Widely Targeted Metabolomics Reveals the Effects of Soil on the Metabolites in Dioscorea opposita Thunb.
Abstract
:1. Introduction
2. Results and Discussion
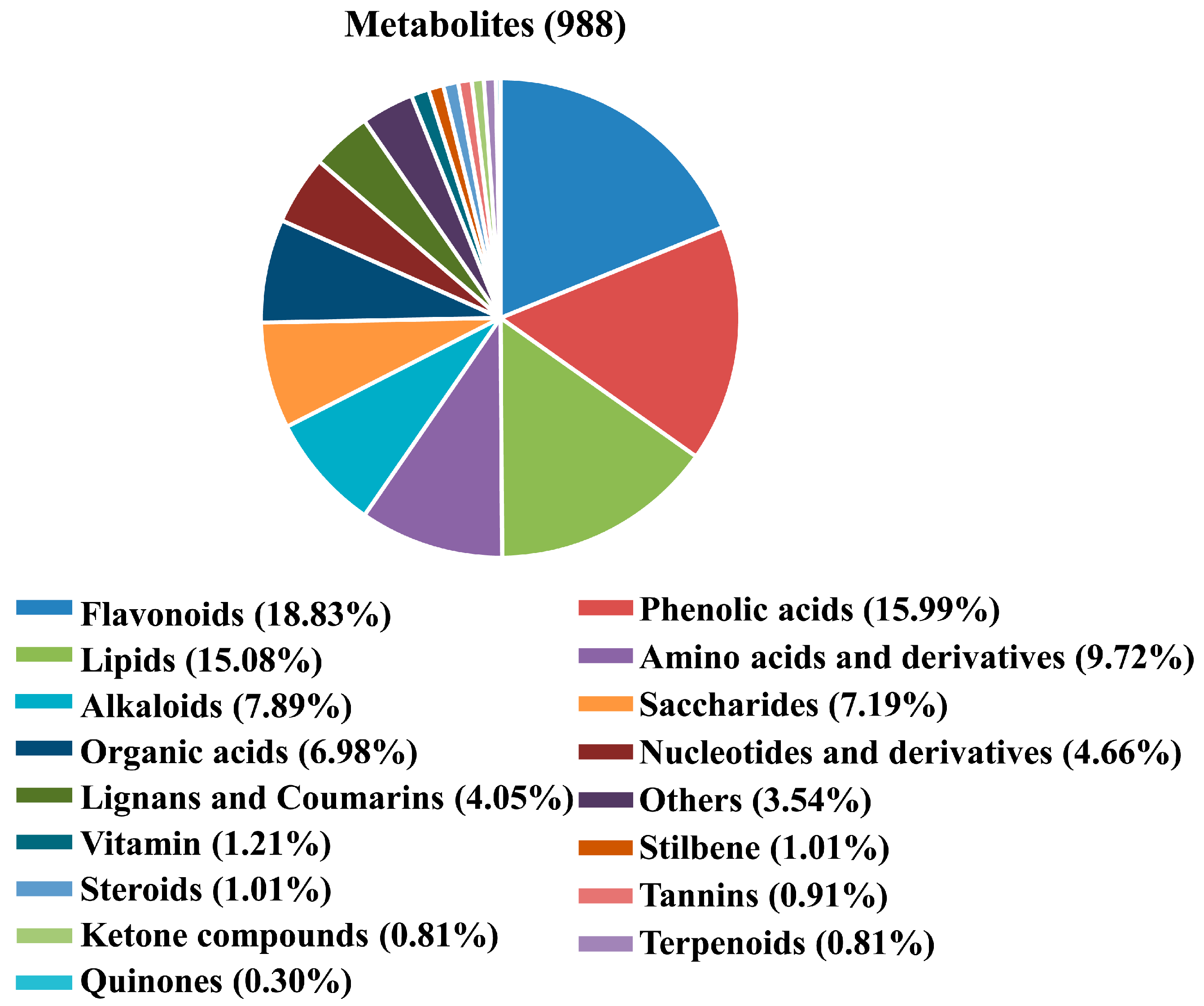
2.1. Widely Targeted Metabolomics Analysis of SCY and LCY
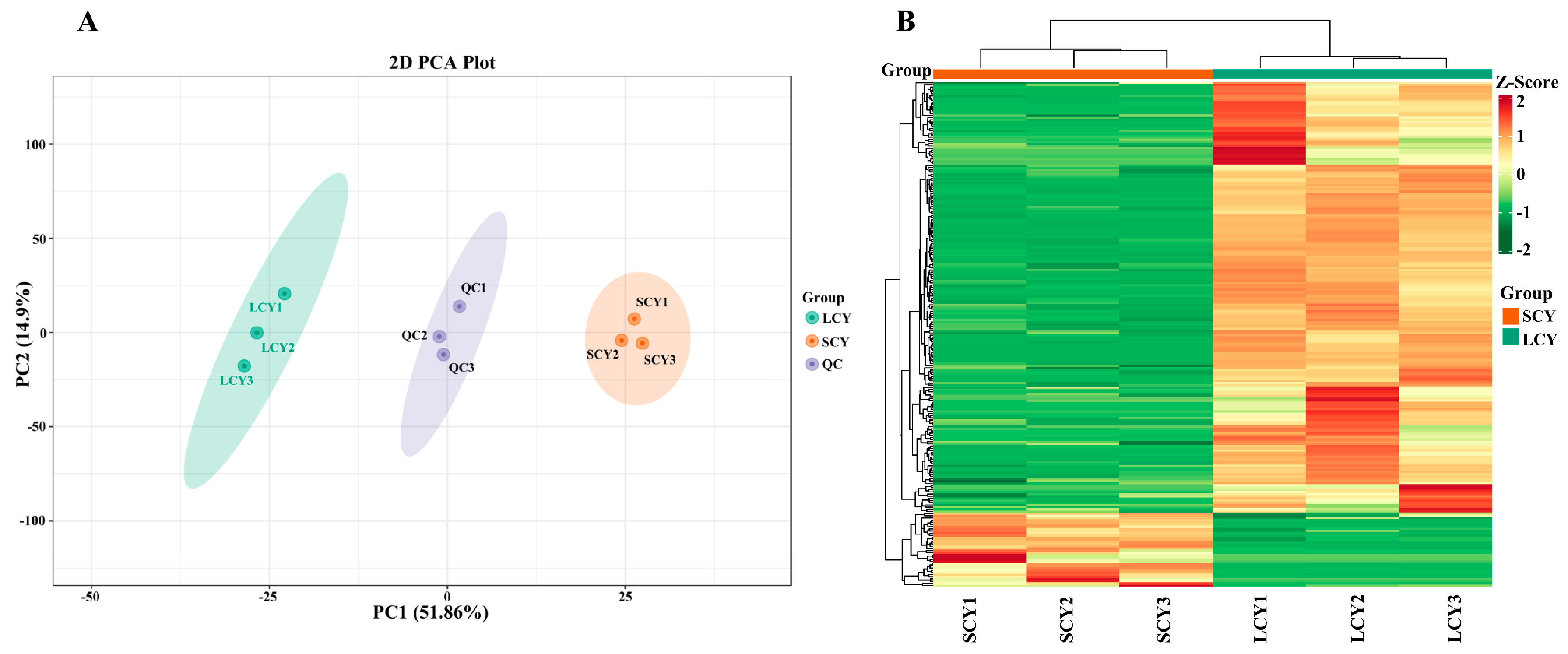
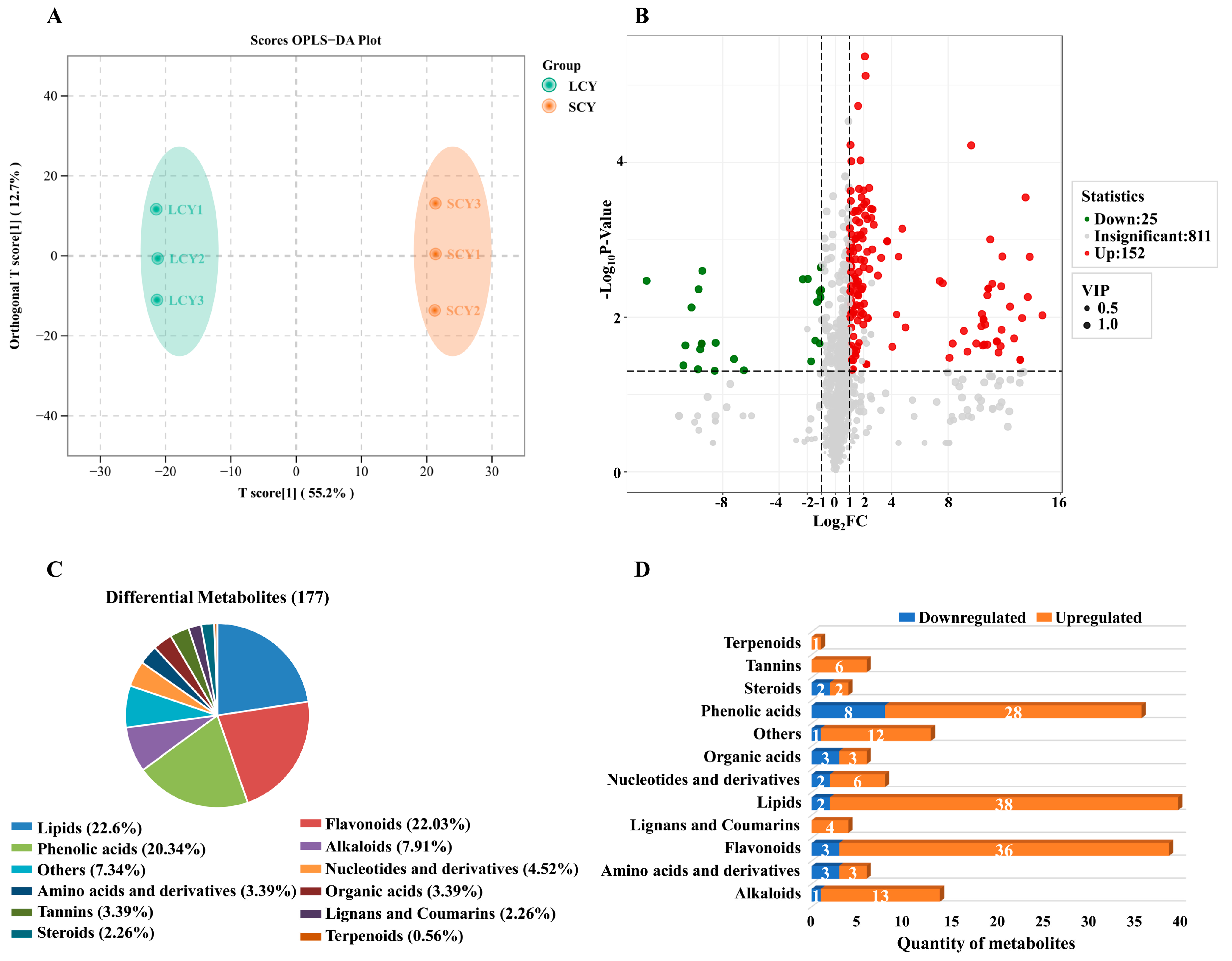
2.2. Multivariate Analysis of Metabolites in SCY and LCY
2.3. Differential Metabolite Analysis between SCY and LCY
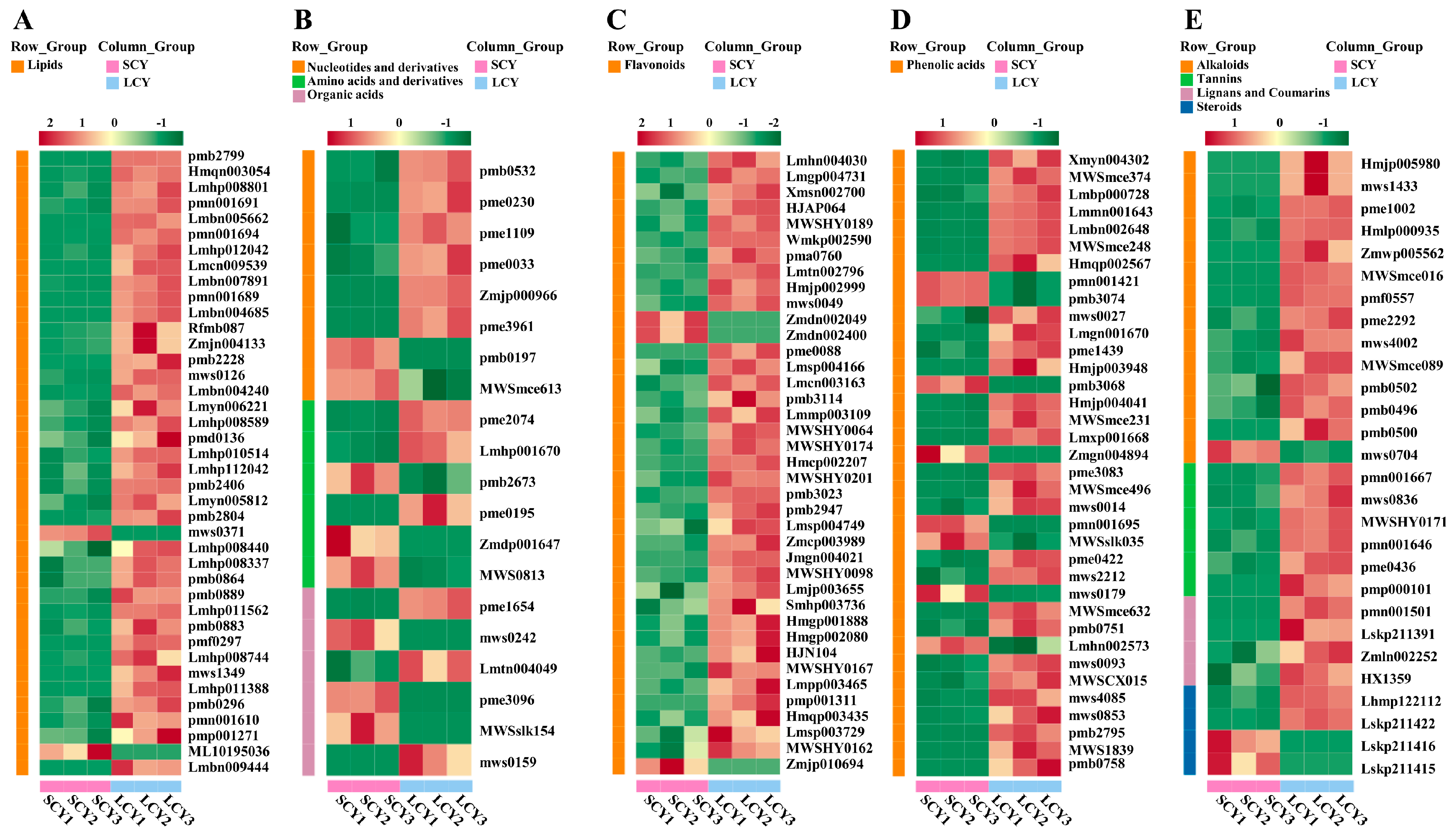
2.3.1. Differences in Primary Metabolites between SCY and LCY
Lipids
Other Differential Primary Metabolites
2.3.2. Differences in Secondary Metabolites between SCY and LCY
Flavonoids
Phenolic Acids
Other Differential Secondary Metabolites
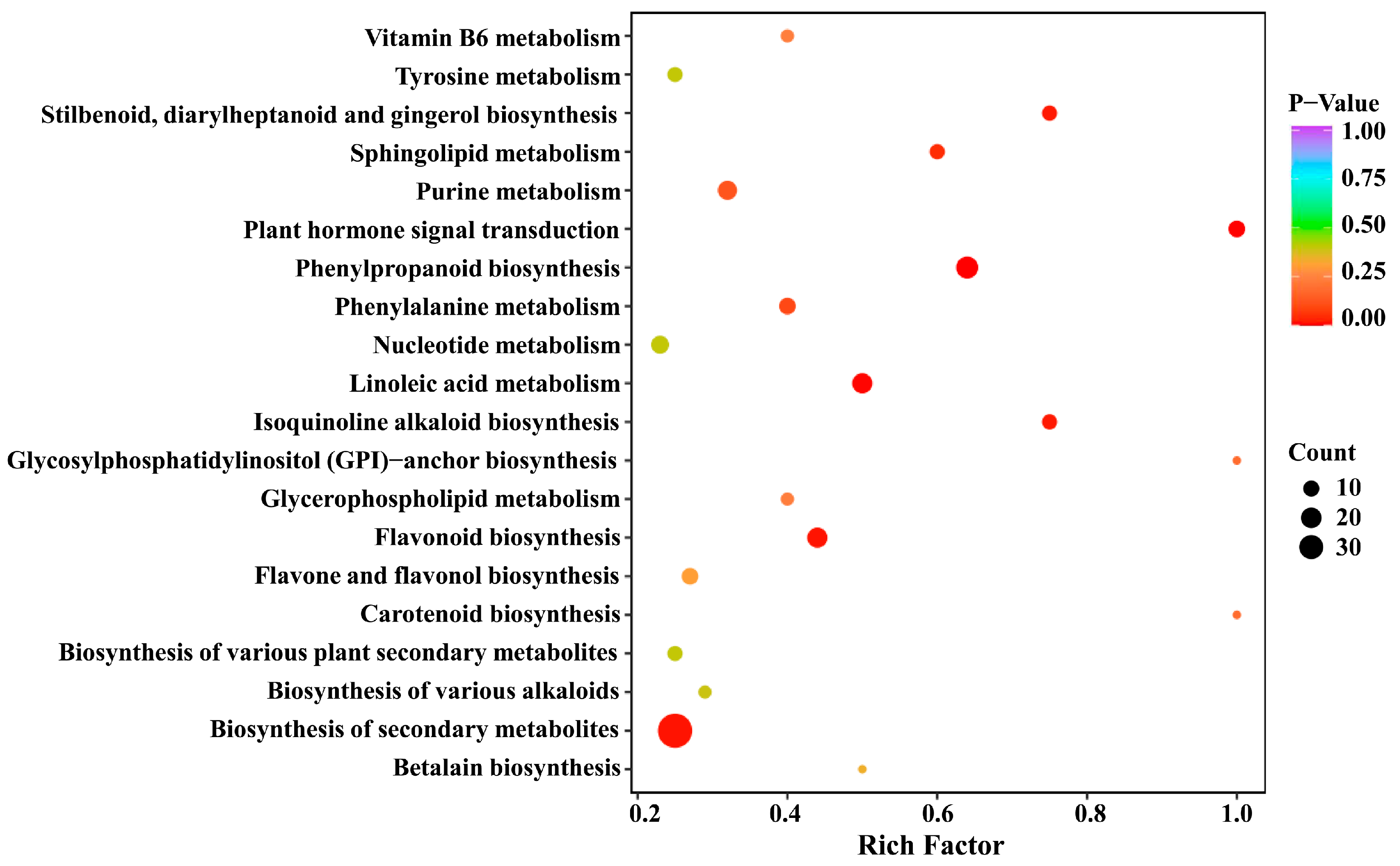
2.4. KEGG Annotation and Enrichment Analysis of Differential Metabolites
3. Materials and Methods
3.1. Sample Preparation and Metabolite Extraction
3.2. UPLC and ESI-Q TRAP-MS/MS Conditions
3.3. Statistical Analysis
3.3.1. Principal Component Analysis
3.3.2. Hierarchical Cluster Analysis
3.3.3. Differential Metabolites Selected
3.3.4. KEGG Annotation and Enrichment Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, P.; Wang, L.; Qian, Y.; Wang, X.; Zhang, S.; Chang, J.; Ruan, Y.; Ma, B. Influences of Extraction Methods on Physicochemical and Functional Characteristics of Three New Bulbil Starches from Dioscorea opposita Thunb. cv. Tiegun. Molecules 2019, 24, 2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, F.; Wang, R.; Zhu, J.; Zhang, Y.; Wang, Y.; Hu, W.; Bell, A.E.; Liu, X. Characterisation comparison of polysaccharides from Dioscorea opposita Thunb. growing in sandy soil, loessial soil and continuous cropping. Int. J. Biol. Macromol. 2019, 126, 776–785. [Google Scholar] [CrossRef]
- An, L.; Yuan, Y.; Ma, J.; Wang, H.; Piao, X.; Ma, J.; Zhang, J.; Zhou, L.; Wu, X. NMR-based metabolomics approach to investigate the distribution characteristics of metabolites in Dioscorea opposita Thunb. cv. Tiegun. Food Chem. 2019, 298, 125063. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhai, Y.; Zhang, Z.; Houa, B.; Liu, Z.; Zhang, B.; Li, J.; Wang, Z.; Sun, Z.; Zhou, J. Liquid chromatography-mass spectrometry-based metabolomics reveals the comprehensive metabolites in Dioscorea opposita Thunb. peel. Sep. Sci. Plus 2023, 6, 2300001. