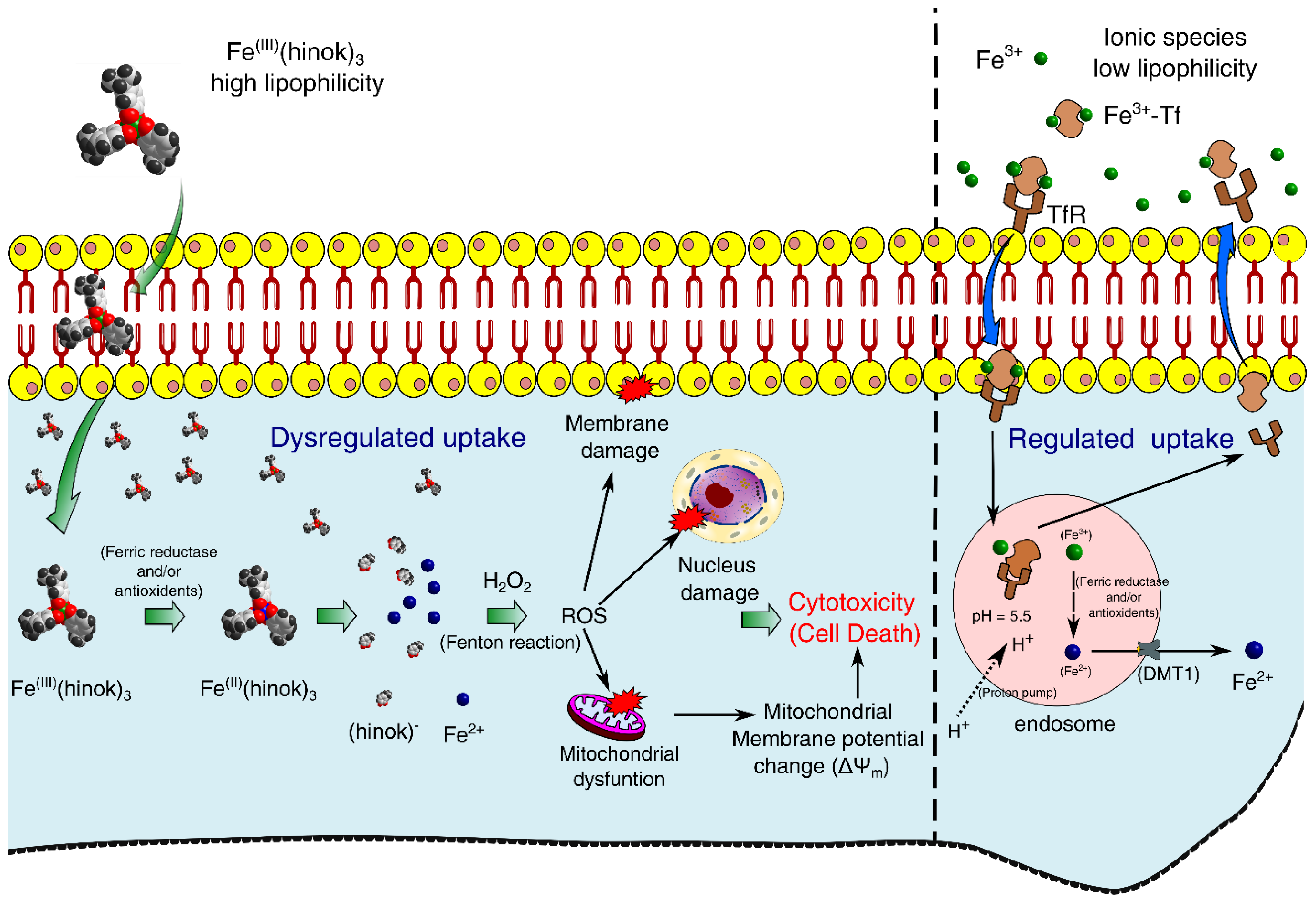
:1. Introduction
2. Results and Discussion
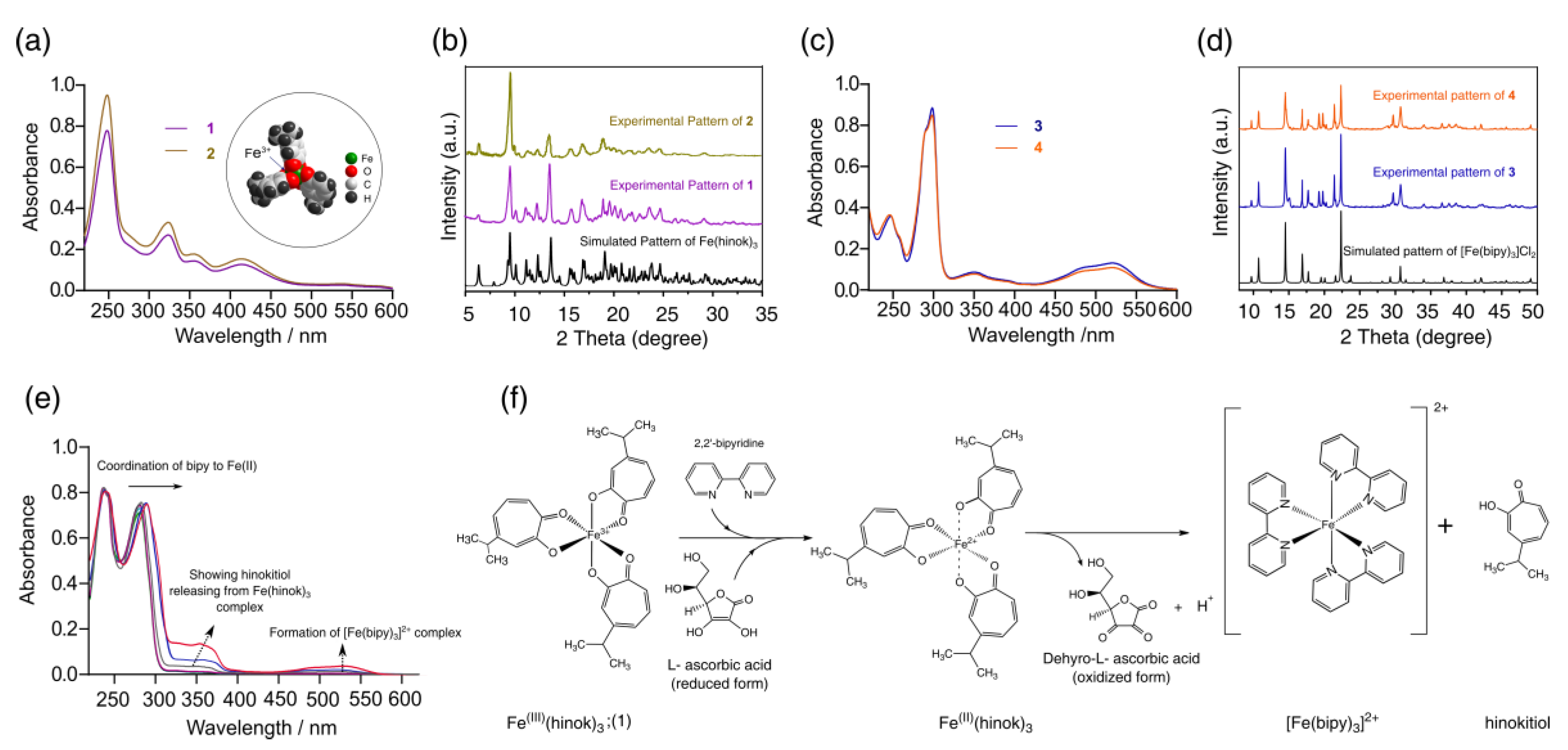
2.1. Synthesis and Characterization of Fe(hinok)3
2.2. Preferential Complexation of Fe(III) by Hinokitiol and Release of Fe(II) upon Reduction
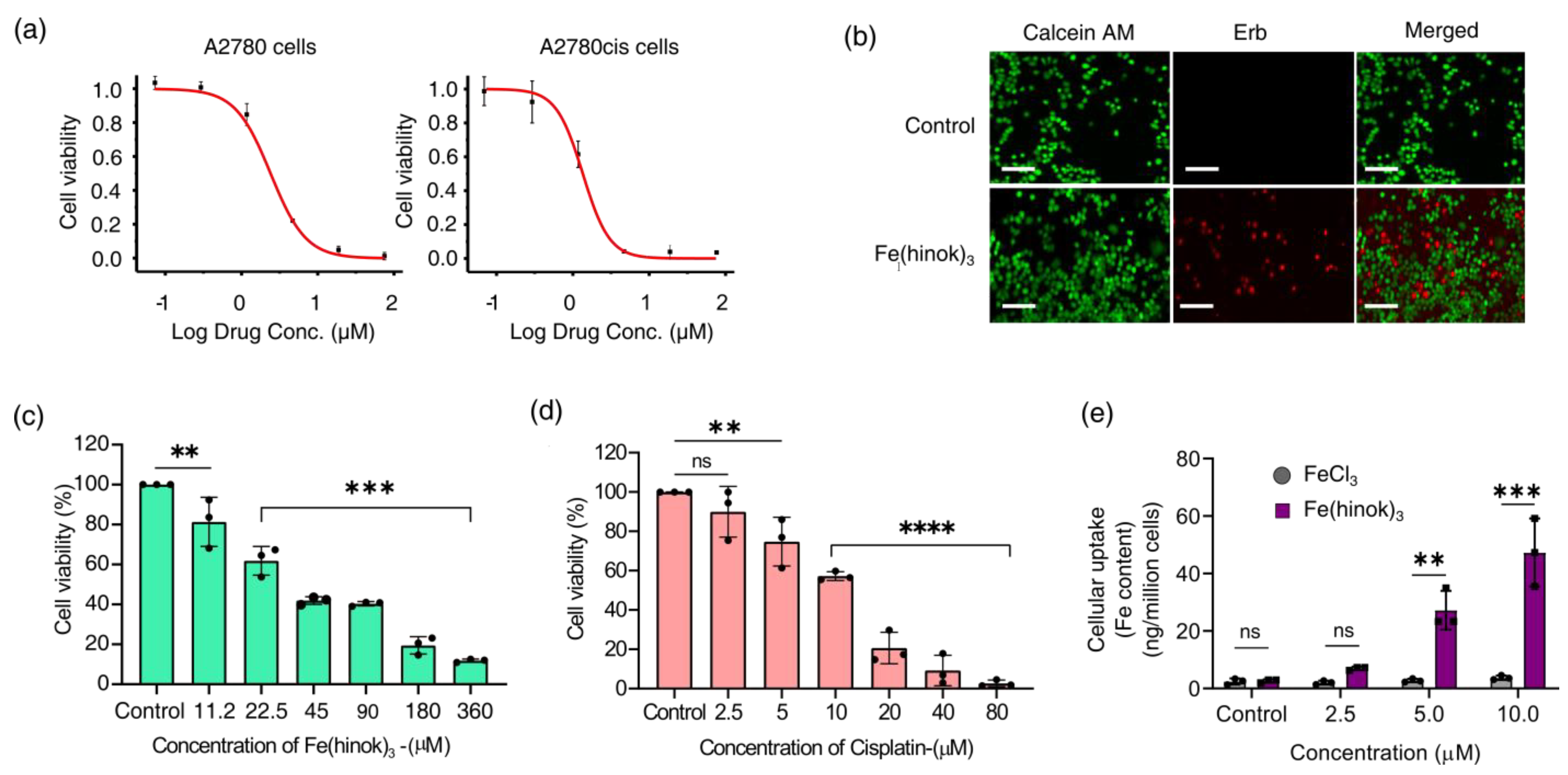
2.3. Cytotoxicity in Different Cancer Cells
2.4. Cytotoxicity in the Human Embryonic Kidney Cells
2.5. Cellular Uptake of Fe(hinok)3
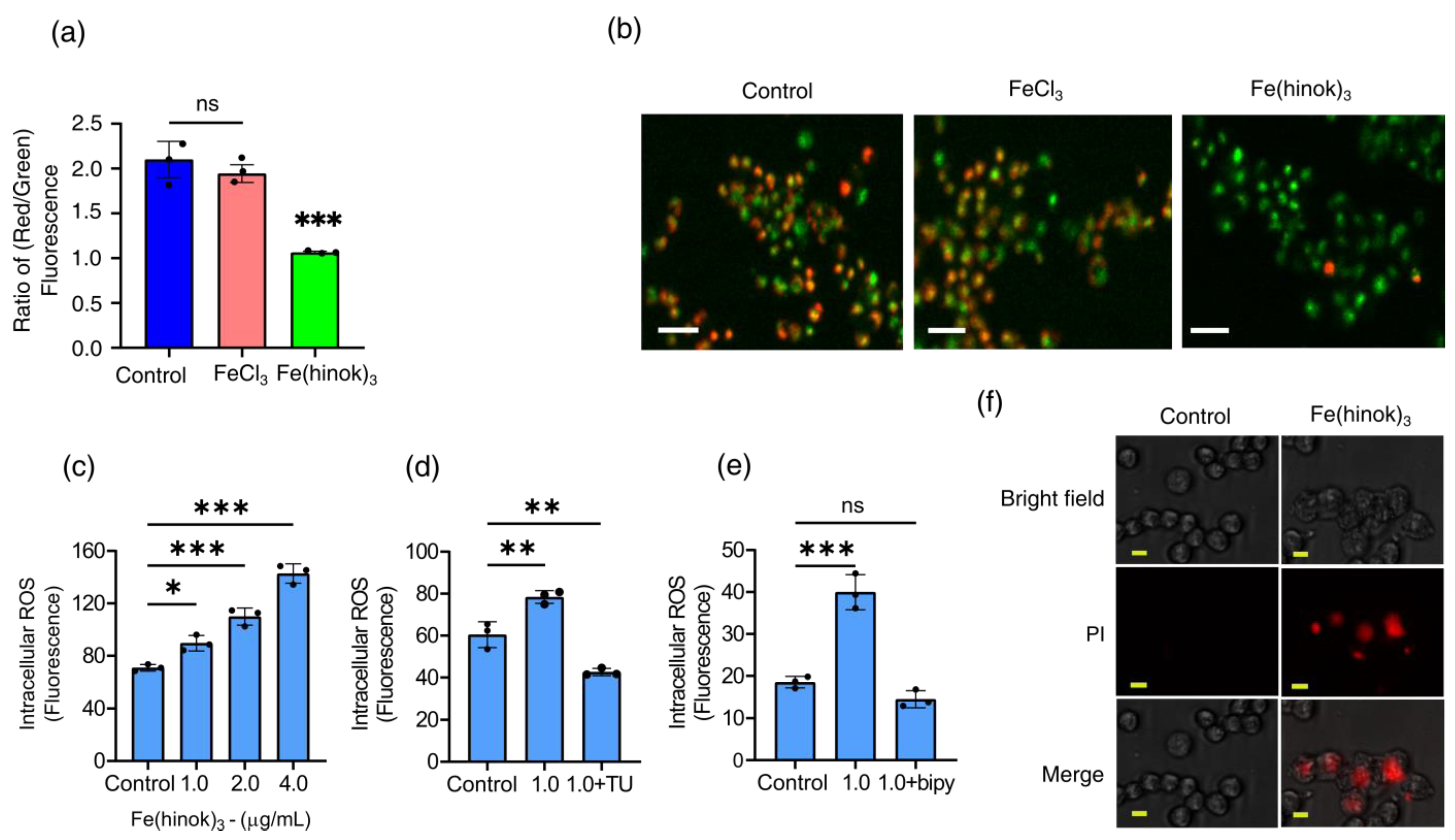
2.6. The Effect of Fe(hinok)3 on the Mitochondrial Membrane Potential (MMP) in A2780cis Cells
2.7. Measurements of Intracellular Generation and Quenching of ROS
2.8. The Effect of Fe(hinok)3 on the Membrane Integrity of A2780cis Cells
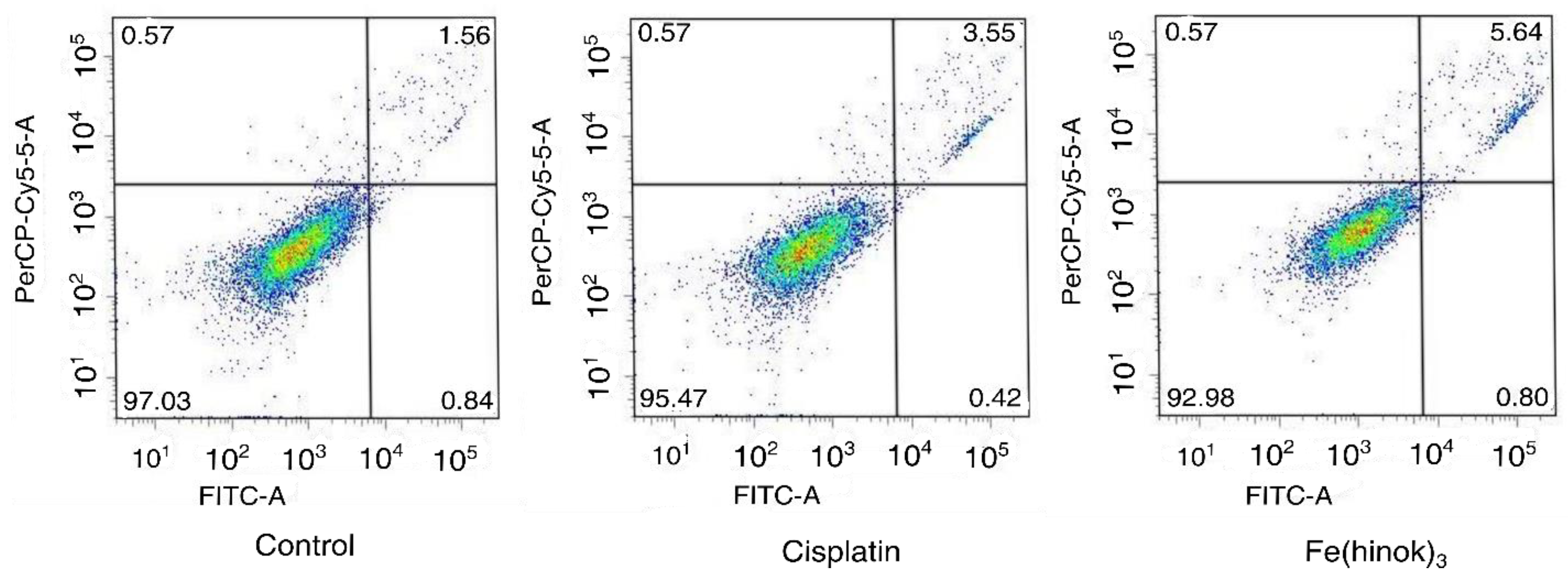
2.9. Apoptosis Assay
3. Conclusions
4. Materials and Methods
4.1. Reaction between FeCl2 and Hinokitiol
4.2. Reaction between FeCl3 and 2,2′-Bipyridine
4.3. Reaction between FeCl2 and 2,2′-Bipyridine
4.4. Reaction between FeCl3, Hinokitiol and 2,2′-Bipyridine
4.5. Reaction between Fe(hinok)3 and 2,2′-Bipyridine in the Presence of Ascorbic Acid
4.6. Cell Viability (MTT) Assays
4.7. LIVE/DEAD Cell Viability Assays
4.8. Cellular Uptake
4.9. Determination of the Change in the Mitochondrial Membrane Potential
4.10. Cell Membrane Permeabilization Assay
4.11. Measurement of the Levels of Intracellular Total ROS
4.12. ROS Scavenge Assay
4.13. Apoptosis Assays
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, E.R.; Lippard, S.J. Structure, recognition, and processing of cisplatin–DNA adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Lippard, S.J. Direct cellular responses to platinum-induced DNA damage. Chem. Rev. 2007, 107, 1387–1407. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt (II) agents, nanoparticle delivery, and Pt (IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Soldevila-Barreda, J.J.; Metzler-Nolte, N. Intracellular catalysis with selected metal complexes and metallic nanoparticles: Advances toward the development of catalytic metallodrugs. Chem. Rev. 2019, 119, 829–869. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque III, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition metal complexes and photodynamic therapy from a tumor-centered approach: Challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev. 2018, 119, 797–828. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Jin, S.; Muhammad, N.; Guo, Z. Stimuli-responsive therapeutic metallodrugs. Chem. Rev. 2018, 119, 1138–1192. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef] [PubMed]
- Köpf-Maier, P.; Köpf, H.; Neuse, E. Ferricenium complexes: A new type of water-soluble antitumor agent. J. Cancer Res. Clin. Oncol. 1984, 108, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Bouché, M.; Hognon, C.; Grandemange, S.; Monari, A.; Gros, P.C. Recent advances in iron-complexes as drug candidates for cancer therapy: Reactivity, mechanism of action and metabolites. Dalton Trans. 2020, 49, 11451–11466. [Google Scholar] [CrossRef]
- Wani, W.A.; Baig, U.; Shreaz, S.; Shiekh, R.A.; Iqbal, P.F.; Jameel, E.; Ahmad, A.; Mohd-Setapar, S.H.; Mushtaque, M.; Hun, L.T. Recent advances in iron complexes as potential anticancer agents. New J. Chem. 2016, 40, 1063–1090. [Google Scholar] [CrossRef]
- Jung, M.; Mertens, C.; Tomat, E.; Brüne, B. Iron as a central player and promising target in cancer progression. Int. J. Mol. Sci. 2019, 20, 273. [Google Scholar] [CrossRef] [Green Version]
- Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [Green Version]
- Mojžišová, G.; Mojžiš, J.; Vašková, J. Organometallic iron complexes as potential cancer therapeutics. Acta Biochim. Pol. 2014, 61. [Google Scholar] [CrossRef]