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Ruan, Y.; Wen, C.; Ge, M.; Qian, Y.; Ma, B. Physicochemical properties and biological activities of polysaccharides from the peel of Dioscorea opposita Thunb. extracted by four different methods. Food Sci. Hum. Well. 2023, 12, 130–139. [Google Scholar] [CrossRef]
- Meng, X.; Hu, W.; Wu, S.; Zhu, Z.; Lu, R.; Yang, G.; Qin, C.; Yang, L.; Nie, G. Chinese yam peel enhances the immunity of the common carp (Cyprinus carpio L.) by improving the gut defence barrier and modulating the intestinal microflora. Fish Shellfish Immunol. 2019, 95, 528–537. [Google Scholar] [CrossRef]
- Zhi, F.; Yang, T.L.; Wang, Q.; Jiang, B.; Wang, Z.P.; Zhang, J.; Chen, Y.Z. Isolation, structure and activity of a novel water-soluble polysaccharide from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2019, 133, 1201–1209. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, D.; Huang, L. Metabolome Profiling of Eight Chinese Yam (Dioscorea polystachya Turcz.) Varieties Reveals Metabolite Diversity and Variety Specific Uses. Life 2021, 11, 687. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Lyons, J.B.; Chilaka, C.A. The Dioscorea Genus (Yam)—An Appraisal of Nutritional and Therapeutic Potentials. Foods 2020, 9, 1304. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhang, X.; Peng, H.; Zhu, S.; You, J.; Zhou, T.; Yu, L.; Song, C.; Yang, B. Exploration of habitat-related chemomarkers for Magnoliae officinalis cortex applying both global and water-soluble components-based metabolomics method. Phytomedicine 2022, 98, 153957. [Google Scholar] [CrossRef]
- Lv, W.; Zhao, N.; Zhao, Q.; Huang, S.; Liu, D.; Wang, Z.; Yang, J.; Zhang, X. Discovery and validation of biomarkers for Zhongning goji berries using liquid chromatography mass spectrometry. J. Chromatogr. B 2020, 1142, 122037. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, C.; Li, K.; Wei, T.; Qi, S.; Wang, Y.; Wang, Q. Comparison of Chemical Constituents in Dioscorea polystachya Truczaninow cv. Tiegun Planted in Different Counties by 1H NMR Metabonomics Approach. J. Instrum. Anal. 2022, 41, 774–780. [Google Scholar]
- Zhang, Q.; Li, P.; Liu, X. Comparison of components from Dioscorea opposita Thunb. growing in loessial soil and sandy soil. J. Pract. Tradit. Chin. Med. 2012, 28, 972–973. [Google Scholar]
- Bambina, P.; Spinella, A.; Lo Papa, G.; Chillura Martino, D.F.; Lo Meo, P.; Corona, O.; Cinquanta, L.; Conte, P. (1)H NMR-Based Metabolomics to Assess the Impact of Soil Type on the Chemical Composition of Nero d’Avola Red Wines. J. Agric. Food Chem. 2023, 71, 5823–5835. [Google Scholar] [CrossRef]
- Xue, G.; Su, S.; Yan, P.; Shang, J.; Wang, J.; Yan, C.; Li, J.; Wang, Q.; Xiong, X.; Xu, H. Integrative analyses of widely targeted metabolomic profiling and derivatization-based LC-MS/MS reveals metabolic changes of Zingiberis Rhizoma and its processed products. Food Chem. 2022, 389, 133068. [Google Scholar] [CrossRef]
- Zhang, D.; Shen, D.; Cao, Y.; Duan, X.; Sun, H. Widely targeted metabolomic approach reveals dynamic changes in non-volatile and volatile metabolites of peanuts during roasting. Food Chem. 2023, 412, 135577. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Ma, P.; Yan, Y.; Huang, L.; Li, Y.; Wang, X. Widely targeted metabolomics analysis reveals the differences in nonvolatile compounds of citronella before and after drying. Biomed. Chromatogr. 2023, 37, e5620. [Google Scholar] [CrossRef]
- Hou, Y.; Mao, H.; Lu, F.; Ma, C.; Zhu, S.; Li, G.; Huang, S.; Zhang, Y.; Lv, C.; Xiao, R. Widely targeted metabolomics and HPLC analysis elaborated the quality formation of Yunnan pickled tea during the whole process at an industrial scale. Food Chem. 2023, 422, 135716. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wu, G.; Guo, L.; Hu, F.; Zhou, L.; Xu, B.; Yin, Q.; Chen, Z. Metabolic Profiling and Potential Taste Biomarkers of Two Rambutans during Maturation. Molecules 2023, 28, 1390. [Google Scholar] [CrossRef] [PubMed]
- Nowak, G.; Grant, D.F.; Moran, J.H. Linoleic acid epoxide promotes the maintenance of mitochondrial function and active Na+ transport following hypoxia. Toxicol. Lett. 2004, 147, 161–175. [Google Scholar] [CrossRef]