- Ponka, P.; Lok, C.N. The transferrin receptor: Role in health and disease. Int. J. Biochem. Cell Biol. 1999, 31, 1111–1137. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Mackenzie, E.L.; Iwasaki, K.; Tsuji, Y. Intracellular iron transport and storage: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 997–1030. [Google Scholar] [CrossRef] [Green Version]
- Steere, A.N.; Byrne, S.L.; Chasteen, N.D.; Mason, A.B. Kinetics of iron release from transferrin bound to the transferrin receptor at endosomal pH. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 326–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Grillo, A.S.; SantaMaria, A.M.; Kafina, M.D.; Cioffi, A.G.; Huston, N.C.; Han, M.; Seo, Y.A.; Yien, Y.Y.; Nardone, C.; Menon, A.V. Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals. Science 2017, 356, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Jordan, I.; Kaplan, J. The mammalian transferrin-independent iron transport system may involve a surface ferrireductase activity. Biochem. J. 1994, 302, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Abeydeera, N.; Yu, B.; Pant, B.D.; Kim, M.-H.; Huang, S.D. Harnessing the toxicity of dysregulated iron uptake for killing Staphylococcus aureus: Reality or mirage? Biomater. Sci. 2022, 10, 474–484. [Google Scholar] [CrossRef]
- Nomiya, K.; Onodera, K.; Tsukagoshi, K.; Shimada, K.; Yoshizawa, A.; Itoyanagi, T.-A.; Sugie, A.; Tsuruta, S.; Sato, R.; Kasuga, N.C. Syntheses, structures and antimicrobial activities of various metal complexes of hinokitiol. Inorg. Chim. Acta 2009, 362, 43–55. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. The early years of 2, 2′-bipyridine—A ligand in its own lifetime. Molecules 2019, 24, 3951. [Google Scholar] [CrossRef] [Green Version]
- Jayawardhana, A.M.; Qiu, Z.; Kempf, S.; Wang, H.; Miterko, M.; Bowers, D.J.; Zheng, Y.-R. Dual-action organoplatinum polymeric nanoparticles overcoming drug resistance in ovarian cancer. Dalton Trans. 2019, 48, 12451–12458. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Gottlieb, E.; Vander Heiden, M.G.; Thompson, C.B. Bcl-xL prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol. Cell. Biol. 2000, 20, 5680–5689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio Protoc. 2019, 9, e3128. [Google Scholar] [CrossRef] [PubMed]