- Tassakka, A.; Sumule, O.; Massi, M.N.; Sulfahri; Manggau, M.; Iskandar, I.W.; Alam, J.F.; Permana, A.D.; Liao, L.M. Potential bioactive compounds as SARS-CoV-2 inhibitors from extracts of the marine red alga Halymenia durvillei (Rhodophyta)—A computational study. Arab. J. Chem. 2021, 14, 103393. [Google Scholar] [CrossRef]
- Kavitha, A.; Prabhakar, P.; Vijayalakshmi, M.; Venkateswarlu, Y. Production of bioactive metabolites by Nocardia levis MK-VL_113. Lett. Appl. Microbiol. 2009, 49, 484–490. [Google Scholar] [CrossRef]
- Figueiredo, C.R.; Matsuo, A.L.; Massaoka, M.H.; Girola, N.; Azevedo, R.A.; Rabaça, A.N.; Farias, C.F.; Pereira, F.V.; Matias, N.S.; Silva, L.P.; et al. Antitumor activity of kielmeyera coriacea leaf constituents in experimental melanoma, tested in vitro and in vivo in syngeneic mice. Adv. Pharm. Bull. 2014, 4, 429–436. [Google Scholar] [PubMed]
- Pabiś, S.; Kula, J. Synthesis and Bioactivity of (R)-Ricinoleic Acid Derivatives: A Review. Curr. Med. Chem. 2016, 23, 4037–4056. [Google Scholar] [CrossRef]
- Gerlach, N.; Mentel, M.; Köhler, T.; Tuchscherer, B.; Garbe, B.; Ülker, J.; Tronnier, H.; Heinrich, U.; Farwick, M. Effect of the multifunctional cosmetic ingredient sphinganine on hair loss in males and females with diffuse hair reduction. Clin. Cosmet. Investig. Dermatol. 2016, 9, 191–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamura, T.; Narumi, K.; Ohata, T.; Satoh, H.; Mori, T.; Furugen, A.; Kobayashi, M.; Iseki, K. Characterization of deoxyribonucleoside transport mediated by concentrative nucleoside transporters. Biochem. Biophys. Res. Commun. 2021, 558, 120–125. [Google Scholar] [CrossRef]
- Iordache, A.M.; Nechita, C.; Podea, P.; Șuvar, N.S.; Mesaroṣ, C.; Voica, C.; Bleiziffer, R.; Culea, M. Comparative Amino Acid Profile and Antioxidant Activity in Sixteen Plant Extracts from Transylvania, Romania. Plants 2023, 12, 2183. [Google Scholar] [CrossRef]
- Yang, L.; Chu, Z.; Liu, M.; Zou, Q.; Li, J.; Liu, Q.; Wang, Y.; Wang, T.; Xiang, J.; Wang, B. Amino acid metabolism in immune cells: Essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, Q.; Liu, R. Widely targeted metabolomics analysis reveals the effect of fermentation on the chemical composition of bee pollen. Food Chem. 2022, 375, 131908. [Google Scholar] [CrossRef]
- Inagaki, H.; Miyamoto, K.; Ando, N.; Murakami, K.; Sugisawa, K.; Morita, S.; Yumoto, E.; Teruya, M.; Uchida, K.; Kato, N.; et al. Deciphering OPDA Signaling Components in the Momilactone-Producing Moss Calohypnum plumiforme. Front. Plant Sci. 2021, 12, 688565. [Google Scholar] [CrossRef]
- Wang, W.; Ling, Y.; Deng, L.; Yao, S.; Zeng, K. Effect of L-cysteine treatment to induce postharvest disease resistance of Monilinia fructicola in plum fruits and the possible mechanisms involved. Pestic. Biochem. Physiol. 2023, 191, 105367. [Google Scholar] [CrossRef]
- Macioszek, V.K.; Jęcz, T.; Ciereszko, I.; Kononowicz, A.K. Jasmonic Acid as a Mediator in Plant Response to Necrotrophic Fungi. Cells 2023, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Lievens, L.; Pollier, J.; Goossens, A.; Beyaert, R.; Staal, J. Abscisic Acid as Pathogen Effector and Immune Regulator. Front. Plant Sci. 2017, 8, 587. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.H.; Lee, S.S.; Zhu, Y.M.; Jin, Z.Q.; Wu, F.B.; Qiu, C.W. Comparative Metabolomic Profiling Reveals Key Secondary Metabolites Associated with High Quality and Nutritional Value in Broad Bean (Vicia faba L.). Molecules 2022, 27, 8995. [Google Scholar] [CrossRef]
- Çetinkaya, M.; Baran, Y. Therapeutic Potential of Luteolin on Cancer. Vaccines 2023, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, F.; Chamkha, M.; Sayadi, S. Protective effect of olive leaves phenolic compounds against neurodegenerative disorders: Promising alternative for Alzheimer and Parkinson diseases modulation. Food Chem. Toxicol. 2022, 159, 112752. [Google Scholar] [CrossRef] [PubMed]
- Rameshrad, M.; Razavi, B.M.; Hosseinzadeh, H. Protective effects of green tea and its main constituents against natural and chemical toxins: A comprehensive review. Food Chem. Toxicol. 2017, 100, 115–137. [Google Scholar] [CrossRef]
- Siebert, D.A.; Paganelli, C.J.; Queiroz, G.S.; Alberton, M.D. Anti-inflammatory activity of the epicuticular wax and its isolated compounds catechin and gallocatechin from Eugenia brasiliensis Lam. (Myrtaceae) leaves. Nat. Prod. Res. 2021, 35, 4720–4723. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, D.; Xiao, S.; Zhang, A.; Deng, Y.; Dai, X.; Zhou, Z.; Ji, Z.; Cao, Q. Comparative Metabolomic and Transcriptomic Analyses of Phytochemicals in Two Elite Sweet Potato Cultivars for Table Use. Molecules 2022, 27, 8939. [Google Scholar] [CrossRef]