- Held, P. An introduction to reactive oxygen species. In Tech Resources-App Guides. 2012; bioteck. com. Volume 802, pp. 5–9. [Google Scholar]
- Al-Anbaky, Q.; Al-Karakooly, Z.; Kilaparty, S.P.; Agrawal, M.; Albkuri, Y.M.; RanguMagar, A.B.; Ghosh, A.; Ali, N. Cytotoxicity of manganese (III) complex in human breast adenocarcinoma cell line is mediated by the generation of reactive oxygen species followed by mitochondrial damage. Int. J. Toxicol. 2016, 35, 672–682. [Google Scholar] [CrossRef] [PubMed]
| IC50 (µM)/(µg/mL) | A2780 | A2780cis | SKOV-3 | MDA-MB-231 | A549 | |
|---|---|---|---|---|---|---|
| Ovarian Cancer Sensitive to cisplatin | Ovarian Cancer Resistant to cisplatin | Ovarian Cancer | Breast Cancer | Lung Cancer | ||
| Cisplatin | 1.60 ± 0.45 µM | 13.19 ± 1.84 µM | 16.31 ± 3.92 µM | 23.68 ± 1.74 µM | 12.03 ± 1.44 µM | 8.20 |
| T.I. Cisplatin | 9.37 | 1.18 | 0.95 | 0.65 | 1.30 | |
| Fe(hinok)3 | 2.05 ± 0.90 µM (1.20 µg/mL) | 0.92 ± 0.73 µM (0.50 µg/mL) | 1.23 ± 0.01 µM (0.67 µg/mL) | 3.83 ± 0.12 µM (2.0 µg/mL) | 1.50 ± 0.32 µM (0.82 µg/mL) | 0.45 |
| T.I. Fe(hinok)3 | 16.67 | 40.00 | 30.78 | 10.20 | 26.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abeydeera, N.; Stilgenbauer, M.; Pant, B.D.; Mudarmah, K.; Dassanayake, T.M.; Zheng, Y.