- Panthiya, L.; Tocharus, J.; Onsa-Ard, A.; Chaichompoo, W.; Suksamrarn, A.; Tocharus, C. Hexahydrocurcumin ameliorates hypertensive and vascular remodeling in L-NAME-induced rats. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2022, 1868, 166317. [Google Scholar] [CrossRef]
- Luo, D.D.; Chen, J.F.; Liu, J.J.; Xie, J.H.; Zhang, Z.B.; Gu, J.Y.; Zhuo, J.Y.; Huang, S.; Su, Z.R.; Sun, Z.H. Tetrahydrocurcumin and octahydrocurcumin, the primary and final hydrogenated metabolites of curcumin, possess superior hepatic-protective effect against acetaminophen-induced liver injury: Role of CYP2E1 and Keap1-Nrf2 pathway. Food Chem. Toxicol. 2019, 123, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, M.; Gu, L.; Yi, W.; Lin, J.; Zhang, L.; Wang, Q.; Qi, Y.; Diao, W.; Chi, M.; et al. The therapeutic role and mechanism of 4-Methoxycinnamic acid in fungal keratitis. Int. Immunopharmacol. 2023, 116, 109782. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Bae, H.J.; Park, K.; Bae, H.J.; Yang, X.; Cho, Y.J.; Jung, S.Y.; Jang, D.S.; Ryu, J.H. 4-Methoxycinnamic acid attenuates schizophrenia-like behaviors induced by MK-801 in mice. J. Ethnopharmacol. 2022, 285, 114864. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Xu, T.Q.; Xu, W.; Zhang, H.X.; Liang, Q.P.; Zhou, G.X. Lyciyunin, a new dimer of feruloyltyramine and five bioactive tyramines from the root of Lycium yunnanense Kuang. Nat. Prod. Res. 2021, 35, 447–454. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Fan, M.; Hu, G.; Guo, M. Potential Antioxidative and Anti-Hyperuricemic Components Targeting Superoxide Dismutase and Xanthine Oxidase Explored from Polygonatum Sibiricum Red. Antioxidants 2022, 11, 1651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, R.; Wang, J. Protective effects of fargesin on cadmium-induced lung injury through regulating aryl hydrocarbon receptor. J. Biochem. Mol. Toxicol. 2022, 36, e23197. [Google Scholar] [CrossRef]
- Lee, W.; Song, G.; Bae, H. Matairesinol Induces Mitochondrial Dysfunction and Exerts Synergistic Anticancer Effects with 5-Fluorouracil in Pancreatic Cancer Cells. Mar. Drugs 2022, 20, 473. [Google Scholar] [CrossRef]
| Compounds | VIP | p-Value | Fold_Change | Type |
|---|---|---|---|---|
| Lipids | ||||
| 12,13-Epoxy-9-octadecenoic acid | 1.35 | 0.00 | 804.07 | Up |
| E,E,Z-1,3,12-nonadecatriene-5,14-diol | 1.35 | 0.01 | 1592.59 | Up |
| 9-Hydroxy-12-oxo-15(Z)-octadecenoic acid | 1.35 | 0.00 | 1859.26 | Up |
| 5S,8R-DiHODE | 1.35 | 0.00 | 1859.26 | Up |
| 1-Eicosanol | 1.35 | 0.00 | 3507.41 | Up |
| Ricinoleic acid | 1.35 | 0.01 | 3574.07 | Up |
| LysoPC 19:0 | 1.35 | 0.02 | 1025.19 | Up |
| Dihydrosphingosine | 1.35 | 0.02 | 1770.37 | Up |
| Nucleotides and derivatives | ||||
| Inosine 5′-monophosphate | 1.33 | 0.00 | 2.11 | Up |
| Adenosine | 1.34 | 0.01 | 4.75 | Up |
| Guanine | 1.31 | 0.00 | 2.45 | Up |
| Hypoxanthine | 1.31 | 0.00 | 2.48 | Up |
| Vidarabine | 1.35 | 0.00 | 4.80 | Up |
| 2′-Deoxyadenosine | 1.35 | 0.01 | 1777.78 | Up |
| Amino acids and derivatives | ||||
| Jasmonoyl-L-isoleucine | 1.32 | 0.00 | 26.92 | Up |
| L-valyl-L-leucine | 1.33 | 0.00 | 2.96 | Up |
| L-cysteine | 1.34 | 0.02 | 1422.22 | Up |
| 6-Hydroxydopaquinone | 1.27 | 0.00 | 0.46 | Down |
| γ-Glutamyl-L-valine | 1.34 | 0.05 | 0.00 | Down |
| 5-Oxoproline | 1.33 | 0.01 | 0.48 | Down |
| Organic acids | ||||
| Jasmonic acid | 1.34 | 0.00 | 8.13 | Up |
| Abscisic acid | 1.28 | 0.01 | 2.16 | Up |
| Phenylpyruvic acid | 1.35 | 0.03 | 3077.78 | Up |
| Suberic acid | 1.34 | 0.02 | 0.00 | Down |
| Aminomalonic acid | 1.35 | 0.00 | 0.00 | Down |
| Triethyl citrate | 1.34 | 0.03 | 0.00 | Down |
| Flavonoids | ||||
| Luteolin (5,7,3′,4′-tetrahydroxyflavone) | 1.35 | 0.01 | 1356.30 | Up |
| Apigenin-7-O-glucoside (cosmosiin) | 1.32 | 0.00 | 2.13 | Up |
| Catechin | 1.31 | 0.00 | 2.16 | Up |
| Epicatechin | 1.34 | 0.00 | 3.08 | Up |
| Gallocatechin | 1.30 | 0.00 | 4.59 | Up |
| Epigallocatechin | 1.33 | 0.00 | 2.51 | Up |
| Phenolic acids | ||||
| Hexahydrocurcumin | 1.35 | 0.01 | 13,000.00 | Up |
| 4-Hydroxycinnamic acid p-hydroxyphenethylamine | 1.35 | 0.02 | 2829.63 | Up |
| Salicylic acid | 1.35 | 0.02 | 6622.22 | Up |
| Dihydrodemethoxy curcumin | 1.34 | 0.02 | 1511.11 | Up |