-R.; Huang, S.D. Lipophilic Fe(III)-Complex with Potent Broad-Spectrum Anticancer Activity and Ability to Overcome Pt Resistance in A2780cis Cancer Cells. Molecules 2023, 28, 4917. https://doi.org/10.3390/molecules28134917
Abeydeera N, Stilgenbauer M, Pant BD, Mudarmah K, Dassanayake TM, Zheng Y-R, Huang SD. Lipophilic Fe(III)-Complex with Potent Broad-Spectrum Anticancer Activity and Ability to Overcome Pt Resistance in A2780cis Cancer Cells. Molecules. 2023; 28(13):4917. https://doi.org/10.3390/molecules28134917
Chicago/Turabian StyleAbeydeera, Nalin, Morgan Stilgenbauer, Bishnu D. Pant, Khalil Mudarmah, Thiloka M. Dassanayake, Yao-Rong Zheng, and Songping D. Huang. 2023. "Lipophilic Fe(III)-Complex with Potent Broad-Spectrum Anticancer Activity and Ability to Overcome Pt Resistance in A2780cis Cancer Cells" Molecules 28, no. 13: 4917. https://doi.org/10.3390/molecules28134917
APA StyleAbeydeera, N., Stilgenbauer, M., Pant, B. D., Mudarmah, K., Dassanayake, T. M., Zheng, Y.-R., & Huang, S. D. (2023). Lipophilic Fe(III)-Complex with Potent Broad-Spectrum Anticancer Activity and Ability to Overcome Pt Resistance in A2780cis Cancer Cells. Molecules, 28(13), 4917. https://doi.org/10.3390/molecules28134917