| 2-Methoxy-4-ethenylphenol | 1.35 | 0.00 | 14,259.26 | Up |
| Vanillin acetate | 1.35 | 0.00 | 2262.96 | Up |
| Anisic acid-O-feruloyl glucoside | 1.34 | 0.03 | 666.67 | Up |
| Ethylparaben | 1.34 | 0.02 | 559.63 | Up |
| 4-Methoxycinnamic acid | 1.35 | 0.00 | 170.74 | Up |
| Octahydrocurcumin | 1.33 | 0.01 | 31.55 | Up |
| Trans-5-O-(p-coumaroyl) shikimate | 1.11 | 0.00 | 22.50 | Up |
| Sinapyl alcohol | 1.32 | 0.02 | 16.51 | Up |
| Alkaloids | ||||
| N-Cis-feruloyltyramine | 1.35 | 0.04 | 8966.67 | Up |
| N-feruloyltyramine | 1.35 | 0.04 | 8966.67 | Up |
| cis-N-p-coumaroyltyramine | 1.35 | 0.02 | 2829.63 | Up |
| Nicotinic acid methyl ester (methyl nicotinate) | 1.35 | 0.00 | 2051.85 | Up |
| N-p-coumaroyl-N’-feruloylputrescine | 1.34 | 0.02 | 322.22 | Up |
| Lignans and coumarins | ||||
| Fargesin | 1.35 | 0.00 | 198.15 | Up |
| Matairesinol (ISO2) | 1.35 | 0.02 | 3392.59 | Up |
| Steroids | ||||
| △5-Pregnene-3β,17α,20(S)-triol glucoside | 1.33 | 0.00 | 2.20 | Up |
| Sileneoside C | 1.34 | 0.00 | 3.98 | Up |
| Nusilsterone | 1.34 | 0.02 | 0.00 | Down |
| 26-Hydroxyintegristerone A (ISO1) | 1.34 | 0.03 | 0.01 | Down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhai, Y.; Zhang, Z.; Liu, Z.; Hou, B.; Zhang, B.; Wang, Z. Widely Targeted Metabolomics Reveals the Effects of Soil on the Metabolites in Dioscorea opposita Thunb. Molecules 2023, 28, 4925. https://doi.org/10.3390/molecules28134925
Yang L, Zhai Y, Zhang Z, Liu Z, Hou B, Zhang B, Wang Z. Widely Targeted Metabolomics Reveals the Effects of Soil on the Metabolites in Dioscorea opposita Thunb. Molecules. 2023; 28(13):4925. https://doi.org/10.3390/molecules28134925
Chicago/Turabian StyleYang, Lanping, Yangyang Zhai, Zhenzhen Zhang, Zhenzhen Liu, Baohua Hou, Baobao Zhang, and Zhenhui Wang. 2023. "Widely Targeted Metabolomics Reveals the Effects of Soil on the Metabolites in Dioscorea opposita Thunb." Molecules 28, no. 13: 4925. https://doi.org/10.3390/molecules28134925
APA StyleYang, L., Zhai, Y., Zhang, Z., Liu, Z., Hou, B., Zhang, B., & Wang, Z. (2023). Widely Targeted Metabolomics Reveals the Effects of Soil on the Metabolites in Dioscorea opposita Thunb. Molecules, 28(13), 4925. https://doi.org/10.3390/molecules28134925





